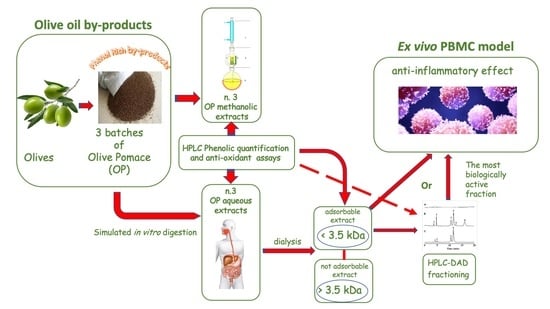
Characterization and Biological Activities of In Vitro Digested Olive Pomace Polyphenols Evaluated on Ex Vivo Human Immune Blood Cells
Abstract
:1. Introduction
2. Results
2.1. Total Phenol Content (TPC) and Phenolic Characterization of OP Extracts
2.1.1. Total Phenol Content (TPC) and Phenolic Characterization of Methanolic OP Extracts
2.1.2. Total Phenol Quantification (TPC) and Phenolic Characterization of Aqueous OP Extracts
2.2. Antioxidant Properties and Reducing Power of OP Extracts
2.2.1. Antioxidant Properties and Reducing Power (ABTS, DPPH and FRAP Assays) of Methanolic OP Extracts
2.2.2. Antioxidant Properties and Reducing Power (ABTS, and FRAP Assays) of Aqueous OP-W n.a. and OP-W Extracts after In Vitro Digestion and Dialysis
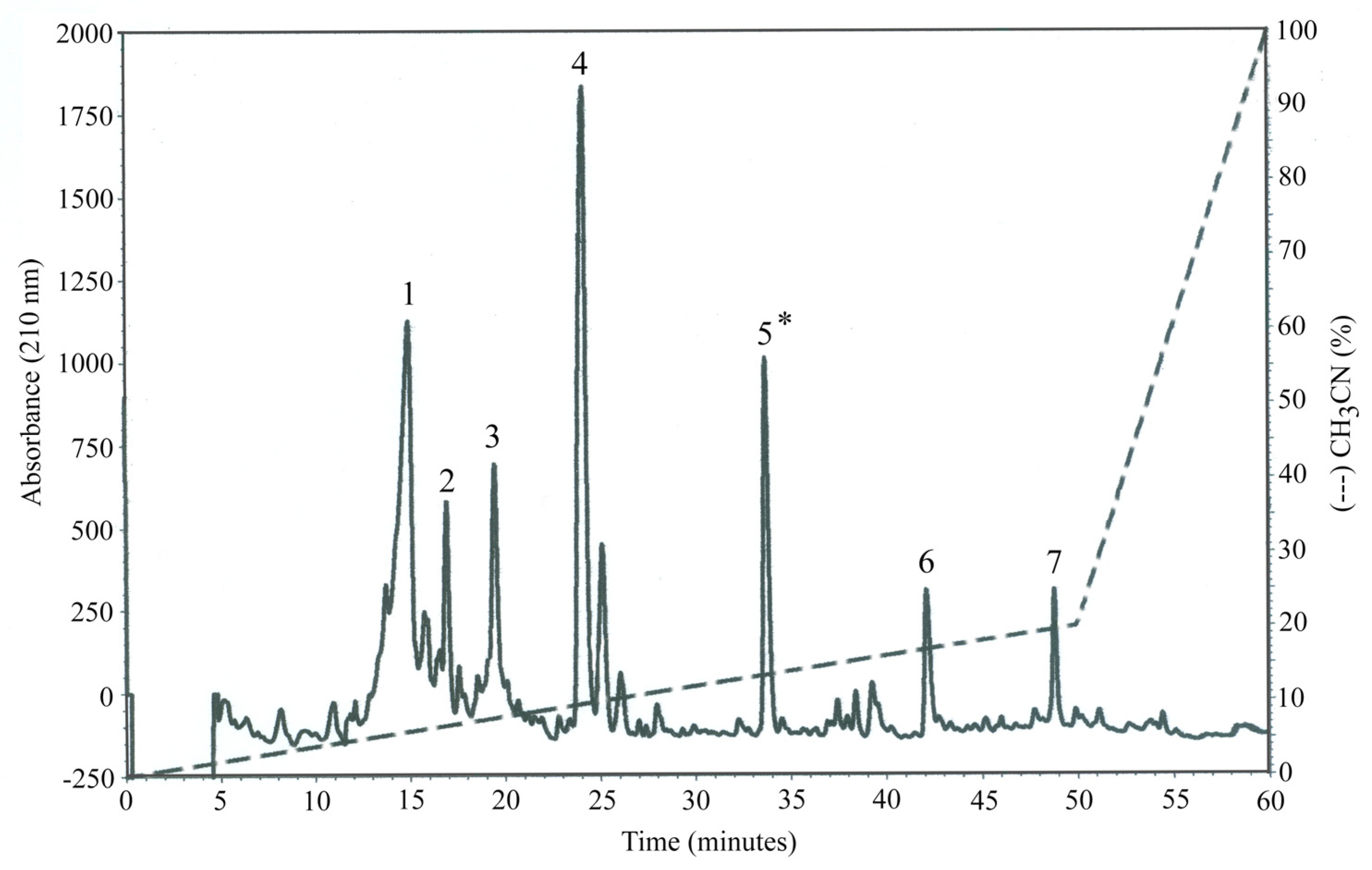
2.2.3. Antioxidant Property (ABTS) and TPC of Aqueous OP-W Fractions (OP-F)
2.3. OP-W Peptide Identification and Possible Bioactivity
2.4. Untargeted Metabolomics of the Most Bioactive OP-F
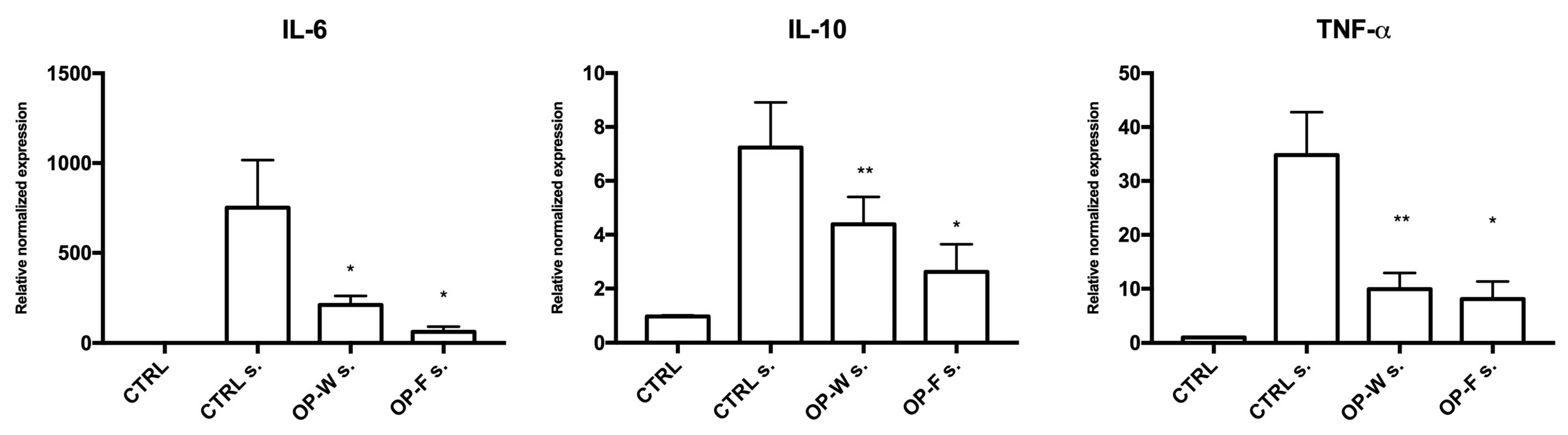
2.5. Cellular Anti-Inflammatory Activities
2.6. Cytokines Concentrations in Conditioned Medium
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Olive Pomace Methanolic Extracts
4.2.1. Radical Scavenging Activity Assays in Methanolic OP Extracts
4.2.2. Ferric Reducing Antioxidant Power (FRAP) Assay in Methanolic OP Extracts
4.2.3. TPC of Methanolic OP Extracts
4.3. OP In Vitro Digestion and OP Aqueous Extracts
4.3.1. Radical Scavenging Activity Assays in Aqueous OP-W n.a. and OP-W Extracts
4.3.2. Ferric Reducing Antioxidant Power (FRAP) Assay in Aqueous OP-W n.a. and OP-W Extracts
4.3.3. TPC in OP-W n.a., OP-W
4.3.4. HPLC-DAD Analysis of Methanolic and Aqueous OP Extracts
4.3.5. HPLC-DAD Fractioning of OP-W (OP-F)
4.3.6. LC-MS/MS Peptide Profiling of OP-W
4.3.7. Search of Potential Biological Activities and Peptide Ranking
4.3.8. Untargeted Metabolomics of OP-F by GCMS Analysis
4.4. Human PBMC Culture, Supplementation and RNA Extraction
4.5. Analysis of mRNA Levels by Real Time Reverse Transcriptase-Polymerase Chain Reaction (Real Time RT-PCR)
4.6. Measurment of PBMC Cytokines in Conditioned Medium
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; Van Woerden, F.; Lonkova, K.; Morton, J.; Poveda, R.A.; Sarraf, M.; Malkawi, F.; Harinath, A.S.; et al. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; Urban Development Series; World Bank Group: Washington, DC, USA, 2018; ISBN 978-1-4648-1347-4. [Google Scholar]
- Tapia-Quirós, P.; Montenegro-Landívar, M.F.; Reig, M.; Vecino, X.; Cortina, J.L.; Saurina, J.; Granados, M. Recovery of Polyphenols from Agri-Food By-Products: The Olive Oil and Winery Industries Cases. Foods 2022, 11, 362. [Google Scholar] [CrossRef] [PubMed]
- Fava, F.; Zanaroli, G.; Vannini, L.; Guerzoni, E.; Bordoni, A.; Viaggi, D.; Robertson, J.; Waldron, K.; Bald, C.; Esturo, A.; et al. New Advances in the Integrated Management of Food Processing By-Products in Europe: Sustainable Exploitation of Fruit and Cereal Processing by-Products with the Production of New Food Products (NAMASTE EU). New Biotechnol. 2013, 30, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Difonzo, G.; Gennaro, G.; Pasqualone, A.; Caponio, F. Potential Use of Plant-based By-products and Waste to Improve the Quality of Gluten-free Foods. J. Sci. Food Agric. 2022, 102, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Malekjani, N.; Jafari, S.M. Valorization of Olive Processing By-Products via Drying Technologies: A Case Study on the Recovery of Bioactive Phenolic Compounds from Olive Leaves, Pomace, and Wastewater. Crit. Rev. Food Sci. Nutr. 2022, 1–19. [Google Scholar] [CrossRef]
