Baricitinib Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice by Inhibiting TGF-β1 Signaling Pathway
Abstract
1. Introduction
2. Results
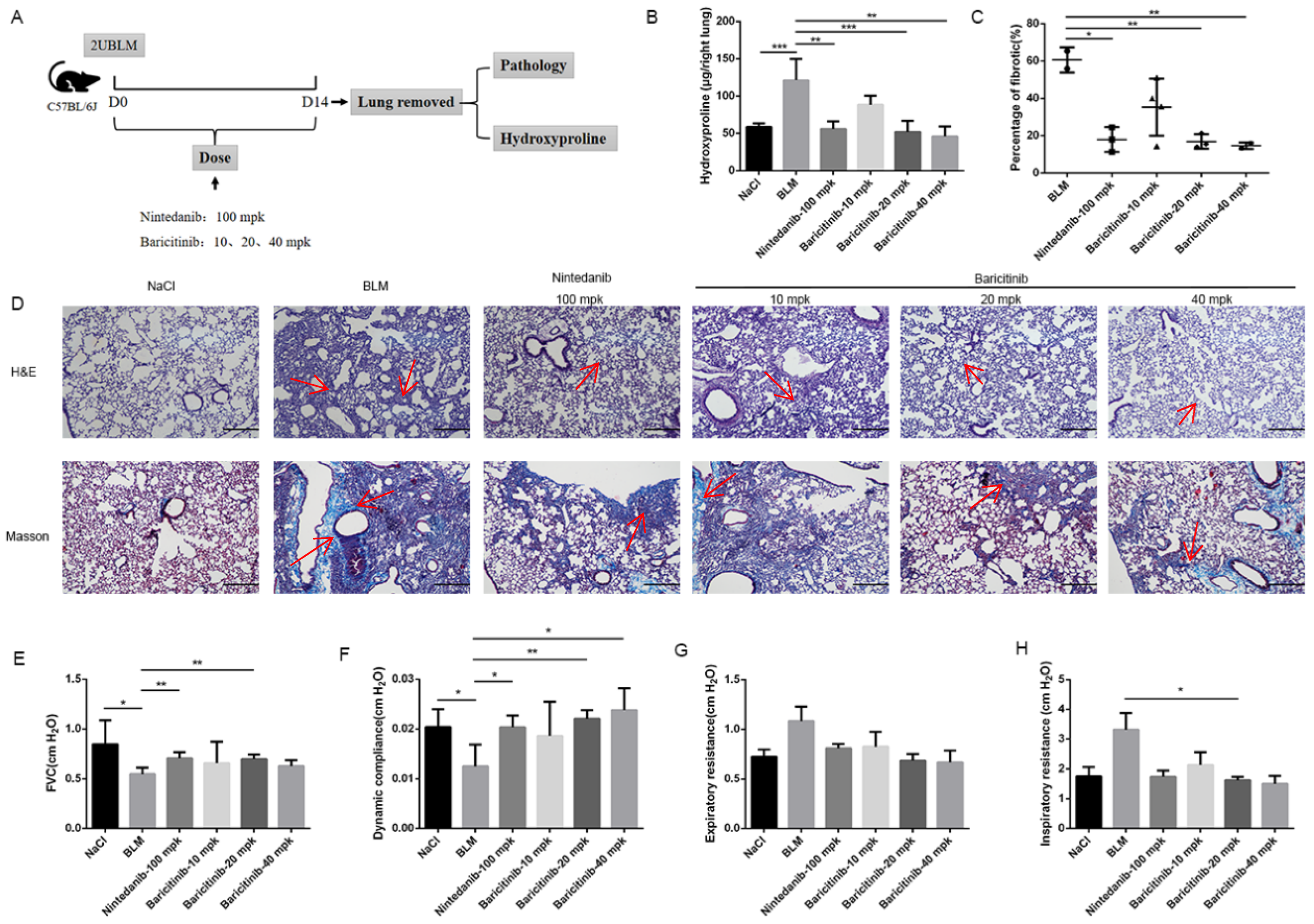
2.1. Baricitinib Attenuates BLM-Induced Pulmonary Fibrosis in Mice
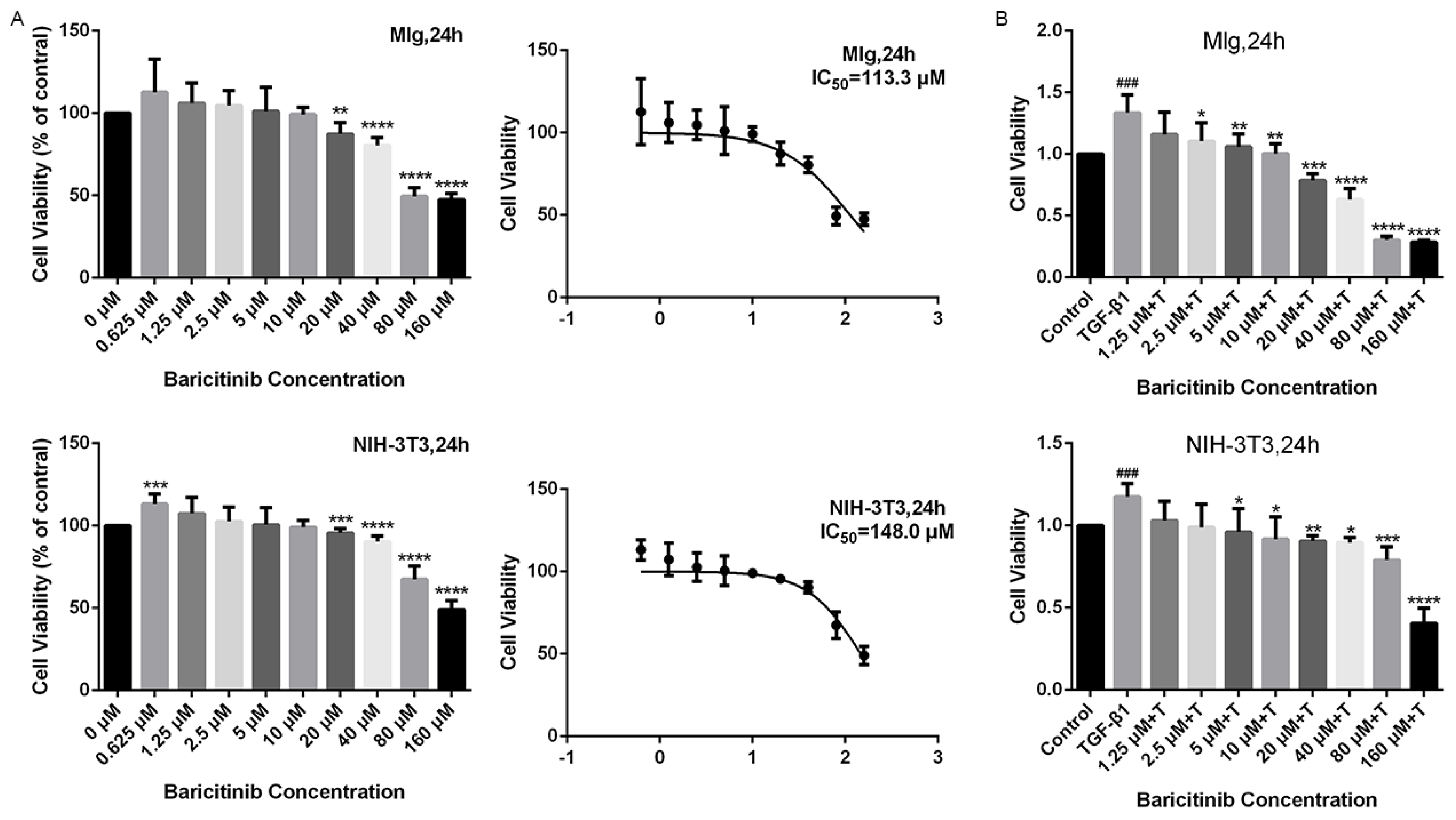
2.2. Baricitinib Suppresses TGF-β1-Induced Proliferation and Migration of Lung Fibroblasts
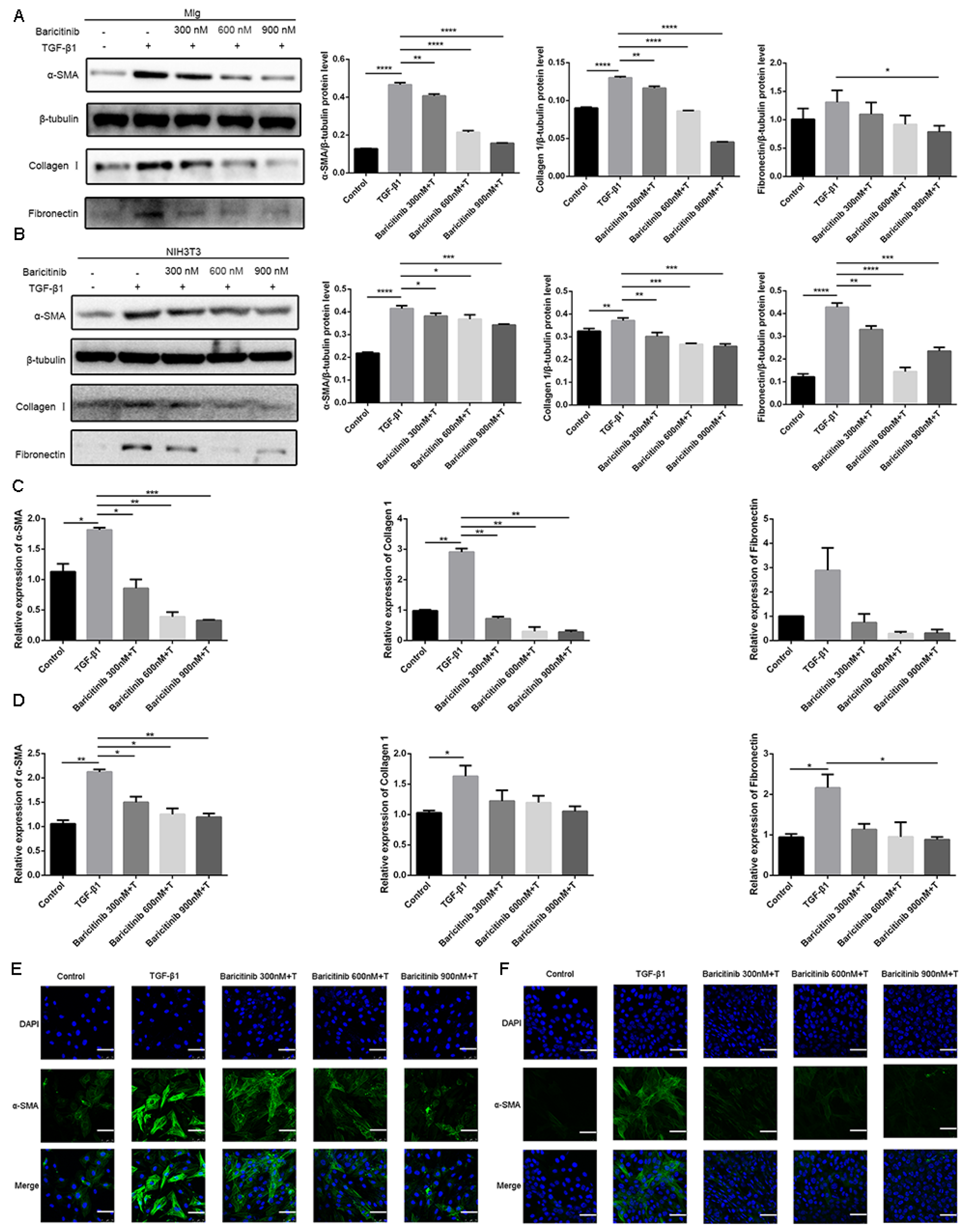
2.3. Baricitinib Attenuates TGF-β1-Induced Activation of Lung Fibroblasts
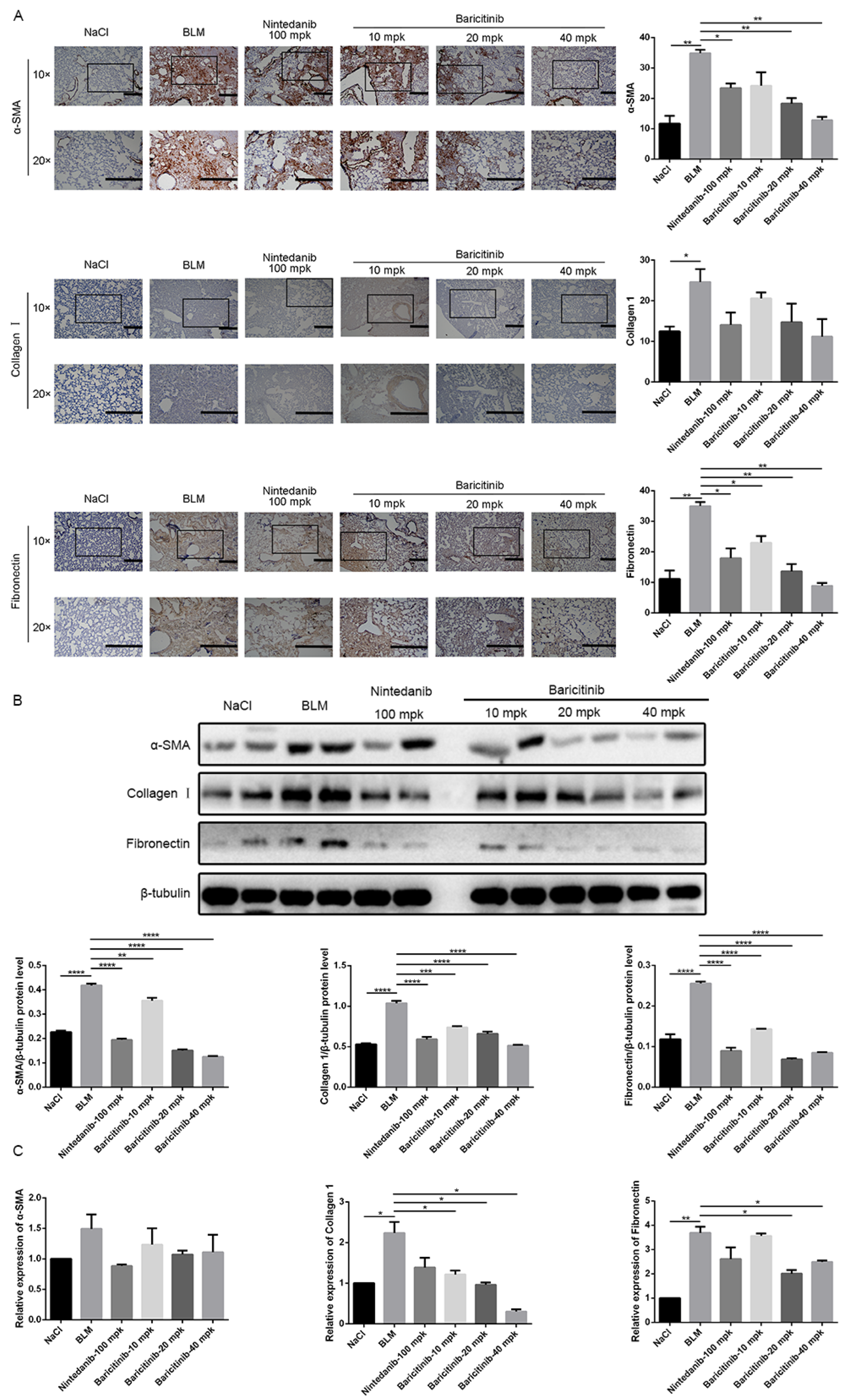
2.4. Baricitinib Inhibits the Activation of Lung Fibroblasts In Vivo
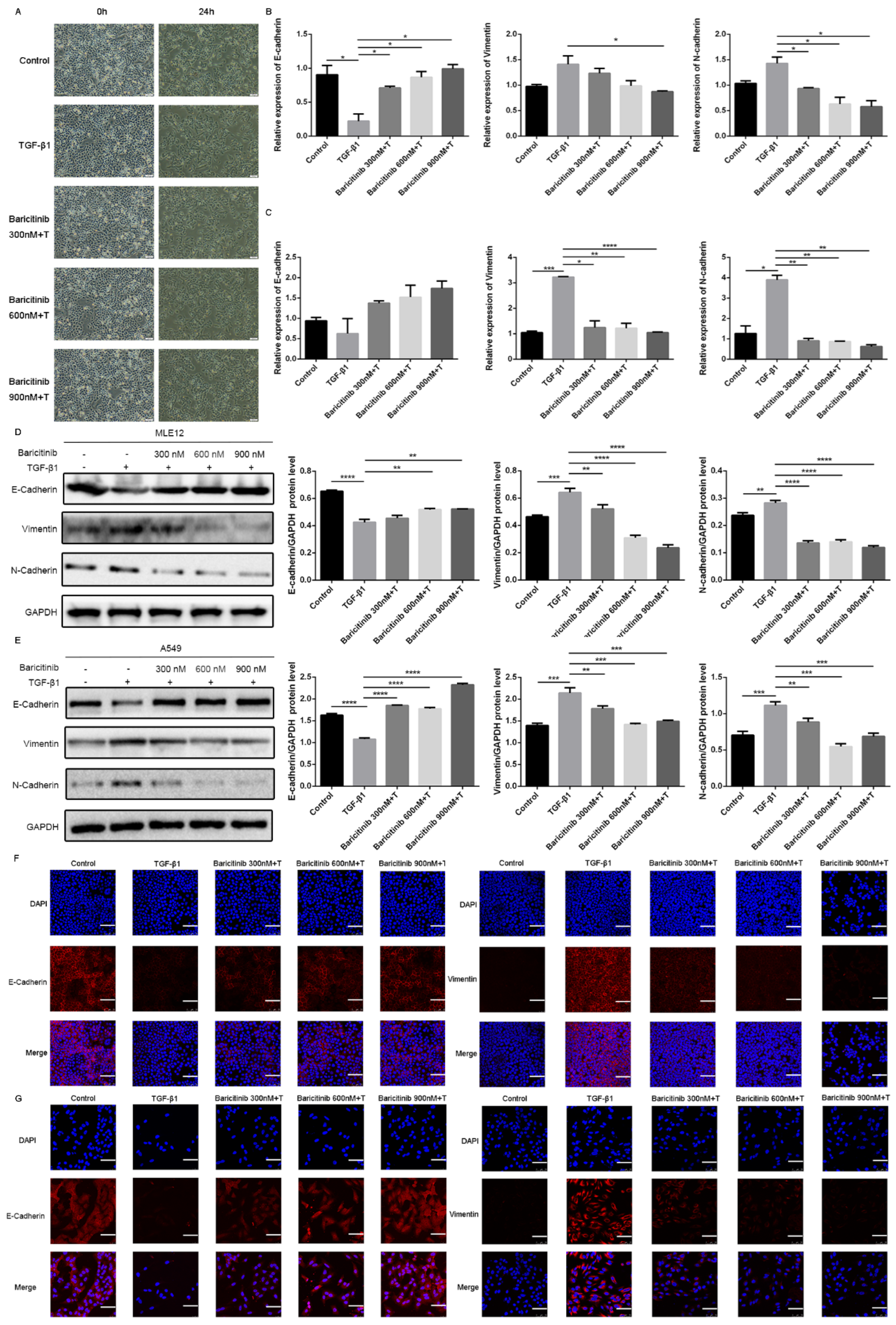
2.5. Baricitinib Inhibits TGF-β1-Induced Epithelial Injury in Alveolar Epithelial Cells
2.6. Baricitinib Alleviates Epithelial Injury In Vivo
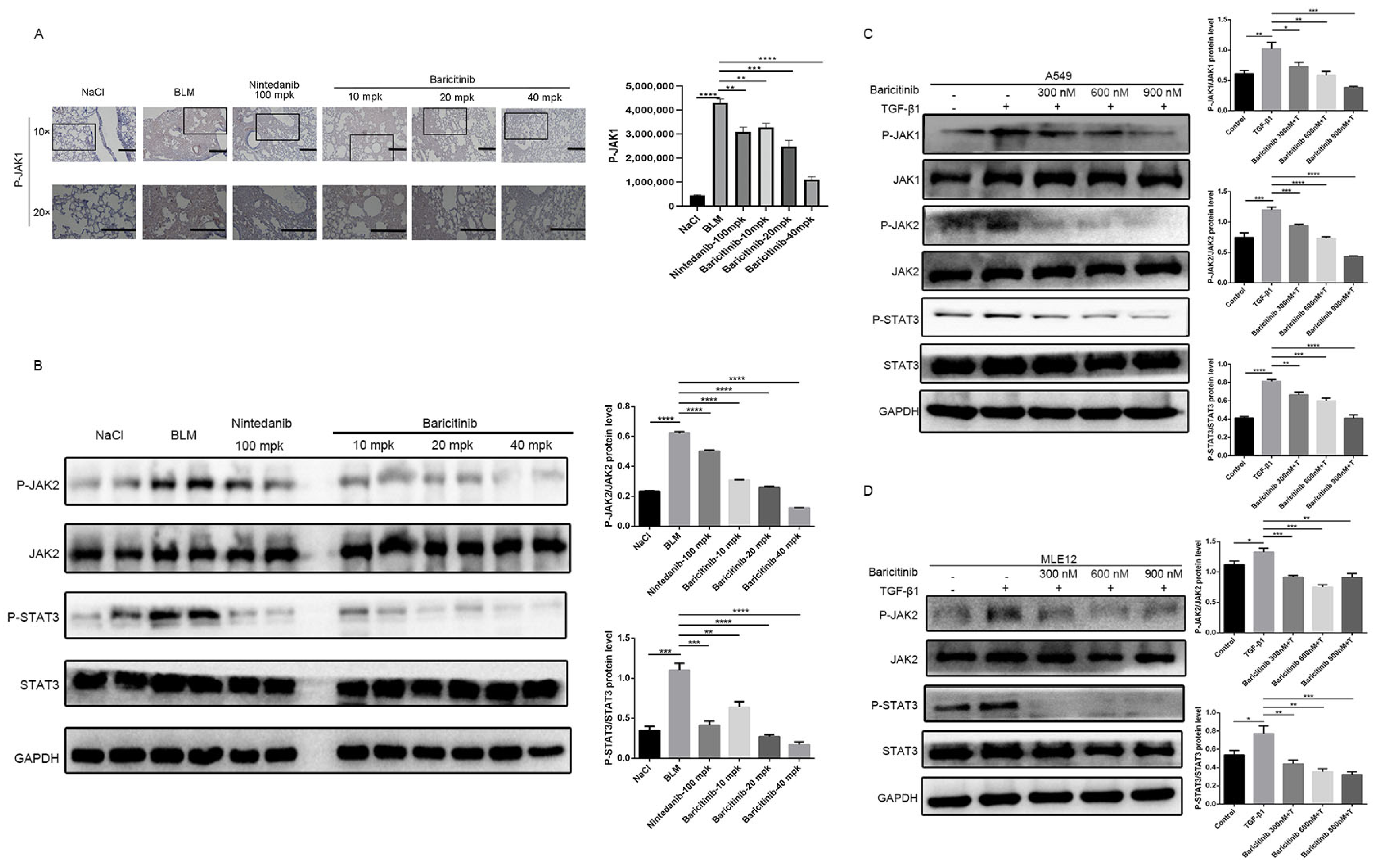
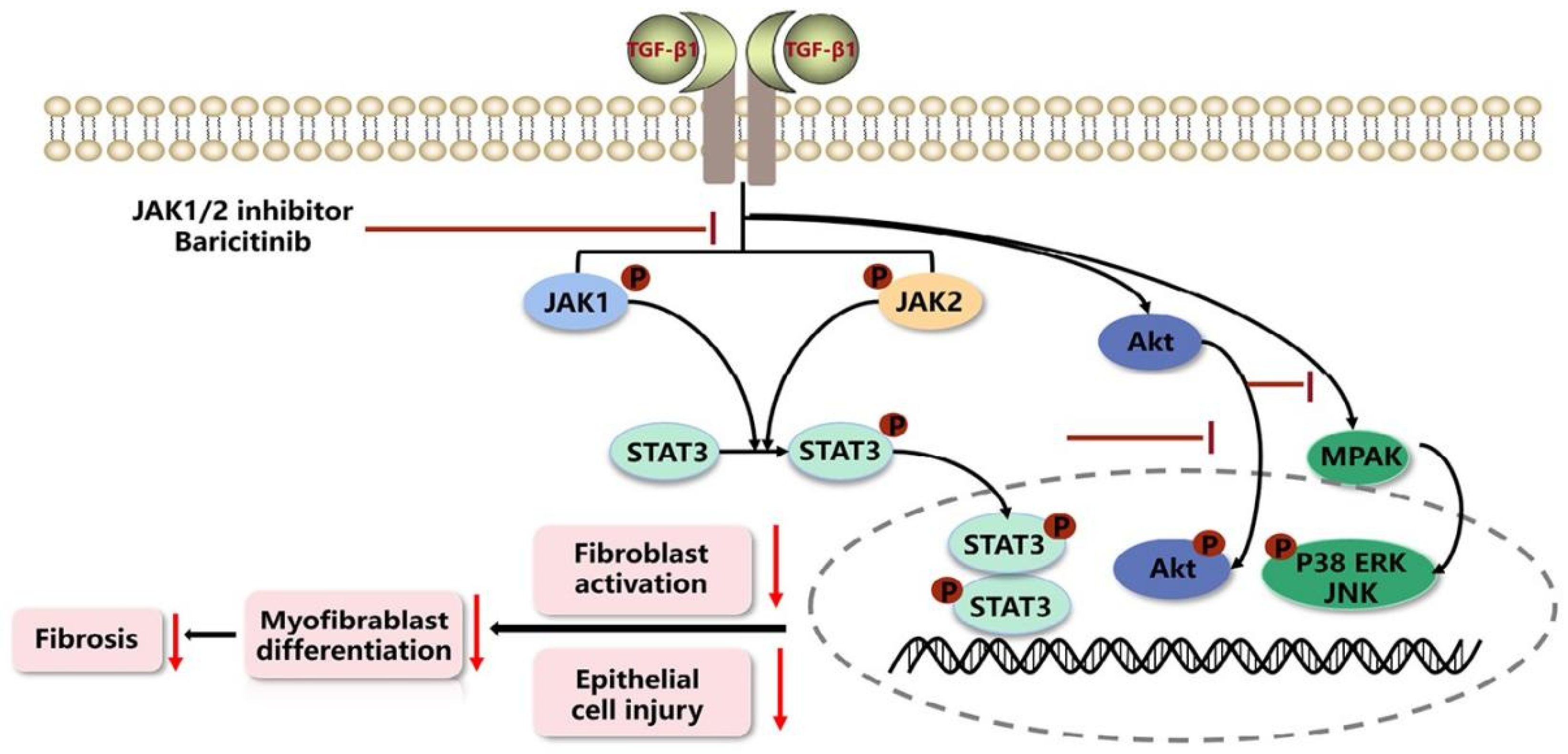
2.7. Baricitinib Alleviates Pulmonary Fibrosis by Inhibiting Activation of JAK-STAT Signaling Pathway
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Animals
3.4. Mouse Models of Pulmonary Fibrosis
3.5. Hydroxyproline Assay
3.6. Histological Examination
3.7. Pulmonary Function Testing
3.8. Cell Viability Analysis
3.9. Wound-Healing Assay
3.10. Quantitative Real-Time PCR (qRT-PCR)
3.11. Western Blot Analysis
3.12. Immunofluorescence
3.13. Immunohistochemistry Staining
3.14. Statistical Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Thannickal, V.J.; Toews, G.B.; White, E.S.; Lynch, J.P.; Martinez, F.J. Mechanisms of pulmonary fibrosis. Annu. Rev. Med. 2004, 55, 395–417. [Google Scholar] [CrossRef] [PubMed]
