Potential Anti-SARS-CoV-2 Prodrugs Activated by Phosphorylation and Their Role in the Aged Population
Abstract
:1. Introduction
2. Chemistry and Synthesis of Anti-SARS-CoV-2 Prodrugs
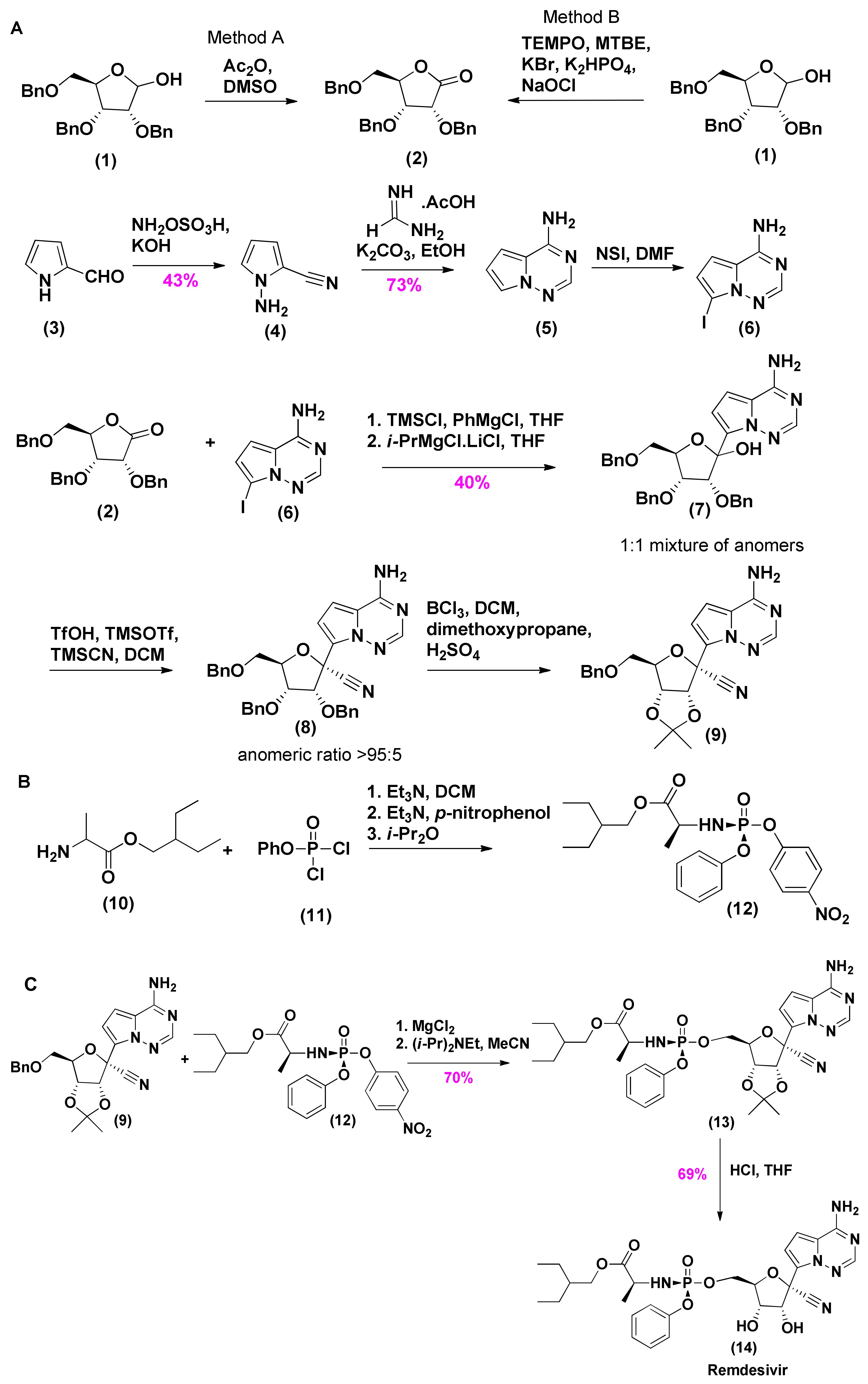
2.1. Synthesis of Remdesivir
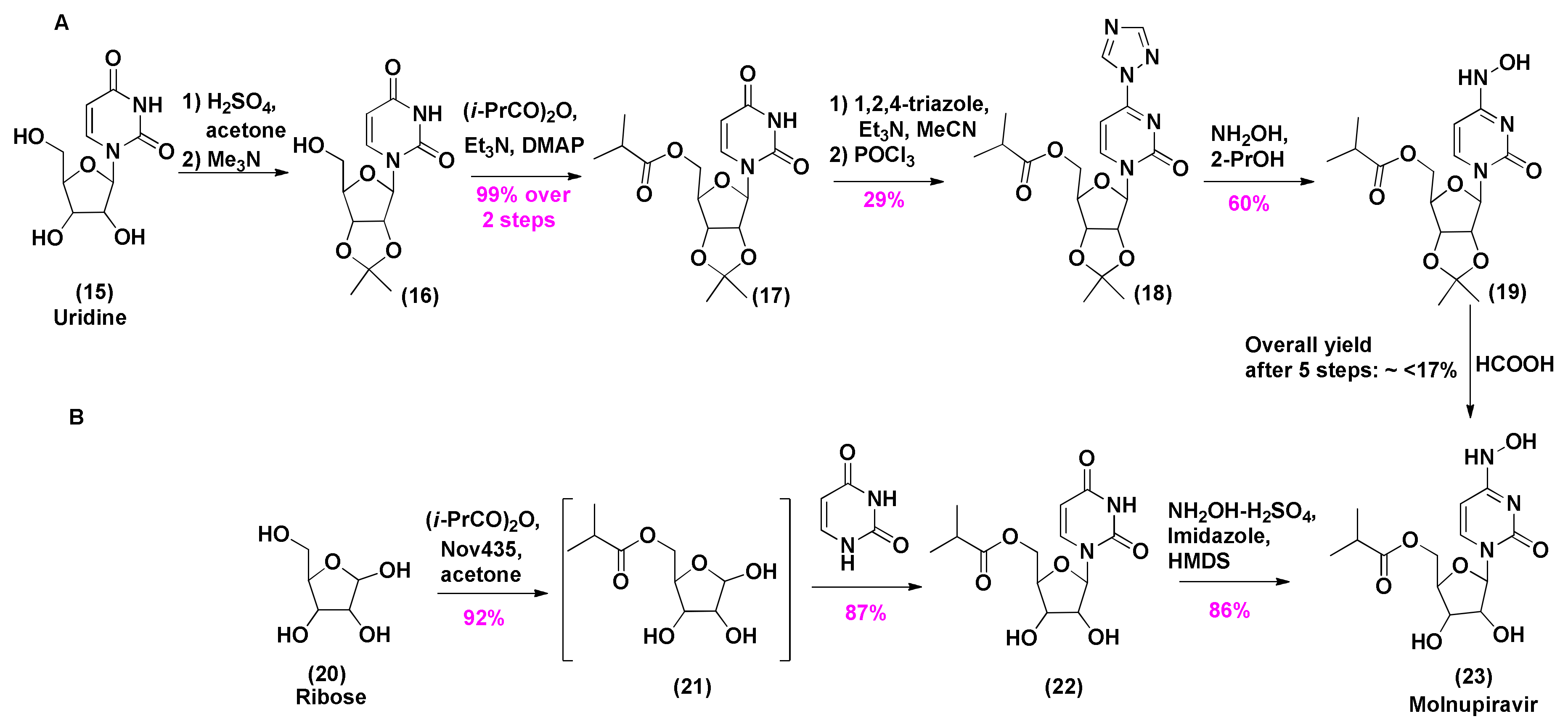
2.2. Synthesis of Molnupiravir
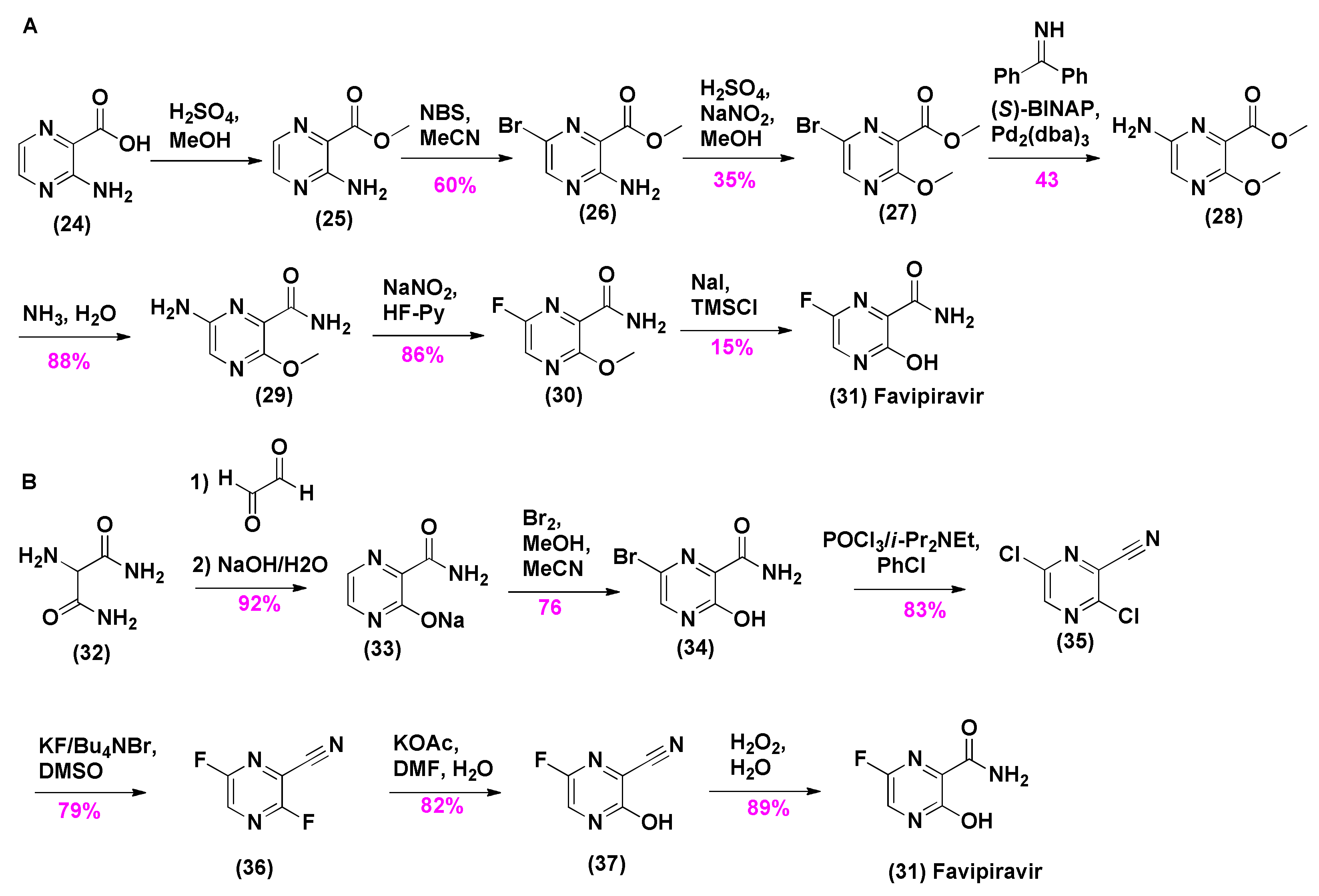
2.3. Synthesis of Favipiravir
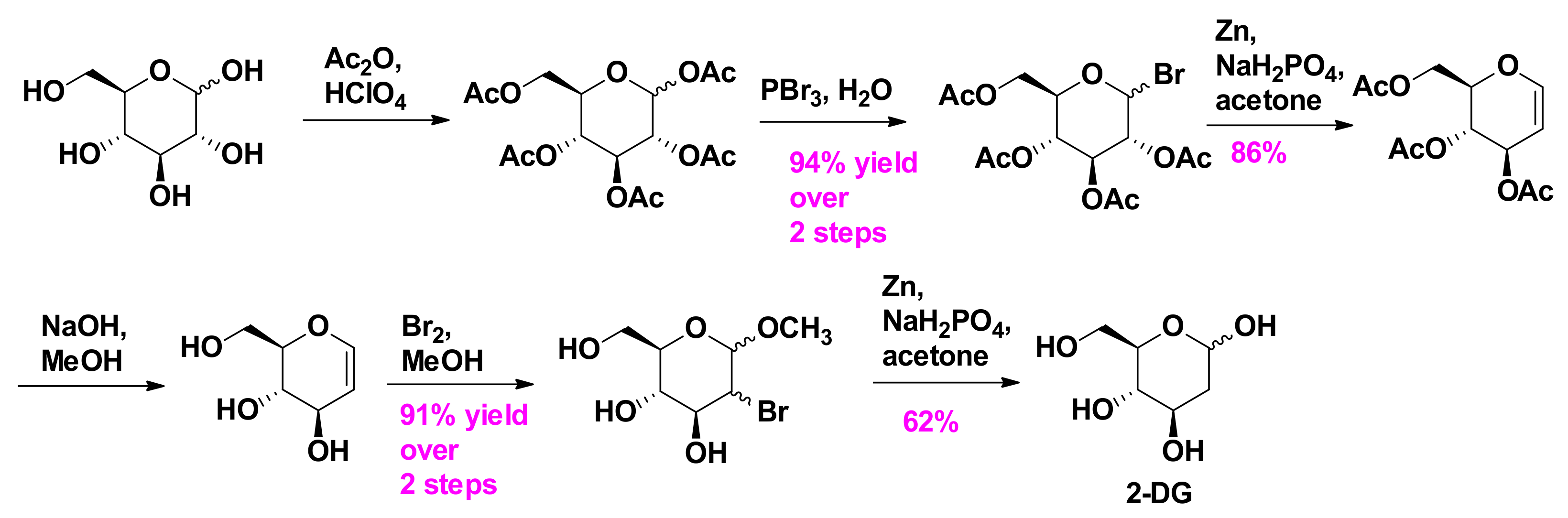
2.4. Synthesis of 2-Deoxy-D-Glucose
3. Clinical Trials of Anti-SARS-CoV-2 Prodrugs for Aged Populations