- Banias, G.; Achillas, C.; Vlachokostas, C.; Moussiopoulos, N.; Stefanou, M. Environmental Impacts in the Life Cycle of Olive Oil: A Literature Review: Environmental Impacts in the Life Cycle of Olive Oil. J. Sci. Food Agric. 2017, 97, 1686–1697. [Google Scholar] [CrossRef] [PubMed]
- Mallamaci, R.; Budriesi, R.; Clodoveo, M.L.; Biotti, G.; Micucci, M.; Ragusa, A.; Curci, F.; Muraglia, M.; Corbo, F.; Franchini, C. Olive Tree in Circular Economy as a Source of Secondary Metabolites Active for Human and Animal Health Beyond Oxidative Stress and Inflammation. Molecules 2021, 26, 1072. [Google Scholar] [CrossRef] [PubMed]
- Barbalace, M.C.; Zallocco, L.; Beghelli, D.; Ronci, M.; Scortichini, S.; Digiacomo, M.; Macchia, M.; Mazzoni, M.R.; Fiorini, D.; Lucacchini, A.; et al. Antioxidant and Neuroprotective Activity of Extra Virgin Olive Oil Extracts Obtained from Quercetano Cultivar Trees Grown in Different Areas of the Tuscany Region (Italy). Antioxidants 2021, 10, 421. [Google Scholar] [CrossRef]
- Cicerale, S.; Lucas, L.; Keast, R. Antimicrobial, Antioxidant and Anti-Inflammatory Phenolic Activities in Extra Virgin Olive Oil. Curr. Opin. Biotechnol. 2012, 23, 129–135. [Google Scholar] [CrossRef]
- Marcelino, G.; Hiane, P.A.; Freitas, K.d.C.; Santana, L.F.; Pott, A.; Donadon, J.R.; de Cássia Avellaneda, G.R. Effects of Olive Oil and Its Minor Components on Cardiovascular Diseases, Inflammation, and Gut Microbiota. Nutrients 2019, 11, 1826. [Google Scholar] [CrossRef] [Green Version]
- Bassani, B.; Rossi, T.; De Stefano, D.; Pizzichini, D.; Corradino, P.; Macrì, N.; Noonan, D.M.; Albini, A.; Bruno, A. Potential Chemopreventive Activities of a Polyphenol Rich Purified Extract from Olive Mill Wastewater on Colon Cancer Cells. J. Funct. Foods 2016, 27, 236–248. [Google Scholar] [CrossRef]
- Omar, S.; Scott, C.; Hamlin, A.; Obied, H. Olive Biophenols Reduces Alzheimer’s Pathology in SH-SY5Y Cells and APPswe Mice. Int. J. Mol. Sci. 2018, 20, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guasch-Ferré, M.; Hruby, A.; Salas-Salvadó, J.; Martínez-González, M.A.; Sun, Q.; Willett, W.C.; Hu, F.B. Olive Oil Consumption and Risk of Type 2 Diabetes in US Women. Am. J. Clin. Nutr. 2015, 102, 479–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camargo, A.; Ruano, J.; Fernandez, J.M.; Parnell, L.D.; Jimenez, A.; Santos-Gonzalez, M.; Marin, C.; Perez-Martinez, P.; Uceda, M.; Lopez-Miranda, J.; et al. Gene Expression Changes in Mononuclear Cells in Patients with Metabolic Syndrome after Acute Intake of Phenol-Rich Virgin Olive Oil. BMC Genom. 2010, 11, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, G.; Spencer, J.; Dessì, M. Extra Virgin Olive Oil Phenolics: Absorption, Metabolism, and Biological Activities in the GI Tract. Toxicol. Ind. Health 2009, 25, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Castrogiovanni, P.; Trovato, F.; Loreto, C.; Nsir, H.; Szychlinska, M.; Musumeci, G. Nutraceutical Supplements in the Management and Prevention of Osteoarthritis. Int. J. Mol. Sci. 2016, 17, 2042. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Chiavaroli, A.; Orlando, G.; Menghini, L.; Ferrante, C.; Di Cesare Mannelli, L.; Ghelardini, C.; Brunetti, L.; Leone, S. Protective Effects Induced by Two Polyphenolic Liquid Complexes from Olive (Olea Europaea, Mainly Cultivar Coratina) Pressing Juice in Rat Isolated Tissues Challenged with LPS. Molecules 2019, 24, 3002. [Google Scholar] [CrossRef] [Green Version]
- Markhali, F.S.; Teixeira, J.A.; Rocha, C.M.R. Olive Tree Leaves—A Source of Valuable Active Compounds. Processes 2020, 8, 1177. [Google Scholar] [CrossRef]
