Molecularly Imprinted Polymer Nanospheres with Hydrophilic Shells for Efficient Molecular Recognition of Heterocyclic Aromatic Amines in Aqueous Solution
Abstract
1. Introduction
2. Results and Discussion
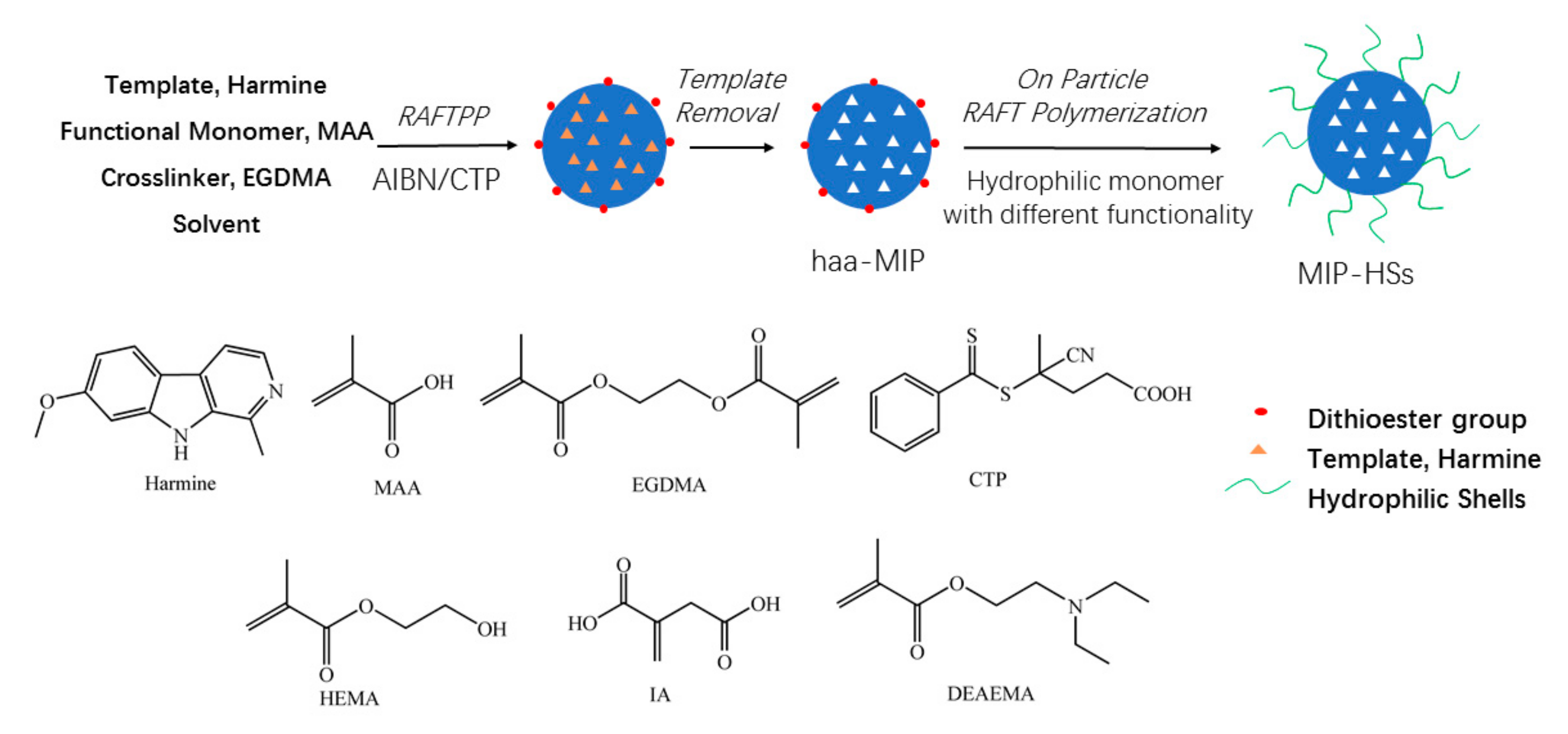
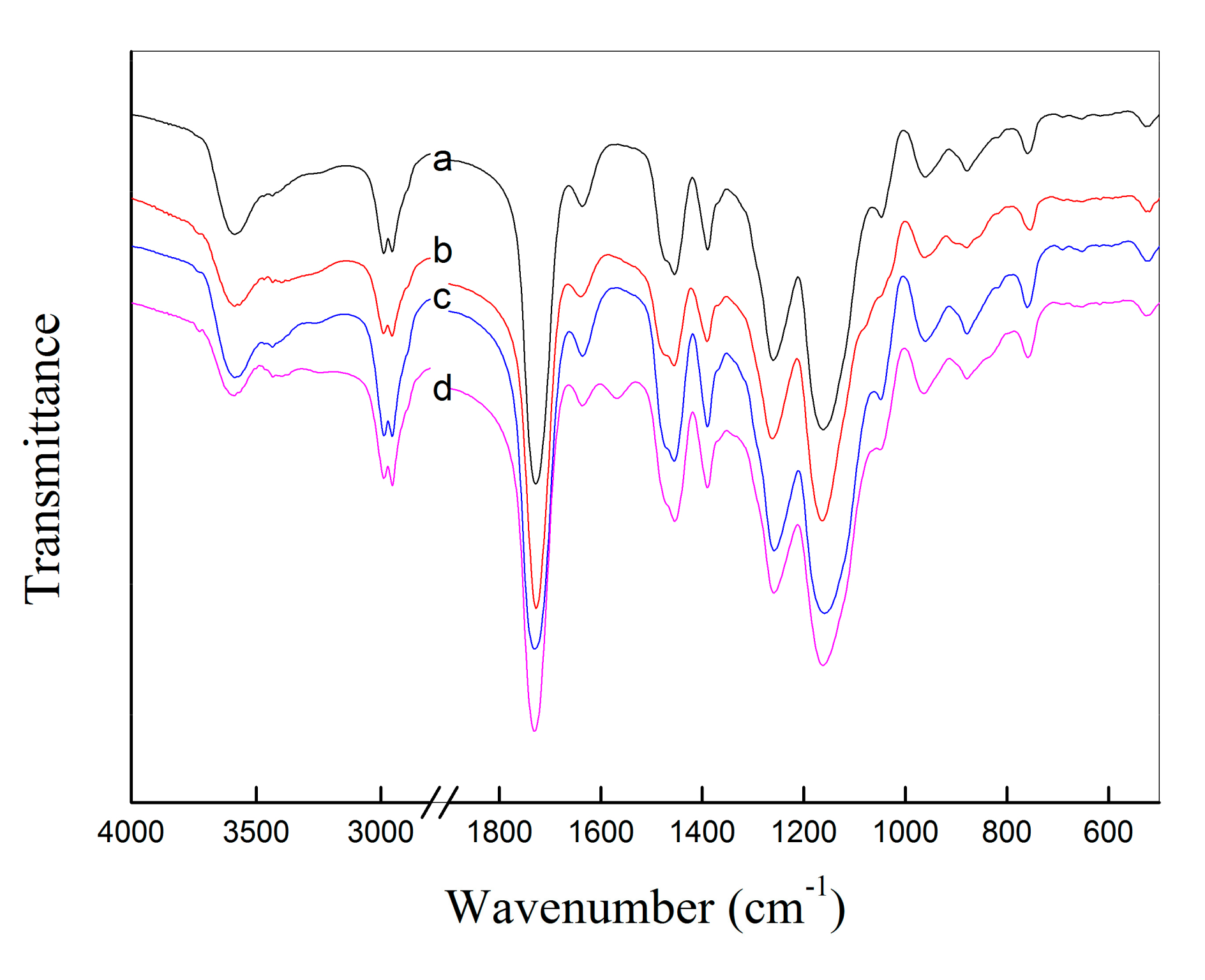
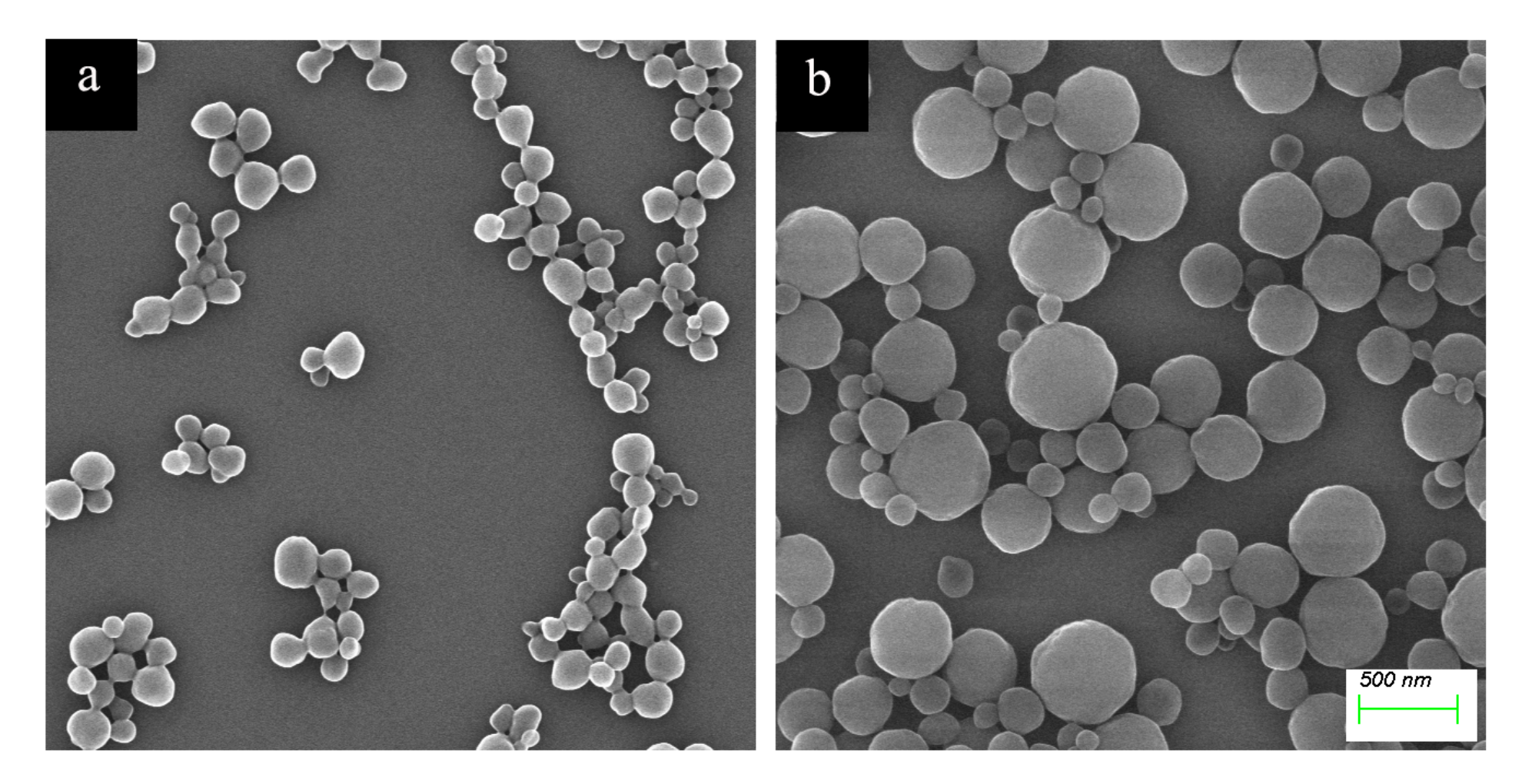
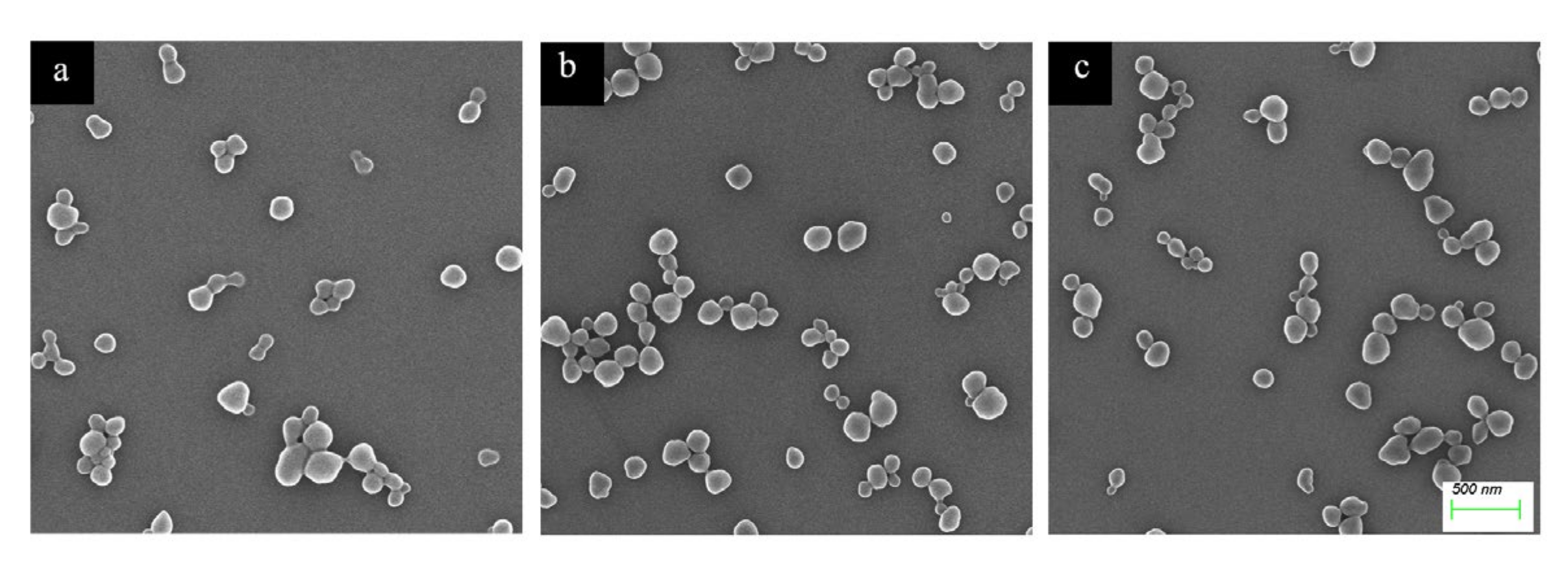
2.1. Preparation and Characterization of Haa-MIP and MIP-HSs
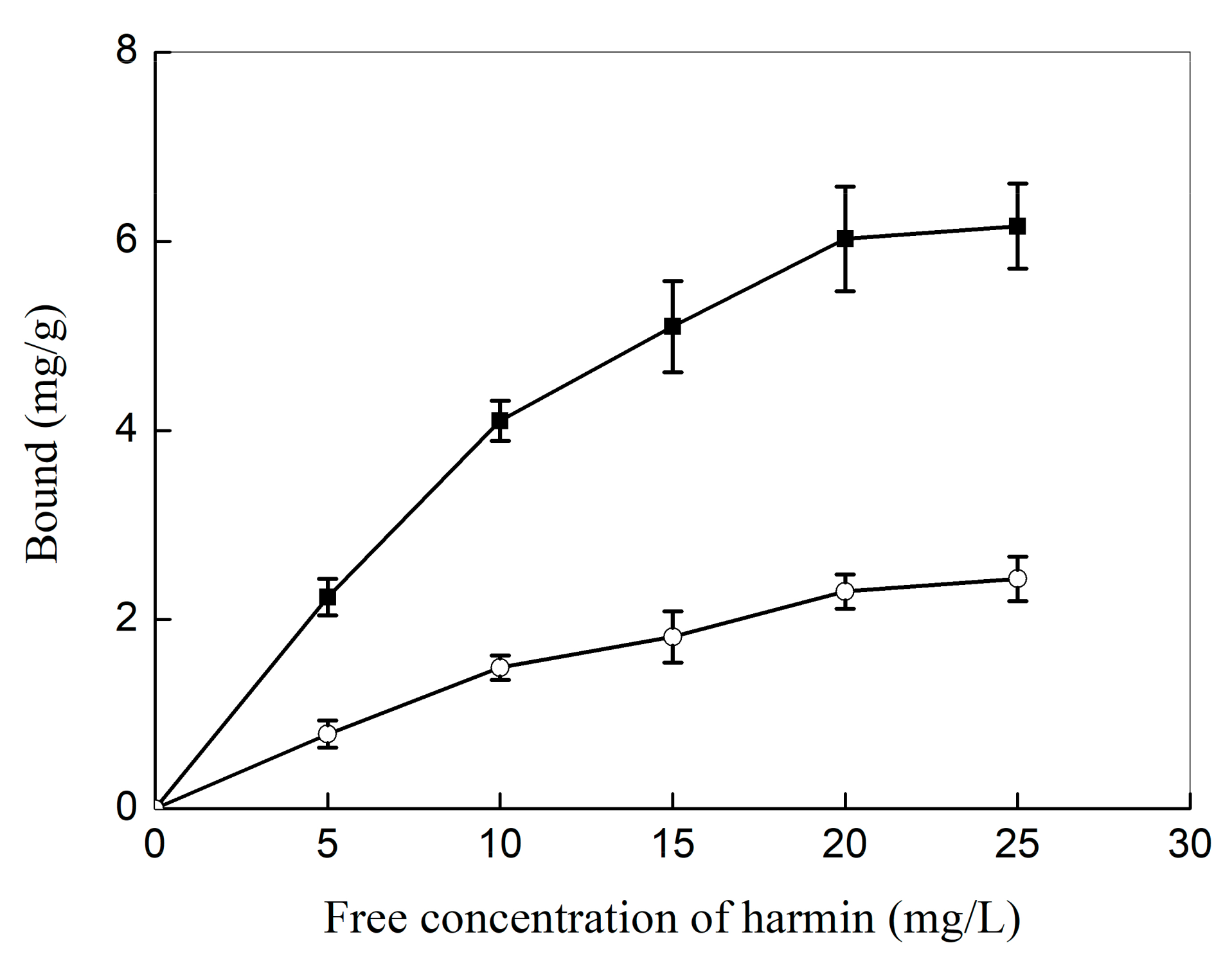
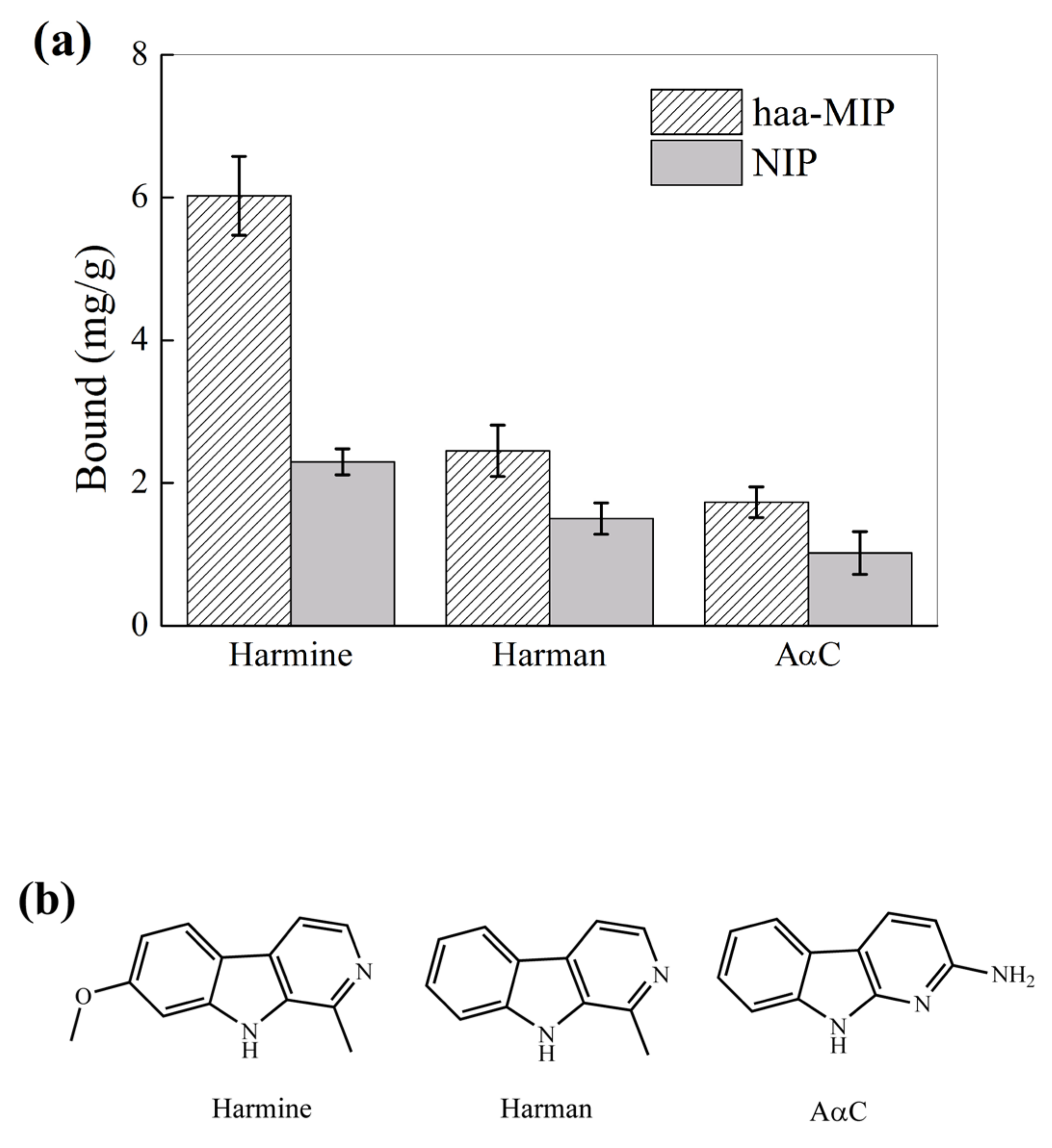
2.2. Molecular Recognition Property of Haa-MIP Particles
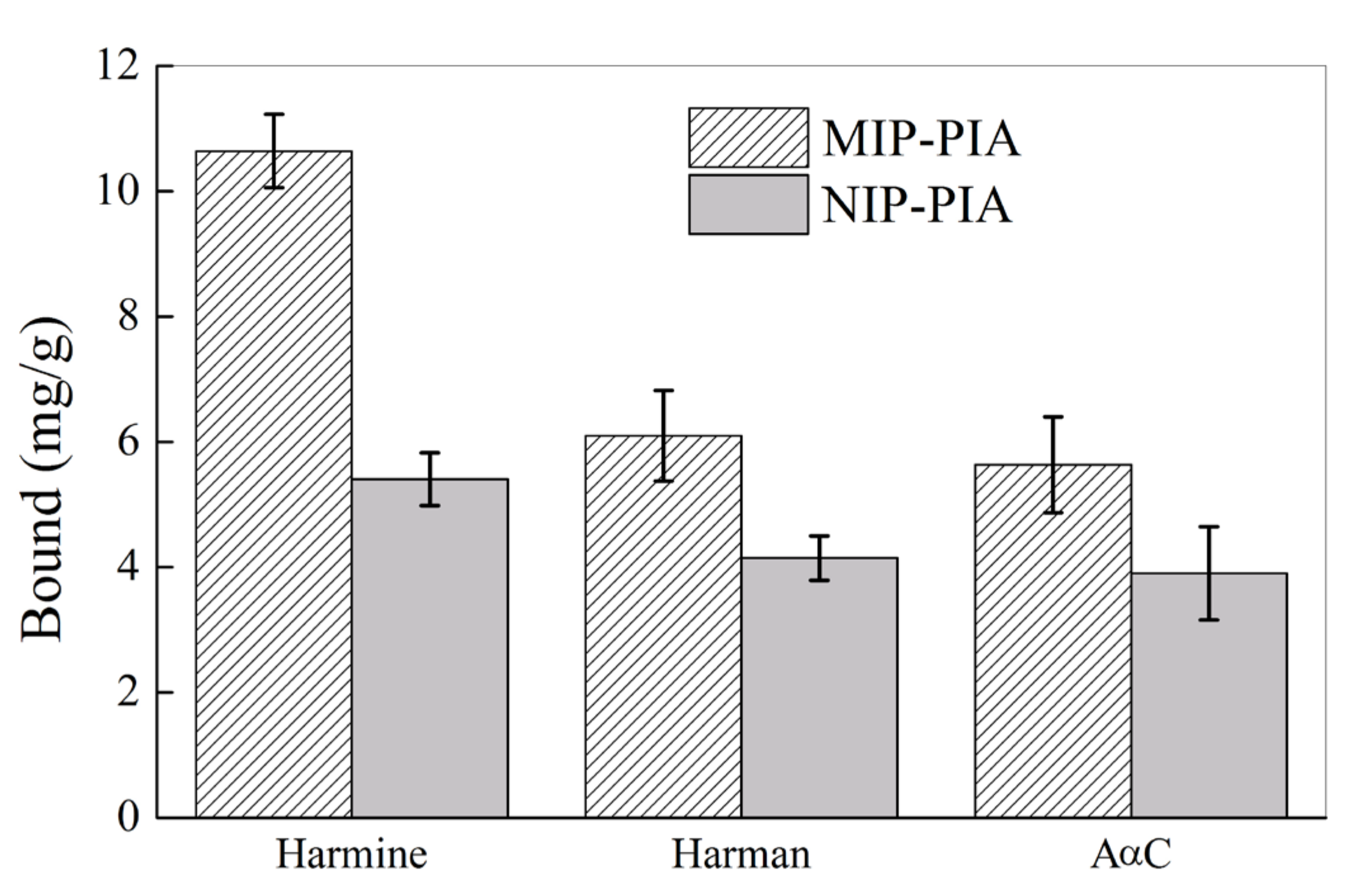
2.3. Molecular Recognition Property of MIP-HSs Particles in Aqueous Solution
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Haa-MIP with Surface-Bound Dithioester Groups
3.3. Synthesis of Core-Shell Structural MIP-HSs with Hydrophilic Shells
3.4. Characterizations
3.5. Equilibrium Binding Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Cheong, W.J.; Yang, S.H.; Ali, F. Molecular imprinted polymers for separation science: A review of reviews. J. Sep. Sci. 2013, 36, 609–628. [Google Scholar] [CrossRef]
- Hu, Y.; Pan, J.; Zhang, K.; Lian, H.; Li, G. Novel applications of molecularly imprinted polymers in sample preparation. Trends Anal. Chem. 2013, 43, 37–52. [Google Scholar] [CrossRef]
