Dyeing Properties, Color Gamut, and Color Evaluation of Cotton Fabrics Dyed with Phellodendron amurense Rupr. (Amur Cork Tree Bark)
Abstract
1. Introduction
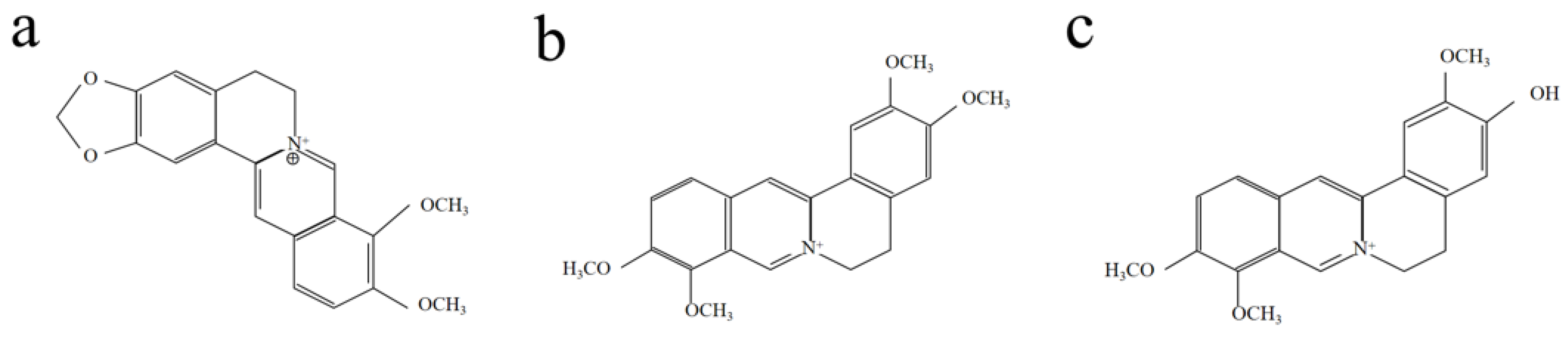
2. Results and Discussion
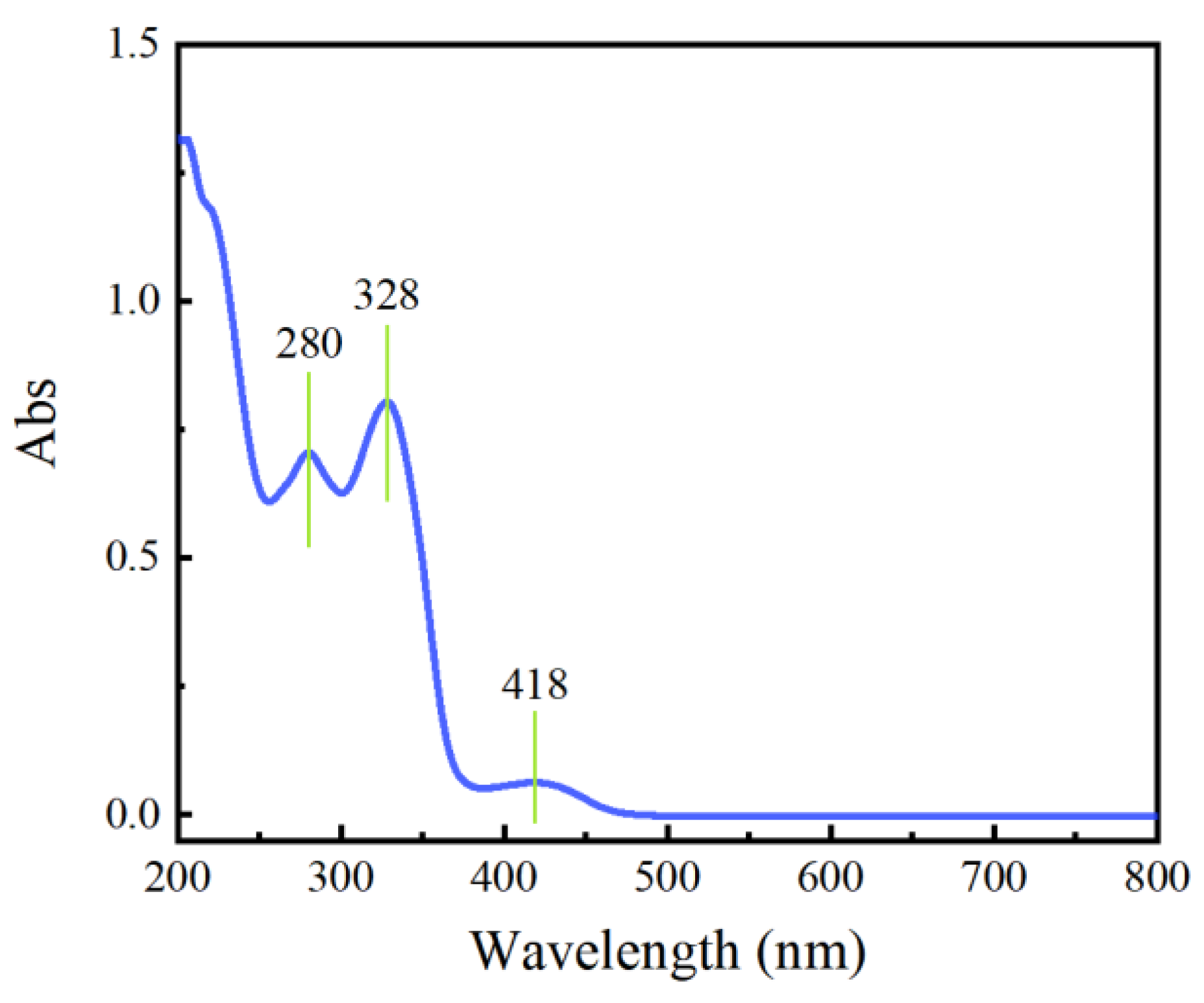
2.1. UV-Visible Spectroscopy of Phellodendron amurense Dye Solution
2.2. Effect of Mordanting on the Color Characteristics of the Cotton Fabrics

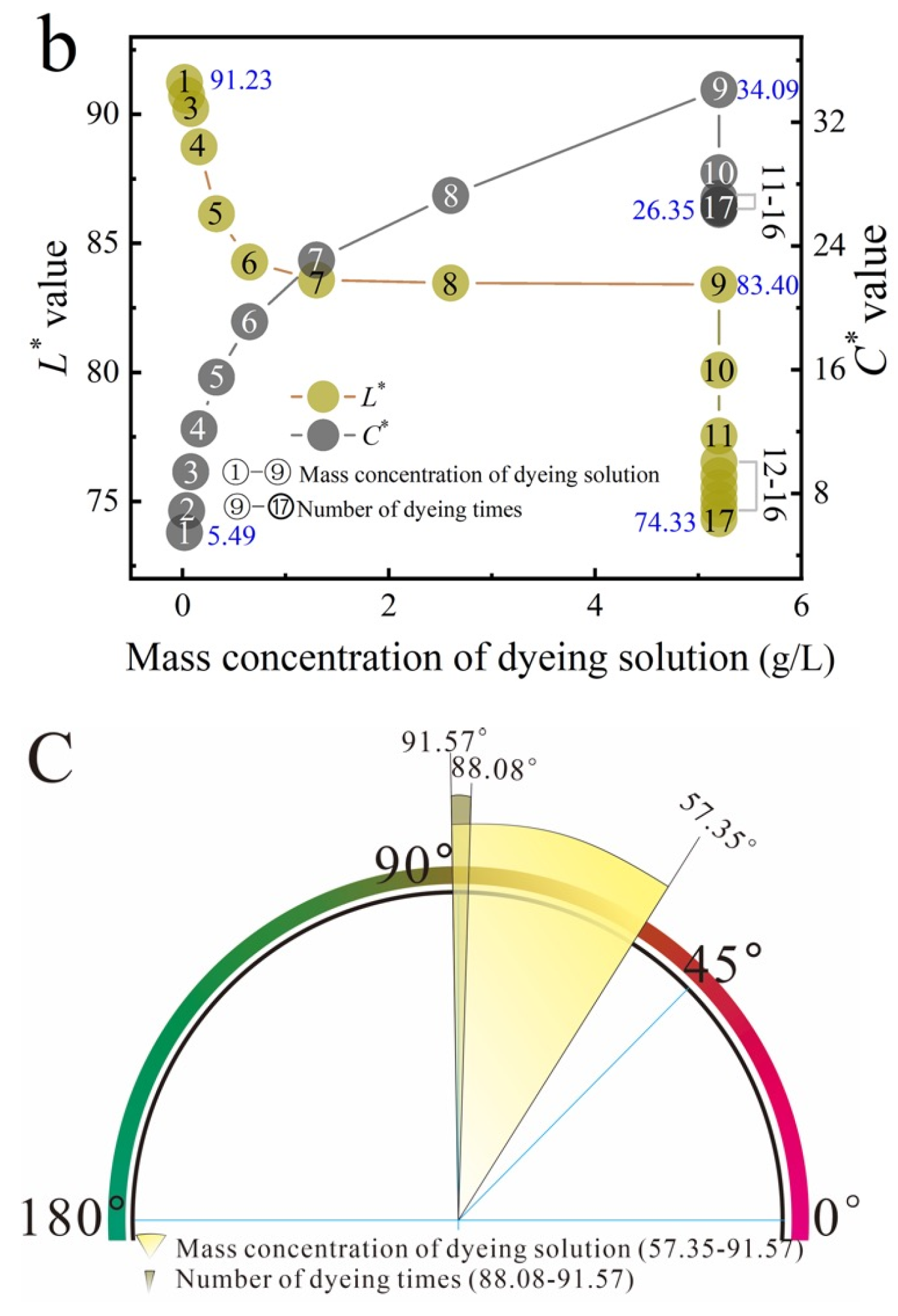
2.3. Effect of Dyeing Parameters on the Color Characteristics of Dyed Cotton Fabrics

2.4. Color Coordinates and Color Gamut Range

2.5. Color Fastness and Color Evaluation of the Cotton Fabrics
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Methods
3.2.1. Cotton Fabrics Pre-Treatment
3.2.2. Dye Extraction
3.2.3. Dyeing Process
- (a)
- Dyeing (without mordant): cotton fabrics were dyed directly in the P. amurense dye solution.
- (b)
- Mordanting:Pre-mordanting: cotton fabrics were treated firstly in aluminum potassium sulfate solution for mordanting. The mordanted fabrics were then wringed out and dyed in the dye solution.Meta-mordanting: Cotton fabrics were dyed in the dyebath containing both dye and the mordant.Post-mordanting: Cotton fabrics were dyed firstly in the dye solution. They were then wringed out and treated within aluminum potassium sulfate mordant solution.
- (c)
- Repeat dyeing: Cotton fabrics were treated firstly in aluminum potassium sulfate solution for mordanting. The dyeing process was then applied and repeated.