- Žugčić, T.; Abdelkebir, R.; Alcantara, C.; Collado, M.C.; García-Pérez, J.V.; Meléndez-Martínez, A.J.; Režek Jambrak, A.; Lorenzo, J.M.; Barba, F.J. From Extraction of Valuable Compounds to Health Promoting Benefits of Olive Leaves through Bioaccessibility, Bioavailability and Impact on Gut Microbiota. Trends Food Sci. Technol. 2019, 83, 63–77. [Google Scholar] [CrossRef]
- Leouifoudi, I.; Harnafi, H.; Zyad, A. Olive Mill Waste Extracts: Polyphenols Content, Antioxidant, and Antimicrobial Activities. Adv. Pharmacol. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef]
- Barbaro, B.; Toietta, G.; Maggio, R.; Arciello, M.; Tarocchi, M.; Galli, A.; Balsano, C. Effects of the Olive-Derived Polyphenol Oleuropein on Human Health. Int. J. Mol. Sci. 2014, 15, 18508–18524. [Google Scholar] [CrossRef] [Green Version]
- Antónia Nunes, M.; Costa, A.S.G.; Bessada, S.; Santos, J.; Puga, H.; Alves, R.C.; Freitas, V.; Oliveira, M.B.P.P. Olive Pomace as a Valuable Source of Bioactive Compounds: A Study Regarding Its Lipid- and Water-Soluble Components. Sci. Total Environ. 2018, 644, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.B.; Oliveira, A.; Campos, D.; Nunes, J.; Vicente, A.A.; Pintado, M. Simulated Digestion of an Olive Pomace Water-Soluble Ingredient: Relationship between the Bioaccessibility of Compounds and Their Potential Health Benefits. Food Funct. 2020, 11, 2238–2254. [Google Scholar] [CrossRef] [PubMed]
- Di Nunzio, M.; Picone, G.; Pasini, F.; Caboni, M.F.; Gianotti, A.; Bordoni, A.; Capozzi, F. Olive Oil Industry By-Products. Effects of a Polyphenol-Rich Extract on the Metabolome and Response to Inflammation in Cultured Intestinal Cell. Food Res. Int. 2018, 113, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullón, P.; Gullón, B.; Astray, G.; Carpena, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Valorization of By-Products from Olive Oil Industry and Added-Value Applications for Innovative Functional Foods. Food Res. Int. 2020, 137, 109683. [Google Scholar] [CrossRef] [PubMed]
- Diab, F.; Khalil, M.; Lupidi, G.; Zbeeb, H.; Salis, A.; Damonte, G.; Bramucci, M.; Portincasa, P.; Vergani, L. Influence of Simulated In Vitro Gastrointestinal Digestion on the Phenolic Profile, Antioxidant, and Biological Activity of Thymbra spicata L. Extracts. Antioxidants 2022, 11, 1778. [Google Scholar] [CrossRef]
- Gómez-Lama Cabanás, C.; Legarda, G.; Ruano-Rosa, D.; Pizarro-Tobías, P.; Valverde-Corredor, A.; Niqui, J.L.; Triviño, J.C.; Roca, A.; Mercado-Blanco, J. Indigenous Pseudomonas Spp. Strains from the Olive (Olea Europaea L.) Rhizosphere as Effective Biocontrol Agents against Verticillium Dahliae: From the Host Roots to the Bacterial Genomes. Front. Microbiol. 2018, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Bartolomei, M.; Capriotti, A.L.; Li, Y.; Bollati, C.; Li, J.; Cerrato, A.; Cecchi, L.; Pugliese, R.; Bellumori, M.; Mulinacci, N.; et al. Exploitation of Olive (Olea Europaea L.) Seed Proteins as Upgraded Source of Bioactive Peptides with Multifunctional Properties: Focus on Antioxidant and Dipeptidyl-Dipeptidase—IV Inhibitory Activities, and Glucagon-like Peptide 1 Improved Modulation. Antioxidants 2022, 11, 1730. [Google Scholar] [CrossRef]
- Aiello, G.; Lammi, C.; Boschin, G.; Zanoni, C.; Arnoldi, A. Exploration of Potentially Bioactive Peptides Generated from the Enzymatic Hydrolysis of Hempseed Proteins. J. Agric. Food Chem. 2017, 65, 10174–10184. [Google Scholar] [CrossRef]