- Pichon, V.; Delaunay, N.; Combes, A. Sample preparation using molecularly imprinted polymers. Anal. Chem. 2020, 92, 16–33. [Google Scholar] [CrossRef]
- Zhou, T.; Jorgensen, L.; Mattebjerg, M.A.; Chronakisc, I.S.; Ye, L. Molecularly imprinted polymer beads for nicotine recognition prepared by RAFT precipitation polymerization: A step forward towards multifunctionalities. RSC Adv. 2014, 4, 30292–30299. [Google Scholar] [CrossRef]
- Nawaz, N.; Bakar, N.K.A.; Mahmud, H.; Jamaludin, N.S. Molecularly imprinted polymers-based DNA biosensors. Anal. Biochem. 2021, 630, 114328. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, M.; Zhang, Y.; Ma, Z.; Xiao, N.; He, X.; Li, W.; Zhang, Y. Preparation of dual-template epitope imprinted polymers for targeted fluorescence imaging and targeted drug delivery to pancreatic cancer BxPC-3 cells. ACS Appl. Mater. Inter. 2019, 11, 32431–32440. [Google Scholar] [CrossRef]
- Haupt, K.; Medina Rangel, P.X.; Bui BT, S. Molecularly imprinted polymers: Antibody mimics for bioimaging and therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Fan, J.P.; Cheng, Y.T.; Zhang, X.H.; Xiao, Z.P.; Liao, D.D.; Chen, H.P.; Huang, K.; Peng, H.L. Preparation of a novel mixed non-covalent and semi-covalent molecularly imprinted membrane with hierarchical pores for separation of genistein in Radix Puerariae Lobatae. React. Funct. Polym. 2020, 146, 104439. [Google Scholar] [CrossRef]
