Effect of Substituent Location on the Relationship between the Transition Dipole Moments, Difference Static Dipole, and Hydrophobicity in Squaraine Dyes for Quantum Information Devices
Abstract
1. Introduction
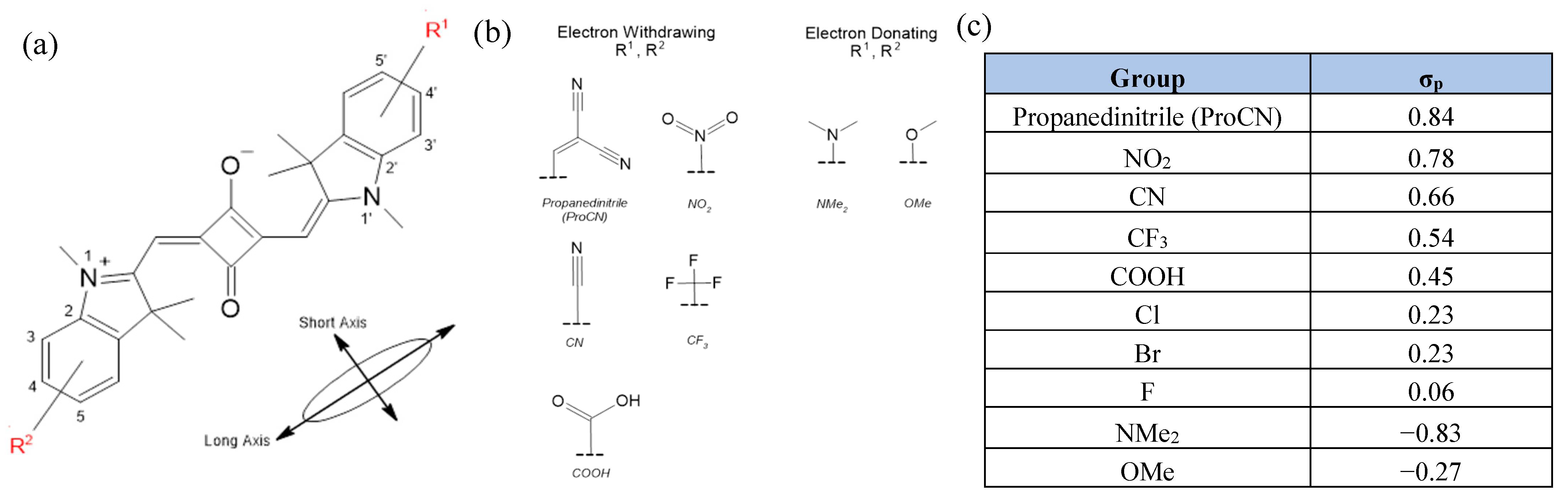
2. Results
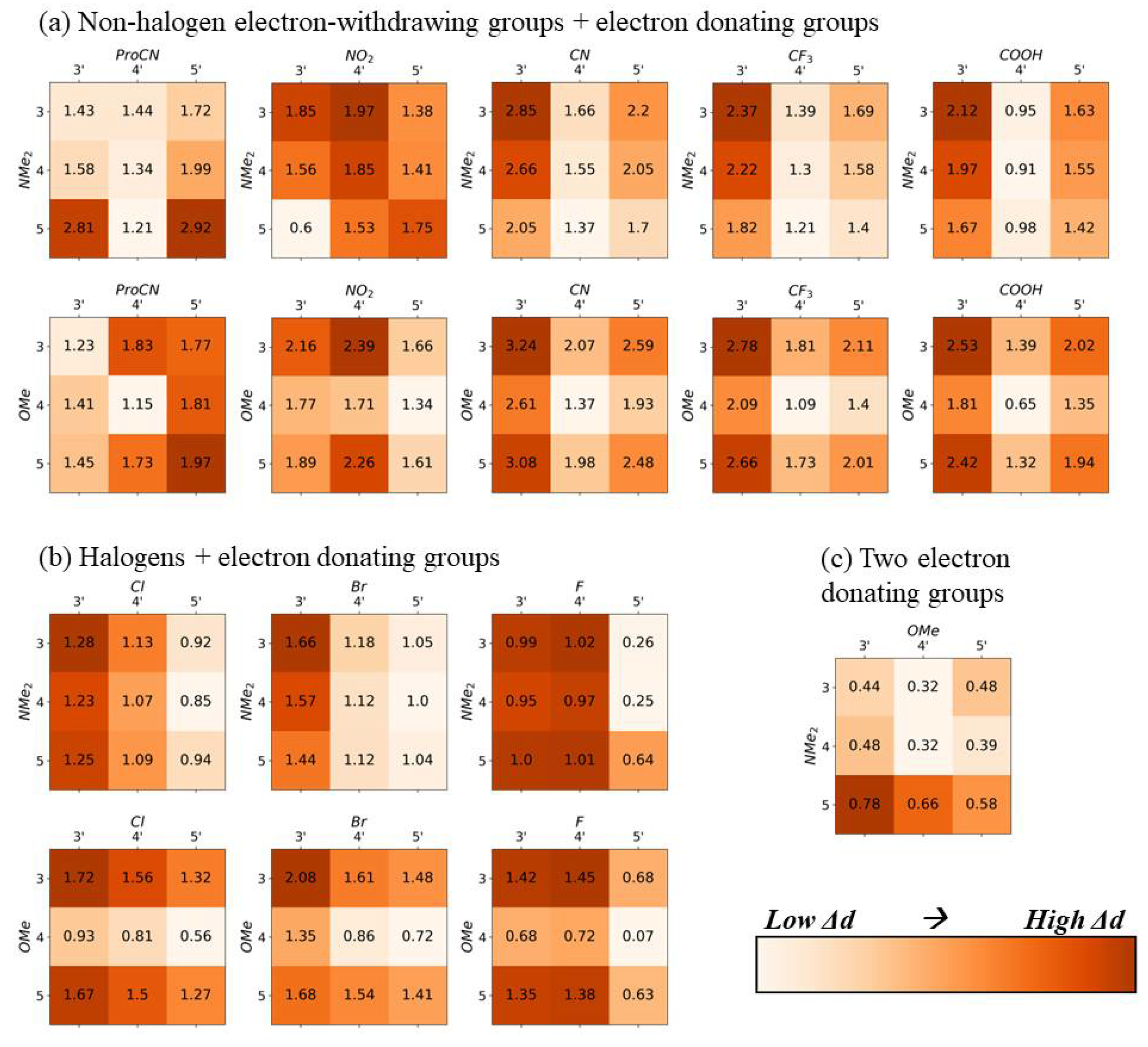
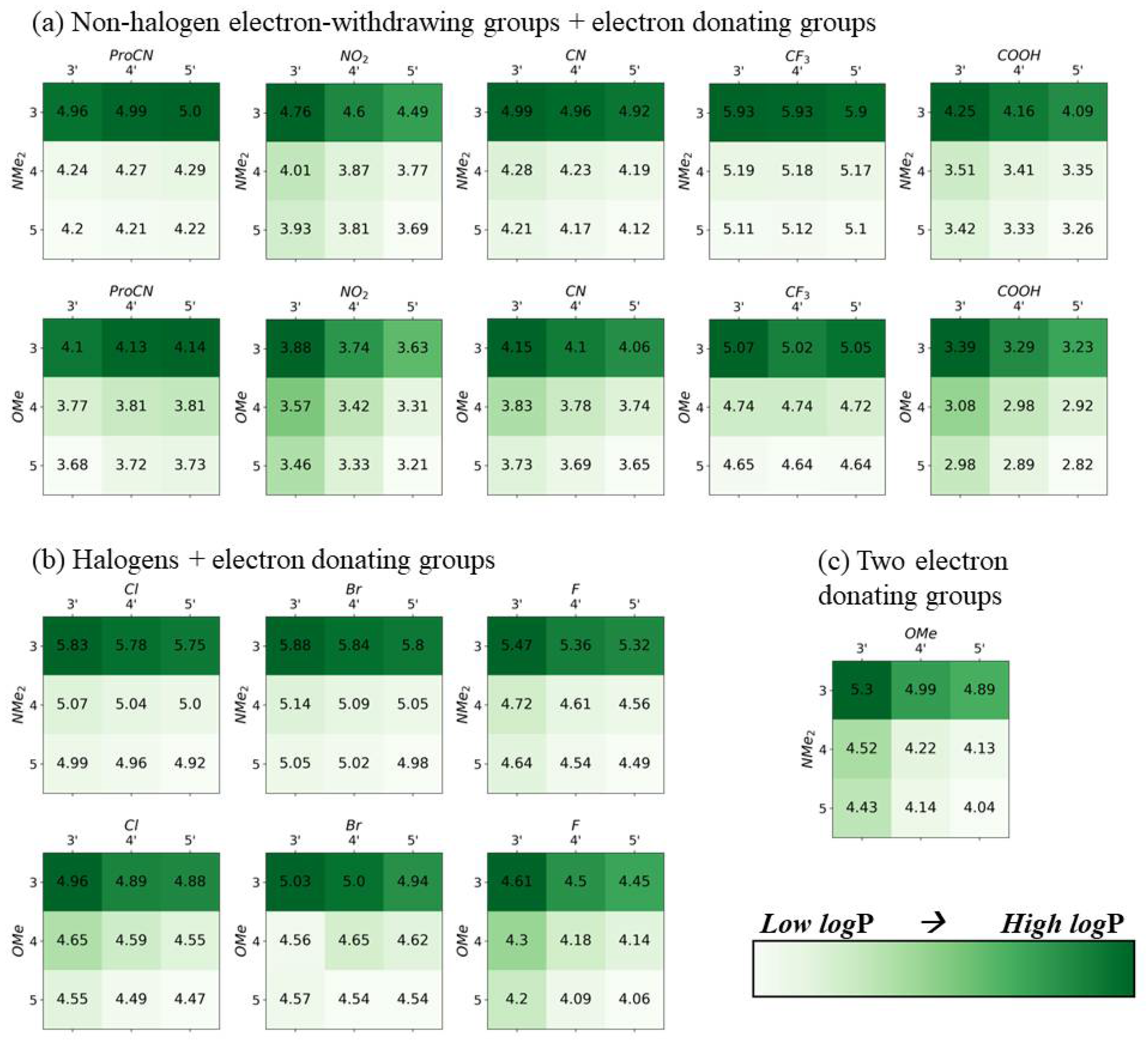
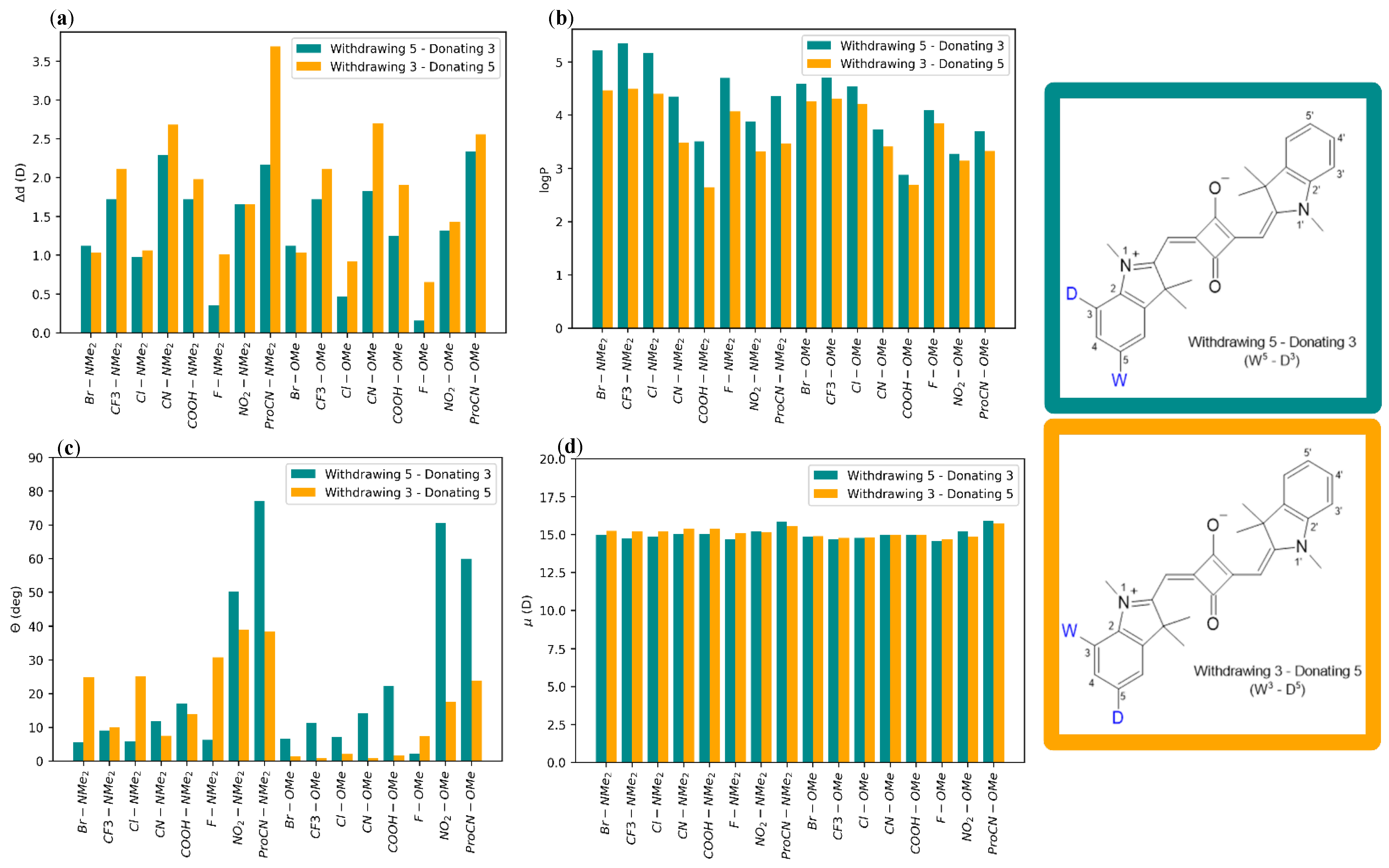
2.1. Squaraine with Substituents on Opposite Sides (“Push-Pull” Dyes)
2.2. Squaraine with Substituents on the Same Side
3. Discussion
4. Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cannon, B.L.; Patten, L.K.; Kellis, D.L.; Davis, P.H.; Lee, J.; Graugnard, E.; Yurke, B.; Knowlton, W.B. Large Davydov Splitting and Strong Fluorescence Suppression: An Investigation of Exciton Delocalization in DNA-Templated Holliday Junction Dye Aggregates. J. Phys. Chem. A 2018, 122, 2086–2095. [Google Scholar] [CrossRef]
- Mirkovic, T.; Ostroumov, E.E.; Anna, J.M.; van Grondelle, R.; Govindjee; Scholes, G.D. Light Absorption and Energy Transfer in the Antenna Complexes of Photosynthetic Organisms. Chem. Rev. 2017, 117, 249–293. [Google Scholar] [CrossRef] [PubMed]
- Monshouwer, R.; Abrahamsson, M.; van Mourik, F.; van Grondelle, R. Superradiance and Exciton Delocalization in Bacterial Photosynthetic Light-Harvesting Systems. J. Phys. Chem. B 1997, 101, 7241–7248. [Google Scholar] [CrossRef]
- Schutte, W.J.; Sluyters-Rehbach, M.; Sluyters, J.H. Aggregation of an Octasubstituted Phthalocyanine in Dodecane Solution. J. Phys. Chem. 1993, 97, 6069–6073. [Google Scholar] [CrossRef]
- Wei, G.; Xiao, X.; Wang, S.; Sun, K.; Bergemann, K.J.; Thompson, M.E.; Forrest, S.R. Functionalized Squaraine Donors for Nanocrystalline Organic Photovoltaics. ACS Nano 2012, 6, 972–978. [Google Scholar] [CrossRef]