- Baj, A.; Moro, E.; Bistoletti, M.; Orlandi, V.; Crema, F.; Giaroni, C. Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis. Int. J. Mol. Sci. 2019, 20, 1482. [Google Scholar] [CrossRef] [Green Version]
- Tomé, D. The Roles of Dietary Glutamate in the Intestine. Ann. Nutr. Metab. 2018, 73, 15–20. [Google Scholar] [CrossRef]
- Araújo, M.; Pimentel, F.B.; Alves, R.C.; Oliveira, M.B.P.P. Phenolic Compounds from Olive Mill Wastes: Health Effects, Analytical Approach and Application as Food Antioxidants. Trends Food Sci. Technol. 2015, 45, 200–211. [Google Scholar] [CrossRef]
- Peralbo-Molina, Á.; Priego-Capote, F.; Luque de Castro, M.D. Tentative Identification of Phenolic Compounds in Olive Pomace Extracts Using Liquid Chromatography–Tandem Mass Spectrometry with a Quadrupole–Quadrupole-Time-of-Flight Mass Detector. J. Agric. Food Chem. 2012, 60, 11542–11550. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J.; Nunes, M.A.; Reszczyński, F.; Costa, A.S.G.; Oliveira, M.B.P.P.; Alves, R.C. Near Infrared (NIR) Spectroscopy as a Tool to Assess Blends Composition and Discriminate Antioxidant Activity of Olive Pomace Cultivars. Waste Biomass Valor 2021, 12, 4901–4913. [Google Scholar] [CrossRef]
- Ryan, D.; Prenzler, P.D.; Lavee, S.; Antolovich, M.; Robards, K. Quantitative Changes in Phenolic Content during Physiological Development of the Olive (Olea Europaea) Cultivar Hardy’s Mammoth. J. Agric. Food Chem. 2003, 51, 2532–2538. [Google Scholar] [CrossRef] [PubMed]
- Nunes, M.A.; Palmeira, J.D.; Melo, D.; Machado, S.; Lobo, J.C.; Costa, A.S.G.; Alves, R.C.; Ferreira, H.; Oliveira, M.B.P.P. Chemical Composition and Antimicrobial Activity of a New Olive Pomace Functional Ingredient. Pharmaceuticals 2021, 14, 913. [Google Scholar] [CrossRef] [PubMed]
- Lesage-Meessen, L.; Navarro, D.; Maunier, S.; Sigoillot, J.-C.; Lorquin, J.; Delattre, M.; Simon, J.-L.; Asther, M.; Labat, M. Simple Phenolic Content in Olive Oil Residues as a Function of Extraction Systems. Food Chem. 2001, 75, 501–507. [Google Scholar] [CrossRef]
- Navajas-Porras, B.; Pérez-Burillo, S.; Morales-Pérez, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship of Quality Parameters, Antioxidant Capacity and Total Phenolic Content of EVOO with Ripening State and Olive Variety. Food Chem. 2020, 325, 126926. [Google Scholar] [CrossRef]
- Visioli, F.; Poli, A.; Gall, C. Antioxidant and Other Biological Activities of Phenols from Olives and Olive Oil. Med. Res. Rev. 2002, 22, 65–75. [Google Scholar] [CrossRef]
- Bayram, B.; Ozcelik, B.; Grimm, S.; Roeder, T.; Schrader, C.; Ernst, I.M.A.; Wagner, A.E.; Grune, T.; Frank, J.; Rimbach, G. A Diet Rich in Olive Oil Phenolics Reduces Oxidative Stress in the Heart of SAMP8 Mice by Induction of Nrf2-Dependent Gene Expression. Rejuvenation Res. 2012, 15, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Torres de Pinedo, A.; Peñalver, P.; Morales, J.C. Synthesis and Evaluation of New Phenolic-Based Antioxidants: Structure–Activity Relationship. Food Chem. 2007, 103, 55–61. [Google Scholar] [CrossRef]
- Haris Omar, S. Oleuropein in Olive and Its Pharmacological Effects. Sci. Pharm. 2010, 78, 133–154. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Yao-Yue, C.; Qin, G.-W.; Guo, L.-H. Luteolin from Purple Perilla Mitigates ROS Insult Particularly in Primary Neurons. Neurobiol. Aging 2012, 33, 176–186. [Google Scholar] [CrossRef]
- de Bock, M.; Thorstensen, E.B.; Derraik, J.G.B.; Henderson, H.V.; Hofman, P.L.; Cutfield, W.S. Human Absorption and Metabolism of Oleuropein and Hydroxytyrosol Ingested as Olive (Olea Europaea L.) Leaf Extract. Mol. Nutr. Food Res. 2013, 57, 2079–2085. [Google Scholar] [CrossRef] [PubMed]
