Control of the Organization of 4,4′-bis(carbazole)-1,1′-biphenyl (CBP) Molecular Materials through Siloxane Functionalization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis
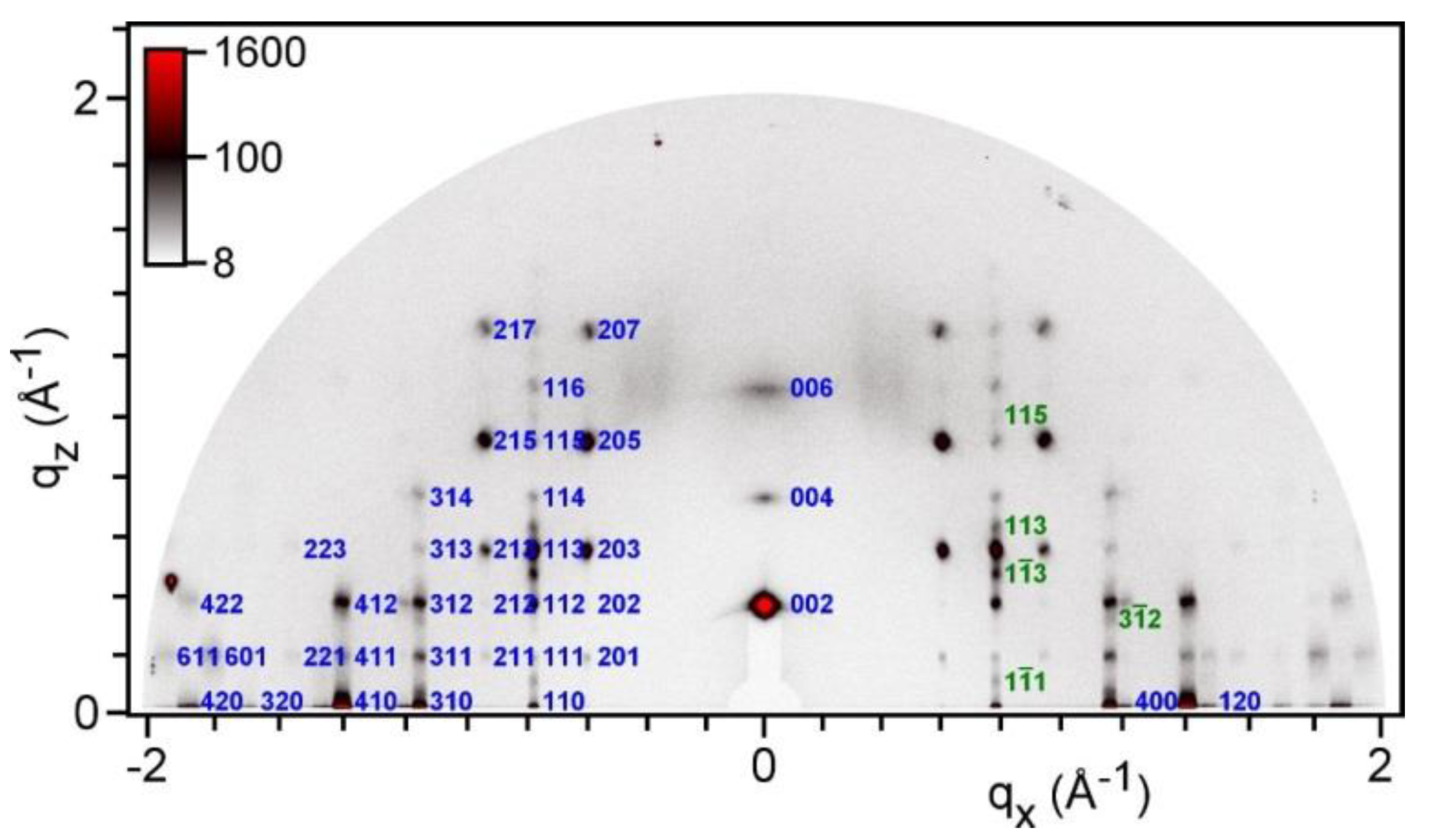
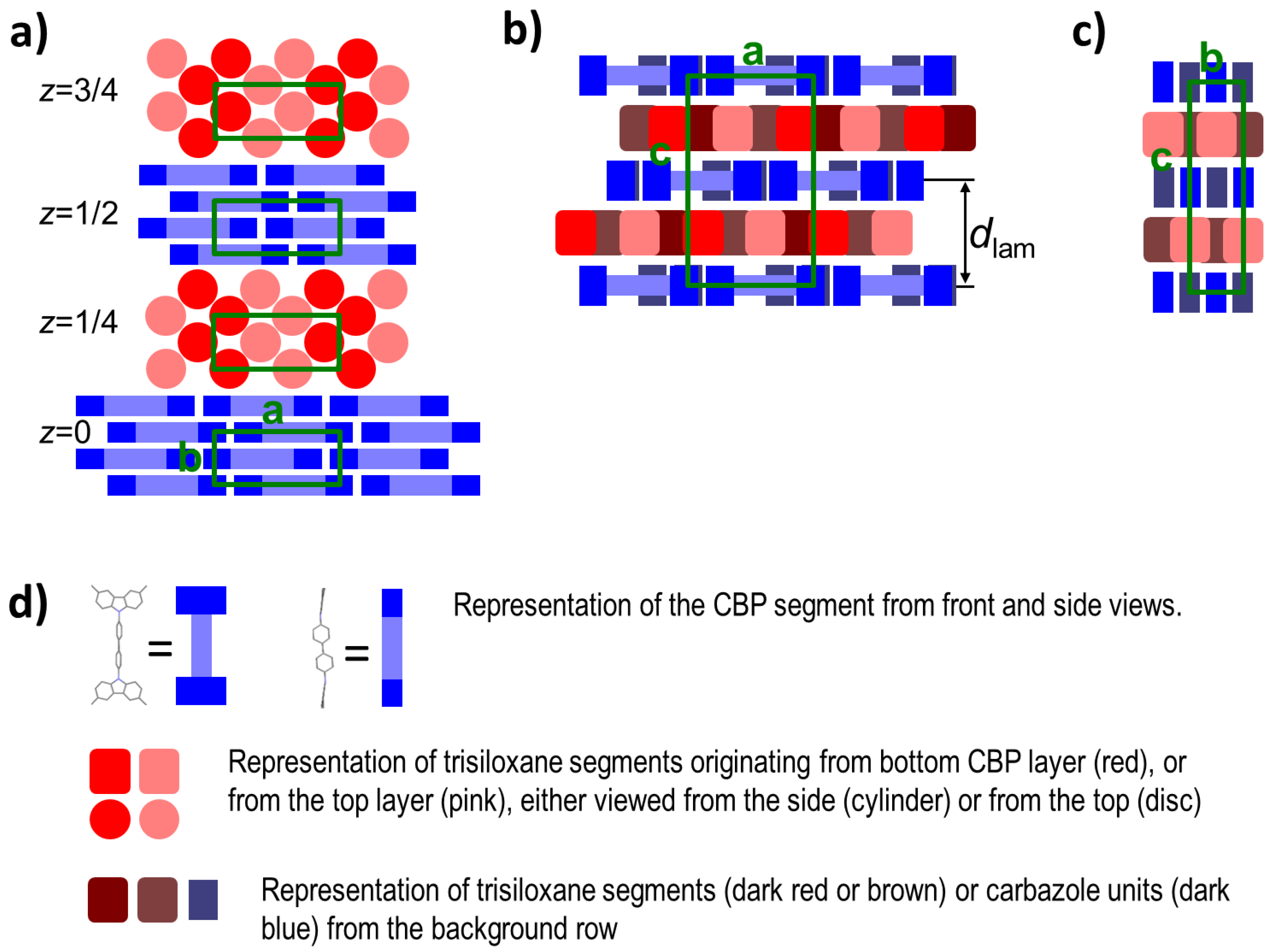
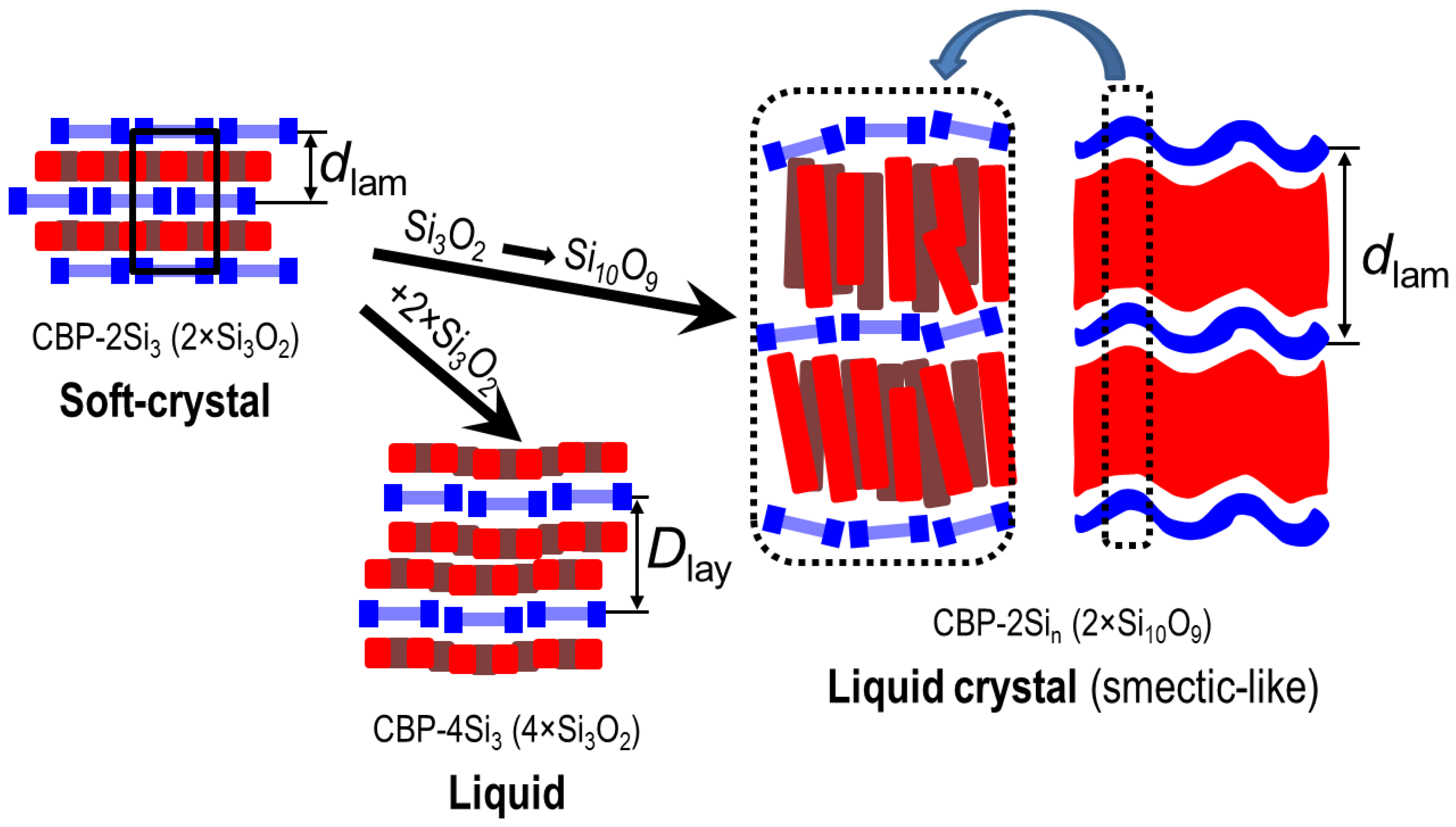
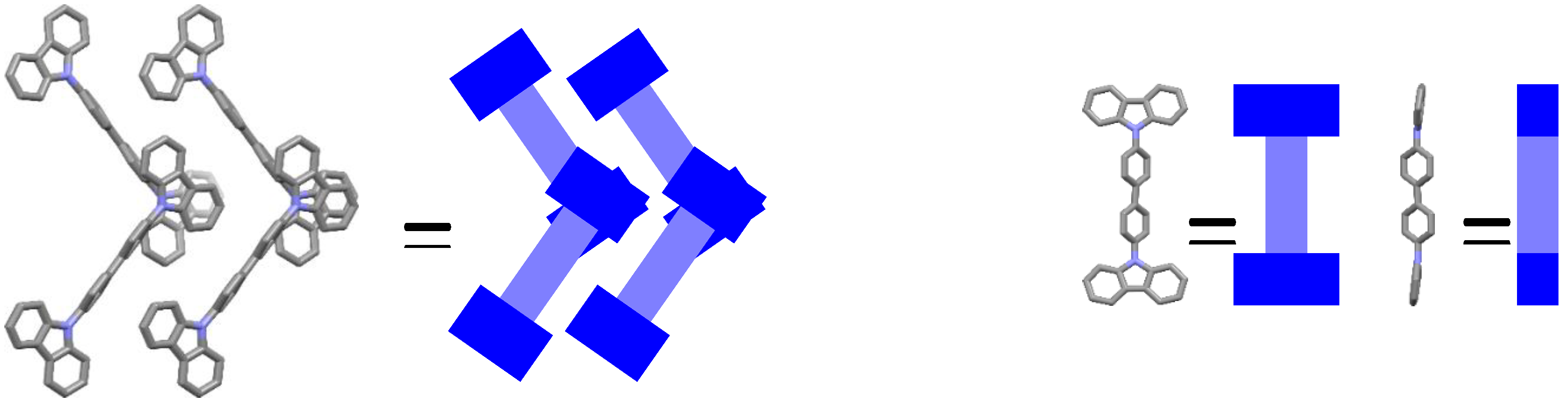
2.2. Thermal and Structural Properties
2.3. Photophysical Properties in Solution
2.4. Photophysical and Charge Transport Properties in Thin Films
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Diao, Y.; Appleton, A.L.; Fang, L.; Bao, Z.J. Integrated Materials Design of Organic Semiconductors for Field-Effect Transistors. Am. Chem. Soc. 2013, 135, 6724–6746. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Tothadi, S.; Desiraju, G.R. Halogen Bonds in Crystal Engineering: Like Hydrogen Bonds yet Different. Acc. Chem. Res. 2014, 47, 2514–2524. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.-F.; Wang, F.-Y.; Pei, J. Control of π–π Stacking via Crystal Engineering in Organic Conjugated Small Molecule Crystals. Cryst Growth Des. 2018, 18, 7–15. [Google Scholar] [CrossRef]
- Lu, F.; Nakanashi, T. Alkyl-π engineering in state control toward versatile optoelectronic soft materials. Sci. Technol. Adv. Mater. 2015, 16, 014805. [Google Scholar] [CrossRef]
- Mei, J.; Bao, Z. Side Chain Engineering in Solution-Processable Conjugated Polymers. Chem. Mater. 2014, 26, 604–615. [Google Scholar] [CrossRef]
- Tschierske, C. Liquid crystal engineering—New complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970. [Google Scholar] [CrossRef]
- Meng, B.; Liu, J.; Wang, L. Oligo(ethylene glycol) as side chains of conjugated polymers for optoelectronic applications. Polym. Chem. 2020, 11, 1261–1270. [Google Scholar] [CrossRef]
- Ren, Z.; Yan, S. Polysiloxanes for optoelectronic applications. Prog. Mater. Sci. 2016, 83, 383–416. [Google Scholar]
- Mark, J.E. Some Interesting Things about Polysiloxanes. Acc. Chem. Res. 2004, 37, 946–953. [Google Scholar] [CrossRef]
