Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis
Abstract
1. Introduction
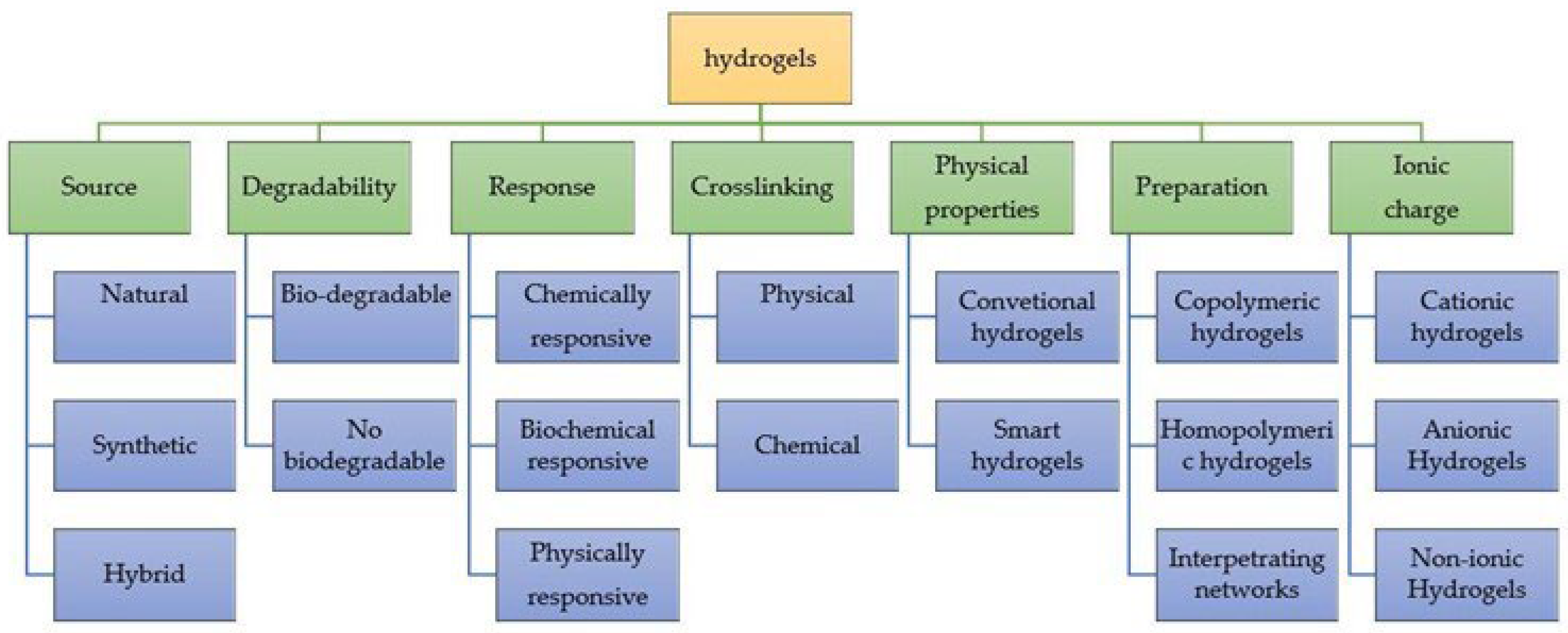
2. Hydrogels
3. Cellulose-Based Hydrogels
3.1. Extraction of Cellulose for the Production of Sustainable Hydrogels
3.2. Nanocellulose
3.3. Cellulose Solubilization
3.4. Crosslinking
3.5. Grafting
3.6. Cellulose Hydrogels with Metal Nanoparticles or Cations
4. Chitosan-Based Hydrogels
4.1. Chitin Deacetylation
4.2. Derivatization of Chitosan
4.3. Derivatization through Ionic Liquids (ILs)
4.4. Click Reactions
4.5. Cycloadditions
4.6. Grafting
4.7. Beads and Coated Beads
4.8. Radiation Synthesis
4.9. Metal–Hydrogel Hybrid Nanoparticles (NPs)
5. Hyaluronic-Acid-Based Hydrogels
5.1. Crosslinking Methods to Create HYA-Based Drug Delivery Systems
5.2. Crosslinking Methods to Create Tissue Engineering Systems
6. Alginate-Based Hydrogels
6.1. Crosslinking to Other Biopolymers [151]

6.2. Nano- and Microparticles
7. Other Natural Hydrogels
7.1. Starch-Based Hydrogels (SHs)
7.2. Lignin-Based Hydrogels
7.3. Inulin-Based Hydrogels
7.4. Linseed Hydrogels
7.5. Gellan-Gum-Based Hydrogels
8. Genipin, a Natural Crosslinker
9. In Situ Forming Injectable Hydrogels
10. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Daly, A.C.; Riley, L.; Segura, T.; Burdick, J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2019, 5, 20–43. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef] [PubMed]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef] [PubMed]
- Caliari, S.R.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Nichol, J.W.; Zhong, X.; Ji, C.; Koshy, S.; Khademhosseini, A.; Dehghani, F. Controlling the Porosity and Microarchitecture of Hydrogels for Tissue Engineering. Tissue Eng. Part B Rev. 2010, 16, 371–383. [Google Scholar] [CrossRef]
- Henderson, T.M.A.; Ladewig, K.; Haylock, D.N.; McLean, K.M.; O’Connor, A.J. Cryogels for biomedical applications. J. Mater. Chem. B 2013, 1, 2682. [Google Scholar] [CrossRef]
- Wade, R.J.; Bassin, E.J.; Rodell, C.B.; Burdick, J.A. Protease-degradable electrospun fibrous hydrogels. Nat. Commun. 2015, 6, 6639. [Google Scholar] [CrossRef]
- Taniguchi, N.; Arakawa, C.; Kobayashi, T. On the basic concept of nano-technology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974. [Google Scholar]
- Feynman, R.P. There’s plenty of room at the bottom. Eng. Sci. 1960, 23, 22–36. [Google Scholar]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2019, 25, 112. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Horvath, I.; Anastas, P.T. Introduction: Green Chemistry. Chem. Rev. 2007, 107, 2167. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Preparatory Study on Food Waste Across Eu 27; European Commission: Brussels, Belgium, 2010; Volume 33, ISBN 9789279221385. [Google Scholar]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Liao, J.; Huang, H. Magnetic chitin hydrogels prepared from Hericium erinaceus residues with tunable characteristics: A novel biosorbent for Cu2+ removal. Carbohydr. Polym. 2019, 220, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.; Kujundzic, M.; John, S.; Bismarck, A. Crab vs. Mushroom: A review of crustacean and fungalchitin in wound treatment. Mar. Drugs 2020, 18, 64. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zhang, L. New solvents and functional materials prepared from cellulose solutions in alkali/urea aqueous system. Food Res. Int. 2010, 52, 387–400. [Google Scholar] [CrossRef]
- Rasli, S.R.A.M.; Ahmad, I.; Lazim, A.M.; Hamzah, A. Extraction and characterization of cellulose from agriculture residue- Oil palm fronds. Malays. J. Anal. Sci. 2017, 21, 1065–1073. [Google Scholar] [CrossRef]
- Haan, T.Y.; Ghani, M.S.H.; Mohammad, A.W. Physical and Chemical Cleaning for Nanofiltration/Reverse Osmosis (NF/RO). J. Kejuruter. 2018, 1, 51–58. [Google Scholar] [CrossRef]
- Haan, T.Y.; Takriff, M.S. Zero waste technologies for sustainable development in palm oil mills. J. Oil Palm Environ. Health 2021, 12, 55–68. [Google Scholar]
- Padzil, F.N.M.; Lee, S.H.; Ainun, Z.M.A.; Lee, C.H.; Abdullah, L.C. Potential of Oil Palm Empty Fruit Bunch Resources in Nanocellulose Hydrogel Production for Versatile Applications: A Review. Materials 2020, 13, 1245. [Google Scholar] [CrossRef]
- Mehanny, S.; Abu-El Magd, E.E.; Ibrahim, M.; Farag, M.; Gil-San-Millan, R.; Navarro, J.; El Habbak, A.E.H.; El-Kashif, E. Extraction and characterization of nanocellulose from three types of palm residues. J. Mater. Res. Technol. 2021, 10, 526–537. [Google Scholar] [CrossRef]
- Teow, Y.H.; Amirudin, S.N.; Ho, K.C. Sustainable approach to the synthesis of cellulose membrane from oil palm empty fruit bunch for dye wastewater treatment. J. Water Process Eng. 2020, 34, 101182. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Caló, E.; Khutoryanskiy, V.V. Biomedical applications of hydrogels: A review of patents and commercial products. Eur. Polym. J. 2015, 65, 252–267. [Google Scholar] [CrossRef]
- Lin, C.C.; Metters, A.T. Hydrogels in controlled release formulations: Network design and mathematical modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379–1408. [Google Scholar] [CrossRef]
- Zainal, S.H.; Mohd, N.H.; Suhaili, N.; Anuar, F.H.; Lazim, A.M.; Othaman, R. Preparation of cellulose-based hydrogel: A review. J. Mater. Res. Technol. 2021, 10, 935–952. [Google Scholar] [CrossRef]
- Haleem, N.; Arshad, M.; Shahid, M.; Tahir, M.A. Synthesis of carboxymethyl cellulose from waste of cotton ginning industry. Carbohydr. Polym. 2014, 113, 249–255. [Google Scholar] [CrossRef]
- Al-Rajabi, M.M.; Teow, Y.H. Green Synthesis of Thermo-Responsive Hydrogel from Oil Palm Empty Fruit Bunches Cellulose for Sustained Drug Delivery. Polymers 2021, 13, 2153. [Google Scholar] [CrossRef]
- Chen, X.; Song, Z.; Li, S.; Tat Thang, N.; Gao, X.; Gong, X.; Guo, M. Facile one-pot synthesis of self-assembled nitrogendoped carbon dots/cellulose nanofibril hydrogel with enhanced fluorescence and mechanical properties. Green Chem. 2020, 22, 3296–3308. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.H.; Ibrahim, I.; Deepak, A.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, C.; Jiang, H.; Liu, F.; Cheng, G.J. Direct ink writing of hierarchically porous cellulose/alginate monolithic hydrogel as a highly effective adsorbent for environmental applications. ACS Appl. Polym. Mater. 2021, 3, 699–709. [Google Scholar] [CrossRef]
- Chinga-Carrasco, G. Cellulose fibres, nanofibrils and microfibrils: The morphological sequence of MFC components from a plant physiology and fibre technology point of view. Nanoscale Res. Lett. 2011, 6, 417. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.S.; Wahjoedi, B.A.; Yussof, A.W.; Abdullah, M.A. Eco-Friendly Extraction and Characterization of Cellulose from Oil Palm Empty Fruit Bunches. BioResources 2013, 8, 2161–2172. [Google Scholar] [CrossRef]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef]