- Zhang, L.; Cole, J.M. Dye Aggregation in Dye-Sensitized Solar Cells. J. Mater. Chem. A 2017, 5, 19541–19559. [Google Scholar] [CrossRef]
- Pastore, M.; De Angelis, F. Aggregation of Organic Dyes on TiO2 in Dye-Sensitized Solar Cells Models: An Ab Initio Investigation. ACS Nano 2010, 4, 556–562. [Google Scholar] [CrossRef]
- Chen, G.; Sasabe, H.; Igarashi, T.; Hong, Z.; Kido, J. Squaraine Dyes for Organic Photovoltaic Cells. J. Mater. Chem. A 2015, 3, 14517–14534. [Google Scholar] [CrossRef]
- Xu, S.; Liu, H.-W.; Huan, S.-Y.; Yuan, L.; Zhang, X.-B. Recent Progress in Utilizing Near-Infrared J-Aggregates for Imaging and Cancer Therapy. Mater. Chem. Front. 2021, 5, 1076–1089. [Google Scholar] [CrossRef]
- Sun, C.; Li, B.; Zhao, M.; Wang, S.; Lei, Z.; Lu, L.; Zhang, H.; Feng, L.; Dou, C.; Yin, D.; et al. J-Aggregates of Cyanine Dye for NIR-II in Vivo Dynamic Vascular Imaging beyond 1500 Nm. J. Am. Chem. Soc. 2019, 141, 19221–19225. [Google Scholar] [CrossRef]
- Zeng, W.; Xu, Y.; Yang, W.; Liu, K.; Bian, K.; Zhang, B. An Ultrasound-Excitable Aggregation-Induced Emission Dye for Enhanced Sonodynamic Therapy of Tumors. Adv. Healthc. Mater. 2020, 9, 2000560. [Google Scholar] [CrossRef]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- Tong, H.; Hong, Y.; Dong, Y.; Häussler, M.; Lam, J.W.Y.; Li, Z.; Guo, Z.; Guo, Z.; Tang, B.Z. Fluorescent “Light-up” Bioprobes Based on Tetraphenylethylene Derivatives with Aggregation-Induced Emission Characteristics. Chem. Commun. 2006, 3705–3707. [Google Scholar] [CrossRef]