- Dvornik, R.P. Silicon-Containing Polymers: The Science and Technology of Their Synthesis and Applications; Jones, R.J., Ando, W., Chojnowski, J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2000; Chapter 7. [Google Scholar]
- Clarson, S.J.; Dodgson, K.; Semlyen, J.A. Studies of cyclic and linear poly(dimethylsiloxanes): 19. Glass transition temperatures and crystallization behavior. Polymer 1985, 26, 930–934. [Google Scholar] [CrossRef]
- Polymer Data Handbook, 4th ed.; Brandrup, J.; Immergut, E.H.; Grulke, E.A. (Eds.) John Wiley & Sons: New York, NY, USA, 1999; Chapter VI. [Google Scholar]
- Pouget, E.; Tonnar, J.; Lucas, P.; Lacroix-Desmazes, P.; Ganachaud, F.; Boutevin, B. Well-Architectured Poly(dimethylsiloxane)-Containing Copolymers Obtained by Radical Chemistry. Chem. Rev. 2010, 110, 1233–1277. [Google Scholar] [CrossRef] [PubMed]
- Kutsumizu, S.; Kawafuchi, A.; Yamamura, Y.; Udagawa, T.; Otaki, T.; Masuda, M.; Miwa, Y.; Saito, K. Stabilization of Bicontinuous Cubic Phase and Its Two-Sided Nature Produced by Use of Siloxane Tails and Introduction of Molecular Nonsymmetry. Chem. Eur. J. 2021, 27, 10293–10302. [Google Scholar] [CrossRef] [PubMed]
- Guillon, D.; Osipov, M.A.; Méry, S.; Siffert, M.; Nicoud, J.-F.; Bourgogne, C.; Sebastião, P.J. Synclinic-anticlinic phase transition in tilted organosiloxane liquid crystals. Mat. Chem. A 2011, 11, 2700–2708. [Google Scholar] [CrossRef]
- Kamatham, N.; Ibraikulov, O.-A.; Durand, P.; Wang, J.; Boyron, O.; Heinrich, B.; Heiser, T.; Lévêque, P.; Leclerc, N.; Méry, S. On the Impact of Linear Siloxanated Side Chains on the Molecular Self-Assembling and Charge Transport Properties of Conjugated Polymers. Adv. Funct. Mater. 2020, 30, 2007734. [Google Scholar] [CrossRef]
- Kamino, B.A.; Bender, T.P. The use of siloxanes, silsesquioxanes, and silicones in organic semiconducting materials. Chem. Soc. Rev. 2013, 42, 5119–5130. [Google Scholar] [CrossRef]
- Sun, D.; Ren, Z.; Bryce, M.R.; Yan, S. Arylsilanes and siloxanes as optoelectronic materials for organic light-emitting diodes (OLEDs). J. Mat. Chem. C 2015, 3, 9496–9508. [Google Scholar] [CrossRef] [Green Version]