3.1. Remdesivir
3.2. Molnupiravir
3.3. Favipiravir
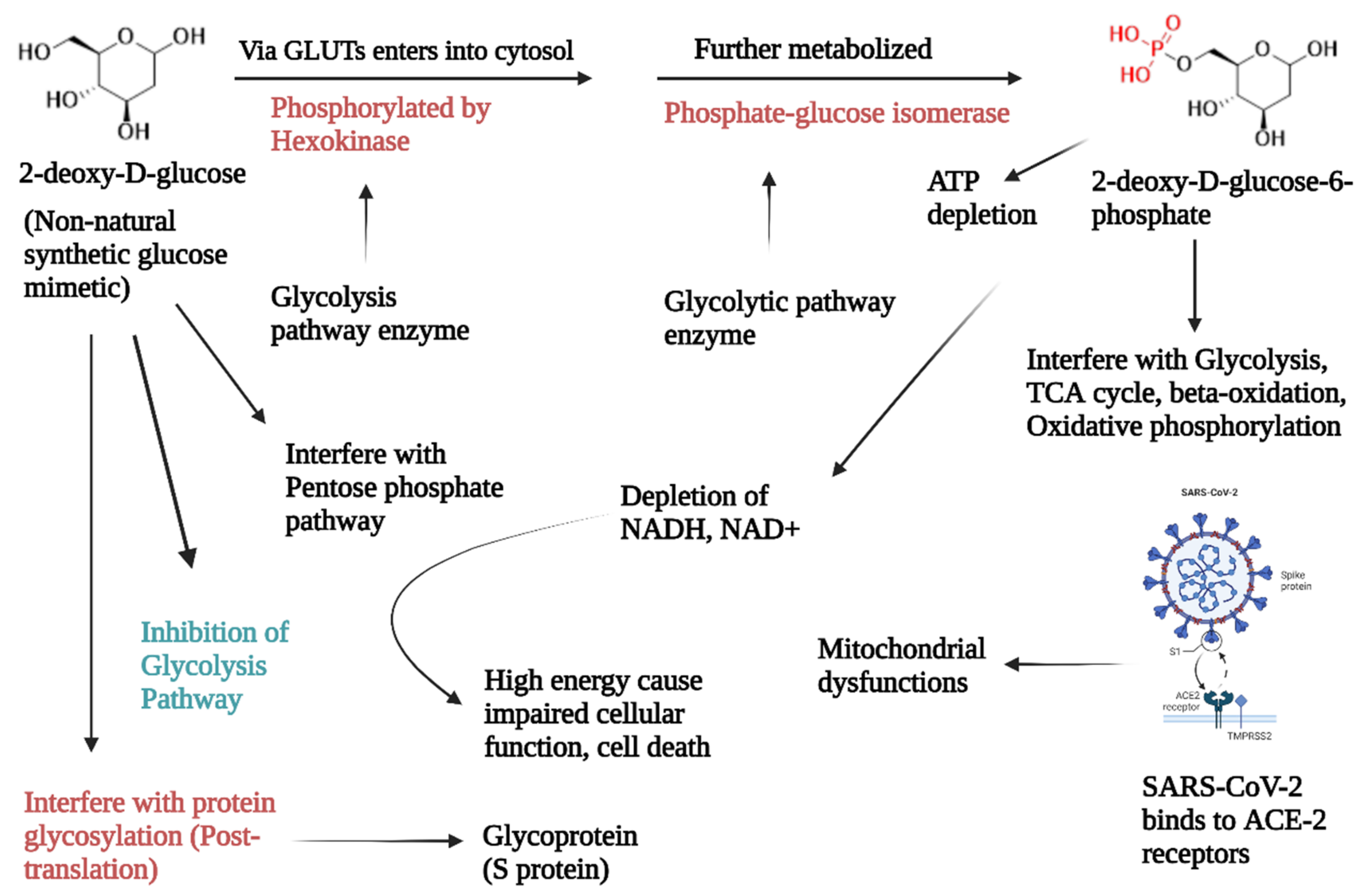
3.4. 2-Deoxy-D-Glucose
4. Impact of Prodrugs on Vaccinated COVID-19 Patients
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Piret, J.; Boivin, G. Pandemics Throughout History. Front. Microbiol. 2021, 11, 631736. [Google Scholar] [CrossRef]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal Vaccines for SARS-CoV-2: From Challenges to Potential in COVID-19 Management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef]
- Chavda, V.P.; Soni, S.; Vora, L.K.; Soni, S.; Khadela, A.; Ajabiya, J. MRNA-Based Vaccines and Therapeutics for COVID-19 and Future Pandemics. Vaccines 2022, 10, 2150. [Google Scholar] [CrossRef]
- Chavda, V.P.; Yao, Q.; Vora, L.K.; Apostolopoulos, V.; Patel, C.A.; Bezbaruah, R.; Patel, A.B.; Chen, Z.-S. Fast-Track Development of Vaccines for SARS-CoV-2: The Shots That Saved the World. Front. Immunol. 2022, 13, 961198. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 23 September 2022).