- Hong, Y.; Liao, J.-Y.; Fu, J.; Kuang, D.-B.; Meier, H.; Su, C.-Y.; Cao, D. Performance of Dye-Sensitized Solar Cells Based on Novel Sensitizers Bearing Asymmetric Double D−π−A Chains with Arylamines as Donors. Dye. Pigment. 2012, 94, 481–489. [Google Scholar] [CrossRef]
- Hong, Y.; Liao, J.-Y.; Cao, D.; Zang, X.; Kuang, D.-B.; Wang, L.; Meier, H.; Su, C.-Y. Organic Dye Bearing Asymmetric Double Donor-π-Acceptor Chains for Dye-Sensitized Solar Cells. J. Org. Chem. 2011, 76, 8015–8021. [Google Scholar] [CrossRef]
- Prokhorenko, V.I.; Steensgaard, D.B.; Holzwarth, A.R. Exciton Dynamics in the Chlorosomal Antennae of the Green Bacteria Chloroflexus Aurantiacus and Chlorobium Tepidum. Biophys. J. 2000, 79, 2105–2120. [Google Scholar] [CrossRef] [PubMed]
- Huber, V.; Sengupta, S.; Würthner, F. Structure—Property Relationships for Self-Assembled Zinc Chlorin Light-Harvesting Dye Aggregates. Chem. A Eur. J. 2008, 14, 7791–7807. [Google Scholar] [CrossRef]
- Thilagam, A. QUANTUM INFORMATION PROCESSING ATTRIBUTES OF J -AGGREGATES. In J-Aggregates; WORLD SCIENTIFIC: Singapore, 2012; pp. 273–308. ISBN 978-981-4365-74-1. [Google Scholar]
- Thilagam, A. Entanglement Dynamics of J-Aggregate Systems. J. Phys. A Math. Theor. 2011, 44, 135306. [Google Scholar] [CrossRef]
- Castellanos, M.A.; Dodin, A.; Willard, A.P. On the Design of Molecular Excitonic Circuits for Quantum Computing: The Universal Quantum Gates. Phys. Chem. Chem. Phys. 2020, 22, 3048–3057. [Google Scholar] [CrossRef] [PubMed]
- Yurke, B.; Elliott, R.; Sup, A. Implementation of a Frenkel Exciton-Based Controlled Phase Shifter. Phys. Rev. A 2022, 107, 012603. [Google Scholar] [CrossRef]
- Kasha, M. Energy Transfer Mechanisms and the Molecular Exciton Model for Molecular Aggregates 1, 2. Radiat. Res. 2012, 178, AV27–AV34. [Google Scholar] [CrossRef]