- Funahashi, M.; Yamaoka, M.; Takenami, K.; Sonoda, A. Liquid-crystalline perylene tetracarboxylic bisimide derivatives bearing cyclotetrasiloxane moieties. J. Mat. Chem. C 2013, 1, 7872–7878. [Google Scholar] [CrossRef]
- Roland, T.; Léonard, J.; Hernandez Ramirez, G.; Méry, S.; Yurchenko, O.; Ludwigs, S.; Haacke, S. Sub-100 fs charge transfer in a novel donor-acceptor-donor triad organized in a smectic film. PCCP 2012, 14, 273–279. [Google Scholar] [CrossRef]
- Polkehn, M.; Tamura, H.; Eisenbrandt, P.; Haacke, S.; Méry, S.; Burghardt, I. Molecular Packing Determines Charge Separation in a Liquid Crystalline Bisthiophene–Perylene Diimide Donor–Acceptor Material. J. Phys. Chem. Lett. 2016, 7, 1327–1334. [Google Scholar] [CrossRef]
- Mei, J.; Kim, D.H.; Ayzner, A.L.; Toney, M.F.; Bao, Z. Siloxane-Terminated Solubilizing Side Chains: Bringing Conjugated Polymer Backbones Closer and Boosting Hole Mobilities in Thin-Film Transistors. J. Am. Chem. Soc. 2011, 133, 20130–20133. [Google Scholar] [CrossRef]
- Han, A.-R.; Lee, J.; Lee, H.R.; Lee, J.; Kang, S.-H.; Ahn, H.; Shin, T.J.; Oh, J.H.; Yang, C. Siloxane Side Chains: A Universal Tool for Practical Applications of Organic Field-Effect Transistors. Macromolecules 2016, 49, 3739–3748. [Google Scholar] [CrossRef]
- Zhao, F.; Yuan, Y.; Ding, Y.; Wang, Y.; Wang, X.; Zhang, G.; Gu, X.; Qiu, L. Taming Charge Transport and Mechanical Properties of Conjugated Polymers with Linear Siloxane Side Chains. Macromolecules 2021, 54, 5440–5450. [Google Scholar] [CrossRef]
- Plint, T.G.; Kamino, B.A.; Bender, T.P. Charge Carrier Mobility of Siliconized Liquid Triarylamine Organic Semiconductors by Time-of-Flight Spectroscopy. J. Phys. Chem. C 2015, 119, 1676–1682. [Google Scholar] [CrossRef]