- Rånby, B.G. Fibrous macromolecular systems. Cellulose and muscle. The colloidal properties of cellulose micelles. Discuss. Faraday Soc. 1951, 11, 158–164. [Google Scholar] [CrossRef]
- Jorfi, M.; Foster, E.J. Recent advances in nanocellulose for biomedical applications. J. Appl. Polym. Sci. 2014, 132, 41719. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Huang, J.; Lin, N.; Ahmad, I.; Dufresne, A.; Thomas, S. Recent developments on nanocellulose reinforced polymer nanocomposites: A review. Polymer 2017, 132, 368–393. [Google Scholar] [CrossRef]
- Chen, W.; Yu, H.; Lee, S.-Y.; Wei, T.; Li, J.; Fan, Z. Nanocellulose: A promising nanomaterial for advanced electrochemical energy storage. Chem. Soc. Rev. 2018, 47, 2837–2872. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in cellulose nanomaterials. Cellulose 2018, 25, 2151–2189. [Google Scholar] [CrossRef]
- Brown, A.J. XLIII.—On an acetic ferment which forms cellulose. J. Chem. Soc. Trans. 1886, 49, 432–439. [Google Scholar] [CrossRef]
- Isogai, A.; Saito, T.; Fukuzumi, H. TEMPO-oxidized cellulose nanofibers. Nanoscale 2011, 3, 71–85. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef] [PubMed]
- Peyre, J.; Pääkkönen, T.; Reza, M.; Kontturi, E. Simultaneous preparation of cellulose nanocrystals and micron-sized porous colloidal particles of cellulose by TEMPO-mediated oxidation. Green Chem. 2015, 17, 808–811. [Google Scholar] [CrossRef]
- Römling, U.; Galperin, M.Y. Bacterial cellulose biosynthesis: Diversity of operons, subunits, products, and functions. Trends Microbiol. 2015, 23, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Campano, C.; Balea, A.; Blanco, A.; Negro, C. Enhancement of the fermentation process and properties of bacterial cellulose: A review. Cellulose 2015, 23, 57–91. [Google Scholar] [CrossRef]
- Iguchi, M.; Yamanaka, S.; Budhiono, A. Bacterial cellulose—A masterpiece of nature’s arts. J. Mater. Sci. 2000, 35, 261–270. [Google Scholar] [CrossRef]
- Müller, A.; Ni, Z.; Hessler, N.; Wesarg, F.; Müller, F.A.; Kralisch, D.; Fischer, D. The Biopolymer Bacterial Nanocellulose as Drug Delivery System: Investigation of Drug Loading and Release using the Model Protein Albumin. J. Pharm. Sci. 2013, 102, 579–592. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Cohen, Y.; Garnier, G.; Garvey, C.J.; Russell, R.A.; Darwish, T.; Garnier, G. Cellulose Dissolution in Ionic Liquid: Ion Binding Revealed by Neutron Scattering. Macromolecules 2018, 51, 7649–7655. [Google Scholar] [CrossRef]
- Piltonen, P.; Hildebrandt, N.C.; Westerlind, B.; Valkama, J.-P.; Tervahartiala, T.; Illikainen, M. Green and efficient method for preparing all-cellulose composites with NaOH/urea solvent. Compos. Sci. Technol. 2016, 135, 153–158. [Google Scholar] [CrossRef]
- Song, Y.; Sun, Y.; Zhang, X.; Zhou, J.; Zhang, L. Homogeneous Quaternization of Cellulose in NaOH/Urea Aqueous Solutions as Gene Carriers. Biomacromolecules 2008, 9, 2259–2264. [Google Scholar] [CrossRef]
- Curvello, R.; Raghuwanshi, V.S.; Garnier, G. Engineering nanocellulose hydrogels for biomedical applications. Adv. Colloid Interface Sci. 2019, 267, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Song, D.; Breedveld, V.; Deng, Y. Rheological study of self-crosslinking and co-crosslinking of ammonium zirconium carbonate and starch in aqueous solutions. J. Appl. Polym. Sci. 2011, 122, 1019–1029. [Google Scholar] [CrossRef]
- Chang, C.; Zhang, L. Cellulose-based hydrogels: Present status and application prospects. Carbohydr. Polym. 2011, 84, 40–53. [Google Scholar] [CrossRef]
- Moberg, T.; Sahlin, K.; Yao, K.; Geng, S.; Westman, G.; Zhou, Q.; Oksman, K.; Rigdahl, M. Rheological properties of nanocellulose suspensions: Effects of fibril/particle dimensions and surface characteristics. Cellulose 2017, 24, 2499–2510. [Google Scholar] [CrossRef]
- Akter, M.; Bhattacharjee, M.; Dhar, A.K.; Rahman, F.B.A.; Haque, S.; Rashid, T.U.; Kabir, S.M.F. Cellulose-Based Hydrogels for Wastewater Treatment: A Concise Review. Gels 2021, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Mohd, N.; Draman, S.F.S.; Salleh, M.S.N.; Yusof, N.B. Dissolution of cellulose in ionic liquid: A review. In Proceedings of the 6th International Advances in Applied Physics and Materials Science Congress & Exhibition: (APMAS 2016), İstanbul, Türkiye, 1–3 June 2016. [Google Scholar] [CrossRef]
- Grignon, J.; Scallan, A.M. Effect of pH and neutral salts upon the swelling of cellulose gels. J. Appl. Polym. Sci. 1980, 25, 2829–2843. [Google Scholar] [CrossRef]
- Lopez-Sanchez, P.; Schuster, E.; Wang, D.; Gidley, M.J.; Strom, A. Diffusion of macromolecules in self-assembled cellulose/hemicellulose hydrogels. Soft Matter 2015, 11, 4002–4010. [Google Scholar] [CrossRef] [PubMed]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, 1705328. [Google Scholar] [CrossRef]
- Liu, J.; Chinga-Carrasco, G.; Cheng, F.; Xu, W.; Willför, S.; Syverud, K.; Xu, C. Hemicellulose-reinforced nanocellulose hydrogels for wound healing application. Cellulose 2016, 23, 3129–3143. [Google Scholar] [CrossRef]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef]
- Das, S.K.; Parandhaman, T.; Dey, M.D. Biomolecule-assisted synthesis of biomimetic nanocomposite hydrogel for hemostatic and wound healing applications. Green Chem. 2021, 23, 629–669. [Google Scholar] [CrossRef]
- Seera, S.D.K.; Kundu, D.; Banerjee, T. Physical and chemical crosslinked microcrystalline cellulose-polyvinyl alcohol hydrogel: Freeze–thaw mediated synthesis, characterization and in vitro delivery of 5-fluorouracil. Cellulose 2020, 27, 6521–6535. [Google Scholar] [CrossRef]
- Peng, N.; Hu, D.; Zeng, J.; Li, Y.; Liang, L.; Chang, C. Superabsorbent cellulose–clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain. Chem. Eng. 2016, 4, 7217–7224. [Google Scholar] [CrossRef]
- Bodin, A.; Ahrenstedt, L.; Fink, H.; Brumer, H.; Risberg, B.; Gatenholm, P. Modification of Nanocellulose with a Xyloglucan–RGD Conjugate Enhances Adhesion and Proliferation of Endothelial Cells: Implications for Tissue Engineering. Biomacromolecules 2007, 8, 3697–3704. [Google Scholar] [CrossRef]
- Bonilla, M.R.; Lopez-Sanchez, P.; Gidley, M.J.; Stokes, J.R. Micromechanical model of biphasic biomaterials with internal adhesion: Application to nanocellulose hydrogel composites. Acta Biomater. 2016, 29, 149–160. [Google Scholar] [CrossRef]
- Abeer, M.M.; Mohd Amin, M.C.I.; Martin, C. A review of bacterial cellulose-based drug delivery systems: Their biochemistry, current approaches and future prospects. J. Pharm. Pharmacol. 2014, 66, 1047–1061. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, M.; Vassalini, I.; Alessandri, I. Green Routes for the Development of Chitin/Chitosan Sustainable Hydrogels. Sustain. Chem. 2020, 1, 325–344. [Google Scholar] [CrossRef]