- De Savi, C.; Hughes, D.L.; Kvaerno, L. Quest for a COVID-19 Cure by Repurposing Small-Molecule Drugs: Mechanism of Action, Clinical Development, Synthesis at Scale, and Outlook for Supply. Org. Process Res. Dev. 2020, 24, 940–976. [Google Scholar] [CrossRef]
- Lei, S.; Chen, X.; Wu, J.; Duan, X.; Men, K. Small Molecules in the Treatment of COVID-19. Signal Transduct. Target. Ther. 2022, 7, 387. [Google Scholar] [CrossRef]
- Laws, M.; Surani, Y.M.; Hasan, M.M.; Chen, Y.; Jin, P.; Al-Adhami, T.; Chowdhury, M.; Imran, A.; Psaltis, I.; Jamshidi, S.; et al. Current Trends and Future Approaches in Small-Molecule Therapeutics for COVID-19. Curr. Med. Chem. 2021, 28, 3803–3824. [Google Scholar] [CrossRef]
- Liu, H.; Iketani, S.; Zask, A.; Khanizeman, N.; Bednarova, E.; Forouhar, F.; Fowler, B.; Hong, S.J.; Mohri, H.; Nair, M.S.; et al. Development of Optimized Drug-like Small Molecule Inhibitors of the SARS-CoV-2 3CL Protease for Treatment of COVID-19. Nat. Commun. 2022, 13, 1891. [Google Scholar] [CrossRef]
- Alam, S.; Sarker, M.M.R.; Afrin, S.; Richi, F.T.; Zhao, C.; Zhou, J.-R.; Mohamed, I.N. Traditional Herbal Medicines, Bioactive Metabolites, and Plant Products Against COVID-19: Update on Clinical Trials and Mechanism of Actions. Front. Pharmacol. 2021, 12, 671498. [Google Scholar] [CrossRef]
- Teli, D.M.; Shah, M.B.; Chhabria, M.T. In Silico Screening of Natural Compounds as Potential Inhibitors of SARS-CoV-2 Main Protease and Spike RBD: Targets for COVID-19. Front. Mol. Biosci. 2021, 7, 599079. [Google Scholar] [CrossRef]
- Demeke, C.A.; Woldeyohanins, A.E.; Kifle, Z.D. Herbal Medicine Use for the Management of COVID-19: A Review Article. Metab. Open 2021, 12, 100141. [Google Scholar] [CrossRef]
- Umeta Chali, B.; Melaku, T.; Berhanu, N.; Mengistu, B.; Milkessa, G.; Mamo, G.; Alemu, S.; Mulugeta, T. Traditional Medicine Practice in the Context of COVID-19 Pandemic: Community Claim in Jimma Zone, Oromia, Ethiopia. Infect. Drug Resist. 2021, 14, 3773–3783. [Google Scholar] [CrossRef]
- COVID-19 Vaccine Resource Center. Available online: https://www.nejm.org/covid-vaccine (accessed on 7 December 2022).
- Moghadas, S.M.; Vilches, T.N.; Zhang, K.; Wells, C.R.; Shoukat, A.; Singer, B.H.; Meyers, L.A.; Neuzil, K.M.; Langley, J.M.; Fitzpatrick, M.C.; et al. The Impact of Vaccination on Coronavirus Disease 2019 (COVID-19) Outbreaks in the United States. Clin. Infect. Dis. 2021, 73, 2257–2264. [Google Scholar] [CrossRef]
- Forni, G.; Mantovani, A.; on behalf of the COVID-19 Commission of Accademia Nazionale dei Lincei, Rome. COVID-19 Vaccines: Where We Stand and Challenges Ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Mistry, P.; Barmania, F.; Mellet, J.; Peta, K.; Strydom, A.; Viljoen, I.M.; James, W.; Gordon, S.; Pepper, M.S. SARS-CoV-2 Variants, Vaccines, and Host Immunity. Front. Immunol. 2022, 12, 809244. [Google Scholar] [CrossRef]
- Krause, P.R.; Fleming, T.R.; Longini, I.M.; Peto, R.; Briand, S.; Heymann, D.L.; Beral, V.; Snape, M.D.; Rees, H.; Ropero, A.-M.; et al. SARS-CoV-2 Variants and Vaccines. N. Engl. J. Med. 2021, 385, 179–186. [Google Scholar] [CrossRef]
- Planas, D.; Veyer, D.; Baidaliuk, A.; Staropoli, I.; Guivel-Benhassine, F.; Rajah, M.M.; Planchais, C.; Porrot, F.; Robillard, N.; Puech, J.; et al. Reduced Sensitivity of SARS-CoV-2 Variant Delta to Antibody Neutralization. Nature 2021, 596, 276–280. [Google Scholar] [CrossRef]
- The Effects of Virus Variants on COVID-19 Vaccines. Available online: https://www.who.int/news-room/feature-stories/detail/the-effects-of-virus-variants-on-covid-19-vaccines (accessed on 25 November 2022).
