Characterization of the Physical Chemistry Properties of Iron-Tailing-Based Ceramsite
Abstract
:1. Introduction
2. Results and Discussion
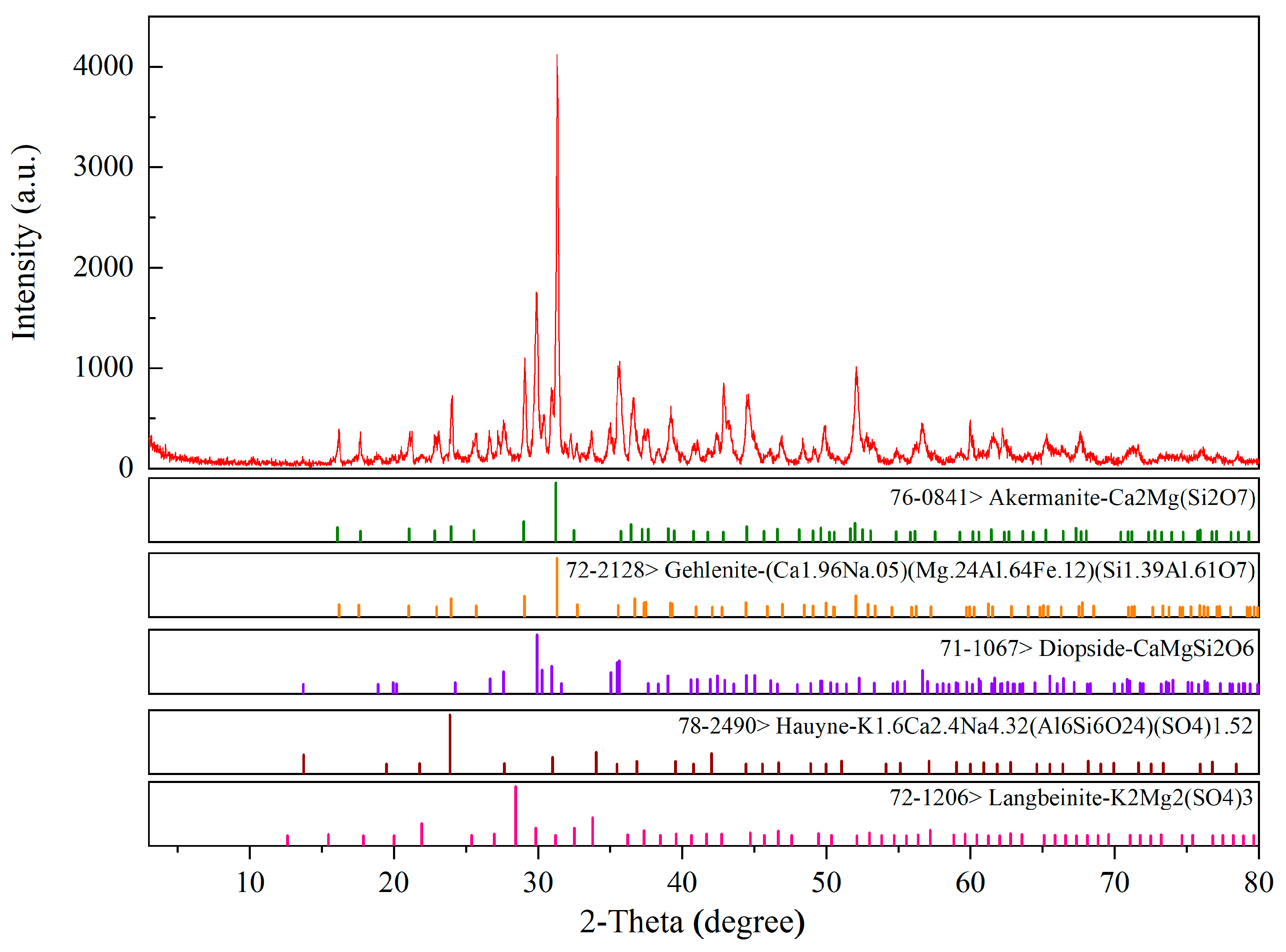
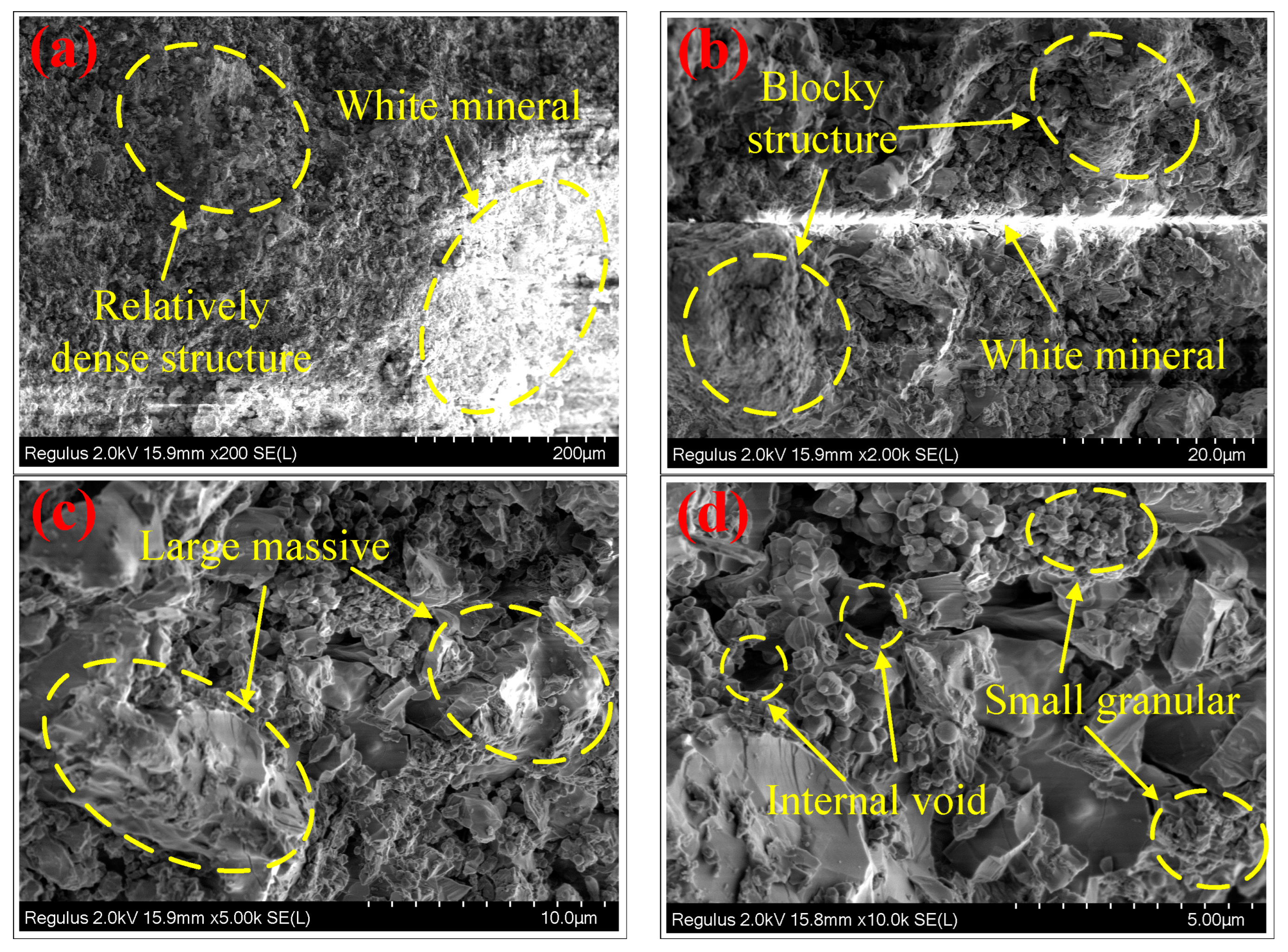
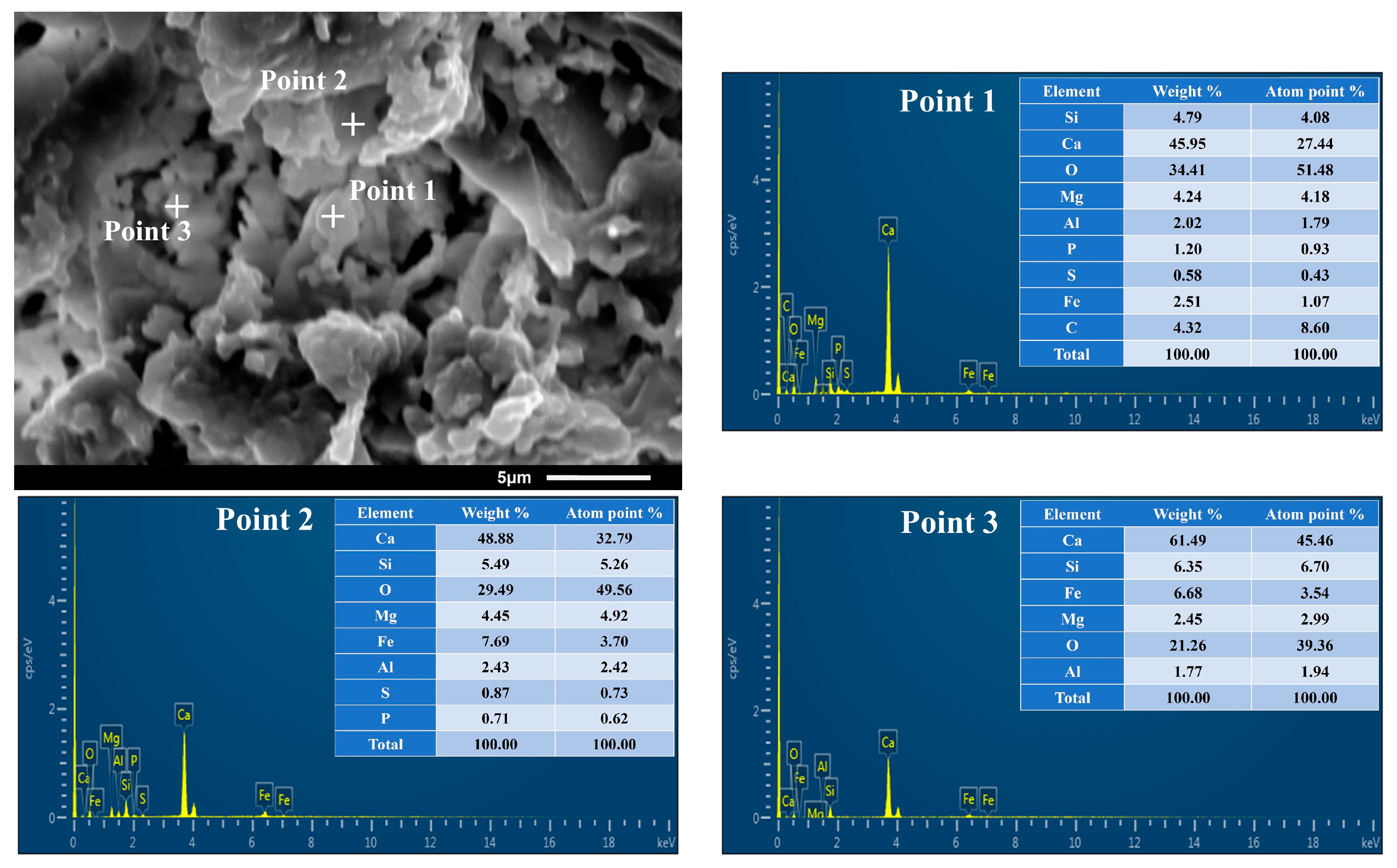
2.1. XRD Analysis and SEM-EDS Analysis
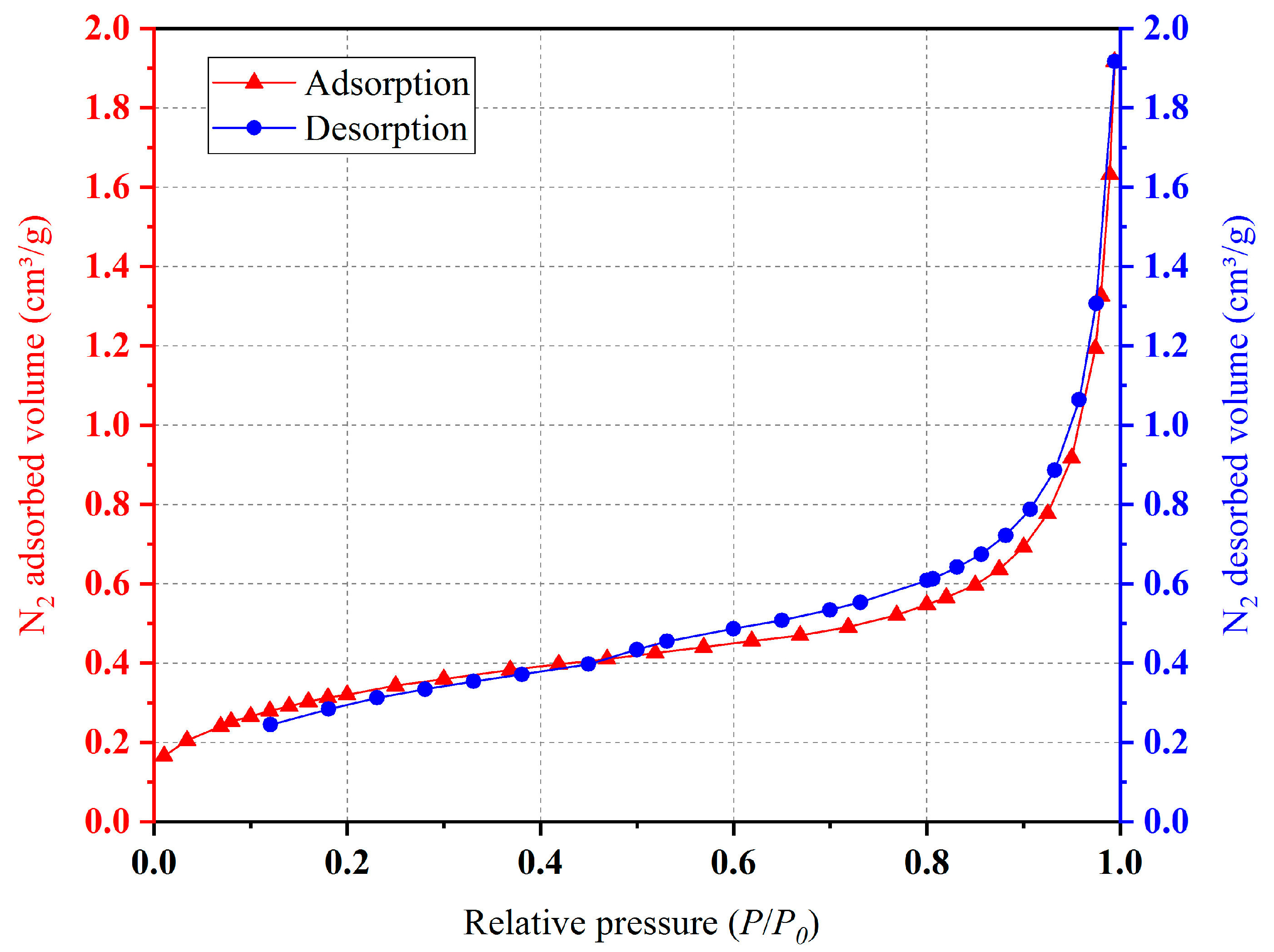
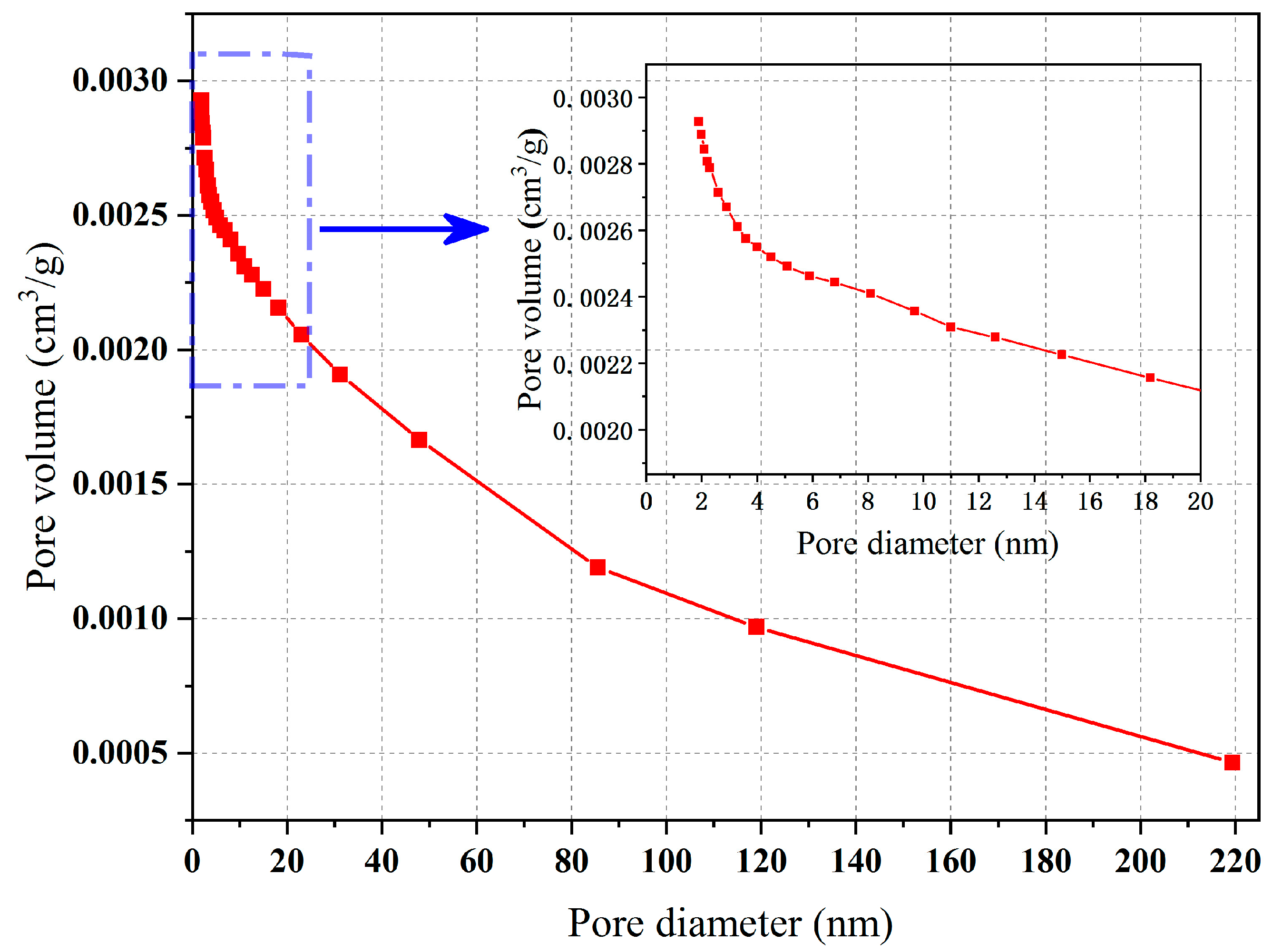
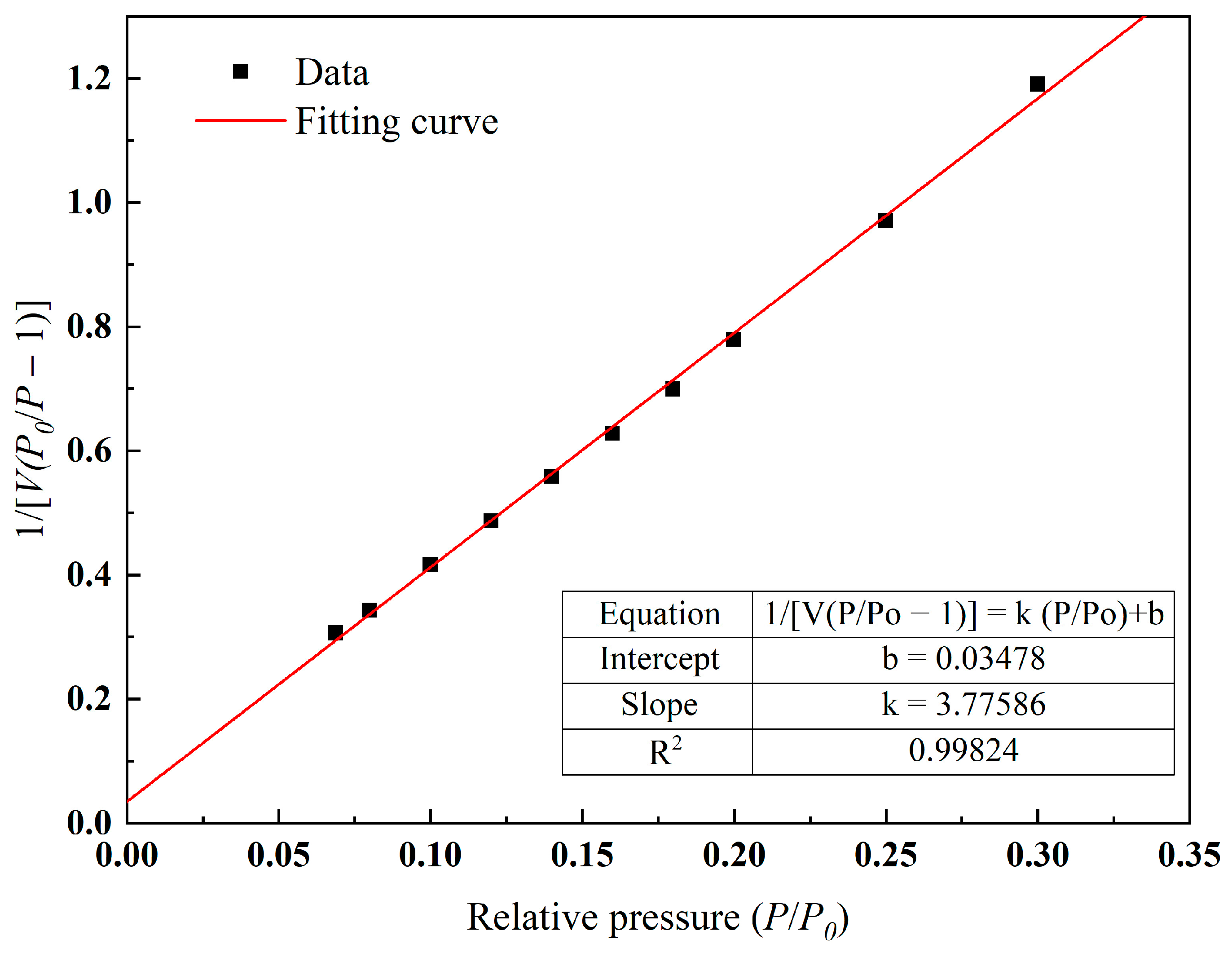
2.2. Specific Surface Area Analysis
2.3. Themogravimetric Analysis
3. Materials and Methods
3.1. Materials
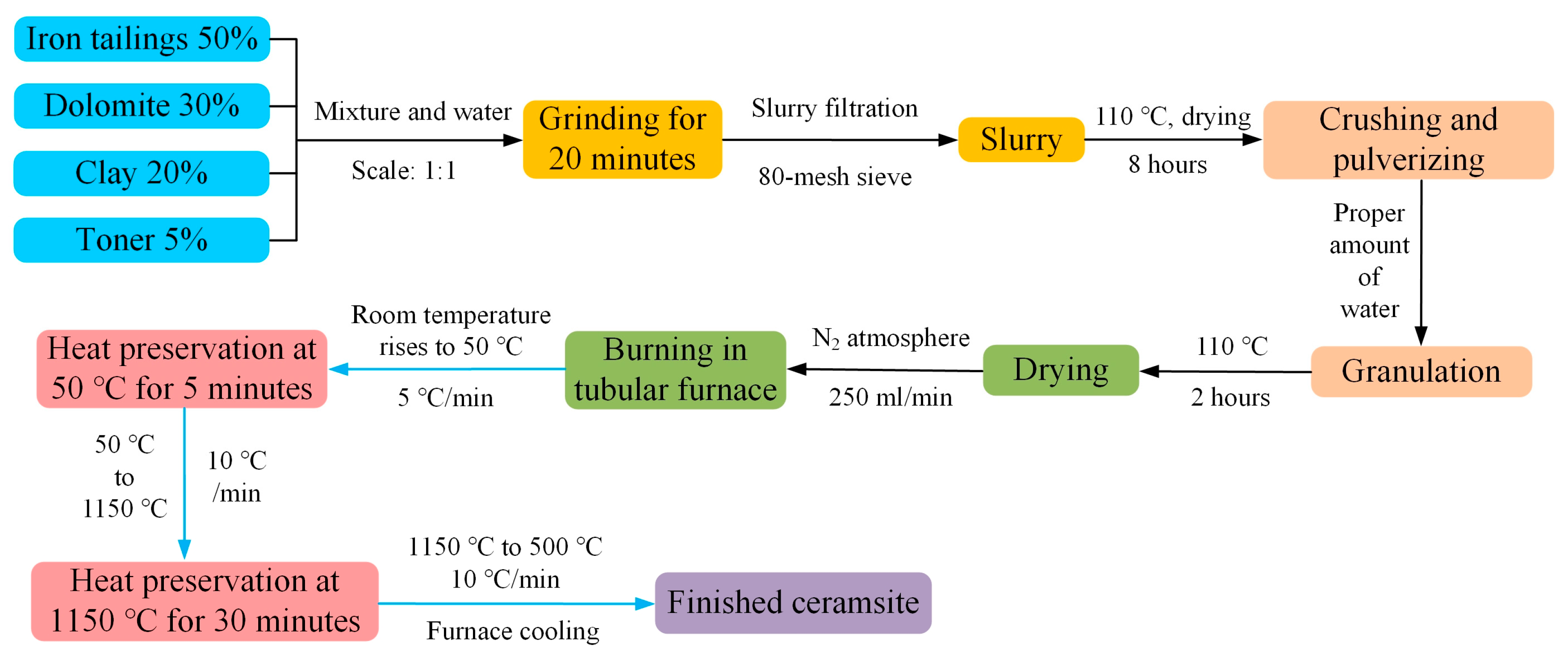
3.2. Ceramsite Preparation Process
3.3. The XRF Method
3.4. The XRD Method
3.5. The SEM-EDS Method
3.6. The TGA Method
3.7. The BET Method
4. Conclusions
- (1)
- By analyzing the experimental results of the XRD, the ceramsite prepared in this paper contained gehlenite and diopside minerals with a high strength, demonstrating that this ceramsite had a higher strength and better hydration resistance than traditional ceramsite. It could be added to traditional building materials to achieve the dual effect of reducing the structural quality and enhancing the material strength;
- (2)