- Ahmad-Qasem, M.H.; Barrajón-Catalán, E.; Micol, V.; Mulet, A.; García-Pérez, J.V. Influence of Freezing and Dehydration of Olive Leaves (Var. Serrana) on Extract Composition and Antioxidant Potential. Food Res. Int. 2013, 50, 189–196. [Google Scholar] [CrossRef]
- Nikou, T.; Sakavitsi, M.E.; Kalampokis, E.; Halabalaki, M. Metabolism and Bioavailability of Olive Bioactive Constituents Based on In Vitro, In Vivo and Human Studies. Nutrients 2022, 14, 3773. [Google Scholar] [CrossRef] [PubMed]
- Galmés, S.; Reynés, B.; Palou, M.; Palou-March, A.; Palou, A. Absorption, Distribution, Metabolism, and Excretion of the Main Olive Tree Phenols and Polyphenols: A Literature Review. J. Agric. Food Chem. 2021, 69, 5281–5296. [Google Scholar] [CrossRef]
- Bender, C.; Strassmann, S.; Golz, C. Oral Bioavailability and Metabolism of Hydroxytyrosol from Food Supplements. Nutrients 2023, 15, 325. [Google Scholar] [CrossRef]
- Mechi, D.; Baccouri, B.; Martín-Vertedor, D.; Abaza, L. Bioavailability of Phenolic Compounds in Californian-Style Table Olives with Tunisian Aqueous Olive Leaf Extracts. Molecules 2023, 28, 707. [Google Scholar] [CrossRef]
- Domínguez-Perles, R.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, A. Gender Differences in Plasma and Urine Metabolites from Sprague–Dawley Rats after Oral Administration of Normal and High Doses of Hydroxytyrosol, Hydroxytyrosol Acetate, and DOPAC. Eur. J. Nutr. 2017, 56, 215–224. [Google Scholar] [CrossRef]
- Sakavitsi, M.E.; Breynaert, A.; Nikou, T.; Lauwers, S.; Pieters, L.; Hermans, N.; Halabalaki, M. Availability and Metabolic Fate of Olive Phenolic Alcohols Hydroxytyrosol and Tyrosol in the Human GI Tract Simulated by the In Vitro GIDM–Colon Model. Metabolites 2022, 12, 391. [Google Scholar] [CrossRef]
- Alemán-Jiménez, C.; Domínguez-Perles, R.; Medina, S.; Prgomet, I.; López-González, I.; Simonelli-Muñoz, A.; Campillo-Cano, M.; Auñón, D.; Ferreres, F.; Gil-Izquierdo, Á. Pharmacokinetics and Bioavailability of Hydroxytyrosol Are Dependent on the Food Matrix in Humans. Eur. J. Nutr. 2021, 60, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Kountouri, A.M.; Mylona, A.; Kaliora, A.C.; Andrikopoulos, N.K. Bioavailability of the Phenolic Compounds of the Fruits (Drupes) of Olea Europaea (Olives): Impact on Plasma Antioxidant Status in Humans. Phytomedicine 2007, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Seiquer, I.; Rueda, A.; Olalla, M.; Cabrera-Vique, C. Assessing the Bioavailability of Polyphenols and Antioxidant Properties of Extra Virgin Argan Oil by Simulated Digestion and Caco-2 Cell Assays. Comparative Study with Extra Virgin Olive Oil. Food Chem. 2015, 188, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, N.P.; Hedemann, M.S.; Lærke, H.N.; Knudsen, K.E.B. Multicompartmental Nontargeted LC–MS Metabolomics: Explorative Study on the Metabolic Responses of Rye Fiber versus Refined Wheat Fiber Intake in Plasma and Urine of Hypercholesterolemic Pigs. J. Proteome Res. 2013, 12, 2818–2832. [Google Scholar] [CrossRef] [PubMed]
- Karković Marković, A.; Torić, J.; Barbarić, M.; Jakobušić Brala, C. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bucciantini, M.; Leri, M.; Nardiello, P.; Casamenti, F.; Stefani, M. Olive Polyphenols: Antioxidant and Anti-Inflammatory Properties. Antioxidants 2021, 10, 1044. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, T.D. Introduction to NF-ΚB: Players, Pathways, Perspectives. Oncogene 2006, 25, 6680–6684. [Google Scholar] [CrossRef] [Green Version]