- King, T.E., Jr.; Pardo, A.; Selman, M. Idiopathic pulmonary fibrosis. Lancet 2011, 378, 1949–1961. [Google Scholar] [CrossRef] [PubMed]
- Hardie, W.D.; Hagood, J.S.; Davé, V.; Perl, A.-K.T.; Whitsett, J.A.; Korfhagen, T.R.; Glasser, S. Signaling pathways in the epithelial origins of pulmonary fibrosis. Cell Cycle 2010, 9, 2769–2776. [Google Scholar] [CrossRef] [PubMed]
- Meyer, K.C. Pulmonary fibrosis, part I: Epidemiology, pathogenesis, and diagnosis. Expert Rev. Respir. Med. 2017, 11, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Glass, D.S.; Grossfeld, D.; Renna, H.A.; Agarwala, P.; Spiegler, P.; Kasselman, L.J.; Glass, A.D.; DeLeon, J.; Reiss, A.B. Idiopathic pulmonary fibrosis: Molecular mechanisms and potential treatment approaches. Respir. Investig. 2020, 58, 320–335. [Google Scholar] [CrossRef]
- Gulati, S.; Thannickal, V.J. The Aging Lung and Idiopathic Pulmonary Fibrosis. Am. J. Med. Sci. 2019, 357, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Györfi, A.H.; Matei, A.E.; Distler, J.H.W. Targeting TGF-β signaling for the treatment of fibrosis. Matrix Biol. 2018, 68–69, 8–27. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Luo, K. Signaling Cross Talk between TGF-β/Smad and Other Signaling Pathways. Cold Spring Harb. Perspect. Biol. 2017, 9, a022137. [Google Scholar] [CrossRef]
- Denton, C.; Merkel, P.; Furst, D.; Khanna, D.; Emery, P.; Hsu, V.; Silliman, N.; Streisand, J.; Powell, J.; Akesson, A.; et al. Recombinant human anti-transforming growth factor beta1 antibody therapy in systemic sclerosis: A multicenter, randomized, placebo-controlled phase I/II trial of CAT-192. Arthritis Rheum. 2007, 56, 323–333. [Google Scholar] [CrossRef]