- SEM-EDS analysis results showed that this type of ceramsite had a relatively dense structure inside, and that the internal structure was dominated by a massive structure. In addition, it contained a small amount of granular, simple minerals and voids. This provided evidence for the high strength of ceramsite and also reflected that the ceramsite had a good adsorbability;
- (3)
- The experimental results of the specific surface area analysis showed that the pore size distribution of the internal pores of this type of ceramsite was relatively uniform, containing mainly medium and large pores. When adsorbing N2, the ceramsite showed “slow at first and then fast” characteristics, and its adsorbability was strong. Therefore, it could be considered for applications in the treatment of polluted wastewater and could play a role in specific environments;
- (4)
- Thermogravimetric analysis showed that the quality of the ceramsite would continue to increase after being heated to 504.6 °C. According to the XRD experimental results and experimental conditions, it was speculated that, in part of the ceramsite ore phase containing Al, Mg or Ca, the elements underwent relatively complex chemical reactions with each other, resulting in the formation of an ore phase with a higher molecular weight.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Yang, C.; Cui, C.; Qin, J.; Cui, X. Characteristics of the fired bricks with low-silicon iron tailings. Constr. Build. Mater. 2014, 70, 36–42. [Google Scholar] [CrossRef]
- Zhan, X.; Wang, L.; Wang, J.; Yue, Z.; Deng, R.; Wang, Y.; Xu, X. Roasting mechanism of lightweight low-aluminum–silicon ceramisite derived from municipal solid waste incineration fly ash and electrolytic manganese residue. Waste Manag. 2022, 153, 267–274. [Google Scholar] [CrossRef]
- Liu, M.; Xu, L.; Zhang, X.; Hao, H.; Di, Y. Preparation of Eco-Friendly Composite Ceramic from Iron Ore Tailings. Mater. Sci. Forum 2009, 610–613, 281–284. [Google Scholar] [CrossRef]
- Qin, J.; Cui, C.; Cui, X.; Hussain, A.; Yang, C. Preparation and characterization of ceramsite from lime mud and coal fly ash. Constr. Build. Mater. 2015, 95, 10–17. [Google Scholar] [CrossRef]
- Li, Y.; Xu, M.; Li, Q.; Gai, A.; Yang, T.; Li, R. Study on the Properties and Heavy Metal Solidification Characteristics of Sintered Ceramsites Composed of Magnesite Tailings, Sewage Sludge, and Coal Gangue. Int. J. Env. Res. Pub. Health 2022, 19, 11128. [Google Scholar] [CrossRef] [PubMed]
- Kwek, S.Y.; Awanga, H.; Cheaha, C.B.; Mohamadb, H. Development of sintered aggregate derived from POFA and silt for lightweight concrete. J. Build. Eng. 2022, 49, 104039. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, X.; Liu, X.; Duan, P.; Yang, H.; Jiang, T.; Wang, D.; Liu, R. Current situation and comprehensive utilization of iron ore tailing resources. J. Min. Sci. 2006, 42, 403–408. [Google Scholar] [CrossRef]