- Kim, E.K.; Choi, E.-J. Pathological Roles of MAPK Signaling Pathways in Human Diseases. Biochim. Et Biophys. Acta (BBA) Mol. Basis Dis. 2010, 1802, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Xia, Y.; Yang, B.; Su, X.; Chen, J.; Li, W.; Jiang, T. Protective Effects of Tyrosol against LPS-Induced Acute Lung Injury via Inhibiting NF-ΚB and AP-1 Activation and Activating the HO-1/Nrf2 Pathways. Biol. Pharm. Bull. 2017, 40, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Martín-Peláez, S.; Castañer, O.; Solà, R.; Motilva, M.; Castell, M.; Pérez-Cano, F.; Fitó, M. Influence of Phenol-Enriched Olive Oils on Human Intestinal Immune Function. Nutrients 2016, 8, 213. [Google Scholar] [CrossRef] [Green Version]
- Bouayed, J.; Bohn, T. Exogenous Antioxidants—Double-Edged Swords in Cellular Redox State: Health Beneficial Effects at Physiologic Doses versus Deleterious Effects at High Doses. Oxidative Med. Cell. Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cea Pavez, I.; Lozano-Sánchez, J.; Borrás-Linares, I.; Nuñez, H.; Robert, P.; Segura-Carretero, A. Obtaining an Extract Rich in Phenolic Compounds from Olive Pomace by Pressurized Liquid Extraction. Molecules 2019, 24, 3108. [Google Scholar] [CrossRef] [Green Version]
- Chanioti, S.; Tzia, C. Extraction of Phenolic Compounds from Olive Pomace by Using Natural Deep Eutectic Solvents and Innovative Extraction Techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239. [Google Scholar] [CrossRef]
- Bionda, A.; Lopreiato, V.; Crepaldi, P.; Chiofalo, V.; Fazio, E.; Oteri, M.; Amato, A.; Liotta, L. Diet Supplemented with Olive Cake as a Model of Circular Economy: Metabolic and Endocrine Responses of Beef Cattle. Front. Sustain. Food Syst. 2022, 6, 1077363. [Google Scholar] [CrossRef]
- Chiofalo, B.; Di Rosa, A.R.; Lo Presti, V.; Chiofalo, V.; Liotta, L. Effect of Supplementation of Herd Diet with Olive Cake on the Composition Profile of Milk and on the Composition, Quality and Sensory Profile of Cheeses Made Therefrom. Animals 2020, 10, 977. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Chandrasekar, M.J.N.; Nanjan, M.J.; Suresh, B. Antioxidant Activity of Caesalpinia Digyna Root. J. Ethnopharmacol. 2007, 113, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Lacanna, A.; Petrucci, R.; Marrosu, G.; Saso, L. Evaluation of the Antioxidant Activity of Flavonoids by “Ferric Reducing Antioxidant Power” Assay and Cyclic Voltammetry. Biochim. Et Biophys. Acta (BBA) Gen. Subj. 2005, 1721, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Nkuimi Wandjou, J.G.; Lancioni, L.; Barbalace, M.C.; Hrelia, S.; Papa, F.; Sagratini, G.; Vittori, S.; Dall’Acqua, S.; Caprioli, G.; Beghelli, D.; et al. Comprehensive Characterization of Phytochemicals and Biological Activities of the Italian Ancient Apple ‘Mela Rosa Dei Monti Sibillini’. Food Res. Int. 2020, 137, 109422. [Google Scholar] [CrossRef]
- Peršurić, Ž.; Saftić Martinović, L.; Zengin, G.; Šarolić, M.; Kraljević Pavelić, S. Characterization of Phenolic and Triacylglycerol Compounds in the Olive Oil By-Product Pâté and Assay of Its Antioxidant and Enzyme Inhibition Activity. LWT 2020, 125, 109225. [Google Scholar] [CrossRef]
- Mooney, C.; Haslam, N.J.; Pollastri, G.; Shields, D.C. Towards the Improved Discovery and Design of Functional Peptides: Common Features of Diverse Classes Permit Generalized Prediction of Bioactivity. PLoS ONE 2012, 7, e45012. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Sample | Ht | T | Ole | Lig | Pin | Myr | Lut | Api | CA | ChlA | GA | TTMI | TPC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OP1 | 224.6 ± 0.4 a | 222.9 ± 1.8 a | 128.9 ± 1.9 a | 103.7 ± 0.5 a | 36.9 ± 0.9 a | 91.9 ± 0.5 a | 599.9 ± 2.7 a | 221.5 ± 5.5 a | 7.8 ± 1.1 a | 48.4 ± 4.3 a | nd | 1686.5 a | 99.8 ± 8.5 A |