- Prud’homme, G.J. Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Investig. 2007, 87, 1077–1091. [Google Scholar] [CrossRef] [PubMed]
- Wolters, P.J.; Collard, H.R.; Jones, K.D. Pathogenesis of idiopathic pulmonary fibrosis. Annu. Rev. Pathol. 2014, 9, 157–179. [Google Scholar] [CrossRef] [PubMed]
- Milara, J.; Hernandez, G.; Ballester, B.; Morell, A.; Roger, I.; Montero, P.; Escrivá, J.; Lloris, J.M.; Molina-Molina, M.; Morcillo, E.; et al. The JAK2 pathway is activated in idiopathic pulmonary fibrosis. Respir. Res. 2018, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Ge, M.; Guo, Z.; He, H.; Zhang, N.; Li, Y.; Song, D. Discovery of 9O-Substituted Palmatine Derivatives as a New Class of anti-COL1A1 Agents via Repressing TGF-β1/Smads and JAK1/STAT3 Pathways. Molecules 2020, 25, 773. [Google Scholar] [CrossRef]
- Xu, S.; Mao, Y.; Wu, J.; Feng, J.; Li, J.; Wu, L.; Yu, Q.; Zhou, Y.; Zhang, J.; Chen, J.; et al. TGF-β/Smad and JAK/STAT pathways are involved in the anti-fibrotic effects of propylene glycol alginate sodium sulphate on hepatic fibrosis. J. Cell Mol. Med. 2020, 24, 5224–5237. [Google Scholar] [CrossRef]
- Chakraborty, D.; Šumová, B.; Mallano, T.; Chen, C.-W.; Distler, A.; Bergmann, C.; Ludolph, I.; Horch, R.E.; Gelse, K.; Ramming, A.; et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat. Commun. 2017, 8, 1130. [Google Scholar] [CrossRef]
- Zehender, A.; Huang, J.; Györfi, A.-H.; Matei, A.-E.; Trinh-Minh, T.; Xu, X.; Li, Y.-N.; Chen, C.-W.; Lin, J.; Dees, C.; et al. The tyrosine phosphatase SHP2 controls TGFβ-induced STAT3 signaling to regulate fibroblast activation and fibrosis. Nat. Commun. 2018, 9, 3259. [Google Scholar] [CrossRef]