- Würthner, F.; Kaiser, T.E.; Saha-Möller, C.R. J-Aggregates: From Serendipitous Discovery to Supramolecular Engineering of Functional Dye Materials. Angew. Chem. Int. Ed. 2011, 50, 3376–3410. [Google Scholar] [CrossRef] [PubMed]
- Childs, A.M.; Gosset, D.; Webb, Z. Universal Computation by Multiparticle Quantum Walk. Science 2013, 339, 791–794. [Google Scholar] [CrossRef] [PubMed]
- Jelley, E.E. Spectral Absorption and Fluorescence of Dyes in the Molecular State. Nature 1936, 138, 1009–1010. [Google Scholar] [CrossRef]
- Jelley, E.E. Molecular, Nematic and Crystal States of I: I·-Diethyl-Ψ-Cyanine Chloride. Nature 1937, 139, 631. [Google Scholar] [CrossRef]
- Zhou, X.; Mandal, S.; Jiang, S.; Lin, S.; Yang, J.; Liu, Y.; Whitten, D.G.; Woodbury, N.W.; Yan, H. Efficient Long-Range, Directional Energy Transfer through DNA-Templated Dye Aggregates. J. Am. Chem. Soc. 2019, 141, 8473–8481. [Google Scholar] [CrossRef] [PubMed]
- Mass, O.A.; Wilson, C.K.; Roy, S.K.; Barclay, M.S.; Patten, L.K.; Terpetschnig, E.A.; Lee, J.; Pensack, R.D.; Yurke, B.; Knowlton, W.B. Exciton Delocalization in Indolenine Squaraine Aggregates Templated by DNA Holliday Junction Scaffolds. J. Phys. Chem. B 2020, 124, 9636–9647. [Google Scholar] [CrossRef]
- Chowdhury, A.; Yu, L.; Raheem, I.; Peteanu, L.; Liu, L.A.; Yaron, D.J. Stark Spectroscopy of Size-Selected Helical H-Aggregates of a Cyanine Dye Templated by Duplex DNA. Effect of Exciton Coupling on Electronic Polarizabilities. J. Phys. Chem. A 2003, 107, 3351–3362. [Google Scholar] [CrossRef]
- Huff, J.S.; Davis, P.H.; Christy, A.; Kellis, D.L.; Kandadai, N.; Toa, Z.S.D.; Scholes, G.D.; Yurke, B.; Knowlton, W.B.; Pensack, R.D. DNA-Templated Aggregates of Strongly Coupled Cyanine Dyes: Nonradiative Decay Governs Exciton Lifetimes. J. Phys. Chem. Lett. 2019, 10, 2386–2392. [Google Scholar] [CrossRef]