- Li, X.; Wang, P.; Qin, J.; Liu, Y.; Qu, Y.; Liu, J.; Cao, R.; Zhang, Y. Mechanical properties of sintered ceramsite from iron ore tailings affected by two-region structure. Constr. Build. Mater. 2020, 240, 117919. [Google Scholar] [CrossRef]
- Wang, M.; Wang, Q.; Mao, J.; Xu, S.; Shi, Z. Study on water-repellent and corrosion-resistant properties of cement mortar using superhydrophobic iron ore tailings. J. Build. Eng. 2022, 62, 105360. [Google Scholar] [CrossRef]
- Warmadewanthi, I.D.A.A.; Chrystiadinia, G.; Kurniawanb, S.B.; Abdullahb, S.R.S. Impact of degraded solid waste utilization as a daily cover for landfill on the formation of methane and leachate. Bioresour. Technol. Rep. 2021, 15, 100797. [Google Scholar] [CrossRef]
- Barik, K.; Prusti, P.; Soren, S.; Meikap, B.C.; Biswal, S.K. Analysis of iron ore pellets properties concerning raw material mineralogy for effective utilization of mining waste. Powder Technol. 2022, 400, 117259. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, W.; Li, F.; Wang, A. Comprehensive Utilization and Resource Utilization of Metal Mine Tailings; Metallurgical Industry Press: Beijing, China, 2002. [Google Scholar]