- Xin, P.; Xu, X.; Deng, C.; Liu, S.; Wang, Y.; Zhou, X.; Ma, H.; Wei, D.; Sun, S. The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol. 2020, 80, 106210. [Google Scholar] [CrossRef]
- Al-Salama, Z.T.; Scott, L.J. Baricitinib: A Review in Rheumatoid Arthritis. Drugs 2018, 78, 761–772. [Google Scholar] [CrossRef]
- Atzeni, F.; Gerratana, E.; Giallanza, M.; La Corte, L.; Nucera, V.; Miceli, G.; Sangari, D.; Masala, I.F. The effect of drugs used in rheumatology for treating SARS-CoV2 infection. Expert Opin. Biol. Ther. 2021, 21, 219–228. [Google Scholar] [CrossRef]
- Cingolani, A.; Tummolo, A.M.; Montemurro, G.; Gremese, E.; Larosa, L.; Cipriani, M.C.; Pasciuto, G.; Liperoti, R.; Murri, R.; Pirronti, T.; et al. Baricitinib as rescue therapy in a patient with COVID-19 with no complete response to sarilumab. Infection 2020, 48, 767–771. [Google Scholar] [CrossRef]
- Hoang, T.N.; Pino, M.; Boddapati, A.K.; Viox, E.G.; Starke, C.E.; Upadhyay, A.A.; Paiardini, M. Baricitinib treatment resolves lower-airway macrophage inflammation and neutrophil recruitment in SARS-CoV-2-infected rhesus macaques. Cell 2021, 184, 460–475.e421. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, Z.; Liu, S.; Gao, S.; Cui, Y.; Huang, K.; Huang, M.; Mao, J.; Li, L.; Gao, J.; et al. Antifibrotic Mechanism of Cinobufagin in Bleomycin-Induced Pulmonary Fibrosis in Mice. Front. Pharmacol. 2019, 10, 1021. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, H.; Liang, L.; Bi, Z.; Wang, Y.; Gao, S.; Wang, M.; Li, H.; Miao, Y.; Deng, R.; et al. Myricetin ameliorates bleomycin-induced pulmonary fibrosis in mice by inhibiting TGF-β signaling via targeting HSP90β. Biochem. Pharmacol. 2020, 178, 114097. [Google Scholar] [CrossRef] [PubMed]