- Ribierre, J.-C.; Bao, L.; Inoue, M.; Schwartz, P.-O.; Kim, J.-H.; Yoshida, K.; Sandanayaka, A.S.D.; Nakanotani, H.; Mager, L.; Méry, S.; et al. Low threshold amplified spontaneous emission and ambipolar charge transport in non-volatile liquid fluorene derivatives. Chem. Commun. 2016, 52, 3103–3106. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Nakanishi, T. Frontiers of solvent-free functional molecular liquids. Chem. Commun. 2017, 53, 10344. [Google Scholar] [CrossRef] [PubMed]
- Lu, F.; Nakanishi, T. Solvent-Free Luminous Molecular Liquids. Adv. Opt. Mater. 2019, 7, 1900176. [Google Scholar] [CrossRef]
- Ribierre, J.-C.; Mizuno, J.; Hattori, R.; Adachi, C. Organic Light Emitting Diodes with Liquid Emitters in Functional Organic Liquids; Nakanishi, T., Ed.; Wiley-VCH: Weinheim, Germany, 2019; Chapter 8. [Google Scholar]
- Wong, M.Y.; Zysman-Colman, E. Purely Organic Thermally Activated Delayed Fluorescence Materials for Organic Light-Emitting Diodes. Adv. Mater. 2017, 29, 1605444. [Google Scholar] [CrossRef] [Green Version]
- Uoyama, H.; Goushi, K.; Shizu, K.; Nomura, H.; Adachi, C. Highly efficient organic light-emitting diodes from delayed fluorescence. Nature 2012, 492, 234. [Google Scholar] [CrossRef]
- Fukagawa, H.; Shimizu, T.; Kamada, T.; Yui, S.; Hasegawa, M.; Morii, K.; Yamamoto, T. Highly efficient and stable organic light-emitting diodes with a greatly reduced amount of phosphorescent emitter. Sci. Rep. 2015, 5, 9855. [Google Scholar] [CrossRef] [Green Version]
- Yamada, T.; Suzuki, F.; Goto, A.; Sato, T.; Tanaka, K.; Kaji, H. Revealing bipolar charge-transport property of 4,4’-N,N’-dicarbazolylbiphenyl (CBP) by quantum chemical calculations. Org. Electron. 2011, 12, 169–178. [Google Scholar] [CrossRef]
- Schrögel, P.; Tomkevičiene, A.; Strohriegl, P.; Hoffmann, S.T.; Köhler, A.; Lennartz, C. A series of CBP-derivatives as host materials for blue phosphorescent organic light-emitting diodes. J. Mat. Chem. 2011, 21, 2266–2273. [Google Scholar] [CrossRef]