3.2.4. UV-Visible Spectroscopy
3.2.5. Color Characteristic Measurement
3.2.6. Color Fastness Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gayathiri, E.; Prakash, P.; Selvam, K.; Awasthi, M.K.; Gobinath, R.; Karri, R.R.; Ragunathan, M.G.; Jayanthi, J.; Mani, V.; Poudineh, M.A.; et al. Plant microbe based remediation approaches in dye removal: A review. Bioengineered 2022, 13, 7798–7828. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Ji, X.; Qian, W.R.; Qiu, X.X.; Yang, Y.D.; Xu, P.H.; Wang, L.L. Environmental Impact Assessment of Discharged Heavy Metals in Textile Production. Fibres Text. East. Eur. 2021, 29, 66–69. [Google Scholar] [CrossRef]
- Odubanjo, G.O.; Oyetibo, G.O.; Ilori, M.O. Ecological Risks of Heavy Metals and Microbiome Taxonomic Profile of a Freshwater Stream Receiving Wastewater of Textile Industry. Front. Environ. Sci. 2021, 9, 554490. [Google Scholar] [CrossRef]
- Li, N.N.; Wang, Q.R.; Zhou, J.N.; Li, S.Q.; Liu, J.Y.; Chen, H.X. Insight into the Progress on Natural Dyes: Sources, Structural Features, Health Effects, Challenges, and Potential. Molecules 2022, 27, 3291. [Google Scholar] [CrossRef]
- Thakker, A.M. Sustainable processing of cotton fabrics with plant-based biomaterials Sapindus mukorossi and Acacia concinna for health-care applications. J. Text. Inst. 2021, 112, 718–726. [Google Scholar] [CrossRef]
- Cui, H.Y.; Xie, W.J.; Hua, Z.J.; Cao, L.H.; Xiong, Z.Y.; Tang, Y.; Yuan, Z.Q. Recent Advancements in Natural Plant Colorants Used for Hair Dye Applications: A Review. Molecules 2022, 27, 8062. [Google Scholar] [CrossRef]
- Rana, R.; Dhiman, K.; Ashawat, M.S. Natural Coloring Agents for Fibers and Their Medicinal Values: A Review. J. Nat. Fibers 2022, 16, 14755–14770. [Google Scholar] [CrossRef]
- Liu, Y. Reflections on the color aesthetics of traditional Chinese Natural dyed Clothing and its contemporary value. Fash. Color 2021, 03, 86–87. [Google Scholar]
- Song, Y. The Tao of Art Could be Expressed by Means of The Technology: The Influence of Plant Dyeing on Color Aesthetic System of Chinese Traditional Garments. Art Des. Res. 2015, 04, 37–42. [Google Scholar]
- Lam, Y.T.; Fan, S.J.; Chae, Y.; Wong, L.H.; He, L.; Bin, F.; Xin, J.H. Heteromolecular pigmentations of plant-derived catechol and their application on textiles. J. Clean. Prod. 2022, 332, 130010. [Google Scholar] [CrossRef]
- Nambela, L.; Haule, L.V.; Mgani, Q. A review on source, chemistry, green synthesis and application of textile colorants. J. Clean. Prod. 2020, 246, 119036. [Google Scholar] [CrossRef]
- Fried, R.; Oprea, I.; Fleck, K.; Rudroff, F. Biogenic colourants in the textile industry—A promising and sustainable alternative to synthetic dyes. Green Chem. 2022, 24, 13–35. [Google Scholar] [CrossRef]
- Gulrajani, M.L. Present status of natural dyes. Indian J. Fibre Text. Res. 2001, 26, 191–201. [Google Scholar]