- Ning, W.; Li, C.-J.; Kaminski, N.; Feghali-Bostwick, C.A.; Alber, S.M.; Di, Y.P.; Otterbein, S.L.; Song, R.; Hayashi, S.; Zhou, Z.; et al. Comprehensive gene expression profiles reveal pathways related to the pathogenesis of chronic obstructive pulmonary disease. Proc. Natl. Acad. Sci. USA 2004, 101, 14895–14900. [Google Scholar] [CrossRef] [PubMed]
- Finnson, K.W.; Almadani, Y.; Philip, A. Non-canonical (non-SMAD2/3) TGF-β signaling in fibrosis: Mechanisms and targets. Semin. Cell Dev. Biol. 2020, 101, 115–122. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-β Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed]
- Salton, F.; Volpe, M.C.; Confalonieri, M. Epithelial-Mesenchymal Transition in the Pathogenesis of Idiopathic Pulmonary Fibrosis. Medicina 2019, 55, 83. [Google Scholar] [CrossRef]
- Zhang, Y.; Liang, R.; Chen, C.-W.; Mallano, T.; Dees, C.; Distler, A.; Reich, A.; Bergmann, C.; Ramming, A.; Gelse, K.; et al. JAK1-dependent transphosphorylation of JAK2 limits the antifibrotic effects of selective JAK2 inhibitors on long-term treatment. Ann. Rheum. Dis. 2017, 76, 1467–1475. [Google Scholar] [CrossRef]
- Kolb, P.; Upagupta, C.; Vierhout, M.; Ayaub, E.; Bellaye, P.S.; Gauldie, J.; Shimbori, C.; Inman, M.; Ask, K.; Kolb, M.R. The importance of interventional timing in the bleomycin model of pulmonary fibrosis. Eur. Respir. J. 2020, 55, 1901105. [Google Scholar] [CrossRef]
- Liu, T.; De Los Santos, F.G.; Phan, S.H. The Bleomycin Model of Pulmonary Fibrosis. Methods Mol. Biol. 2017, 1627, 27–42. [Google Scholar] [PubMed]