- Chavda, V.P.; Apostolopoulos, V. Is Booster Dose Strategy Sufficient for Omicron Variant of SARS-CoV-2? Vaccines 2022, 10, 367. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Global Impact of Delta plus Variant and Vaccination. Expert Rev. Vaccines 2022, 21, 597–600. [Google Scholar] [CrossRef]
- Chavda, V.P.; Bezbaruah, R.; Deka, K.; Nongrang, L.; Kalita, T. The Delta and Omicron Variants of SARS-CoV-2: What We Know So Far. Vaccines 2022, 10, 1926. [Google Scholar] [CrossRef]
- Chavda, V.P. Nanobased Nano Drug Delivery: A Comprehensive Review. In Applications of Targeted Nano Drugs and Delivery Systems; Micro and Nano Technologies; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 4; pp. 69–92. ISBN 978-0-12-814029-1. [Google Scholar]
- Chavda, V.P. Nanotherapeutics and Nanobiotechnology. In Applications of Targeted Nano Drugs and Delivery Systems; Micro and Nano Technologies; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 1; pp. 1–13. ISBN 978-0-12-814029-1. [Google Scholar]
- Wang, Z.; Yang, L. Broad-spectrum Prodrugs with Anti-SARS-CoV-2 Activities: Strategies, Benefits, and Challenges. J. Med. Virol. 2022, 94, 1373–1390. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Meanwell, N.A.; Di, L.; Hageman, M.J. The Expanding Role of Prodrugs in Contemporary Drug Design and Development. Nat. Rev. Drug Discov. 2018, 17, 559–587. [Google Scholar] [CrossRef] [PubMed]
- Yasri, S.; Wiwanitki, V. Molnupiravir, Favipiravir and Other Antiviral Drugs with Proposed Potentials for Management of COVID-19: A Concern on Antioxidant Aspect. Int. J. Biochem. Mol. Biol. 2022, 13, 1–4. [Google Scholar]
- Hashemian, S.M.R.; Pourhanifeh, M.H.; Hamblin, M.R.; Shahrzad, M.K.; Mirzaei, H. RdRp Inhibitors and COVID-19: Is Molnupiravir a Good Option? Biomed. Pharmacother. 2022, 146, 112517. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Padmanabhan, S. A Novel Property of Hexokinase Inhibition by Favipiravir and Proposed Advantages over Molnupiravir and 2 Deoxy d Glucose in Treating COVID-19. Biotechnol. Lett. 2022, 44, 831–843. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Chavda, V.P.; Vora, L.K.; Gajjar, N.; Apostolopoulos, V.; Shah, N.; Chen, Z.-S. 2-Deoxy-D-Glucose and Its Derivatives for the COVID-19 Treatment: An Update. Front. Pharmacol. 2022, 13, 899633. [Google Scholar] [CrossRef]
- Eastman, R.T.; Roth, J.S.; Brimacombe, K.R.; Simeonov, A.; Shen, M.; Patnaik, S.; Hall, M.D. Remdesivir: A Review of Its Discovery and Development Leading to Emergency Use Authorization for Treatment of COVID-19. ACS Cent. Sci. 2020, 6, 672–683. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. FDA’s Approval of Veklury (Remdesivir) for the Treatment of COVID-19—The Science of Safety and Effectiveness; FDA: Silver Spring, MD, USA, 2021. [Google Scholar]
- Yang, L.; Wang, Z. Natural Products, Alone or in Combination with FDA-Approved Drugs, to Treat COVID-19 and Lung Cancer. Biomedicines 2021, 9, 689. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, L. GS-5734: A Potentially Approved Drug by FDA against SARS-Cov-2. New J. Chem. 2020, 44, 12417–12429. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Jureka, A.S.; Edwards, M.R.; Lohan, S.; Williams, C.G.; Keiser, P.T.; Davey, R.A.; Totonchy, J.; Tiwari, R.K.; Basler, C.F.; et al. Synthesis and Antiviral Activity of Fatty Acyl Conjugates of Remdesivir against Severe Acute Respiratory Syndrome Coronavirus 2 and Ebola Virus. Eur. J. Med. Chem. 2021, 226, 113862. [Google Scholar] [CrossRef]
- Li, Y.; Liu, M.; Yan, Y.; Wang, Z.; Dai, Q.; Yang, X.; Guo, X.; Li, W.; Chen, X.; Cao, R.; et al. Molnupiravir and Its Active Form, EIDD-1931, Show Potent Antiviral Activity against Enterovirus Infections In Vitro and In Vivo. Viruses 2022, 14, 1142. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, L. Turning the Tide: Natural Products and Natural-Product-Inspired Chemicals as Potential Counters to SARS-CoV-2 Infection. Front. Pharmacol. 2020, 11, 1013. [Google Scholar] [CrossRef]
- Abdelnabi, R.; Foo, C.S.; Kaptein, S.J.F.; Zhang, X.; Do, T.N.D.; Langendries, L.; Vangeel, L.; Breuer, J.; Pang, J.; Williams, R.; et al. The Combined Treatment of Molnupiravir and Favipiravir Results in a Potentiation of Antiviral Efficacy in a SARS-CoV-2 Hamster Infection Model. EBioMedicine 2021, 72, 103595. [Google Scholar] [CrossRef]