- Gupta, V.K. Fundamentals of Natural Dyes and Its Application on Textile Substrates. In Chemistry and Technology of Natural and Synthetic Dyes and Pigments; IntechOpen Publisher: London, UK, 2019. [Google Scholar]
- Chen, F.L.; Ji, X.; Chu, J.; Xu, P.H.; Wang, L.L. A review: Life cycle assessment of cotton textiles. Ind. Text. 2021, 72, 19–29. [Google Scholar] [CrossRef]
- Zhang, Y.; Guo, Q.; Wei, C.; Wang, J.K.; Gong, J.X. Research progress of plant dyeing cotton fabric fastness. Cotton Text. Technol. 2023, 51, 80–84. [Google Scholar]
- Mun, J.S.; Kim, H.C.; Mun, S.P. Black Color Expression of Silk and Cotton Fabrics Using Neutral Extract from Pinus radiata Bark and Various Iron Mordants. Fiber. Polym. 2022, 23, 1008–1017. [Google Scholar] [CrossRef]
- Baek, N.W.; Zhang, X.; Lou, J.F.; Fan, X.R. Dyeing Fabrics with a Colorant Extracted from Blue-Green Algae. AATCC J. Res. 2022, 9, 223–230. [Google Scholar] [CrossRef]
- Ke, G.Z.; Zhu, K.D.; Chowdhury, M.H. Dyeing of Cochineal Natural Dye on Cotton Fabrics Treated with Oxidant and Chitosan. J. Nat. Fibers 2021, 18, 317–329. [Google Scholar] [CrossRef]
- Mansour, R.; Ben Ali, H. Investigating the Use of Chitosan: Toward Improving the Dyeability of Cotton Fabrics Dyed with Roselle (Hibiscus sabdariffa L.). J. Nat. Fibers 2021, 18, 1007–1016. [Google Scholar] [CrossRef]
- Giacomini, F.; de Souza, A.A.U.; de Barros, M. Cationization of cotton with ovalbumin to improve dyeing of modified cotton with cochineal natural dye. Text. Res. J. 2020, 90, 1805–1822. [Google Scholar] [CrossRef]
- Pisitsak, P.; Hutakamol, J.; Thongcharoen, R.; Phokaew, P.; Kanjanawan, K.; Saksaeng, N. Improving the dyeability of cotton with tannin-rich natural dye through pretreatment with whey protein isolate. Ind. Crop. Prod. 2016, 79, 47–56. [Google Scholar] [CrossRef]
- Feng, Y.; Guo, L.; Chang, Y.Q.; Zheng, Y.G.; Zhang, Q.Q. Research on difference of Phellodendron amurense and Phellodendron chinense based on fingerprint and multicomponent content determination. Chin. Tradit. Herb. Drugs 2022, 53, 5179–5184. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, A.; Chen, Y.; Lei, Y. Rapid screening of dyes for Kesi hanging panels in the Palace Museum. Sci. Conserv. Archaeol. 2020, 01, 70–76. [Google Scholar]
- Zhang, H. The Study on the Ultrasonic Extraction of Phellodendron Dye and Its Dyeing Performance. Master’s Thesis, Wuhan Textile University, Wuhan, China, 2021. [Google Scholar]
- Chen, A.H.; Lee, Y.T.; Chen, C.Y. Microwave accelerated extraction and capillary electrophoresis analysis of Berberine from the cortices of Phellodendron wilsonii and Phellodendron amurense. J. Chin. Chem. Soc. 2005, 52, 781–784. [Google Scholar] [CrossRef]
- Balazova, L.; Kurhajec, S.; Kello, M.; Bedlovicova, Z.; Zigova, M.; Petrovova, E.; Benova, K.; Mojzis, J.; Eftimova, J. Antiproliferative Effect of Phellodendron amurense Rupr. Based on Angiogenesis. Life 2022, 12, 767. [Google Scholar] [CrossRef]