- Moore, B.B.; Hogaboam, C.M. Murine models of pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L152–L160. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Rubio, G.A.; Limper, A.H.; Williams, K.; Elliot, S.J.; Ninou, I.; Aidinis, V.; Tzouvelekis, A.; Glassberg, M.K. Exploring Animal Models That Resemble Idiopathic Pulmonary Fibrosis. Front. Med. 2017, 4, 118. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Xie, Y.; Abel, P.W.; Huang, Y.; Ma, Q.; Li, L.; Hao, J.; Wolff, D.W.; Wei, T.; Tu, Y. Transforming growth factor (TGF)-β1-induced miR-133a inhibits myofibroblast differentiation and pulmonary fibrosis. Cell Death Dis. 2019, 10, 670. [Google Scholar] [CrossRef]
- Tang, L.-Y.; Heller, M.; Meng, Z.; Yu, L.-R.; Tang, Y.; Zhou, M.; Zhang, Y.E. Transforming Growth Factor-β (TGF-β) Directly Activates the JAK1-STAT3 Axis to Induce Hepatic Fibrosis in Coordination with the SMAD Pathway. J. Biol. Chem. 2017, 292, 4302–4312. [Google Scholar] [CrossRef]
- Goker Bagca, B.; Biray Avci, C. The potential of JAK/STAT pathway inhibition by ruxolitinib in the treatment of COVID-19. Cytokine Growth Factor Rev. 2020, 54, 51–62. [Google Scholar] [CrossRef]
- Meletiadis, J.; Tsiodras, S.; Tsirigotis, P. Interleukin-6 Blocking vs. JAK-STAT Inhibition for Prevention of Lung Injury in Patients with COVID-19. Infect. Dis. Ther. 2020, 9, 707–713. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]



| Reagents | Manufacturer |
|---|---|
| Recombinant Human TGF-β1 | Peprotech (Cranbury, NJ, USA) |
| Collagen Ⅰ, Fibronectin, P-JAK1/JAK1, P-JAK2/JAK2, P-STAT3/STAT3, P-AKT/AKT, P-ERK/ERK, P-JNK/JNK, P-P38/P38, GAPDH, E-cadherin, Vimentin, N-cadherin antibodies | Cell Signaling Technology (Boston, MA, USA) |