- Wang, H.; Xu, J.; Liu, Y.; Sheng, L. Preparation of ceramsite from municipal sludge and its application in water treatment: A review. J. Environ. Manag. 2021, 287, 112374. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Zou, J.; Li, G. Effect of sintering temperature on the characteristics of sludge ceramsite. J. Hazard. Mater. 2008, 150, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Xu, G.; Li, G. Ceramsite obtained from water and wastewater sludge and its characteristics affected by Fe2O3, CaO, and MgO. J. Hazard. Mater. 2009, 165, 995–1001. [Google Scholar] [CrossRef]
- Xie, J.; Liu, J.; Liu, F.; Wang, J.; Huang, P. Investigation of a new lightweight green concrete containing sludge ceramsite and recycled fine aggregates. J. Clean. Prod. 2019, 235, 1240–1254. [Google Scholar] [CrossRef]
- Shao, Q.; Zhang, Y.; Liu, Z.; Long, L.; Liu, Z.; Chen, Y.; Hu, X.; Lu, M.; Huang, L. Phosphorus and nitrogen recovery from wastewater by ceramsite: Adsorption mechanism, plant cultivation and sustainability analysis. Sci. Total Environ. 2022, 805, 150288. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.R.; Zou, J.L.; Dai, Y. Utilization of dried sludge for making ceramsite. Water Sci. Technol. 2006, 54, 69–79. [Google Scholar] [CrossRef]