- Babu, S.S.; Hollamby, M.J.; Aimi, J.; Ozawa, H.; Saeki, A.; Seki, S.; Kobayashi, K.; Hagiwara, K.; Yoshizawa, M.; Möhwald, H.; et al. Nonvolatile liquid anthracenes for facile full-colour luminescence tuning at single blue-light excitation. Nature Commun. 2013, 4, 1969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasahara, T.; Matsunami, S.; Edura, T.; Oshima, J.; Adachi, C.; Shoji, S.; Mizuno, J. Fabrication and performance evaluation of microfluidic organic light emitting diode. Sens. Actuators A 2013, 195, 219–223. [Google Scholar] [CrossRef]
- Shaya, J.; Correia, G.; Heinrich, B.; Ribierre, J.-C.; Polychronopoulou, K.; Mager, L.; Méry, S. Functionalization of Biphenylcarbazole (CBP) with Siloxane-Hybrid Chains for Solvent-Free Liquid Materials. Molecules 2022, 27, 89. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C. Micro-segregation, molecular shape and molecular topology—Partners of the design of liquid crystalline materials with complex mesophase morphologies. J. Mater. Chem. 2001, 11, 2647–2671. [Google Scholar] [CrossRef]
- Da Rosa, R.R.; Brose, I.S.; Vilela, G.D.; Merlo, A.A. 3,5-diarylisoxazoles: A New Entry to Soft Crystal Phase. Mol. Cryst. Liq. Cryst. 2015, 612, 158–168. [Google Scholar] [CrossRef]
- Mandle, R.J.; Goodby, J.W. Intercalated soft-crystalline mesophase exhibited by an unsymmetrical twist-bend nematogen. CrystEngComm 2016, 18, 8794–8802. [Google Scholar] [CrossRef] [Green Version]
- Kaafarani, A.O.; El-Ballouli, R.; Trattnig, A.; Fonari Sax, S.; Wex, B.; Risko, C.; Khnayzer, R.S.; Barlow, S.; Patra, D.; Timofeeva, T.V.; et al. Bis(carbazolyl) derivatives of pyrene and tetrahydropyrene: Synthesis, structures, optical properties, electrochemistry, and electroluminescence. J. Mater. Chem. C 2013, 1, 1638–1650. [Google Scholar] [CrossRef] [Green Version]
- Xiao, y.; Su, X.; Sosa-Vargas, L.; Lacaze, E.; Heinrich, B.; Donnio, B.; Kreher, D.; Mathevet, F.; Attias, A.-J. Chemical engineering of donor–acceptor liquid crystalline dyads and triads for the controlled nanostructuration of organic semiconductors. CrystEngComm 2016, 18, 4787–4798. [Google Scholar] [CrossRef]