- Seifert, J.L.; Connor, R.E.; Kushon, S.A.; Wang, M.; Armitage, B.A. Spontaneous Assembly of Helical Cyanine Dye Aggregates on DNA Nanotemplates. J. Am. Chem. Soc. 1999, 121, 2987–2995. [Google Scholar] [CrossRef]
- Asanuma, H.; Fujii, T.; Kato, T.; Kashida, H. Coherent Interactions of Dyes Assembled on DNA. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 124–135. [Google Scholar] [CrossRef]
- Nicoli, F.; Roos, M.K.; Hemmig, E.A.; Di Antonio, M.; de Vivie-Riedle, R.; Liedl, T. Proximity-Induced H-Aggregation of Cyanine Dyes on DNA-Duplexes. J. Phys. Chem. A 2016, 120, 9941–9947. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.D.; Kim, Y.C.; Díaz, S.A.; Buckhout-White, S.; Mathur, D.; Medintz, I.L.; Melinger, J.S. Optical Properties of Vibronically Coupled Cy3 Dimers on DNA Scaffolds. J. Phys. Chem. B 2018, 122, 5020–5029. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, J.W.; Hernandez, A.C.; Knowlton, W.B.; Yurke, B.; Li, L. Ab Initio Studies of Exciton Interactions of Cy5 Dyes. J. Phys. Chem. A 2018, 122, 8989–8997. [Google Scholar] [CrossRef] [PubMed]
- Königstein, C.; Bauer, R. Charge Separation in J-Aggregates of Covalently Linked Cyanine Dye-Viologen Systems. Sol. Energy Mater. Sol. Cells 1994, 31, 535–539. [Google Scholar] [CrossRef]
- Mass, O.A.; Wilson, C.K.; Barcenas, G.; Terpetschnig, E.A.; Obukhova, O.M.; Kolosova, O.S.; Tatarets, A.L.; Li, L.; Yurke, B.; Knowlton, W.B.; et al. Influence of Hydrophobicity on Excitonic Coupling in DNA-Templated Indolenine Squaraine Dye Aggregates. J. Phys. Chem. C 2022, 126, 3475–3488. [Google Scholar] [CrossRef] [PubMed]