- Choi, J.; Moon, M.Y.; Han, G.Y.; Chang, M.S.; Yang, D.; Cha, J. Phellodendron amurense Extract Protects Human Keratinocytes from PM2.5-Induced Inflammation via PAR-2 Signaling. Biomolecules 2021, 11, 23. [Google Scholar] [CrossRef]
- Alam, S.; Mandal, P.; Jagdale, P.R.; Ayanur, A.; Ansari, K.M. Safety studies of Nexrutine, bark extract of Phellodendron amurense through repeated oral exposure to rats for 28 days. Heliyon 2021, 7, e07654. [Google Scholar] [CrossRef]
- Lee, Y.H.; Hwang, E.K.; Jung, Y.J.; Do, S.K.; Kim, H.D. Dyeing and Deodorizing Properties of Cotton, Silk, Wool Fabrics Dyed with Amur Corktree, Dryopteris crassirhizoma, Chrysanthemum boreale, Artemisia Extracts. J. Appl. Polym. Sci. 2010, 115, 2246–2253. [Google Scholar] [CrossRef]
- Corak, I.; Brlek, I.; Sutlovic, A.; Tarbuk, A. Natural Dyeing of Modified Cotton Fabric with Cochineal Dye. Molecules 2022, 27, 1100. [Google Scholar] [CrossRef]
- Hu, Y.L.; Liu, J.; Zhao, F.; Hu, Z.W.; Wu, Z.Y.; Peng, Z.Q. Fading of silk fabrics dyed with vegetable dyes based on a self-designed miniature fiber optic spectrometer under oxygen and anoxic conditions. J. Silk 2019, 56, 1–7. [Google Scholar] [CrossRef]
- Motaghi, Z. An Economical Dyeing Process for Cotton and Wool Fabrics and Improvement their Antibacterial Properties and UV protection. J. Nat. Fibers 2018, 15, 777–788. [Google Scholar] [CrossRef]
- Liu, J. Identification of Ancient Fibers and Dyes—Take Archaeological Textiles of Yingpan as an Example. Master’s Thesis, Zhejiang Sci-Tech University, Hangzhou, China, 2011. [Google Scholar]
- Ren, H.; Liu, J.; Ma, S. Introduction to Inorganic Chenistry of Energetic Materials, 2nd ed.; Beijing Institute of Technology Press: Beijing, China, 2020; p. 317. [Google Scholar]
- Ke, G.Z.; Yu, W.D. Extraction of natural dye rhizoma coptidis and dyeing on acrylic fibers. Wool Text. J. 2011, 39, 22–26. [Google Scholar] [CrossRef]
- Xiao, L. Extraction of red pigment from red rice and its application in cotton/linen fabrics. Shanghai Text. Sci. Technol. 2020, 48, 8–12. [Google Scholar] [CrossRef]
- Cui, Y.Z.; Zhang, C.; Yu, Y. Study on cotton fabrics ecological dyeing process with cork tree bark based on loess mordant. J. Dalian Dalian Polytech. Univ. 2008, 02, 183–187. [Google Scholar]
- Zhang, D.B.; Zhao, F.; Liu, J.; Zhou, Y.; Chao, X.H.; Zhao, J.; Wu, Z.Y.; Peng, Z.Q.; Hu, Z.W. Study on Light aging of madder on silk. J. Text. Res. 2011, 32, 67–71. [Google Scholar] [CrossRef]
- Karadag, R. Establishing a New International Standard for Natural Dyed Textile Goods Natural Organic Dye Standard (NODS). J. Nat. Fibers 2023, 20, 2162187. [Google Scholar] [CrossRef]