- Jin, T.; Liu, T.; Jiang, S.; Kurdyla, D.; Klein, B.A.; Michaelis, V.K.; Lam, E.; Li, J.; Moores, A. Chitosan nanocrystals synthesis via aging and application towards alginate hydrogels for sustainable drug release. Green Chem. 2021, 23, 6527–6537. [Google Scholar] [CrossRef]
- Chelu, M.; Moreno, J.C.; Atkinson, I.; Cusu, J.P.; Rusu, A.; Bratan, V.; Aricov, L.; Anastasescu, M.; Seciu-Grama, A.-M.; Musuc, A.M. Green synthesis of bioinspired chitosan-ZnO-based polysaccharide gums hydrogels with propolis extract as novel functional natural biomaterials. Int. J. Biol. Macromol. 2022, 211, 410–424. [Google Scholar] [CrossRef]
- Mohd Amin, M.C.I.; Ahmad, N.; Halib, N.; Ahmad, I. Synthesis and characterization of thermo- and pH-responsive bacterial cellulose/acrylic acid hydrogels for drug delivery. Carbohydr. Polym. 2012, 88, 465–473. [Google Scholar] [CrossRef]
- García-Astrain, C.; González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar] [CrossRef]
- Gulsonbi, M.; Parthasarathy, S.; Raj, K.B.; Jaisankar, V. Green synthesis, characterization and drug delivery applications of a novel silver/carboxymethylcellulose–poly (acrylamide) hydrogel nanocomposite. Ecotoxicol. Environ. Saf. 2016, 134, 421–426. [Google Scholar] [CrossRef]
- Basu, A.; Strømme, M.; Ferraz, N. Towards Tunable Protein-Carrier Wound Dressings Based on Nanocellulose Hydrogels Crosslinked with Calcium Ions. Nanomaterials 2018, 8, 550. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Zheng, J.; Guo, W.; Zhu, Z.; Wang, Z.; Dong, B.; Lin, C.; Huang, B.; Lu, B. Smart cellulose-derived magnetic hydrogel with rapid swelling and deswelling properties for remotely controlled drug release. Cellulose 2019, 26, 6861–6877. [Google Scholar] [CrossRef]
- Argüelles-Monal, W.; Lizardi-Mendoza, J.; Fernández-Quiroz, D.; Recillas-Mota, M.; Montiel-Herrera, M. Chitosan Derivatives: Introducing New Functionalities with a Controlled Molecular Architecture for Innovative Materials. Polymers 2018, 10, 342. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lavall, R.L.; Assis, O.B.G.; Campana-Filho, S.P. β-Chitin from the pens of Loligo sp.: Extraction and characterization. Bioresour. Technol. 2007, 98, 2465–2472. [Google Scholar] [CrossRef]
- Roy, J.C.; Salaün, F.; Giraud, S.; Ferri, A.; Chen, G.; Guan, J. Solubility of Chitin: Solvents, Solution Behaviors and Their Related Mechanisms. Solubility Polysacch. 2017, 3, 20–60. [Google Scholar]
- Baklagina, Y.G.; Klechkovskaya, V.V.; Kononova, S.V.; Petrova, V.A.; Poshina, D.N.; Orekhov, A.S.; Skorik, Y.A. Polymorphic Modifications of Chitosan. Crystallogr. Rep. 2018, 63, 303–313. [Google Scholar] [CrossRef]
- Tsigos, I.; Martinou, A.; Kafetzopoulos, D.; Bouriotis, V. Chitin deacetylases: New, versatile tools in biotechnology. Trends Biotechnol. 2000, 18, 305–312. [Google Scholar] [CrossRef]
- Kim, Y.J.; Zhao, Y.; Oh, K.T.; Nguyen, V.N.; Park, R.D. Enzymatic deacetylation of chitin by extracellular chitin deacetylase from a newly screened Mortierella sp. DY-52. J. Microbiol. Biotechnol. 2008, 18, 759–766. [Google Scholar]
- Zhao, Y.; Park, R.D.; Muzzarelli, R.A.A. Chitin deacetylases: Properties and applications. Mar. Drugs 2010, 8, 24–46. [Google Scholar] [CrossRef]
- Liu, C.; Wang, G.; Sui, W.; An, L.; Si, C. Preparation and Characterization of Chitosan by a Novel Deacetylation Approach Using Glycerol as Green Reaction Solvent. ACS Sustain. Chem. Eng. 2017, 5, 4690–4698. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, J.W.; Moretti, A.; Uhrich, K.E. Designing polymers with sugar-based advantages for bioactive delivery applications. J. Control. Release 2015, 219, 355–368. [Google Scholar] [CrossRef]
- Kurita, K. Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans. Mar. Biotechnol. 2006, 8, 203–226. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F.M. Chitin and Chitosan: Major Sources, Properties and Applications. In Monomers, Polymers and Composites from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2008; pp. 517–542. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar] [CrossRef]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohydr. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef]
- Rocha, M.A.M.; Coimbra, M.A.; Nunes, C. Applications of chitosan and their derivatives in beverages: A critical review. Curr. Opin. Food Sci. 2017, 15, 61–69. [Google Scholar] [CrossRef]
- Sudarshan, N.R.; Hoover, D.G.; Knorr, D. Antibacterial action of chitosan. Food Biotechnol. 1992, 6, 257–272. [Google Scholar] [CrossRef]
- Fei Liu, X.; Lin Guan, Y.; Zhi Yang, D.; Li, Z.; De Yao, K. Antibacterial action of chitosan and carboxymethylated chitosan. J. Appl. Polym. Sci. 2001, 79, 1324–1335. [Google Scholar] [CrossRef]
- Costa, A.; Walkowiak, B.; Campanella, L.; Gupta, B.; Albertini, M.C.; Teodori, L. Tissue Engineering Between Click Chemistry and Green Chemistry. Substantia 2019, 3, 29–38. [Google Scholar]
- van Rantwijk, F.; Madeira Lau, R.; Sheldon, R.A. Biocatalytic transformations in ionic liquids. Trends Biotechnol. 2003, 21, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Petkovic, M.; Seddon, K.R.; Rebelo, L.P.N.; Silva Pereira, C. Ionic liquids: A pathway to environmental acceptability. Chem. Soc. Rev. 2011, 40, 1383–1403. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiao, C.; Li, Y.; Li, T. Dissolution and resource fulization of biopolymers in ionic liquids. React. Funct. Polym. 2016, 100, 181–190. [Google Scholar] [CrossRef]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Ackova, D.G.; Kanjevac, T.; Rimondini, L.; Bosnakovski, D. Perspectives in engineered mesenchymal stem/stromal cells based anti-cancer drug delivery systems. Recent Pat. Anti Cancer Drug Discov. 2016, 11, 98–111. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, H.I.; Na, J.H.; Jeon, S.; Lim, S.; Koo, H.; Han, S.; Kang, S.; Park, S.; Moon, S.; et al. In vivo stem cell tracking with imageable nanoparticles that bind bioorthogonal chemical receptors on the stem cell surface. Biomaterials 2017, 139, 12–29. [Google Scholar] [CrossRef]
- Lee, S.; Jung, S.; Koo, H.; Na, J.H.; Yoon, H.Y.; Shim, M.K.; Park, J.; Kim, J.-H.; Lee, S.; Pomper, M.G.; et al. Nano-sized metabolic precursors for heterogeneous tumor-targeting strategy using bioorthogonal click chemistry in vivo. Biomaterials 2017, 148, 1–15. [Google Scholar] [CrossRef]
- Mongis, A.; Piller, F.; Piller, V. Coupling of Immunostimulants to Live Cells through Metabolic Glycoengineering and Bioorthogonal Click Chemistry. Bioconjugate Chem. 2017, 28, 1151–1165. [Google Scholar] [CrossRef]
- Linthorst, J.A. An overview: Origins and development of green chemistry. Found. Chem. 2010, 12, 55–68. [Google Scholar] [CrossRef]