- Biaggne, A.; Knowlton, W.B.; Yurke, B.; Lee, J.; Li, L. Substituent Effects on the Solubility and Electronic Properties of the Cyanine Dye Cy5: Density Functional and Time-Dependent Density Functional Theory Calculations. Molecules 2021, 26, 524. [Google Scholar] [CrossRef]
- Markova, L.I.; Malinovskii, V.L.; Patsenker, L.D.; Häner, R. J- vs. H-Type Assembly: Pentamethine Cyanine (Cy5) as a near-IR Chiroptical Reporter. Chem. Commun. 2013, 49, 5298–5300. [Google Scholar] [CrossRef] [PubMed]
- Markova, L.I.; Terpetschnig, E.A.; Patsenker, L.D. Comparison of a Series of Hydrophilic Squaraine and Cyanine Dyes for Use as Biological Labels. Dye. Pigment. 2013, 99, 561–570. [Google Scholar] [CrossRef]
- Kolosova, O.S.; Shishkina, S.V.; Marks, V.; Gellerman, G.; Hovor, I.V.; Tatarets, A.L.; Terpetschnig, E.A.; Patsenker, L.D. Molecular Structure and Spectral Properties of Indolenine Based Norsquaraines versus Squaraines. Dye. Pigment. 2019, 163, 318–329. [Google Scholar] [CrossRef]
- Mayerhöffer, U.; Fimmel, B.; Würthner, F. Bright Near-Infrared Fluorophores Based on Squaraines by Unexpected Halogen Effects. Angew. Chem. Int. Ed. Engl. 2012, 51, 164–167. [Google Scholar] [CrossRef]
- Bamgbelu, A.; Wang, J.; Leszczynski, J. TDDFT Study of the Optical Properties of Cy5 and Its Derivatives. J. Phys. Chem. A 2010, 114, 3551–3555. [Google Scholar] [CrossRef]
- Cao, J.; Fan, J.; Sun, W.; Guo, Y.; Wu, H.; Peng, X. The Photoprocess Effects of an Amino Group Located at Different Positions along the Polymethine Chain in Indodicarbocyanine Dyes. RSC Adv. 2017, 7, 30740–30746. [Google Scholar] [CrossRef]