- Fan, J.P.; Yu, J.X.; Yang, X.M.; Zhang, X.H.; Yuan, T.T.; Peng, H.L. Preparation, characterization, and application of multiple stimuli-responsive rattle-type magnetic hollow molecular imprinted poly (ionic liquids) nanospheres (Fe3O4@void@PILMIP) for specific recognition of protein. Chem. Eng. J. 2018, 337, 722–732. [Google Scholar] [CrossRef]
- Zhang, H. Controlled/“living” radical precipitation polymerization: A versatile polymerization technique for advanced functional polymers. Eur. Polym. J. 2013, 49, 579–600. [Google Scholar] [CrossRef]
- Peng, H.; Luo, M.; Xiong, H.; Yu, N.; Ning, F.; Fan, J.; Zeng, Z.; Li, J.; Chen, L. Preparation of photonic-magnetic responsive molecularly imprinted microspheres and their application to fast and selective extraction of 17β-estradiol. J. Chromatogr. A 2016, 1442, 1–11. [Google Scholar] [CrossRef]
- Lasakova, M.; Jandera, P. Molecularly imprinted polymers and their application in solid phase extraction. J. Sep. Sci. 2009, 32, 799–812. [Google Scholar] [CrossRef]
- Azizi, A.; Shahhoseini, F.; Bottaro, C.S. Magnetic molecularly imprinted polymers prepared by reversible addition fragmentation chain transfer polymerization for dispersive solid phase extraction of polycyclic aromatic hydrocarbons in water. J. Chromatogr. A 2020, 1610, 460534. [Google Scholar] [CrossRef]
- Liang, W.; Lu, Y.; Li, N.; Li, H.; Zhu, F. Microwave-assisted synthesis of magnetic surface molecular imprinted polymer for adsorption and solid phase extraction of 4-nitrophenol in wastewater. Microchem. J. 2020, 159, 105316. [Google Scholar] [CrossRef]
- Xu, L.; Qiao, X.; Ma, Y.; Zhang, X.; Xu, X. Preparation of a hydrophilic molecularly imprinted polymer and its application in solid-phase extraction to determine of trace acrylamide in foods coupled with high-performance liquid chromatography. Food Anal. Methods 2013, 6, 838–844. [Google Scholar] [CrossRef]
- Zhu, W.; Zhou, Y.; Liu, S.; Luo, M.; Du, J.; Fan, J.; Xiong, H.; Peng, H. A novel magnetic fluorescent molecularly imprinted sensor for highly selective and sensitive detection of 4-nitrophenol in food samples through a dual-recognition mechanism. Food Chem. 2021, 348, 129126. [Google Scholar] [CrossRef]
- Soleimani, M.; Daryasari, A.; Joshani, P. Molecularly imprinted polymer nanoparticles for selective solid phase extraction of fluvoxamine in human urine and plasma. J. Chromatogr. Sci. 2020, 58, 274–279. [Google Scholar] [CrossRef]