- Jahangirian, H.; Lemraski, E.G.; Rafiee-Moghaddam, R.; Webster, T.J. A review of using green chemistry methods for biomaterials in tissue engineering. Int. J. Nanomed. 2018, 13, 5953. [Google Scholar] [CrossRef]
- Soares, R.M.; Siqueira, N.M.; Prabhakaram, M.P.; Ramakrishna, S. Electrospinning and electrospray of bio-based and natural polymers for biomaterials development. Mater. Sci. Eng. C 2018, 92, 969–982. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Jain, P.; Peng, H.; Rahimi, K.; Singh, S.; Pich, A. Dual-Degradable Biohybrid Microgels by Direct Cross-Linking of Chitosan and Dextran Using Azide–Alkyne Cycloaddition. Biomacromolecules 2020, 21, 4933–4944. [Google Scholar] [CrossRef] [PubMed]
- Larsen, C.; Harboe, E.; Johansen, M.; Olesen, H.P. Macromolecular prodrugs. XVI. Colon-targeted delivery—Comparison of the rate of release of naproxen from dextran ester prodrugs in homogenates of various segments of the pig gastrointestinal (GI) tract. Pharm. Res. 1989, 6, 995–999. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wu, L.; Yao, J.; Vogt, C.; Richnow, H.-H. Carbon and hydrogen isotopic fractionation during abiotic hydrolysis and aerobic biodegradation of phthalate esters. Sci. Total Environ. 2019, 660, 559–566. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Liu, D.; Luo, W.; Liu, Y.; Zhu, M.; Li, X.; Liu, M. Functionalization of chitosan via single electron transfer living radical polymerization in an ionic liquid and its antimicrobial activity. J. Appl. Polym. Sci. 2015, 132, 42754. [Google Scholar] [CrossRef]
- Chen, H.; Cui, S.; Zhao, Y.; Zhang, C.; Zhang, S.; Peng, X. Grafting Chitosan with Polyethylenimine in an Ionic Liquid for Efficient Gene Delivery. PLoS ONE 2015, 10, e0121817. [Google Scholar] [CrossRef]
- Zhou, Y.; Fan, M.; Luo, X.; Huang, L.; Chen, L. Acidic ionic liquid catalyzed crosslinking of oxycellulose with chitosan for advanced biocomposites. Carbohydr. Polym. 2014, 113, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Lu, S.; Hu, Z.; Zhang, B.; Li, S.; Hong, P. Marine collagen peptide grafted carboxymethyl chitosan: Optimization preparation and coagulation evaluation. Int. J. Biol. Macromol. 2020, 164, 3953–3964. [Google Scholar] [CrossRef]
- Logigan, C.-L.; Delaite, C.; Tiron, C.-E.; Peptu, C.; Popa, M.; Peptu, C.A. Chitosan Grafted Poly (Ethylene Glycol) Methyl Ether Acrylate Particulate Hydrogels for Drug Delivery Applications. Gels 2022, 8, 494. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, J.; Yin, R.; Ma, G.; Yang, D.; Niea, J. Electrospinning of methoxy poly(ethylene glycol)-grafted chitosan and poly(ethylene oxide) blend aqueous solution. Carbohydr. Polym. 2011, 83, 270–276. [Google Scholar] [CrossRef]
- Mahanta, A.K.; Maiti, P. Injectable hydrogel through hydrophobic grafting on chitosan for controlled drug delivery. ACS Appl. Bio Mater. 2019, 2, 5415–5426. [Google Scholar] [CrossRef]
- Horo, H.; Bhattacharyya, S.; Mandal, B.; Kundu, L.M. Synthesis of functionalized silk-coated chitosan-gold nanoparticles and microparticles for target-directed delivery of antitumor agents. Carbohydr. Polym. 2021, 258, 117659. [Google Scholar] [CrossRef] [PubMed]
- King, C.A.; Shamshina, J.L.; Zavgorodnya, O.; Cutfield, T.; Block, L.E.; Rogers, R.D. Porous Chitin Microbeads for More Sustainable Cosmetics. ACS Sustain. Chem. Eng. 2017, 5, 11660–11667. [Google Scholar] [CrossRef]
- Risbud, M.V.; Hardikar, A.A.; Bhat, S.V.; Bhonde, R.R. Hydrogels As controlled release system for antibiotic delivery. J. Control. Release 2000, 68, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Gobin, A.S.; Rhea, R.; Newman, R.A.; Mathur, A.B. Silk-fibroin-coated liposomes for long-term and targeted drug delivery. Int. J. Nanomed. 2006, 1, 81–87. [Google Scholar] [CrossRef]
- Wang, X.; Hu, X.; Daley, A.; Rabotyagova, O.; Cebe, P.; Kaplan, D.L. Nanolayer biomaterial coatings of silk fibroin for controlled release. J. Control. Release 2007, 121, 190–199. [Google Scholar] [CrossRef]
- Wang, X.; Wenk, E.; Hu, X.; Castro, G.R.; Meinel, L.; Wang, X.; Kaplan, D.L. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials 2007, 28, 4161–4169. [Google Scholar] [CrossRef]
- Zhou, J.; Yang, X.; Wright, J.F.; High, K.A.; Couto, L.; Qu, G. PEG-modulated column chromatography for purification of recombinant adeno-associated virus serotype 9. J. Virol. Methods 2011, 173, 99–107. [Google Scholar] [CrossRef]
- Song, M.-H.; Pham, T.P.T.; Yun, Y.-S. Ionic liquid-assisted cellulose coating of chitosan hydrogel beads and their application as drug carriers. Sci. Rep. 2020, 10, 13905. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Liu, L. Microwave-assisted preparation of temperature sensitive poly (N-isopropylacrylamide) hydrogels in poly (ethylene oxide)-600. J. Appl. Polym. Sci. 2006, 102, 4177–4184. [Google Scholar] [CrossRef]
- Cook, J.P.; Goodall, G.W.; Khutoryanskaya, O.V.; Khutoryanskiy, V.V. Microwave-Assisted Hydrogel Synthesis: A New Method for Crosslinking Polymers in Aqueous Solutions. Macromol. Rapid Commun. 2012, 33, 332–336. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. Ultrasound assisted in situ emulsion polymerization for polymer nanocomposite: A review. Chem. Eng. Process. Process Intensif. 2014, 85, 86–107. [Google Scholar] [CrossRef]
- Cass, P.; Knower, W.; Pereeia, E.; Holmes, N.P.; Hughes, T. Preparation of hydrogels via ultrasonic polymerization. Ultrason. Sonochem. 2010, 17, 326–332. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H.; Pinjari, D.V.; Gogate, P.R.; Pandit, A.B. Kinetic studies of semibatch emulsion copolymerization of methyl methacrylate and styrene in the presence of high intensity ultrasound and initiator. Chem. Eng. Process. Process Intensif. 2014, 85, 168–177. [Google Scholar] [CrossRef]
- Li, D.; Mu, C.; Cai, S.; Lin, W. Ultrasonic irradiation in the enzymatic extraction of collagen. Ultrason. Sonochem. 2009, 16, 605–609. [Google Scholar] [CrossRef]
- Jin, J.; Tu, H.; Chen, J.; Cheng, G.; Shi, X.; Deng, H.; Li, Z.; Du, Y. Rectorite-intercalated nanoparticles for improving controlled release of doxorubicin hydrochloride. Int. J. Biol. Macromol. 2017, 101, 815–822. [Google Scholar] [CrossRef]
- Xie, Y.; Liao, X.; Zhang, J.; Yang, F.; Fan, Z. Novel chitosan hydrogels reinforced by silver nanoparticles with ultrahigh mechanical and high antibacterial properties for accelerating wound healing. Int. J. Biol. Macromol. 2018, 119, 402–412. [Google Scholar] [CrossRef]
- Trombino, S.; Servidio, C.; Curcio, F.; Cassano, R. Strategies for Hyaluronic Acid-Based Hydrogel Design in Drug Delivery. Pharmaceutics 2019, 11, 407. [Google Scholar] [CrossRef]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X. Hyaluronic Acid-Based Hydrogels: From a Natural Polysaccharide to Complex Networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef]