| β-tubulin antibody | Proteintech (Wuhan, China) |
| α-SMA antibody | Santa Cruz Biotechnology (Beijing, China) |
| Goat pAb to Rb IgG (HRP) and Rb pAb to Ms IgG | ImmunoWay (Beijing, China) |
| TRIzol reagent | Ambion Life Technology (Beijing, China) |
| DEPC Treated H2O and RNAse Away H2O | Life Technologies (Shanghai, China) |
| Fastking gDNA Dispelling RT SuperMix | TIANGEN (Beijing, China) |
| UNICON® Qpcr SYBR Green Master Mix and Liposomal transfection reagent | YEVSEN (Shanghai, China) |
| BLM | Nippon Kayaku (Tokyo, Japan) |
| Masson’s Trichrome Stain Kit | Solarbio (Beijing, China) |
| Cell lines | Culture Conditions |
|---|---|
| Mouse pulmonary epithelial cells (MLE12, ATCC) | DMEM/F-12 (1:1) basic (1×) (Gibco) with 10% fetal bovine serum (FBS, ExCell Bio, Shanghai, China) and 1% antibiotics (gibco) |
| Human pulmonary epithelial cells (A549, ATCC) | RPMI-1640 (KeyGEN BioTECH, Beijing, China) with 10% FBS |
| Mouse lung fibroblast cells (Mlg, ATCC) | DMEM (KeyGEN BioTECH, Nanjing, China) with 10% FBS |
| Mouse embryonic fibroblast cells (NIH-3T3, ATCC) | DMEM (KeyGEN BioTECH, Nanjing, China) with 10% FBS |
| For Mouse | ||
| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
| GAPDH | TGGA TTTGGACGCA TTGGTC | TTTGCACTGGTACGTGTTGAT |
| α-SMA | TGGGTGAACTCCATCGCTGTA | GTCGAATGCAACAAGGAAGCC |
| Collagen I | AAGCCGGAGGACAACCTTTTA | GCGAAGAGAATGACCAGATCC |
| Fibronectin | GTGCCCGGAATACGCATGTA | CTGGTGGACGGGATCATCCT |
| E-cadherin | CAGCCTTCTTTTCGGAAGACT | GGTAGACAGCTCCCTATGACTG |
| Vimentin | GCTGCGAGAGAAATTGCAGGA | CCACTTTCCGTTCAAGGTCAAG |