| OP2 | 36.9 ± 0.5 b | 46.7 ± 2.5 b | 44.9 ± 1.7 b | 27.1 ± 0.1 b | 24.0 ± 1.3 b | 70.8 ± 1.2 b | 474.1 ± 1.7 b | 175.5 ± 0.2 b | 9.8 ± 0.1 b | 28.9 ± 3.2 b | nd | 938.7 b | 26.3 ± 3.9 B |
| OP3 | 4.9 ± 0.3 c | 8.2 ± 0.5 c | 29.4 ± 0.1 c | 24.1 ± 0.6 c | 17.8 ± 0.7 c | 72.9 ± 1.1 b | 354.7 ± 3.2 c | 129.9 ± 1.1 c | 7.5 ± 0.5 a | 11.4 ± 1.5 c | nd | 660.8 c | 14.5 ± 2.4 C |
| Sample | Ht | T | Ole | Lig | Pin | Myr | Lut | Api | CA | ChlA | GA | TTMI | TPC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OP1-W n.a. | 138.8 ± 0.4 A | 241.6 ± 6.7 A | 41.7 ± 3.0 A | 28.2 ± 2.2 | 50.6 ± 0.8 A | 201.0 ± 0.1 A | 212.2 ± 1.9 A | nd | 30.5 ± 2.8 A | 32.7 ± 1.3 A | 76.2 ± 1.4 A | 1053.5 | 127.9 ± 4.1 A |
| OP2-W n.a. | 59.4 ± 1.1 B | 101.0 ± 4.5 B | 37.8 ± 1.2 B | nd | 46.8 ± 3.4 B | 188.7 ± 2.1 B | 197.5 ± 1.6 B | nd | 24.8 ± 0.6 B | 28.9 ± 3.0 A | 93.8 ± 1.3 B | 778.7 | 96.8 ± 10.6 B |
| OP3-W n.a. | nd | nd | 12.8 ± 1.0 C | nd | 48.1 ± 1.3 AB | 183.0 ± 2.3 B | 192.2 ± 1.0 B | nd | 18.0 ± 1.2 C | 18.4 ± 1.1 B | 295.4 ± 2.1 C | 767.9 | 88.5 ± 21.7 B |
| OP1-W | 386.7 ± 3.1 a | 627.8 ± 3.4 a | 129.8 ± 4.8 a | 23.9 ± 1.3 a | 41.4 ± 1.7 a | 161.6 ± 1.5 a | 168.4 ± 1.0 a | nd | 36.2 ± 0.5 a | 57.5 ± 2.1 a | 56.0 ± 0.6 a | 1527.7 | 96.1 ± 3.0 a |
| OP2-W | 62.5 ± 1.4 b | 77.7 ± 1.8 b | 13.1 ± 1.1 b | 15.4 ± 0.9 b | 28.7 ± 0.8 b | 118.9 ± 0.3 b | 124.4 ± 0.7 b | nd | 14.1 ± 0.8 b | 26.9 ± 1.4 b | 34.9 ± 0.7 b | 516.6 | 72.4 ± 13.9 b |
| OP3-W | nd | nd | 23.9 ± 1.1 c | 20.7 ± 1.7 a | 26.3 ± 1.8 b | 100.5 ± 0.3 c | 105.1 ± 0.1 c | nd | 12.8 ± 1.7 b | 15.0 ± 1.3 c | 34.1 ± 3.1 b | 338.4 | 68.5 ± 7.6 b |
| Sample | Ht | T | Ole | Lig | Pin | Myr | Lut | Api | CA | ChlA | GA |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OP1-W | 86.1 | 140.8 | 50.3 | 11.5 | 56.1 | 87.9 | 14.0 | - | 231.2 | 59.4 | - |
| OP2-W | 84.5 | 83.2 | 14.6 | 28.3 | 60.0 | 84.0 | 13.1 | - | 71.5 | 46.6 | - |
| OP3-W | - | - | 40.7 | 43.0 | 74.0 | 68.9 | 14.8 | - | 84.9 | 66.1 | - |
| Radical Scavenging Assays | |||
|---|---|---|---|
| ABTS | DPPH | FRAP | |
| Samples | Trolox Equivalent ± SD | Trolox Equivalent ± SD | Trolox Equivalent ± SD |
| OP1 | 124.6 ± 4.2 a | 44.5 ± 2.5 a | 74.6 ± 3.5 a |
| OP2 | 55.6 ± 4.1 b | 19.6 ± 2.4 b | 31.5 ± 1.5 b |
| OP3 | 19.5 ± 1.2 c | 9.22 ± 0.7 c | 6.3 ± 0.07 c |
| ABTS | FRAP | |
|---|---|---|
| Samples | Trolox Equivalent ± SD | Trolox Equivalent ± SD |
| OP-W n.a. 1 | ||
| 1 | 127.3 ± 7.4 a | 88.3 ± 3.1 a |
| 2 | 79.5 ± 6.5 b | 45.1 ± 2.2 b |
| 3 | 74.3 ± 10.5 b | 40 ± 0.1 b |
| OP-W 2 | ||
| 1 | 82.5 ± 4.5 a | 44.8 ± 0.8 a |
| 2 | 68.9 ± 3.0 b | 22.7 ± 0.6 b |
| 3 | 73.6 ± 2.2 b | 14.4 ± 1.0 c |
| ABTS | TPC | |
|---|---|---|
| OP-F | Trolox Equivalent ± SD | mg of GAE/g Extract ± SD |
| 1 | 63.3 ± 5.3 AB | 156.7 ± 0.7 A |
| 2 | 26.5 ± 0.6 A | 127.3 ± 3.1 A |
| 3 | 369.7 ± 4.0 C | 719.5 ± 10.9 B |
| 4 | 342.2 ± 5.8 C | 761.8 ± 10.0 B |
| 5 | 1036.7 ± 35.5 D | 1199.4 ± 69.9 C |
| 6 | 76.5 ± 3.2 BE | 197.4 ± 9.7 A |
| 7 | 253.7 ± 8.3 F | 450.5 ± 28.6 D |
| OP-W1 Proteins | ||||
|---|---|---|---|---|
| ID | Protein | Organism | MW | Peptides |
| J9XLG0 | Putative polyphenol oxidase | Olea europaea | 53658 | 2 |
| E3TJS3 | 50S ribosomal protein L16 | Olea europaea | 15346 | 2 |
| A0A0N9LRR6 | Amine oxidase | Olea europaea | 87207 | 4 |
| B2VPR8 | Pectin esterase 2 | Olea europaea | 39856 | 2 |
| A0A0G3FBC7 | LIP (fragment) | Olea europaea | 11938 | 2 |
| A4GE45 | Profilin-1 | Olea europaea | 14520 | 2 |
| A0A649ZUF2 | 4-coumarate-CoA ligase | Olea europaea | 59898 | 3 |
| Q5DTB7 | Ole e 3 allergen | Olea europaea | 5795 | 2 |