- Serini, S.; Cassano, R.; Bruni, M.; Servidio, C.; Calviello, G.; Trombino, S. Characterization of a hyaluronic acid and folic acid-based hydrogel for cisplatin delivery: Antineoplastic effect in human ovarian cancer cells in vitro. Int. J. Pharm. 2021, 606, 120899–120912. [Google Scholar] [CrossRef] [PubMed]
- Prestwich, G.D. Biomaterials from Chemically Modified Hyaluronan. Glycoforum 2001, 5, A4. [Google Scholar]
- Bulpitt, P.; Aeschlimann, D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. J. Biomed. Mater. Res. 1999, 47, 152–169. [Google Scholar] [CrossRef]
- Low, P.S.; Antony, A.C. Folate receptor-targeted drugs for cancer and inflammatory diseases. Adv. Drug Deliv. Rev. 2004, 56, 1055–1058. [Google Scholar] [CrossRef]
- Christian, S.L.; Berry, M.D. Trace Amine-Associated Receptors as Novel Therapeutic Targets for Immunomodulatory Disorders. Front. Pharmacol. 2018, 9, 680. [Google Scholar] [CrossRef]
- Keming, X.; Fan, L.; Shu, J.G.; Joo, E.C.; Hirohisa, Y.; Motoichi, K. Injectable hyaluronic acid-tyramine hydrogels incorporating interferon-α2a for liver cancer therapy. J. Control. Release 2013, 166, 203–210. [Google Scholar]
- Pérez, L.A.; Hernández, R.; Alonso, J.M.; Pérez-González, R.; Sáez-Martínez, V. Hyaluronic Acid Hydrogels Crosslinked in Physiological Conditions: Synthesis and Biomedical Applications. Biomedicines 2021, 9, 1113. [Google Scholar] [CrossRef]
- Lee, F.; Chung, J.E.; Kurisawa, M. An injectable enzymatically crosslinked hyaluronic acid–tyramine hydrogel system with independent tuning of mechanical strength and gelation rate. Soft Matter 2008, 4, 880–887. [Google Scholar] [CrossRef]
- Larrañeta, E.; Henry, M.; Irwin, N.J.; Trotter, J.; Perminova, A.A.; Donnelly, R.F. Synthesis and characterization of hyaluronic acid hydrogels crosslinked using a solvent-free process for potential biomedical applications. Carbohydr. Polym. 2018, 181, 1194–1205. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand diels-Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.J.; Gulfam, M.; Jo, S.-H.; Gal, Y.-S.; Oh, C.-W.; Park, S.-H.; Lim, K.T. Multi-stimuli responsive hydrogels derived from hyaluronic acid for cancer therapy application. Carbohydr. Polym. 2018, 286, 119303. [Google Scholar] [CrossRef] [PubMed]
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Alini, M.; Eglin, D.; Grad, S. Optimization of hyaluronic acid-tyramine/silk-fibroin composite hydrogels for cartilage tissue engineering and delivery of anti-inflammatory and anabolic drugs. Mater. Sci. Eng. C 2021, 120, 111701–111714. [Google Scholar] [CrossRef]
- Raia, N.R.; Partlow, B.P.; McGill, M.; Kimmerling, E.P.; Ghezzi, C.E.; Kaplan, D.L. Enzymatically crosslinked silk-hyaluronic acid hydrogels. Biomaterials 2017, 131, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin–alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef] [PubMed]
- Tortelli, F.; Cancedda, R. Three-dimensional cultures of osteogenic and chondrogenic cells: A tissue engineering approach to mimic bone and cartilage in vitro. Eur. Cells Mater. 2009, 17, 1–14. [Google Scholar] [CrossRef]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef]
- Koh, L.-D.; Cheng, Y.; Teng, C.-P.; Khin, Y.-W.; Loh, X.-J.; Tee, S.-Y.; Low, M.; Ye, E.; Yu, H.-D.; Zhang, Y.-W.; et al. Structures, mechanical properties and applications of silk fibroin materials. Prog. Polym. Sci. 2015, 46, 86–110. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Fatahi, Y.; Khademhosseini, A.; Kaplan, D.L. Overview of Silk Fibroin Use in Wound Dressings. Trends Biotechnol. 2018, 36, 907–922. [Google Scholar] [CrossRef]
- Niu, C.; Liu, X.; Wang, Y.; Li, X.; Shi, J. Photothermal-modulated drug release from a composite hydrogel based on silk fibroin and sodium alginate. Eur. Polym. J. 2021, 146, 110267. [Google Scholar] [CrossRef]
- Mandru, M.; Bercea, M.; Gradinaru, L.M.; Ciobanu, C.; Drobota, M.; Vlad, S.; Albulescu, R. Polyurethane/poly(vinyl alcohol) hydrogels: Preparation, characterization and drug delivery. Eur. Polym. J. 2019, 118, 137–145. [Google Scholar] [CrossRef]
- Cong, Z.; Shi, Y.; Wang, Y.; Wang, Y.; Niu, J.; Chen, N.; Xue, H. A novel controlled drug delivery system based on alginate hydrogel/chitosan micelle composites. Int. J. Biol. Macromol. 2018, 107, 855–864. [Google Scholar] [CrossRef]
- Birla, S.S.; Tiwari, V.V.; Gade, A.K.; Ingle, A.P.; Yadav, A.P.; Rai, M.K. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 2009, 48, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Han, J.W.; Kwon, D.-N.; Kim, J.-H. Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 2014, 9, 373. [Google Scholar] [CrossRef]
- Abid, J.P.; Wark, A.W.; Brevet, P.F.; Girault, H.H. Preparation of silver nanoparticles in solution from a silver salt by laser irradiation. Chem. Commun. 2002, 7, 792–793. [Google Scholar] [CrossRef]
- Fernanda Andrade, P.; de Faria, A.F.; da Silva, D.S.; Alves Bonacin, J.; Gonçalves, M.D.C. Structural and Morphological Investigations of β-Cyclodextrin-Coated Silver Nanoparticles. Microsc. Microanal. 2014, 20, 2114–2115. [Google Scholar] [CrossRef]
- Pérez-Díaz, M.; Alvarado-Gomez, E.; Magaña-Aquino, M.; Sánchez-Sánchez, R.; Velasquillo, C.; Gonzalez, C.; Ganem-Rondero, A.; Martínez-Castañon, G.; Zavala-Alonso, N.; Martinez-Gutierrez, F. Anti-biofilm activity of chitosan gels formulated with silver nanoparticles and their cytotoxic effect on human fibroblasts. Mater. Sci. Eng. C 2016, 60, 317–323. [Google Scholar] [CrossRef]
- Keat, C.L.; Aziz, A.; Eid, A.M.; Elmarzugi, N.A. Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2015, 2, 47. [Google Scholar] [CrossRef]
- Rahisuddin; AL-Thabaiti, S.A.; Khan, Z.; Manzoor, N. Biosynthesis of silver nanoparticles and its antibacterial and antifungal activities towards Gram-positive, Gram-negative bacterial strains and different species of Candida fungus. Bioprocess Biosyst. Eng. 2015, 38, 1773–1781. [Google Scholar] [CrossRef]
- Jadhav, K.; Dhamecha, D.; Bhattacharya, D.; Patil, M. Green and ecofriendly synthesis of silver nanoparticles: Characterization, biocompatibility studies and gel formulation for treatment of infections in burns. J. Photochem. Photobiol. B Biol. 2016, 155, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; Deepak, V.; Ramkumarpandian, S.; Nellaiah, H.; Sangiliyandi, G. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 2008, 62, 4411–4413. [Google Scholar] [CrossRef]
- Das, A.; Kumar, A.; Patil, N.B.; Viswanathan, C.; Ghosh, D. Preparation and characterization of silver nanoparticle loaded amorphous hydrogel of carboxymethylcellulose for infected wounds. Carbohydr. Polym. 2015, 130, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Naraginti, S.; Kumari, P.L.; Das, R.K.; Sivakumar, A.; Patil, S.H.; Andhalkar, V.V. Amelioration of excision wounds by topical application of green synthesized, formulated silver and gold nanoparticles in albino Wistar rats. Mater. Sci. Eng. C 2016, 62, 293–300. [Google Scholar] [CrossRef]