- Du, B.; Qu, T.; Chen, Z.; Cao, X.; Han, S.; Shen, G.; Wang, L. A novel restricted access material combined to molecularly imprinted polymers for selective solid-phase extraction and high performance liquid chromatography determination of 2-methoxyestradiol in plasma samples. Talanta 2014, 129, 465–472. [Google Scholar] [CrossRef]
- Yang, J.; Li, Y.; Huang, C.; Jiao, Y.; Chen, J. A phenolphthalein-dummy template molecularly imprinted polymer for highly selective extraction and clean-up of bisphenol a in complex biological, environmental and food samples. Polymers 2018, 10, 1150. [Google Scholar] [CrossRef]
- Liang, X.; Liu, F.; Wan, Y.; Yin, X.; Liu, W. Facile synthesis of molecularly imprinted polymers for selective extraction of tyrosine metabolites in human urine. J. Chromatogr. A 2019, 1587, 34–41. [Google Scholar] [CrossRef]
- Lu, H.; Chen, Z.; Xu, S. Surface molecularly imprinted polymers on multi-function mno2-decorated magnetic carbon nanotubes for dispersive solid-phase extraction of three fluoroquinolones from milk samples. ACS Food Sci. Technol. 2022, 2, 1612–1621. [Google Scholar] [CrossRef]
- Zhang, H. Water-compatible molecularly imprinted polymers: Promising synthetic substitutes for biological receptors. Polymer 2014, 55, 699–714. [Google Scholar] [CrossRef]