- Pei, D.; Li, Y.; Cang, D. Na+-solidification behavior of SiO2-Al2O3-CaO-MgO (10 wt%) ceramics prepared from red mud. Ceram. Int. 2017, 43, 16936–16942. [Google Scholar] [CrossRef]
- Zhai, Z.; Jin, J.; Wang, Y.; Hou, F.; Yang, H.; Li, H. Study on formation mechanism of akermanite in Zhundong coal ash. CIESC J. 2018, 69, 5266–5275. (In Chinese) [Google Scholar]
- Qin, J.; Yang, S.; Bao, Y.; Wu, Y.; Cai, L.; Wen, Q. Modification of gehlenite ceramsite and its treatment efficiency on manganese-containing wastewater. Environ. Eng. 2022, 40, 47–54. (In Chinese) [Google Scholar]
- Chang, Z.; Huang, F.; Zhang, Z.; Wang, X.; Wen, X.; Song, G.; Li, M. Effect of Melting Temperature on the Crystal Growth of Diopside. J. Ceram. 2021, 42, 977–983. (In Chinese) [Google Scholar]
- Zhao, L.; Li, Y.; Zhang, L.; Cang, D. Effects of CaO and Fe2O3 on the microstructure and mechanical properties of SiO2–CaO–MgO–Fe2O3 ceramics from steel slag. ISIJ Int. 2017, 57, 15–22. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Li, Y.; Zhou, Y.; Cang, D. Preparation of novel ceramics with high CaO content from steel slag. Mater. Des. 2014, 64, 608–613. [Google Scholar] [CrossRef]
- Pei, D.; Li, Y.; Cang, D. In Situ XRD Study on Sintering Mechanism of SiO2-Al2O3-CaO-MgO Ceramics from Red Mud. Mater. Lett. 2019, 240, 229–232. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Cai, S.; Liu, X.; Xie, X.; Xu, S. Research on Preparation of High Strength Ceramic by Using East Lake Silt. J. Wuhan Univ. Technol. 2015, 37, 21–25. (In Chinese) [Google Scholar]