- Pieper, P.; Russo, V.; Heinrich, B.; Donnio, D.; Deschenaux, R. Liquid-Crystalline Tris[60]fullerodendrimers. J. Org. Chem. 2018, 83, 3208–3219. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Heinrich, B.; Kirscher, G.; Chaumont, A.; Henry, M.; Kyritsakas, N.; Haketa, Y.; Maeda, H.; Mobian, P. Dipyrrolyldiketonato Titanium(IV) Complexes from Monomeric to Multinuclear Architectures: Synthesis, Stability, and Liquid-Crystal Properties. Inorg. Chem. 2020, 59, 12802–12816. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, N.; L’Her, M.; Scrafton, E.; Atoini, Y.; Gentile, G.; Heinrich, B.; Berthiot, R.; Aliprandi, A.; Douce, L. Luminescent Ionic Liquid Crystals Based on Naphthalene-Imidazolium Unit. Eur. J Org. Chem. 2021, 2091–2098. [Google Scholar] [CrossRef]
- Low, P.J.; Paterson, M.A.J.; Yufit, D.S.; Howard, J.A.K.; Cherryman, J.C.; Tackley, D.R.; Brook, R.; Brown, B. Towards an understanding of structure–property relationships in hole-transport materials: The influence of molecular conformation on oxidation potential in poly(aryl)amines. J. Mat. Chem. 2005, 15, 2304–2315. [Google Scholar] [CrossRef]
- Kawakami, Y.; Aoki, T.; Yamashita, Y.; Hirose, M.; Ishitani, A. Surface modification of poly(methyl methacrylate) film by siloxane graft copolymers. Macromolecules 1985, 18, 580–582. [Google Scholar] [CrossRef]
- Houe, Y.; Tulevski, G.S.; Valint, P.L.; Gardella, J.A. Synthesis and Surface Analysis of Siloxane-Containing Amphiphilic Graft Copolymers, Poly(2-hydroxyethyl methacrylate-g-dimethylsiloxane) and Poly(2,3-dihydroxypropyl methacrylate-g-dimethylsiloxane). Macromolecules 2002, 35, 5953–5962. [Google Scholar] [CrossRef]
- Bagnich, S.A.; Rudnick, A.; Schroegel, P.; Strohriegl, P.; Köhler, A. Triplet energies and excimer formation in meta- and para-linked carbazolebiphenyl matrix materials. Phil. Trans. R. Soc. A 2015, 373, 20140446. [Google Scholar] [CrossRef] [Green Version]
- Ab Rani, M.A.; Borduas, N.; Colquhoun, V.; Hanley, R.; Johnson, H.; Larger, S.; Lickiss, P.D.; Llopis-Mestre, V.; Luu, S.; Mogstad, M.; et al. The potential of methylsiloxanes as solvents for synthetic chemistry applications. Green Chem. 2014, 16, 1282–1296. [Google Scholar] [CrossRef] [Green Version]