- Ma, Y.; Pan, G.; Zhang, Y.; Guo, X.; Zhang, H. Narrowly dispersed hydrophilic molecularly imprinted polymer nanoparticles for efficient molecular recognition in real aqueous samples including river water, milk, and bovine serum. Angew. Chem. Int. Ed. 2013, 52, 1511–1514. [Google Scholar] [CrossRef]
- Zhao, M.; Chen, X.; Zhang, H.; Yan, H.; Zhang, H. Well-defined hydrophilic molecularly imprinted polymer microspheres for efficient molecular recognition in real biological samples by facile raft coupling chemistry. Biomacromolecules 2014, 15, 1663–1675. [Google Scholar] [CrossRef]
- Barzegar, B.; Kamankesh, M.; Mohammadi, A. Heterocyclic aromatic amines in cooked food: A review on formation, health risk-toxicology and their analytical techniques. Food Chem. 2019, 280, 240–254. [Google Scholar] [CrossRef]
- Pouzou, J.G.; Costard, S.; Zagmutt, F.J. Probabilistic assessment of dietary exposure to heterocyclic amines and polycyclic aromatic hydrocarbons from consumption of meats and breads in the United States. Food Chem. Toxicol. 2018, 114, 361–374. [Google Scholar] [CrossRef]
- Liu, S.; Taylor, L.T.; Borgerding, M.F.; Coleman, W.M.; Bombick, B.R. Trace analysis of mutagenic heterocyclic aromatic amines in cigarette smoke condensate and its base fractions via silylation-GC-MS. Contrib. Tob. Res. 2013, 25, 550–562. [Google Scholar] [CrossRef]