- Xu, Z.; Liu, J.; Song, M.; Zhang, Z. Effect of temperature on properties of sintered ceramsite from sludge and bottom sludge. Chin. J. Environ. Eng. 2013, 7, 1894–1900. (In Chinese) [Google Scholar]
- Zhao, P.; Liu, C.; Srinivasakannan, C.; Zhang, L.; Wang, F.; Gao, J. Basic research on the microwave absorbing properties and microwave roasting mechanism of stibnite concentrate. Powder Technol. 2021, 379, 630–640. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, M.; Li, S.; Zeng, H.; Du, J.; Chen, C.; Pan, Y. Surface modification of phosphorus-containing hydrotalcite using rare-earth coupling agent and its application in polypropylene. Powder Technol. 2019, 342, 555–561. [Google Scholar] [CrossRef]
- Liu, J.; Liu, C.; Hong, Y.; Zhang, L. Basic study on microwave carbon-thermal reduction senarmontite (Sb2O3) to produce antimony: High-temperature dielectric properties and a microwave reduction mechanism. Powder Technol. 2021, 389, 482–492. [Google Scholar] [CrossRef]
- Allibert, M. Verein Deutscher Eisenhüttenleute. Slag Atlas; Verlag Stahleisen: Düsseldorf, Germany, 1995. [Google Scholar]


| Oxide Types | SiO2 | CaO | Al2O3 | MgO | Fe2O3 | SO3 | Na2O | K2O | Total |