- Bagnich, S.A.; Athanasopoulos, S.; Rudnick, A.; Schroegel, P.; Bauer, I.; Greenham, N.C.; Strohriegl, P.; Köhler, A. Excimer Formation by Steric Twisting in Carbazole and Triphenylamine-Based Host Materials. .J. Phys. Chem. C 2015, 119, 2380–2899. [Google Scholar] [CrossRef]
- Gong, S.; He, X.; Chen, Y.; Jiang, Z.; Zhong, C.; Ma, D.; Qin, J.; Yan, C. Simple CBP isomers with high triplet energies for highly efficient blue electrophosphorescence. J. Mat. Chem. 2012, 22, 2894–2899. [Google Scholar] [CrossRef]
- Ribierre, J.-C.; Aoyama, T.; Muto, T.; Imase, Y.; Wada, T. Charge transport properties in liquid carbazole. Org. Elect. 2008, 9, 396–400. [Google Scholar] [CrossRef]
- Ribierre, J.-C.; Ruseckas, A.; Gaudin, O.P.M.; Samuel, I.D.W.; Barcena, H.; Staton, S.V.; Burn, P.L. Effects of thermal annealing on the photophysical properties of bisfluorene-cored dendrimer films. Org. Elect. 2009, 10, 803–808. [Google Scholar] [CrossRef]
- Spano, F.C. The Spectral Signatures of Frenkel Polarons in H- and J-Aggregates. Acc. Chem. Res. 2010, 43, 429–439. [Google Scholar] [CrossRef]
- Adachi, C.; Baldo, M.A.; Forrest, S.R. Electroluminescence mechanisms in organic light emitting devices employing a europium chelate doped in a wide energy gap bipolar conducting host. J. Appl. Phys. 2000, 87, 8049. [Google Scholar] [CrossRef]
- Matsushima, H.; Naka, S.; Okada, H.; Onnagawa, H. Organic electrophosphorescent devices with mixed hole transport material as emission layer. Curr. Appl. Phys. 2005, 5, 305–308. [Google Scholar] [CrossRef]
- Matsusue, N.; Suzuki, Y.; Naito, H. Charge Carrier Transport in Neat Thin Films of Phosphorescent Iridium Complexes. Jap. J. Appl. Phys. 2005, 44, 3691. [Google Scholar] [CrossRef]

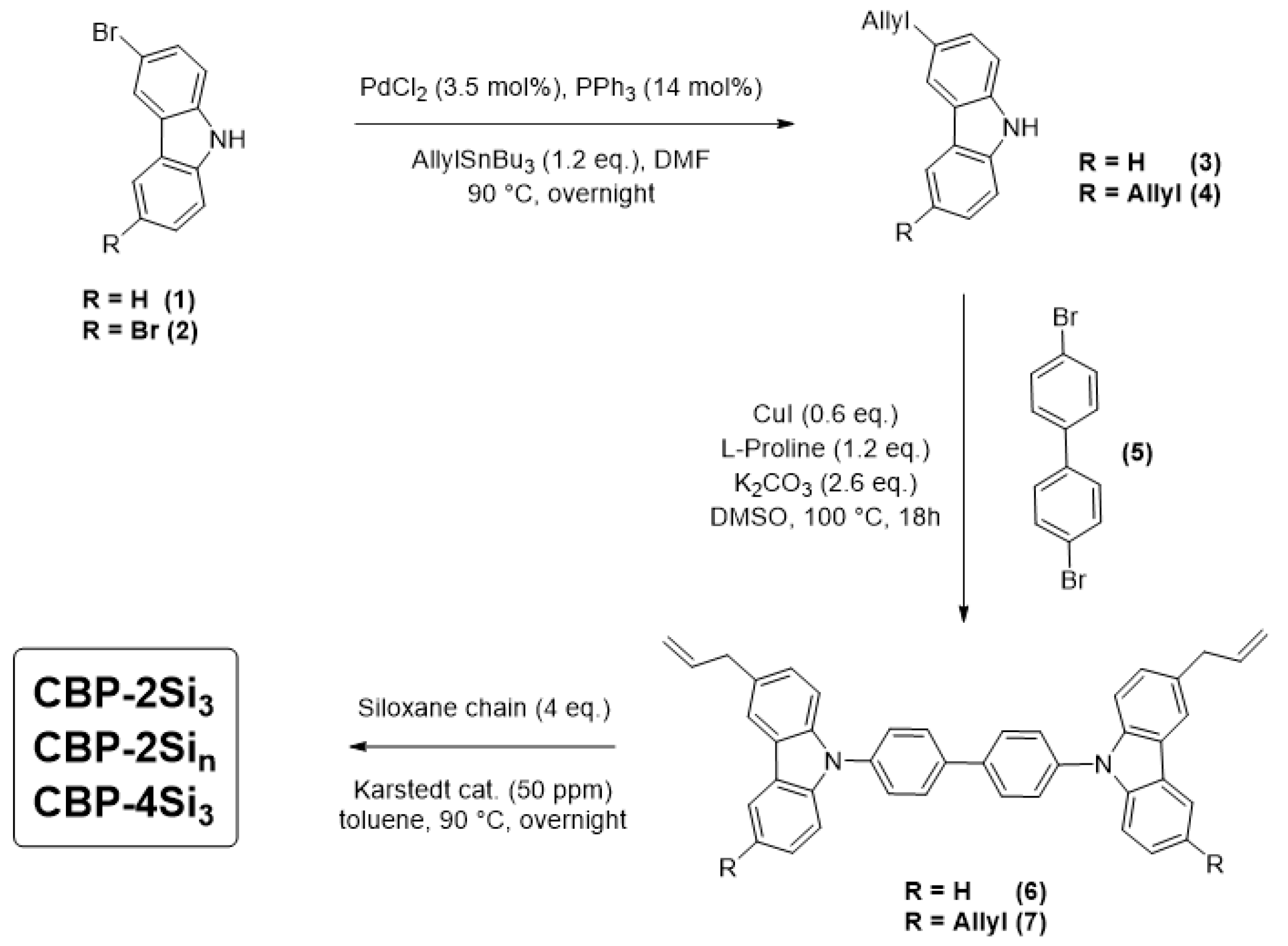
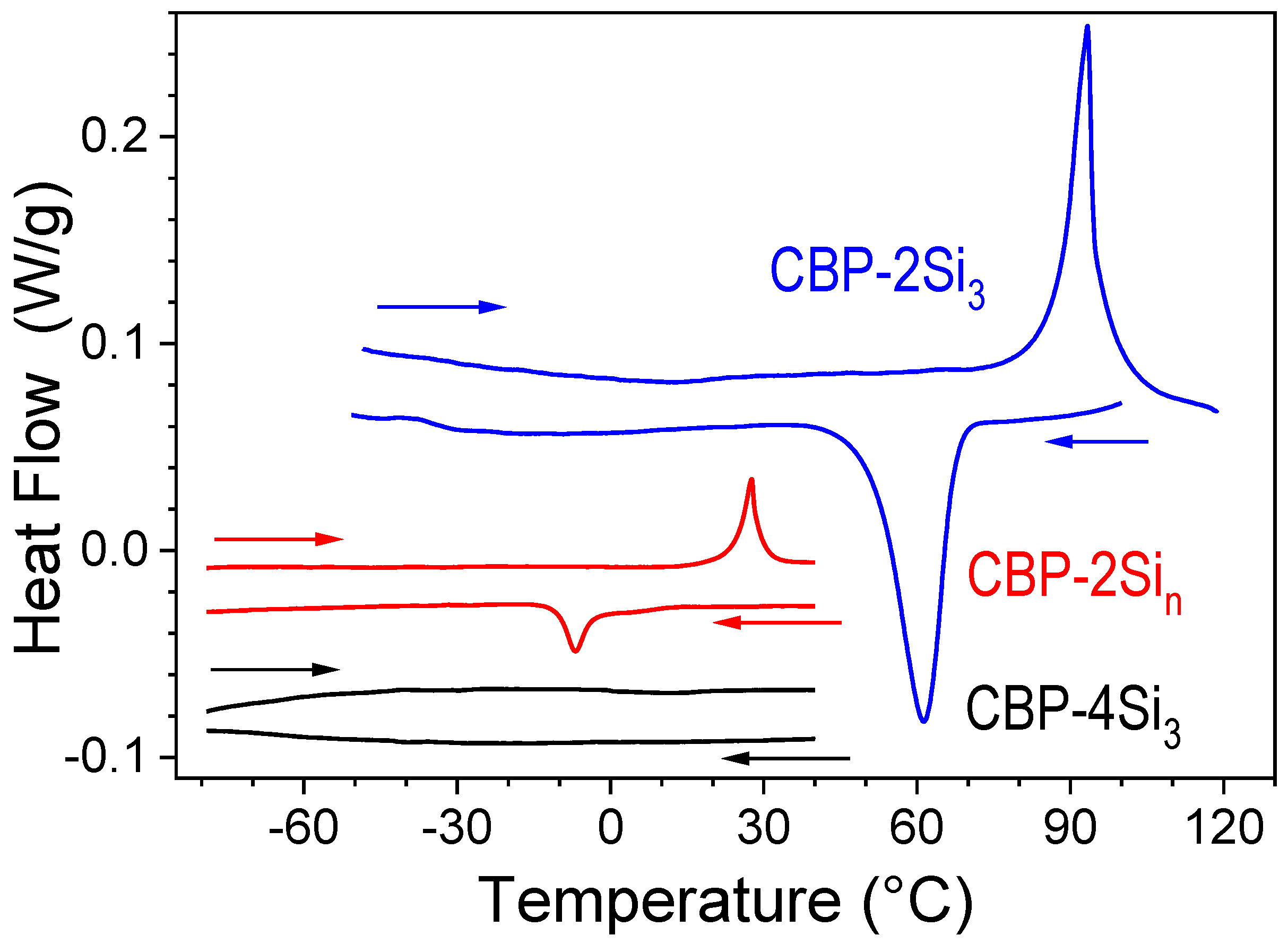
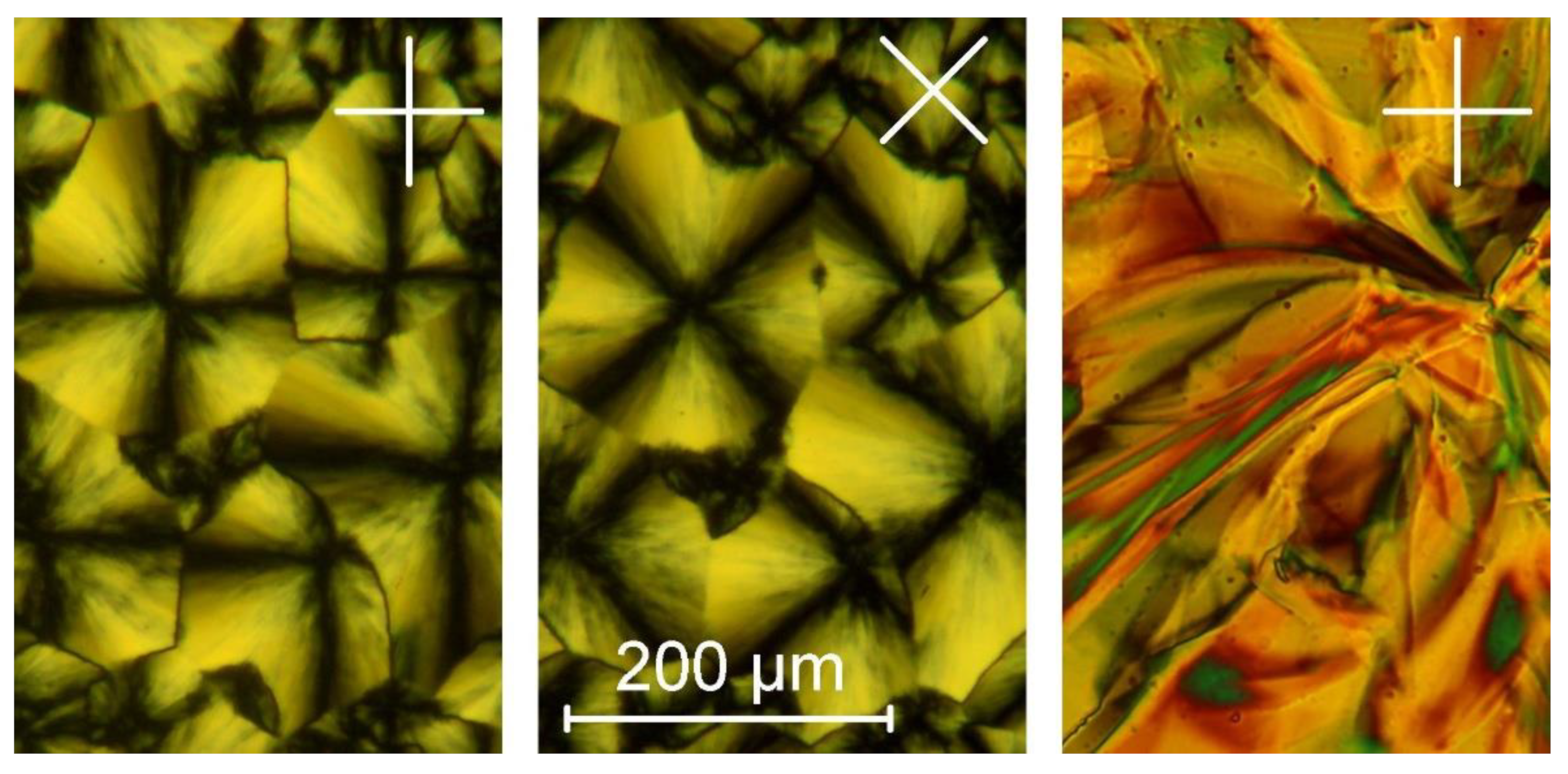
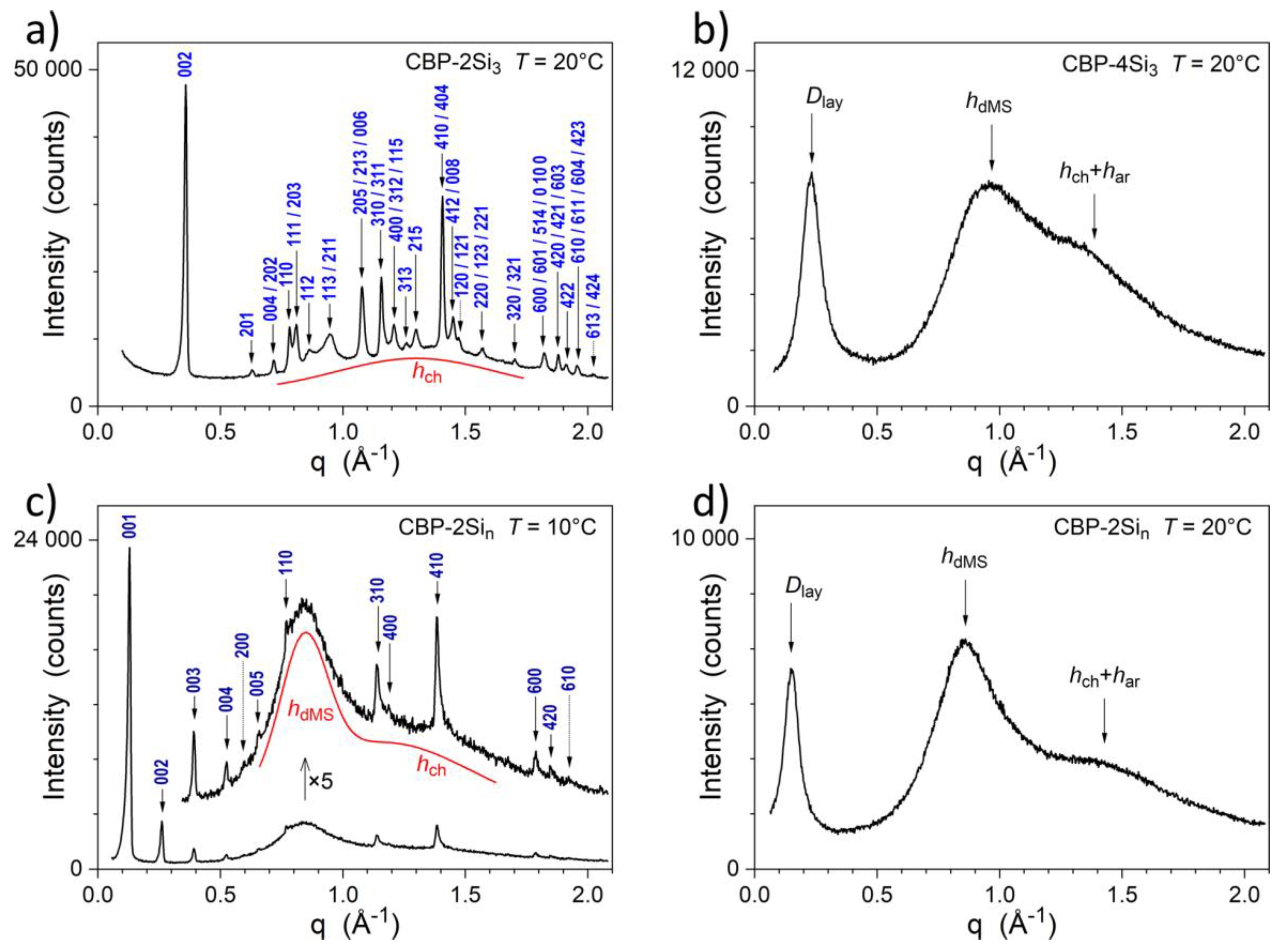
| Compound | Tg (°C) | Tm (°C) | TLC→Iso (°C) | ΔH→Iso (kJ/mol) | Tdeg (°C) |
|---|---|---|---|---|---|
| CBP [35] | (62) | 282 | - | 40 | 365 |
| CBP-2Si3 | - | 87 | - | 16 | 250 |