- Syed, Y.Y. Molnupiravir: First Approval. Drugs 2022, 82, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, U.; Raju, R.; Udwadia, Z.F. Favipiravir: A New and Emerging Antiviral Option in COVID-19. Med. J. Armed. India 2020, 76, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Baranovich, T.; Wong, S.-S.; Armstrong, J.; Marjuki, H.; Webby, R.J.; Webster, R.G.; Govorkova, E.A. T-705 (Favipiravir) Induces Lethal Mutagenesis in Influenza A H1N1 Viruses in Vitro. J. Virol. 2013, 87, 3741–3751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, H.T.; Hwang, E.S. 2-Deoxyglucose: An Anticancer and Antiviral Therapeutic, but Not Any More a Low Glucose Mimetic. Life Sci. 2006, 78, 1392–1399. [Google Scholar] [CrossRef]
- Sahu, K.K.; Kumar, R. Role of 2-Deoxy-D-Glucose (2-DG) in COVID-19 Disease: A Potential Game-Changer. J. Fam. Med. Prim. Care 2021, 10, 3548–3552. [Google Scholar] [CrossRef]
- Sharun, K.; Tiwari, R.; Dhama, K. Protease Inhibitor GC376 for COVID-19: Lessons Learned from Feline Infectious Peritonitis. Ann. Med. Surg. 2021, 61, 122–125. [Google Scholar] [CrossRef]
- Owen, D.R.; Allerton, C.M.N.; Anderson, A.S.; Aschenbrenner, L.; Avery, M.; Berritt, S.; Boras, B.; Cardin, R.D.; Carlo, A.; Coffman, K.J.; et al. An Oral SARS-CoV-2 Mpro Inhibitor Clinical Candidate for the Treatment of COVID-19. Science 2021, 374, 1586–1593. [Google Scholar] [CrossRef]
- Narayanan, A.; Narwal, M.; Majowicz, S.A.; Varricchio, C.; Toner, S.A.; Ballatore, C.; Brancale, A.; Murakami, K.S.; Jose, J. Identification of SARS-CoV-2 Inhibitors Targeting Mpro and PLpro Using in-Cell-Protease Assay. Commun. Biol. 2022, 5, 169. [Google Scholar] [CrossRef]
- Lee, M.J.; Nori, A.; Fidler, S.; Kulasegaram, R.; Fox, J.; Smith, C. RECEDE-C19 study Response to ‘The Use of Tenofovir in Patients with COVID-19’. HIV Med. 2022, 23, 929–930. [Google Scholar] [CrossRef]
- Shannon, A.; Fattorini, V.; Sama, B.; Selisko, B.; Feracci, M.; Falcou, C.; Gauffre, P.; El Kazzi, P.; Delpal, A.; Decroly, E.; et al. A Dual Mechanism of Action of AT-527 against SARS-CoV-2 Polymerase. Nat. Commun. 2022, 13, 621. [Google Scholar] [CrossRef] [PubMed]
- Rabie, A.M. Cyanorona-20: The First Potent Anti-SARS-CoV-2 Agent. Int. Immunopharmacol. 2021, 98, 107831. [Google Scholar] [CrossRef] [PubMed]
- Jornada, D.H.; dos Santos Fernandes, G.F.; Chiba, D.E.; de Melo, T.R.F.; dos Santos, J.L.; Chung, M.C. The Prodrug Approach: A Successful Tool for Improving Drug Solubility. Molecules 2015, 21, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and Clinical Applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef]
- Testa, B. Prodrugs: Bridging Pharmacodynamic/Pharmacokinetic Gaps. Curr. Opin. Chem. Biol. 2009, 13, 338–344. [Google Scholar] [CrossRef]
- Siegel, D.; Hui, H.C.; Doerffler, E.; Clarke, M.O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][Triazin-4-Amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60, 1648–1661. [Google Scholar] [CrossRef] [Green Version]
- Hughes, D.L. Quest for a Cure: Potential Small-Molecule Treatments for COVID-19, Part 2. Org. Process Res. Dev. 2021, 25, 1089–1111. [Google Scholar] [CrossRef]
- Painter, G.R.; Guthrie, D.B.; Bluemling, G.R.; Natchus, M.G. N4-Hydroxycytidine and Derivatives and Anti-Viral Uses Related Thereto 2016. WO Patent WO2016106050A1, 30 June 2016. [Google Scholar]
- Benkovics, T.; McIntosh, J.; Silverman, S.; Kong, J.; Maligres, P.; Itoh, T.; Yang, H.; Huffman, M.; Verma, D.; Pan, W.; et al. Evolving to an Ideal Synthesis of Molnupiravir, an Investigational Treatment for COVID-19. ChemRxiv 2020. [Google Scholar]
- Furuta, Y.; Egawa, H. Nitrogenous Heterocyclic Carboxamide Derivatives or Salts Thereof and Antiviral Agents Containing Both. WO Patent WO2000010569A1, 2 March 2000. [Google Scholar]
- Hara, T.; Norimatsu, N.; Kurushima, H.; Kano, T. Method for Producing Dichloropyrazine Derivative. U.S. Patent US8835636B2, 16 September 2014. [Google Scholar]
- Marzabadi, C.H.; Franck, R.W. The Synthesis of 2-Deoxyglycosides: 1988–1999. Tetrahedron 2000, 56, 8385–8417. [Google Scholar] [CrossRef]
- Wijayasinghe, Y.S.; Bhansali, M.P.; Borkar, M.R.; Chaturbhuj, G.U.; Muntean, B.S.; Viola, R.E.; Bhansali, P.R. A Comprehensive Biological and Synthetic Perspective on 2-Deoxy-d-Glucose (2-DG), A Sweet Molecule with Therapeutic and Diagnostic Potentials. J. Med. Chem. 2022, 65, 3706–3728. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, H.; Liu, Y.; Hua, Y.; He, B.; Ning, X.; Qin, Z.; Liu, H.-M.; Liu, F.-W. Facile Approaches to 2-Deoxy-d-Glucose and 2-Deoxy-α-d-Glucopyranonucleosides from d-Glucal. Synthesis 2017, 49, 3686–3691. [Google Scholar] [CrossRef]
- Indian Patents. 187908: “An Improved Process for Preparation of 2-Deoxy-D-glucose”. Available online: https://www.allindianpatents.com/patents/187908-an-improved-process-for-preparation-of-2-deoxy-d-glucose (accessed on 12 January 2023).
- Commissioner of the FDA Approves First Treatment for COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-covid-19 (accessed on 26 November 2022).