- Bridhkoti, J.P.; Gahlaut, R.; Joshi, H.C.; Pant, S. Effect of Positional Substitution of Amino Group on Excited State Dipole Moments of Quinoline. J. Lumin. 2011, 131, 1869–1873. [Google Scholar] [CrossRef]
- Barcenas, G.; Biaggne, A.; Mass, O.A.; Wilson, C.K.; Obukhova, O.M.; Kolosova, O.S.; Tatarets, A.L.; Terpetschnig, E.; Pensack, R.D.; Lee, J.; et al. First-Principles Studies of Substituent Effects on Squaraine Dyes. RSC Adv. 2021, 11, 19029–19040. [Google Scholar] [CrossRef] [PubMed]
- Jacquemin, D.; Zhao, Y.; Valero, R.; Adamo, C.; Ciofini, I.; Truhlar, D.G. Verdict: Time-Dependent Density Functional Theory “Not Guilty” of Large Errors for Cyanines. J. Chem. Theory Comput. 2012, 8, 1255–1259. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.C.; Okamoto, Y. Electrophilic Substituent Constants. J. Am. Chem. Soc. 1958, 80, 4979–4987. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A Survey of Hammett Substituent Constants and Resonance and Field Parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Abramavicius, D.; Palmieri, B.; Mukamel, S. Extracting Single and Two-Exciton Couplings in Photosynthetic Complexes by Coherent Two-Dimensional Electronic Spectra. Chem. Phys. 2009, 357, 79–84. [Google Scholar] [CrossRef]
- Kee, H.L.; Kirmaier, C.; Tang, Q.; Diers, J.R.; Muthiah, C.; Taniguchi, M.; Laha, J.K.; Ptaszek, M.; Lindsey, J.S.; Bocian, D.F.; et al. Effects of Substituents on Synthetic Analogs of Chlorophylls. Part 2: Redox Properties, Optical Spectra and Electronic Structure. Photochem. Photobiol. 2007, 83, 1125–1143. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev., C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J. GaussView Version 6 2016. Available online: https://gaussian.com/gaussview6/ (accessed on 11 February 2023).