| N-cadherin | CTCCAACGGGCATCTTCATTAT | CAAGTGAAACCGGGCTATCAG |
| For Human | ||
| Gene | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
| β-actin | AGGCCAACCGTGAAAAGATG | AGAGCATAGCCCTCGTAGATGG |
| E-cadherin | A TTTTTCCCTCGACACCCGAT | TCCCAGGCGTAGACCAAGA |
| Vimentin | AGTCCACTGAGTACCGGAGAC | CA TTTCACGCA TCTGGCGTTC |
| N-cadherin | TTTGATGGAGGTCTCCTAACACC | ACGTTTAACACGTTGGAAATGTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, S.; Liang, J.; Zhang, J.; Liu, Z.; Miao, Y.; Wei, Y.; Li, S.; Gu, J.; Cui, Y.; Xiao, T.; et al. Baricitinib Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice by Inhibiting TGF-β1 Signaling Pathway. Molecules 2023, 28, 2195. https://doi.org/10.3390/molecules28052195
Gu S, Liang J, Zhang J, Liu Z, Miao Y, Wei Y, Li S, Gu J, Cui Y, Xiao T, et al. Baricitinib Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice by Inhibiting TGF-β1 Signaling Pathway. Molecules. 2023; 28(5):2195. https://doi.org/10.3390/molecules28052195
Chicago/Turabian StyleGu, Songtao, Jingjing Liang, Jianwei Zhang, Zhichao Liu, Yang Miao, Yuli Wei, Shimeng Li, Jinying Gu, Yunyao Cui, Ting Xiao, and et al. 2023. "Baricitinib Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice by Inhibiting TGF-β1 Signaling Pathway" Molecules 28, no. 5: 2195. https://doi.org/10.3390/molecules28052195
APA StyleGu, S., Liang, J., Zhang, J., Liu, Z., Miao, Y., Wei, Y., Li, S., Gu, J., Cui, Y., Xiao, T., Li, X., & Yang, C. (2023). Baricitinib Attenuates Bleomycin-Induced Pulmonary Fibrosis in Mice by Inhibiting TGF-β1 Signaling Pathway. Molecules, 28(5), 2195. https://doi.org/10.3390/molecules28052195