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic Efficacy of the Small Molecule GS-5734 against Ebola Virus in Rhesus Monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulangu, S.; Dodd, L.E.; Davey, R.T.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Yin, W.; Mao, C.; Luan, X.; Shen, D.-D.; Shen, Q.; Su, H.; Wang, X.; Zhou, F.; Zhao, W.; Gao, M.; et al. Structural Basis for Inhibition of the RNA-Dependent RNA Polymerase from SARS-CoV-2 by Remdesivir. Science 2020, 368, 1499–1504. [Google Scholar] [CrossRef]
- Malin, J.J.; Suárez, I.; Priesner, V.; Fätkenheuer, G.; Rybniker, J. Remdesivir against COVID-19 and Other Viral Diseases. Clin. Microbiol. Rev. 2020, 34, e00162-20. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Won, J.J.; Graham, R.L.; Dinnon, K.H.; Sims, A.C.; Feng, J.Y.; Cihlar, T.; Denison, M.R.; Baric, R.S.; Sheahan, T.P. Broad Spectrum Antiviral Remdesivir Inhibits Human Endemic and Zoonotic Deltacoronaviruses with a Highly Divergent RNA Dependent RNA Polymerase. Antivir. Res. 2019, 169, 104541. [Google Scholar] [CrossRef] [PubMed]
- Frediansyah, A.; Nainu, F.; Dhama, K.; Mudatsir, M.; Harapan, H. Remdesivir and Its Antiviral Activity against COVID-19: A Systematic Review. Clin. Epidemiol. Glob. Health 2021, 9, 123–127. [Google Scholar] [CrossRef]
- Pizzorno, A.; Padey, B.; Dubois, J.; Julien, T.; Traversier, A.; Dulière, V.; Brun, P.; Lina, B.; Rosa-Calatrava, M.; Terrier, O. In Vitro Evaluation of Antiviral Activity of Single and Combined Repurposable Drugs against SARS-CoV-2. Antivir. Res. 2020, 181, 104878. [Google Scholar] [CrossRef]
- Spinner, C.D.; Gottlieb, R.L.; Criner, G.J.; Arribas López, J.R.; Cattelan, A.M.; Soriano Viladomiu, A.; Ogbuagu, O.; Malhotra, P.; Mullane, K.M.; Castagna, A.; et al. Effect of Remdesivir vs Standard Care on Clinical Status at 11 Days in Patients With Moderate COVID-19: A Randomized Clinical Trial. JAMA 2020, 324, 1048. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zhang, D.; Du, G.; Du, R.; Zhao, J.; Jin, Y.; Fu, S.; Gao, L.; Cheng, Z.; Lu, Q.; et al. Remdesivir in Adults with Severe COVID-19: A Randomised, Double-Blind, Placebo-Controlled, Multicentre Trial. Lancet 2020, 395, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Poliseno, M.; Colpani, A.; Zauli, B.; Puci, M.V.; Santantonio, T.; Meloni, M.C.; Fois, M.; Fanelli, C.; Saderi, L.; et al. Reduced Risk of Death in People with SARS-CoV-2 Infection Treated with Remdesivir: A Nested Case–Control Study. Curr. Med. Res. Opin. 2022, 38, 2029–2033. [Google Scholar] [CrossRef]
- Veklury® (Remdesivir) Retains Antiviral Activity Against Omicron, Delta and Other Emergent SARS-CoV-2 Variants in Multiple In Vitro Studies. Available online: https://www.gilead.com/news-and-press/press-room/press-releases/2022/2/veklury-remdesivir-retains-antiviral-activity-against-omicron-delta-and-other-emergent-sarscov2-variants-in-multiple-in-vitro-studies (accessed on 7 December 2022).
- Ramos-Rincon, J.-M.; López-Carmona, M.-D.; Cobos-Palacios, L.; López-Sampalo, A.; Rubio-Rivas, M.; Martín-Escalante, M.-D.; de-Cossio-Tejido, S.; Taboada-Martínez, M.-L.; Muiño-Miguez, A.; Areses-Manrique, M.; et al. Remdesivir in Very Old Patients (≥80 Years) Hospitalized with COVID-19: Real World Data from the SEMI-COVID-19 Registry. J. Clin. Med. 2022, 11, 3769. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ding, M.; Dong, X.; Zhang, J.; Kursat Azkur, A.; Azkur, D.; Gan, H.; Sun, Y.; Fu, W.; Li, W.; et al. Risk Factors for Severe and Critically Ill COVID-19 Patients: A Review. Allergy 2021, 76, 428–455. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. [Google Scholar] [CrossRef]
- Mohd, I..; Kumar Arora, M.; Asdaq, S.M.B.; Khan, S.A.; Alaqel, S.I.; Alshammari, M.K.; Alshehri, M.M.; Alshrari, A.S.; Mateq Ali, A.; Al-shammeri, A.M.; et al. Discovery, Development, and Patent Trends on Molnupiravir: A Prospective Oral Treatment for COVID-19. Molecules 2021, 26, 5795. [Google Scholar] [CrossRef]
- Tian, L.; Pang, Z.; Li, M.; Lou, F.; An, X.; Zhu, S.; Song, L.; Tong, Y.; Fan, H.; Fan, J. Molnupiravir and Its Antiviral Activity Against COVID-19. Front. Immunol. 2022, 13, 855496. [Google Scholar] [CrossRef] [PubMed]
- Gordon, C.J.; Tchesnokov, E.P.; Schinazi, R.F.; Götte, M. Molnupiravir Promotes SARS-CoV-2 Mutagenesis via the RNA Template. J. Biol. Chem. 2021, 297, 100770. [Google Scholar] [CrossRef]
- Kabinger, F.; Stiller, C.; Schmitzová, J.; Dienemann, C.; Kokic, G.; Hillen, H.S.; Höbartner, C.; Cramer, P. Mechanism of Molnupiravir-Induced SARS-CoV-2 Mutagenesis. Nat. Struct. Mol. Biol. 2021, 28, 740–746. [Google Scholar] [CrossRef]
- Maisonnasse, P.; Aldon, Y.; Marc, A.; Marlin, R.; Dereuddre-Bosquet, N.; Kuzmina, N.A.; Freyn, A.W.; Snitselaar, J.L.; Gonçalves, A.; Caniels, T.G.; et al. COVA1-18 Neutralizing Antibody Protects against SARS-CoV-2 in Three Preclinical Models. Nat. Commun. 2021, 12, 6097. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Zhou, S.; Graham, R.L.; Pruijssers, A.J.; Agostini, M.L.; Leist, S.R.; Schäfer, A.; Dinnon, K.H.; Stevens, L.J.; et al. An Orally Bioavailable Broad-Spectrum Antiviral Inhibits SARS-CoV-2 in Human Airway Epithelial Cell Cultures and Multiple Coronaviruses in Mice. Sci. Transl. Med. 2020, 12, eabb5883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troth, S.; Butterton, J.; DeAnda, C.S.; Escobar, P.; Grobler, J.; Hazuda, D.; Painter, G. Letter to the Editor in Response to Zhou et Al. J. Infect. Dis. 2021, 224, 1442–1443. [Google Scholar] [CrossRef] [PubMed]