| J9XH65 | Putative geraniol 10-hydroxylase | Olea europaea | 46724 | 5 |
| A0A126X2X6 | Putative LOV domain-containing | Olea europaea | 70139 | 2 |
| A0A7G7YFM0 | Ribulose bisphosphate carboxylase | Olea europaea | 53072 | 2 |
| Q1W4C7 | Hexosyltransferase | Olea europaea | 31071 | 2 |
| J9XLE5 | Isopentenyl-diphospate Delta-isomerase | Olea europaea | 25822 | 2 |
| A0A1B1V5C3 | Putative transcriptional corepressor | Olea europaea | 54598 | 2 |
| Proteins from Organisms in OP-W1 Sample | ||||
|---|---|---|---|---|
| ID | Protein | Organism | MW | Peptides |
| A0A2A2DN42 | Chemotaxis protein | Pseudomonas sp. PIC141 | 57171 | 2 |
| A0A2A2DPP3 | Short chain dehydrogenase | Pseudomonas sp. PIC 125 | 28887 | 3 |
| A0A2A2E801 | Poly(A) polymerase | Pseudomonas sp. PIC 125 | 53512 | 2 |
| A0A2A2DPY9 | PhoH family protein | Pseudomonas sp. PIC 141 | 38446 | 2 |
| A0A2A2E7V3 | UvrABC system protein C | Pseudomonas sp. PIC125 | 67237 | 2 |
| A0A2A2E543 | Amine oxidase | Pseudomonas sp. PIC141 | 62484 | 2 |
| A0A2A2DX44 | Serine hydrolase | Pseudomonas sp. PIC141 | 40686 | 2 |
| A0A2A2DU16 | Haemagglutinin | Pseudomonas sp. PIC125. | 9783 | 2 |
| A0A2A2DPN3 | DUF1329 domain | Pseudomonas sp. PIC125 | 50476 | 2 |
| A0A2A2DJ68 | TonB-dep. siderophore receptor | Pseudomonas sp. PIC125 | 78117 | 3 |
| A0A2A2EB40 | Tail-specific protease | Pseudomonas sp. PIC125 | 77756 | 3 |
| A0A2A2E740 | RNA helicase | Pseudomonas sp. PIC125 | 48800 | 3 |
| A0A2A2E9Q7 | Coproporphyrinogen-III oxidase | Pseudomonas sp. PIC125 | 53148 | 2 |
| Metabolites | % |
|---|---|
| Trietanolamine | 3.07 |
| Propanamine,N(2fluorophenyl)3(4morpholyl) | 0.18 |
| Glycerol | 3.2 |
| Ciclopenthylamine | 2.26 |
| 1 propanamine N,N diproyl | 2.26 |
| Cystathyonine | 1.04 |
| Tris,N-acetyl | 1.08 |
| Homocisteine | 7.1 |
| Tyrosol | 20.49 |
| Glutamic acid | 7.61 |
| 4 Hexylphenol | 4.52 |
| Phenol,3 butanol | 0.58 |
| Bis oxyethylthiosulfide | 2.74 |
| Linoleic acid | 0.23 |
| Sugar | 3.25 |
| Gene | 5′-Forward-3′ | 5′-Reverse-3′ |
|---|---|---|
| IL-6 | GCAGAAAAAGGCAAAGAATC | CTACATTTGCCGAAGAGC |
| IL-10 | GCCTTTAATAAGCTCCAAGAG | ATCTTCATTGTCATGTAGGC |
| TNF-α | AGGCAGTCAGATCATCTTC | TTATCTCTCAGCTCCACG |
| 18S | GTAACCCGTTGAACCCCATT | CCATCCAATCGGTAGTAGCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alimenti, C.; Lianza, M.; Antognoni, F.; Giusti, L.; Bistoni, O.; Liotta, L.; Angeloni, C.; Lupidi, G.; Beghelli, D. Characterization and Biological Activities of In Vitro Digested Olive Pomace Polyphenols Evaluated on Ex Vivo Human Immune Blood Cells. Molecules 2023, 28, 2122. https://doi.org/10.3390/molecules28052122
Alimenti C, Lianza M, Antognoni F, Giusti L, Bistoni O, Liotta L, Angeloni C, Lupidi G, Beghelli D. Characterization and Biological Activities of In Vitro Digested Olive Pomace Polyphenols Evaluated on Ex Vivo Human Immune Blood Cells. Molecules. 2023; 28(5):2122. https://doi.org/10.3390/molecules28052122
Chicago/Turabian StyleAlimenti, Claudio, Mariacaterina Lianza, Fabiana Antognoni, Laura Giusti, Onelia Bistoni, Luigi Liotta, Cristina Angeloni, Giulio Lupidi, and Daniela Beghelli. 2023. "Characterization and Biological Activities of In Vitro Digested Olive Pomace Polyphenols Evaluated on Ex Vivo Human Immune Blood Cells" Molecules 28, no. 5: 2122. https://doi.org/10.3390/molecules28052122
APA StyleAlimenti, C., Lianza, M., Antognoni, F., Giusti, L., Bistoni, O., Liotta, L., Angeloni, C., Lupidi, G., & Beghelli, D. (2023). Characterization and Biological Activities of In Vitro Digested Olive Pomace Polyphenols Evaluated on Ex Vivo Human Immune Blood Cells. Molecules, 28(5), 2122. https://doi.org/10.3390/molecules28052122