- Mekkawy, A.; El-Mokhtar, M.; Nafady, N.; Yousef, N.; Hamad, M.; El-Shanawany, S.; Ibrahim, E.; Elsabahy, M. In vitro and in vivo evaluation of biologically synthesized silver nanoparticles for topical applications: Effect of surface coating and loading into hydrogels. Int. J. Nanomed. 2017, 12, 759–777. [Google Scholar] [CrossRef]
- Rial, R.; Hassan, N.; Liu, Z.; Ruso, J.M. The design and green nanofabrication of noble hydrogel systems with encapsulation of doped bioactive hydroxyapatite toward sustained drug delivery. J. Mol. Liq. 2021, 343, 117598. [Google Scholar] [CrossRef]
- Benedini, L.; Laiuppa, J.; Santillán, G.; Baldini, M.; Messina, P. Antibacterial alginate/nano-hydroxyapatite composites for bone tissue engineering: Assessment of their bioactivity, biocompatibility, and antibacterial activity. Mater. Sci. Eng. C 2020, 115, 111101. [Google Scholar] [CrossRef]
- Netz, D.J.A.; Sepulveda, P.; Pandolfelli, V.C.; Spadaro, A.C.C.; Alencastre, J.B.; Bentley, M.V.L.B.; Marchetti, J.M. Potential use of gelcasting hydroxyapatite porous ceramic as an implantable drug delivery system. Int. J. Pharm. 2001, 213, 117–125. [Google Scholar] [CrossRef]
- Uchida, A.; Shinto, Y.; Araki, N.; Ono, K. Slow release of anticancer drugs from porous calcium hydroxyapatite ceramic. J. Orthop. Res. 1992, 10, 440–445. [Google Scholar] [CrossRef]
- Rivas, M.; del Valle, L.J.; Rodríguez-Rivero, A.M.; Turon, P.; Puiggalí, J.; Alemán, C. Loading of Antibiotic into Biocoated Hydroxyapatite Nanoparticles: Smart Antitumor Platforms with Regulated Release. ACS Biomater. Sci. Eng. 2018, 4, 3234–3245. [Google Scholar] [CrossRef]
- Zou, Q.; Li, Y.; Zhang, L.; Zuo, Y.; Li, J.; Li, J. Antibiotic delivery system using nano-hydroxyapatite/chitosan bone cement consisting of berberine. J. Biomed. Mater. Res. Part A 2009, 89, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Li, C.; Huang, S.; Hou, Z.; Cheng, Z.; Yang, P.; Peng, C.; Lin, J. Self-activated luminescent and mesoporous strontium hydroxyapatite nanorods for drug delivery. Biomaterials 2010, 31, 3374–3383. [Google Scholar] [CrossRef] [PubMed]
- Gadow, R.; Killinger, A.; Stiegler, N. Hydroxyapatite coatings for biomedical applications deposited by different thermal spray techniques. Surf. Coat. Technol. 2010, 205, 1157–1164. [Google Scholar] [CrossRef]
- Guesmi, Y.; Agougui, H.; Lafi, R.; Jabli, M.; Hafiane, A. Synthesis of hydroxyapatite-sodium alginate via a co-precipitation technique for efficient adsorption of Methylene Blue dye. J. Mol. Liq. 2018, 249, 912–920. [Google Scholar] [CrossRef]
- Rial, R.; Tahoces, P.G.; Hassan, N.; Cordero, M.L.; Liu, Z.; Ruso, J.M. Noble microfluidic system for bioceramic nanoparticles engineering. Mater. Sci. Eng. C 2019, 102, 221–227. [Google Scholar] [CrossRef]
- Mendes, S.C.; Reis, R.L.; Bovell, Y.P.; Cunha, A.M.; van Blitterswijk, C.A.; de Bruijn, J.D. Biocompatibility testing of novel starch-based materials with potential application in orthopaedic surgery: A preliminary study. Biomaterials 2001, 22, 2057–2064. [Google Scholar] [CrossRef]
- Stephen, S.; Gorain, B.; Choudhury, H.; Chatterjee, B. Exploring the role of mesoporous silica nanoparticle in the development of novel drug delivery systems. Drug Deliv. Transl. Res. 2021, 12, 105–123. [Google Scholar] [CrossRef]
- Wu, K.; Liao, Y.-T.; Liu, C.-H.; Yu, J. Liver cancer cells: Targeting and prolonged-release drug carriers consisting of mesoporous silica nanoparticles and alginate microspheres. Int. J. Nanomed. 2014, 9, 2767. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2021, 30, 19–50. [Google Scholar] [CrossRef]
- Marques, A.P.; Reis, R.L.; Hunt, J.A. The biocompatibility of novel starch-based polymers and composites: In vitro studies. Biomaterials 2002, 23, 1471–1478. [Google Scholar] [CrossRef]
- Azevedo, H.S.; Gama, F.M.; Reis, R.L. In Vitro Assessment of the Enzymatic Degradation of Several Starch Based Biomaterials. Biomacromolecules 2003, 4, 1703–1712. [Google Scholar] [CrossRef] [PubMed]
- Defaye, J.; Wong, E. Structural studies of gum arabic, the exudate polysaccharide from Acacia senegal. Carbohydr. Res. 1986, 150, 221–231. [Google Scholar] [CrossRef]
- Reddy, S.M. Novel oral colon-specific drug delivery systems for pharmacotherapy of peptides and nonpeptide drugs. Drugs Today 1999, 35, 537. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Simões, S. Modular Hydrogels for Drug Delivery. J. Biomater. Nanobiotechnol. 2012, 3, 185–199. [Google Scholar] [CrossRef]
- Whistler, R.L.; Daniel, J.R. Starch. In Kirk–Othmer Encyclopedia of Chemical Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Robyt, J.F. Starch: Structure, Properties, Chemistry, and Enzymology. In Glycoscience; Springer: Berlin/Heidelberg, Germany, 2008; pp. 1437–1472. [Google Scholar] [CrossRef]
- Zhang, L.M.; Yang, C.; Yan, L. Perspectives on: Strategies to Fabricate Starch-based Hydrogels with Potential Biomedical Applications. J. Bioact. Compat. Polym. 2005, 20, 297–314. [Google Scholar] [CrossRef]
- Tester, R.F.; Karkalas, J.; Qi, X. Starch—Composition, fine structure and architecture. J. Cereal Sci. 2004, 39, 151–165. [Google Scholar] [CrossRef]
- Nasri-Nasrabadi, B.; Mehrasa, M.; Rafienia, M.; Bonakdar, S.; Behzad, T.; Gavanji, S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr. Polym. 2014, 108, 232–238. [Google Scholar] [CrossRef]
- Dey, P.M. Methods in Plant Biochemistry: Plant Phenolics (Methods in Plant Biochemistry); Academic Press: Cambridge, MA, USA, 1989. [Google Scholar]
- French, D. Fine Structure of Starch and its Relationship to the Organization of Starch Granules. J. Jpn. Soc. Starch Sci. 1972, 19, 8–25. [Google Scholar] [CrossRef]
- Mua, J.P.; Jackson, D.S. Fine Structure of Corn Amylose and Amylopectin Fractions with Various Molecular Weights†. J. Agric. Food Chem. 1997, 45, 3840–3847. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Biliaderis, C.G. Structures and Phase Transitions of Starch Polymers. In Polysaccharide Association Structures in Food; CRC Press: Boca Raton, FL, USA, 1998; pp. 71–182. [Google Scholar] [CrossRef]
- Ellis, R.P.; Cochrane, M.P.; Dale, M.F.B.; Duffus, C.M.; Lynn, A.; Morrison, I.M.; Prentice, R.D.M.; Swanston, J.S.; Tiller, S.A. Starch production and industrial use. J. Sci. Food Agric. 1998, 77, 289–311. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef] [PubMed]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant starch—A review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.-J.; Kim, C.-H.; Park, J.-K. Synthesis and Characterization of Starch-g-Polycaprolactone Copolymer. Macromolecules 1999, 32, 7402–7408. [Google Scholar] [CrossRef]