|---|---|---|---|---|---|---|---|---|---|
| Percentage (%) | 41.74 | 20.59 | 13.49 | 8.18 | 8.52 | 1.98 | 2.13 | 1.67 | 98.30 |
| Raw Material | SiO2 | CaO | Al2O3 | MgO | Fe2O3 | SO3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Iron tailings | 43.01 | 8.86 | 16.73 | 3.89 | 15.25 | 3.90 | 4.00 | 1.43 |
| Dolomite | 1.97 | 70.26 | 0.35 | 26.98 | 0.26 | 0.04 | 0.02 | 0.01 |
| Clay | 66.13 | 7.02 | 16.87 | 2.81 | 2.82 | 0.08 | 0.41 | 3.16 |
| Sample | Iron Tailings | Dolomite | Clay |
|---|---|---|---|
| #1 | 50% | 30% | 20% |
| #2 | 60% | 25% | 15% |
| #3 | 70% | 20% | 10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hua, S.; Wu, D.; Wu, J.; Li, S.; Liu, G.; Pei, D. Characterization of the Physical Chemistry Properties of Iron-Tailing-Based Ceramsite. Molecules 2023, 28, 2258. https://doi.org/10.3390/molecules28052258
Hua S, Wu D, Wu J, Li S, Liu G, Pei D. Characterization of the Physical Chemistry Properties of Iron-Tailing-Based Ceramsite. Molecules. 2023; 28(5):2258. https://doi.org/10.3390/molecules28052258
Chicago/Turabian StyleHua, Shaoguang, Dun Wu, Jian Wu, Shuqin Li, Guijian Liu, and Dejian Pei. 2023. "Characterization of the Physical Chemistry Properties of Iron-Tailing-Based Ceramsite" Molecules 28, no. 5: 2258. https://doi.org/10.3390/molecules28052258