| Dye Process | Mass Concentration of Dye Solution (g/L) | Number of Dyeing Times | Soap Washing Fastness | Rubbing Fastness | Light Fastness | PANTONE (TPX) | Compare with PANTONE (ΔE) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Color Change | Color Staining | Dry | Wet | Color Number | Color Name | |||||
| Without mordant | 5.20 | 1 | 2–3 | 2–3 | 3 | 3 | 1–2 | 13-0917 | Italian Straw | 0.976 |
| Pre-mordanting | 0.33 | 1 | 3 | 3 | 4 | 3 | 3 | 12-0709 | Macadamia | 3.647 |
| 0.65 | 1 | 3 | 3 | 4 | 3 | 3 | 13-0613 | Chino Green | 1.514 | |
| 1.30 | 1 | 3 | 3 | 4 | 3 | 3 | 13-0915 | Reed Yellow | 0.425 | |
| 2.60 | 1 | 3 | 3 | 4 | 3 | 3 | 13-0917 | Italian Straw | 1.556 | |
| 5.20 | 1 | 3 | 3 | 4 | 3 | 3 | 13-0922 | Straw | 1.718 | |
| 5.20 | 2 | 3 | 3 | 4 | 3 | 3 | 14-0925 | Parsnip | 1.818 | |
| 5.20 | 3 | 3 | 3 | 4 | 3 | 3 | 2.263 | |||
| 5.20 | 4 | 3 | 3 | 4 | 3 | 3 | 14-0721 | Hemp | 2.711 | |
| 5.20 | 5 | 3 | 3 | 4 | 3 | 3 | 2.744 | |||
| 5.20 | 6 | 3 | 3 | 4 | 3 | 3 | 2.824 | |||
| 5.20 | 7 | 3 | 3 | 4 | 3 | 3 | 15-0719 | Silver Fern | 2.524 | |
| 5.20 | 8 | 3 | 3 | 4 | 3 | 3 | 2.448 | |||
| 5.20 | 9 | 3 | 3 | 4 | 3 | 3 | 2.375 | |||
| No. | Dyeing Process | Mass Concentration of Dye Solution (g/L) | Dyeing Temperature (°C) | Dyeing Time (min) | Mordant Concentration (g/L) | pH | Number of Dyeing Times |
|---|---|---|---|---|---|---|---|
| 1 | Dyeing | 5.2 | 70 | 30 | —— | 5 | 1 |
| 2 | Pre-mordanting | 5.2 | 70 | 30 | 5 | 5 | 1 |
| 3 | Meta-mordanting | 5.2 | 70 | 30 | 5 | 5 | 1 |
| 4 | Post-mordanting | 5.2 | 70 | 30 | 5 | 5 | 1 |
| 5 | Pre-mordanting | 5.2 | 20~80 | 30 | 5 | 5 | 1 |
| 6 | Pre-mordanting | 5.2 | 70 | 1~60 | 5 | 5 | 1 |
| 7 | Pre-mordanting | 5.2 | 70 | 30 | 0.31~20 | 5 | 1 |
| 8 | Pre-mordanting | 5.2 | 70 | 30 | 5 | 3~9 | 1 |
| 9 | Pre-mordanting | 0.02~5.2 | 70 | 30 | 5 | 5 | 1 |
| 10 | Pre-mordanting | 5.2 | 70 | 30 | 5 | 5 | 2~10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, X.; Zhao, Z.; Ren, Y.; Xu, F.; Liu, J. Dyeing Properties, Color Gamut, and Color Evaluation of Cotton Fabrics Dyed with Phellodendron amurense Rupr. (Amur Cork Tree Bark). Molecules 2023, 28, 2220. https://doi.org/10.3390/molecules28052220
Ji X, Zhao Z, Ren Y, Xu F, Liu J. Dyeing Properties, Color Gamut, and Color Evaluation of Cotton Fabrics Dyed with Phellodendron amurense Rupr. (Amur Cork Tree Bark). Molecules. 2023; 28(5):2220. https://doi.org/10.3390/molecules28052220
Chicago/Turabian StyleJi, Xinyu, Zhijun Zhao, Yulu Ren, Fei Xu, and Jianhong Liu. 2023. "Dyeing Properties, Color Gamut, and Color Evaluation of Cotton Fabrics Dyed with Phellodendron amurense Rupr. (Amur Cork Tree Bark)" Molecules 28, no. 5: 2220. https://doi.org/10.3390/molecules28052220
APA StyleJi, X., Zhao, Z., Ren, Y., Xu, F., & Liu, J. (2023). Dyeing Properties, Color Gamut, and Color Evaluation of Cotton Fabrics Dyed with Phellodendron amurense Rupr. (Amur Cork Tree Bark). Molecules, 28(5), 2220. https://doi.org/10.3390/molecules28052220