- Peidayesh, H.; Ahmadi, Z.; Khonakdar, H.A.; Abdouss, M.; Chodák, I. Baked hydrogel from corn starch and chitosan blends cross-linked by citric acid: Preparation and properties. Polym. Adv. Technol. 2020, 31, 1256–1269. [Google Scholar] [CrossRef]
- Ghosh, S.; Viana, J.C.; Reis, R.L.; Mano, J.F. The double porogen approach as a new technique for the fabrication of interconnected poly(L-lactic acid) and starch based biodegradable scaffolds. J. Mater. Sci. Mater. Med. 2007, 18, 185–193. [Google Scholar] [CrossRef]
- Fan, Y.; Picchioni, F. Modification of starch: A review on the application of “green” solvents and controlled functionalization. Carbohydr. Polym. 2020, 241, 116350. [Google Scholar] [CrossRef]
- Thakore, I.M.; Desai, S.; Sarawade, B.D.; Devi, S. Studies on biodegradability, morphology and thermo-mechanical properties of LDPE/modified starch blends. Eur. Polym. J. 2001, 37, 151–160. [Google Scholar] [CrossRef]
- Hashim, K.; Dahlan, K.Z.; Noordin, N.M.; Yoshii, F. Hydrogel of sago starch/water-soluble polymers by electron beam irradiation technique. In Proceedings of the Takasaki Workshop on Bilateral Cooperations, Radiation Processing of Natural Polymers, No. IAEA-SM—365. Takasaki, Japan, 1–2 November 1999; Kume, T., Maekawa, Y., Eds.; Functional Material Laboratory: Zurich, Switzerland, 2000. [Google Scholar]
- Geresh, S.; Gilboa, Y.; Peisahov-Korol, J.; Gdalevsky, G.; Voorspoels, J.; Remon, J.P.; Kost, J. Preparation and characterization of bioadhesive grafted starch copolymers as platforms for controlled drug delivery. J. Appl. Polym. Sci. 2002, 86, 1157–1162. [Google Scholar] [CrossRef]
- Zhai, M.; Yoshii, F.; Kume, T.; Hashim, K. Syntheses of PVA/starch grafted hydrogels by irradiation. Carbohydr. Polym. 2002, 50, 295–303. [Google Scholar] [CrossRef]
- Yoshii, F.; Zhao, L.; Wach, R.A.; Nagasawa, N.; Mitomo, H.; Kume, T. Hydrogels of polysaccharide derivatives crosslinked with irradiation at paste-like condition. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2003, 208, 320–324. [Google Scholar] [CrossRef]
- Peppas, N.A.; Khare, A.R. Preparation, structure and diffusional behavior of hydrogels in controlled release. Adv. Drug Deliv. Rev. 1993, 11, 1–35. [Google Scholar] [CrossRef]
- Rosiak, J.M.; Ulański, P. Synthesis of hydrogels by irradiation of polymers in aqueous solution. Radiat. Phys. Chem. 1999, 55, 139–151. [Google Scholar] [CrossRef]
- Chin, S.F.; Romainor, A.N.B.; Pang, S.C.; Lihan, S. Antimicrobial starch-citrate hydrogel for potential applications as drug delivery carriers. J. Drug Deliv. Sci. Technol. 2019, 54, 101239. [Google Scholar] [CrossRef]
- Ma, C.; Gerhard, E.; Lu, D.; Yang, J. Citrate chemistry and biology for biomaterials design. Biomaterials 2018, 178, 383–400. [Google Scholar] [CrossRef]
- Larrañeta, E.; Imízcoz, M.; Toh, J.X.; Irwin, N.J.; Ripolin, A.; Perminova, A.; Domínguez-Robles, J.; Rodríguez, A.; Donnelly, R.F. Synthesis and Characterization of Lignin Hydrogels for Potential Applications as Drug Eluting Antimicrobial Coatings for Medical Materials. ACS Sustain. Chem. Eng. 2018, 6, 9037–9046. [Google Scholar] [CrossRef]
- Larrañeta, E.; Lutton, R.E.M.; Brady, A.J.; Vicente-Pérez, E.M.; Woolfson, A.D.; Thakur, R.R.S.; Donnelly, R.F. Microwave-Assisted Preparation of Hydrogel-Forming Microneedle Arrays for Transdermal Drug Delivery Applications. Macromol. Mater. Eng. 2015, 300, 586–595. [Google Scholar] [CrossRef]
- Espinoza-Acosta, J.L.; Torres-Chávez, P.I.; Ramírez-Wong, B.; López-Saiz, C.M.; Montaño-Leyva, B. Antioxidant, Antimicrobial, and Antimutagenic Properties of Technical Lignins and Their Applications. BioResources 2016, 11, 5452–5481. [Google Scholar] [CrossRef]
- Liu, D.; Li, Y.; Qian, Y.; Xiao, Y.; Du, S.; Qiu, X. Synergistic Antioxidant Performance of Lignin and Quercetin Mixtures. ACS Sustain. Chem. Eng. 2017, 5, 8424–8428. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Fouladian, P.; Parikh, A.; Barclay, T.G.; Song, Y.; Garg, S. Preparation and Characterization of Oxidized Inulin Hydrogel for Controlled Drug Delivery. Pharmaceutics 2019, 11, 356. [Google Scholar] [CrossRef]
- Mahmood, A.; Erum, A.; Mumtaz, S.; Tulain, U.R.; Malik, N.S.; Alqahtani, M.S. Preliminary Investigation of Linum usitatissimum Mucilage-Based Hydrogel as Possible Substitute to Synthetic Polymer-Based Hydrogels for Sustained Release Oral Drug Delivery. Gels 2022, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, F.A.; Hussain, M.A.; Ashraf, M.U.; Haseeb, M.T.; Farid-ul-Haq, M. Linseed hydrogel based floating drug delivery system for fluoroquinolone antibiotics: Design, in vitro drug release and in vivo real-time floating detection. Saudi Pharm. J. 2020, 28, 538–549. [Google Scholar] [CrossRef] [PubMed]
- Imran, M.; Hussain, S.; Mehmood, K.; Saeed, Z.; Parvaiz, M.; Younas, U.; Nadeem, H.A.; Ghalani, S.P.; Saleem, S. Optimization of ecofriendly synthesis of Ag Nanoparticles by Linum usitatissimum Hydrogel using Response Surface Methodology and its biological applications. Mater. Today Commun. 2021, 29, 102789. [Google Scholar] [CrossRef]
- Muthukumar, T.; Song, J.E.; Khang, G. Biological Role of Gellan Gum in Improving Scaffold Drug Delivery, Cell Adhesion Properties for Tissue Engineering Applications. Molecules 2019, 24, 4514. [Google Scholar] [CrossRef]
- Shen, X.; Shamshina, J.L.; Berton, P.; Gurau, G.; Rogers, R.D. Hydrogels based on cellulose and chitin: Fabrication, properties, and applications. Green Chem. 2016, 18, 53–75. [Google Scholar] [CrossRef]
- Pacelli, S.; Paolicelli, P.; Moretti, G.; Petralito, S.; Di Giacomo, S.; Vitalone, A.; Casadei, M.A. Gellan gum methacrylate and laponite as an innovative nanocomposite hydrogel for biomedical applications. Eur. Polym. J. 2016, 77, 114–123. [Google Scholar] [CrossRef]
- Srisuk, P.; Berti, F.V.; da Silva, L.P.; Marques, A.P.; Reis, R.L.; Correlo, V.M. Electroactive Gellan Gum/Polyaniline Spongy-Like Hydrogels. ACS Biomater. Sci. Eng. 2018, 4, 1779–1787. [Google Scholar] [CrossRef]
- Berti, F.V.; Srisuk, P.; da Silva, L.P.; Marques, A.P.; Reis, R.L.; Correlo, V.M. Synthesis and Characterization of Electroactive Gellan Gum Spongy-Like Hydrogels for Skeletal Muscle Tissue Engineering Applications. Tissue Eng. Part A 2017, 23, 968–979. [Google Scholar] [CrossRef]
- Salas, B.M.S.; Bustamante, G.A.G.; Quiroz, D.F.; Ortega, M.M.C.; Encinas, J.C.; Franco, P.J.H.; del Castillo Castro, T. Nanocomposite hydrogels of gellan gum and polypyrrole for electro-stimulated ibuprofen release application. React. Funct. Polym. 2022, 176, 105296. [Google Scholar] [CrossRef]