| CBP-2Sin | - | - | 27 | 11 | 270 |
| CBP-4Si3 | −60 | - | - | 0 | 250 |
| CBP | CBP-2prop | CBP-4prop | |
|---|---|---|---|
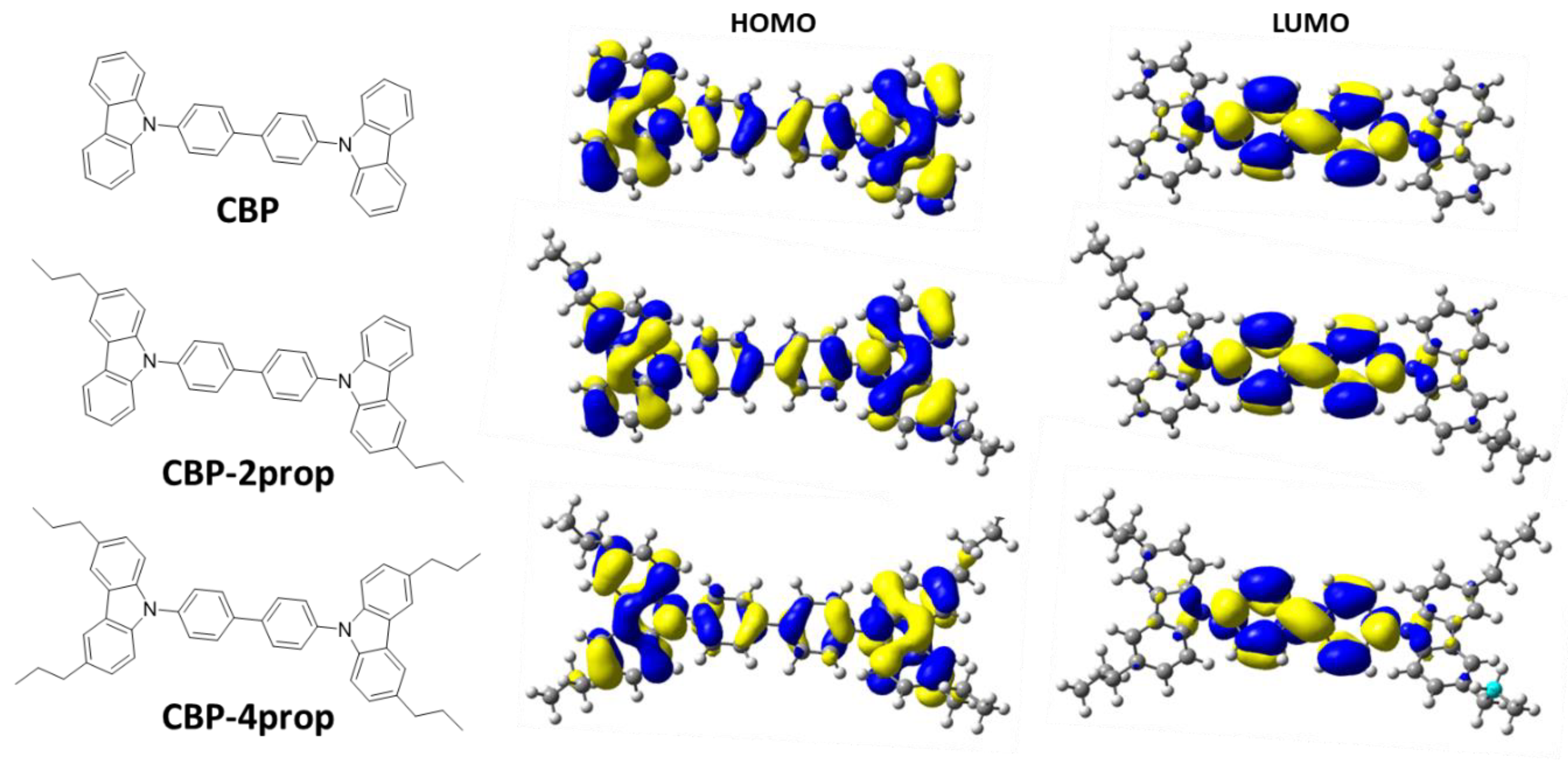
| LUMO (eV) | 1.23 | 1.20 | 1.16 |
| HOMO (eV) | 5.32 | 5.22 | 5.13 |
| S1 (eV) | 3.56 * | 3.50 * | 3.46 * |
| λS0-S1 (nm) | 348 | 354 | 359 |
| Oscillator strength | 0.6417 | 0.6918 | 0.7463 |
| Compound | Absorption | Emission | ||||
|---|---|---|---|---|---|---|
| Name | State | λmax (sol.) a (nm) | λmax (film) (nm) | λmax (sol.) a,b (nm) | λmax (film) b (nm) | PLQYc (%) |
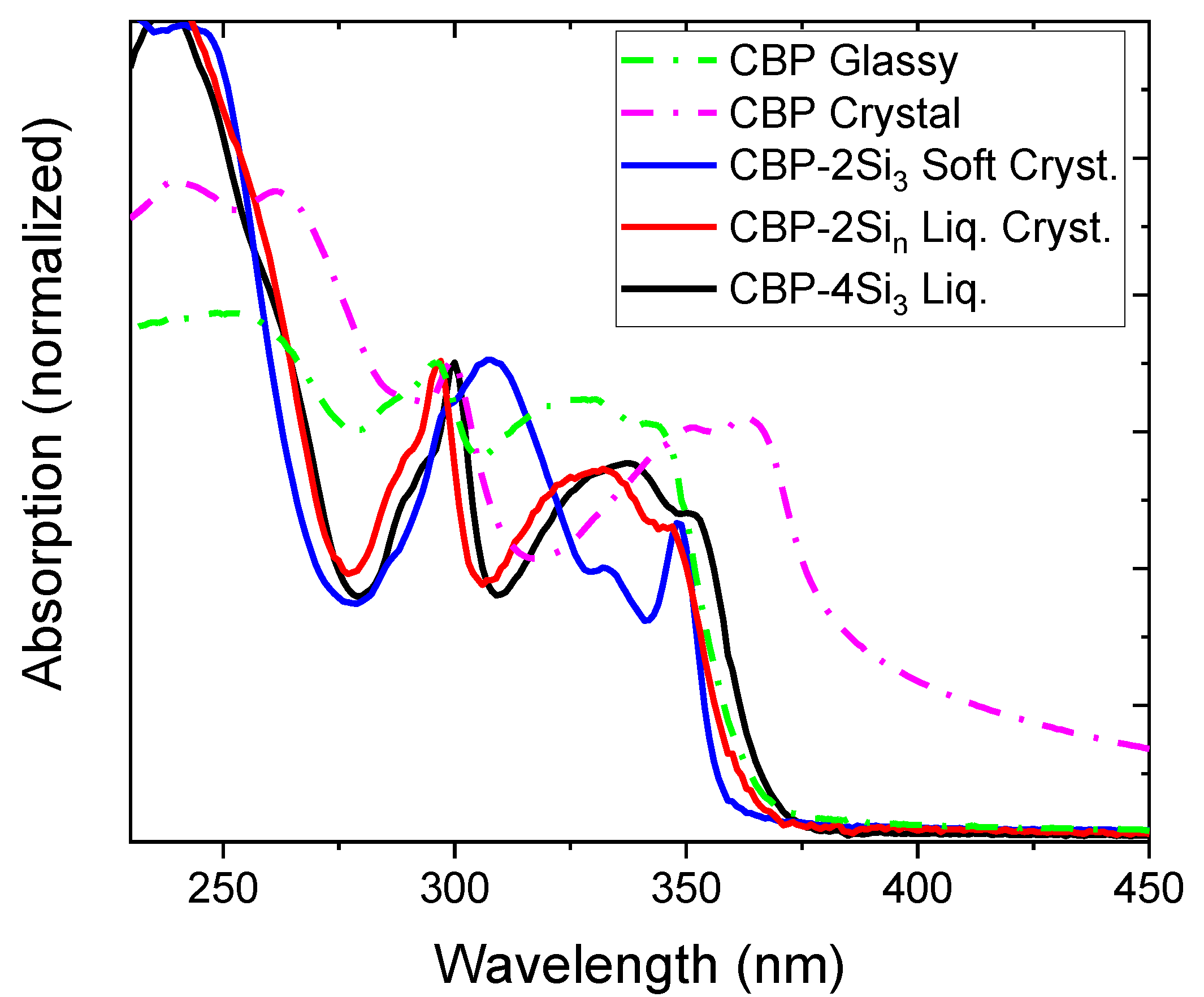
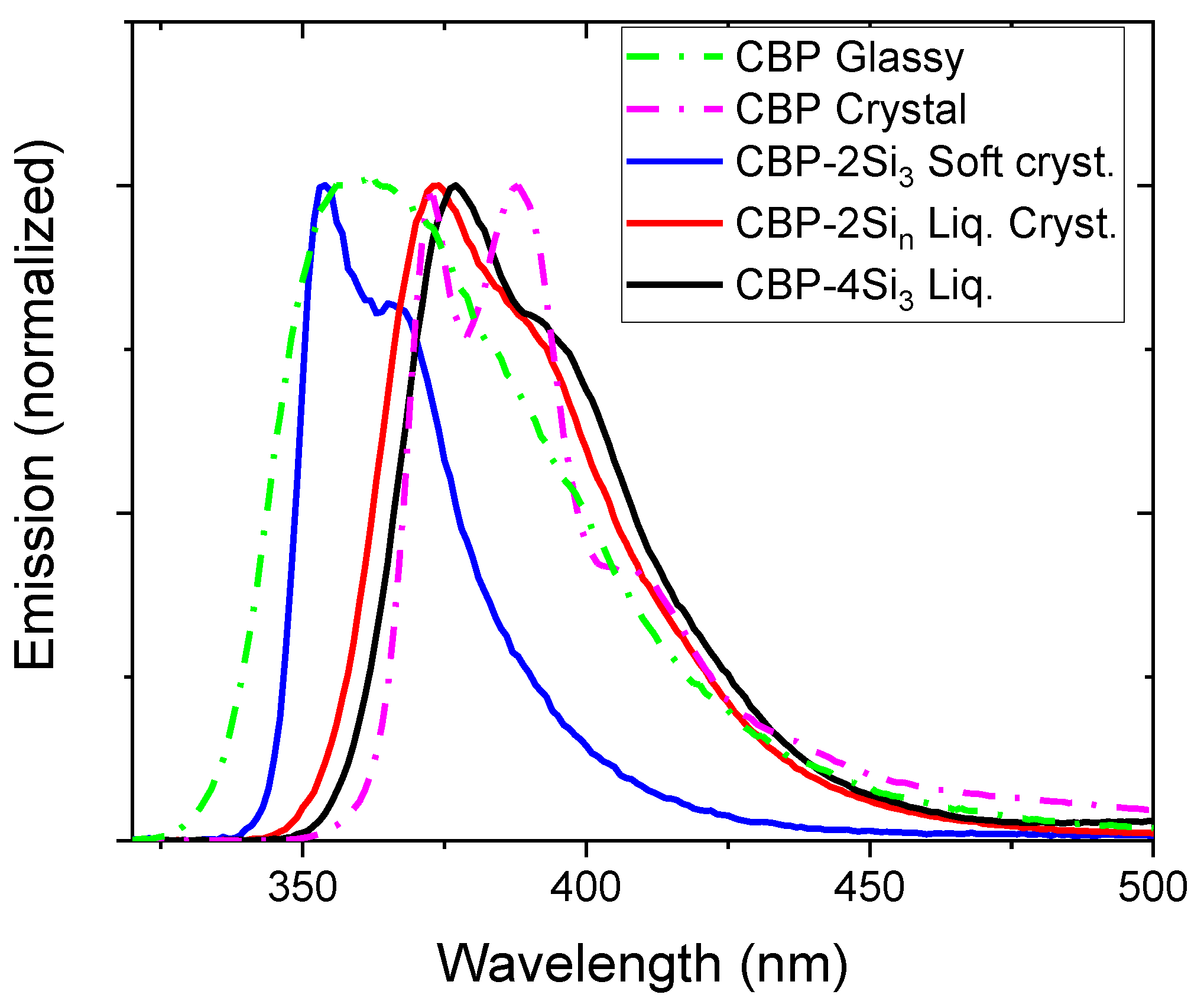
| CBP | Glassy | 293, 317, 340 * | 299, 351, 363 | 372 | 362, 384 * | 0.51 |
| Crystal | 299, 351, 363 | 372, 388, 409 * | 0.36 | |||
| CBP-4Si3 | Liquid | 300, 333, 351 * | 308, 333, 348 * | 395 | 377, 392 * | 0.58 |
| CBP-2Si10 | (smectic-like) liquid crystal | 296, 326, 346 * | 297, 332, 347 * | 385 | 374, 389 * | 0.35 |
| CBP-2Si3 | Soft crystal | 296, 325, 347 * | 300, 338, 352 | 376 | 354, 366 | 0.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaya, J.; Ribierre, J.-C.; Correia, G.; Dappe, Y.J.; Mathevet, F.; Mager, L.; Heinrich, B.; Méry, S. Control of the Organization of 4,4′-bis(carbazole)-1,1′-biphenyl (CBP) Molecular Materials through Siloxane Functionalization. Molecules 2023, 28, 2038. https://doi.org/10.3390/molecules28052038
Shaya J, Ribierre J-C, Correia G, Dappe YJ, Mathevet F, Mager L, Heinrich B, Méry S. Control of the Organization of 4,4′-bis(carbazole)-1,1′-biphenyl (CBP) Molecular Materials through Siloxane Functionalization. Molecules. 2023; 28(5):2038. https://doi.org/10.3390/molecules28052038
Chicago/Turabian StyleShaya, Janah, Jean-Charles Ribierre, Gabriel Correia, Yannick J. Dappe, Fabrice Mathevet, Loïc Mager, Benoît Heinrich, and Stéphane Méry. 2023. "Control of the Organization of 4,4′-bis(carbazole)-1,1′-biphenyl (CBP) Molecular Materials through Siloxane Functionalization" Molecules 28, no. 5: 2038. https://doi.org/10.3390/molecules28052038