- Chevolleau, S.; Bouville, A.; Debrauwer, L. Development and validation of a modified QuEChERS protocol coupled to UHPLC-APCI-MS/MS for the simple and rapid quantification of 16 heterocyclic aromatic amines in cooked beef. Food Chem. 2020, 316, 126327. [Google Scholar] [CrossRef]
- Sentellas, S.; Moyano, E.; Puignou, L.; Galceran, M.T. Optimization of a clean-up procedure for the determination of heterocyclic aromatic amines in urine by field-amplified sample injection–capillary electrophoresis–mass spectrometry. J. Chromatogr. A 2004, 1032, 193–201. [Google Scholar] [CrossRef]
- Fu, Y.; Zhao, G.; Wang, S.; Yu, J.; Xie, F.; Wang, H.; Xie, J. Simultaneous determination of fifteen heterocyclic aromatic amines in the urine of smokers and nonsmokers using ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2014, 1333, 45–53. [Google Scholar] [CrossRef]
- Jhu, S.C.; Wang, J.Y.; Wang, H.T.; Chen, S.F. Analysis of heterocyclic aromatic amines using selective extraction by magnetic molecularly imprinted polymers coupled with liquid chromatography—Mass spectrometry. J. Food Drug Anal. 2021, 29, 14. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, P.; Wang, Y.; Yang, S.; Sun, X.; Peng, B.; Pan, L.; Jia, Y.; Zhang, X.; Nie, C. Molecularly Imprinted Polymer Nanospheres with Hydrophilic Shells for Efficient Molecular Recognition of Heterocyclic Aromatic Amines in Aqueous Solution. Molecules 2023, 28, 2052. https://doi.org/10.3390/molecules28052052
Sun P, Wang Y, Yang S, Sun X, Peng B, Pan L, Jia Y, Zhang X, Nie C. Molecularly Imprinted Polymer Nanospheres with Hydrophilic Shells for Efficient Molecular Recognition of Heterocyclic Aromatic Amines in Aqueous Solution. Molecules. 2023; 28(5):2052. https://doi.org/10.3390/molecules28052052
Chicago/Turabian StyleSun, Peijian, Yipeng Wang, Song Yang, Xuehui Sun, Bin Peng, Lining Pan, Yunzhen Jia, Xiaobing Zhang, and Cong Nie. 2023. "Molecularly Imprinted Polymer Nanospheres with Hydrophilic Shells for Efficient Molecular Recognition of Heterocyclic Aromatic Amines in Aqueous Solution" Molecules 28, no. 5: 2052. https://doi.org/10.3390/molecules28052052
APA StyleSun, P., Wang, Y., Yang, S., Sun, X., Peng, B., Pan, L., Jia, Y., Zhang, X., & Nie, C. (2023). Molecularly Imprinted Polymer Nanospheres with Hydrophilic Shells for Efficient Molecular Recognition of Heterocyclic Aromatic Amines in Aqueous Solution. Molecules, 28(5), 2052. https://doi.org/10.3390/molecules28052052