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Ratner, M.A.; Pople, J.A.; Redfern, P.C.; Curtiss, L.A. 6-31G* Basis Set for Third-Row Atoms. J. Comput. Chem. 2001, 22, 976–984. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Jacquemin, D. Excited-State Dipole and Quadrupole Moments: TD-DFT versus CC2. J. Chem. Theory Comput. 2016, 12, 3993–4003. [Google Scholar] [CrossRef] [PubMed]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Alvarado-González, M.; Flores-Holguín, N.; Gallo, M.; Orrantia-Borunda, E.; Glossman-Mitnik, D. TD-DFT/IEFPCM Determination of the Absorption and Emission Spectra of DABCYL. J. Mol. Struct. THEOCHEM 2010, 945, 101–103. [Google Scholar] [CrossRef]
- Cao, H.-Y.; Si, D.-H.; Tang, Q.; Zheng, X.-F.; Hao, C. Electronic Structures and Solvent Effects of Unsymmetrical Neo-Confused Porphyrin: DFT and TDDFT–IEFPCM Investigations. Comput. Theor. Chem. 2016, 1081, 18–24. [Google Scholar] [CrossRef]
- Kostjukova, L.O.; Leontieva, S.V.; Kostjukov, V.V. The Vibronic Absorption Spectrum and Electronic Properties of Azure B in Aqueous Solution: TD-DFT/DFT Study. J. Mol. Graph. Model. 2021, 107, 107964. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, H.; Wu, T.; Wang, Q.; van der Spoel, D. Comparison of Implicit and Explicit Solvent Models for the Calculation of Solvation Free Energy in Organic Solvents. J. Chem. Theory Comput. 2017, 13, 1034–1043. [Google Scholar] [CrossRef] [PubMed]
- Nedyalkova, M.A.; Madurga, S.; Tobiszewski, M.; Simeonov, V. Calculating the Partition Coefficients of Organic Solvents in Octanol/Water and Octanol/Air. J. Chem. Inf. Model. 2019, 59, 2257–2263. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ketteridge, M.; Biaggne, A.; Rau, R.; Barcenas, G.; Mass, O.A.; Knowlton, W.B.; Yurke, B.; Li, L. Effect of Substituent Location on the Relationship between the Transition Dipole Moments, Difference Static Dipole, and Hydrophobicity in Squaraine Dyes for Quantum Information Devices. Molecules 2023, 28, 2163. https://doi.org/10.3390/molecules28052163
Ketteridge M, Biaggne A, Rau R, Barcenas G, Mass OA, Knowlton WB, Yurke B, Li L. Effect of Substituent Location on the Relationship between the Transition Dipole Moments, Difference Static Dipole, and Hydrophobicity in Squaraine Dyes for Quantum Information Devices. Molecules. 2023; 28(5):2163. https://doi.org/10.3390/molecules28052163
Chicago/Turabian StyleKetteridge, Maia, Austin Biaggne, Ryan Rau, German Barcenas, Olga A. Mass, William B. Knowlton, Bernard Yurke, and Lan Li. 2023. "Effect of Substituent Location on the Relationship between the Transition Dipole Moments, Difference Static Dipole, and Hydrophobicity in Squaraine Dyes for Quantum Information Devices" Molecules 28, no. 5: 2163. https://doi.org/10.3390/molecules28052163
APA StyleKetteridge, M., Biaggne, A., Rau, R., Barcenas, G., Mass, O. A., Knowlton, W. B., Yurke, B., & Li, L. (2023). Effect of Substituent Location on the Relationship between the Transition Dipole Moments, Difference Static Dipole, and Hydrophobicity in Squaraine Dyes for Quantum Information Devices. Molecules, 28(5), 2163. https://doi.org/10.3390/molecules28052163