- Ilkar Erdagi, S.; Ngwabebhoh, A.F.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Feng, R.; Li, J.; Wang, Y.; Song, Y.; Tan, G.; Liu, D.; Liu, W.; Yang, X.; Pan, H.; et al. A hybrid genipin-crosslinked dual-sensitive hydrogel/nanostructured lipid carrier ocular drug delivery platform. Asian J. Pharm. Sci. 2019, 14, 423–434. [Google Scholar] [CrossRef]
- Yu, Y.; Feng, R.; Yu, S.; Li, J.; Wang, Y.; Song, Y.; Yang, X.; Pan, W.; Li, S. Nanostructured lipid carrier-based pH and temperature dual-responsive hydrogel composed of carboxymethyl chitosan and poloxamer for drug delivery. Int. J. Biol. Macromol. 2018, 114, 462–469. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, S.; Yu, S.; Li, J.; Tan, G.; Li, S.; Pan, W. A Hybrid Genipin-Cross-Linked Hydrogel/Nanostructured Lipid Carrier for Ocular Drug Delivery: Cellular, ex Vivo, and in Vivo Evaluation. ACS Biomater. Sci. Eng. 2020, 6, 1543–1552. [Google Scholar] [CrossRef]
- Ng, V.W.L.; Chan, J.M.W.; Sardon, H.; Ono, R.J.; García, J.M.; Yang, Y.Y.; Hedrick, J.L. Antimicrobial hydrogels: A new weapon in the arsenal against multidrug-resistant infections. Adv. Drug Deliv. Rev. 2014, 78, 46–62. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Han, Q.; Chen, B.; Zheng, Y.; Zhang, K.; Li, Q.; Wang, J. Antimicrobial hydrogels: Promising materials for medical application. Int. J. Nanomed. 2018, 13, 2217–2263. [Google Scholar] [CrossRef]
- Wu, F.; Meng, G.; He, J.; Wu, Y.; Wu, F.; Gu, Z. Antibiotic-Loaded Chitosan Hydrogel with Superior Dual Functions: Antibacterial Efficacy and Osteoblastic Cell Responses. ACS Appl. Mater. Interfaces 2014, 6, 10005–10013. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.C.; Chin, S.F.; Tay, S.H.; Tchong, F.M. Starch–maleate–polyvinyl alcohol hydrogels with controllable swelling behaviors. Carbohydr. Polym. 2011, 84, 424–429. [Google Scholar] [CrossRef]
- Mackiewicz, M.; Romanski, J.; Drozd, E.; Gruber-Bzura, B.; Fiedor, P.; Stojek, Z.; Karbarz, M. Nanohydrogel with N, N′-bis(acryloyl)cystine crosslinker for high drug loading. Int. J. Pharm. 2017, 523, 336–342. [Google Scholar] [CrossRef]
- Jain, S.; Bajpai, A.K. Designing polyethylene glycol (PEG)–Plasticized membranes of poly (vinyl alcohol-g-methyl methacrylate) and investigation of water sorption and blood compatibility behaviors. Des. Monomers Polym. 2012, 16, 436–446. [Google Scholar] [CrossRef]
- Xu, Z.; Li, J.; Zhou, H.; Jiang, X.; Yang, C.; Wang, F.; Pan, Y.; Li, N.; Li, X.; Shi, L.; et al. Morphological and swelling behavior of cellulose nanofiber (CNF)/poly(vinyl alcohol) (PVA) hydrogels: Poly(ethylene glycol) (PEG) as porogen. RSC Adv. 2016, 6, 43626–43633. [Google Scholar] [CrossRef]
- Liu, J.; Qi, C.; Tao, K.; Zhang, J.; Zhang, J.; Xu, L.; Jiang, X.; Zhang, Y.; Huang, L.; Li, Q.; et al. Sericin/Dextran Injectable Hydrogel as an Optically Trackable Drug Delivery System for Malignant Melanoma Treatment. ACS Appl. Mater. Interfaces 2016, 8, 6411–6422. [Google Scholar] [CrossRef] [PubMed]
- Guaresti, O.; Basasoro, S.; González, K.; Eceiza, A.; Gabilondo, N. In situ cross–linked chitosan hydrogels via Michael addition reaction based on water–soluble thiol–maleimide precursors. Eur. Polym. J. 2019, 119, 376–384. [Google Scholar] [CrossRef]
- Lu, M.; Liu, Y.; Huang, Y.-C.; Huang, C.-J.; Tsai, W.-B. Fabrication of photo-crosslinkable glycol chitosan hydrogel as a tissue adhesive. Carbohydr. Polym. 2018, 181, 668–674. [Google Scholar] [CrossRef]
- Davachi, S.M.; Haramshahi, S.M.A.; Akhavirad, S.A.; Bahrami, N.; Hassanzadeh, S.; Ezzatpour, S.; Hassanzadeh, N.; Malekzadeh Kebria, M.; Khanmohammadi, M.; Bagher, Z. Development of chitosan/hyaluronic acid hydrogel scaffolds via enzymatic reaction for cartilage tissue engineering. Mater. Today Commun. 2022, 30, 103230. [Google Scholar] [CrossRef]
- Khoshfetrat, A.B.; Khanmohammadi, M.; Sakai, S.; Taya, M. Enzymatically gellable galactosylated chitosan: Hydrogel characteristics and hepatic cell behavior. Int. J. Biol. Macromol. 2016, 92, 892–899. [Google Scholar] [CrossRef]






| Natural Polymer | Technological Process | Refs. |
|---|---|---|
| Cellulose | Production of Sustainable Cellulose (OPEFB) | [20,21,22,23] |
| Cellulose Solubilization | [52,53] | |
| Nanocellulose: CNF, CNC, BNC | [35,36,37,38,39,40,41,42,43,44,45,46,47,48] | |
| Crosslinking: CMC + PVA, hemicellulose adsorption onto CNF, BNC hydrogels | [54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] | |
| Grafting: Acrylic acid on BNC fibres | [55,75] | |
| Grafting to CNCs surface | [76] | |
| CNF + Ag NPs, CNF+ Ca2+, Fe3O4 nanoparticles + β-CD/cellulose | [77,78,79] | |
| Chitosan | Chitin deacetylation | [85,86,87,88] |
| Click reactions | [103,104,105,106] | |
| Diels–Alder Cycloaddition | [105,108,109] | |
| Huisgen’s reaction | [107,108,109,110] | |
| Derivatization through IL | [99,100,101,102] | |
| Grafting: CMCS + MCP, Michael reaction by PEGA, esterdiol polyurethane’ hydrophilic chitosan | [116,117,118,119] | |
| Beads (coated by cellulose, Silk Fibroin), microbeads through ILs, chitin microbeads | [120,121,122,123,124,125,126,127] | |
| Radiation Synthesis | [128,129,130,131,132,133] | |
| Metal–Hydrogel NPs | [73,98,134,135] | |
| Hyaluronic Acid | Modifications for hydrophobicity and bioactivity | [138,139] |
| Coupling HYA-Tyramine mediated by Carbodiimide | [141,142,143,144,145] | |
| Crosslinking in solid phase | [146] | |
| IEDDA click reaction | [147,148] | |
| Crosslinking aimed for Tissue Engineering | [149,150] | |
| Alginate | Crosslinking by cations to biopolymers | [151,152,153,154,155,156,157,158,159] |
| Metal Nano- and Microparticles (Ag-NPs, HA, MSN) | [160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184] | |
| Lignin | Lignin + PEG + GAN and MW radiation | [219,220,221,222] |
| Inulin | Oxidation by periodate, then crosslinking with AAD, accelerated by MW | [223] |
| Gellan Gum | Electroactive GG crosslinked to amines, such as polyaniline, and incorporating PPy to realize EDRDs | [227,228,229,230,231,232] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombino, S.; Sole, R.; Di Gioia, M.L.; Procopio, D.; Curcio, F.; Cassano, R. Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis. Molecules 2023, 28, 2107. https://doi.org/10.3390/molecules28052107
Trombino S, Sole R, Di Gioia ML, Procopio D, Curcio F, Cassano R. Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis. Molecules. 2023; 28(5):2107. https://doi.org/10.3390/molecules28052107
Chicago/Turabian StyleTrombino, Sonia, Roberta Sole, Maria Luisa Di Gioia, Debora Procopio, Federica Curcio, and Roberta Cassano. 2023. "Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis" Molecules 28, no. 5: 2107. https://doi.org/10.3390/molecules28052107
APA StyleTrombino, S., Sole, R., Di Gioia, M. L., Procopio, D., Curcio, F., & Cassano, R. (2023). Green Chemistry Principles for Nano- and Micro-Sized Hydrogel Synthesis. Molecules, 28(5), 2107. https://doi.org/10.3390/molecules28052107