- Abdelnabi, R.; Foo, C.S.; De Jonghe, S.; Maes, P.; Weynand, B.; Neyts, J. Molnupiravir Inhibits Replication of the Emerging SARS-CoV-2 Variants of Concern in a Hamster Infection Model. J. Infect. Dis. 2021, 224, 749–753. [Google Scholar] [CrossRef]
- Takashita, E.; Kinoshita, N.; Yamayoshi, S.; Sakai-Tagawa, Y.; Fujisaki, S.; Ito, M.; Iwatsuki-Horimoto, K.; Chiba, S.; Halfmann, P.; Nagai, H.; et al. Efficacy of Antibodies and Antiviral Drugs against COVID-19 Omicron Variant. N. Engl. J. Med. 2022, 386, 995–998. [Google Scholar] [CrossRef]
- Rosenke, K.; Okumura, A.; Lewis, M.C.; Feldmann, F.; Meade-White, K.; Bohler, W.F.; Griffin, A.; Rosenke, R.; Shaia, C.; Jarvis, M.A.; et al. Molnupiravir Inhibits SARS-CoV-2 Variants Including Omicron in the Hamster Model. JCI Insight 2022, 7, e160108. [Google Scholar] [CrossRef] [PubMed]
- De Vito, A.; Colpani, A.; Bitti, A.; Zauli, B.; Meloni, M.C.; Fois, M.; Denti, L.; Bacciu, S.; Marcia, C.; Maida, I.; et al. Safety and Efficacy of Molnupiravir in SARS-CoV-2-infected Patients: A Real-life Experience. J. Med. Virol. 2022, 94, 5582–5588. [Google Scholar] [CrossRef] [PubMed]
- Flisiak, R.; Zarębska-Michaluk, D.; Rogalska, M.; Kryńska, J.A.; Kowalska, J.; Dutkiewicz, E.; Dobrowolska, K.; Jaroszewicz, J.; Moniuszko-Malinowska, A.; Rorat, M.; et al. Real-World Experience with Molnupiravir during the Period of SARS-CoV-2 Omicron Variant Dominance. Pharmacol. Rep. 2022, 74, 1279–1285. [Google Scholar] [CrossRef]
- Gonda, K.; Suzuki, K.; Kono, K.; Takenoshita, S. Safety of Oral Administration of Molnupiravir for Hospitalized Elderly People Aged 80 Years Old or Older with COVID-19. Res. Sq. 2022. preprint. [Google Scholar] [CrossRef]
- Joshi, S.; Parkar, J.; Ansari, A.; Vora, A.; Talwar, D.; Tiwaskar, M.; Patil, S.; Barkate, H. Role of Favipiravir in the Treatment of COVID-19. Int. J. Infect. Dis. 2021, 102, 501–508. [Google Scholar] [CrossRef]
- Basu, D.; Chavda, V.P.; Mehta, A.A. Therapeutics for COVID-19 and Post COVID-19 Complications: An Update. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100086. [Google Scholar] [CrossRef]
- Hassanipour, S.; Arab-Zozani, M.; Amani, B.; Heidarzad, F.; Fathalipour, M.; Martinez-de-Hoyo, R. The Efficacy and Safety of Favipiravir in Treatment of COVID-19: A Systematic Review and Meta-Analysis of Clinical Trials. Sci Rep 2021, 11, 11022. [Google Scholar] [CrossRef]
- Cai, Q.; Yang, M.; Liu, D.; Chen, J.; Shu, D.; Xia, J.; Liao, X.; Gu, Y.; Cai, Q.; Yang, Y.; et al. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering 2020, 6, 1192–1198. [Google Scholar] [CrossRef]
- McMahon, J.H.; Lau, J.S.Y.; Coldham, A.; Roney, J.; Hagenauer, M.; Price, S.; Bryant, M.; Garlick, J.; Paterson, A.; Lee, S.J.; et al. Favipiravir in Early Symptomatic COVID-19, a Randomised Placebo-Controlled Trial. eClinicalMedicine 2022, 54, 101703. [Google Scholar] [CrossRef] [PubMed]
- Udwadia, Z.F.; Singh, P.; Barkate, H.; Patil, S.; Rangwala, S.; Pendse, A.; Kadam, J.; Wu, W.; Caracta, C.F.; Tandon, M. Efficacy and Safety of Favipiravir, an Oral RNA-Dependent RNA Polymerase Inhibitor, in Mild-to-Moderate COVID-19: A Randomized, Comparative, Open-Label, Multicenter, Phase 3 Clinical Trial. Int. J. Infect. Dis. 2021, 103, 62–71. [Google Scholar] [CrossRef]
- Yuan, Y.; Lu, Q.-B.; Yao, W.-S.; Zhao, J.; Zhang, X.-A.; Cui, N.; Yuan, C.; Yang, T.; Peng, X.-F.; Lv, S.-M.; et al. Clinical Efficacy and Safety Evaluation of Favipiravir in Treating Patients with Severe Fever with Thrombocytopenia Syndrome. eBioMedicine 2021, 72, 103591. [Google Scholar] [CrossRef]
- Papp, H.; Lanszki, Z.; Keserű, G.M.; Jakab, F. Favipiravir for the Treatment of COVID-19 in Elderly Patients—What Do We Know after 2 Years of COVID-19? GeroScience 2022, 44, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Ivashchenko, A.A.; Dmitriev, K.A.; Vostokova, N.V.; Azarova, V.N.; Blinow, A.A.; Egorova, A.N.; Gordeev, I.G.; Ilin, A.P.; Karapetian, R.N.; Kravchenko, D.V.; et al. AVIFAVIR for Treatment of Patients With Moderate Coronavirus Disease 2019 (COVID-19): Interim Results of a Phase II/III Multicenter Randomized Clinical Trial. Clin. Infect. Dis. 2021, 73, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, M.; Tsushima, K.; Tanaka, S.; Hagiwara, E.; Tarumoto, N.; Kawada, I.; Hirai, Y.; Fujiwara, S.; Komase, Y.; Saraya, T.; et al. Efficacy and Safety of Favipiravir in Moderate COVID-19 Pneumonia Patients without Oxygen Therapy: A Randomized, Phase III Clinical Trial. Infect. Dis. Ther. 2021, 10, 2489–2509. [Google Scholar] [CrossRef]
- Karatas, E.; Aksoy, L.; Ozaslan, E. Association of Early Favipiravir Use with Reduced COVID-19 Fatality among Hospitalized Patients. Infect. Chemother. 2021, 53, 300–307. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, G.; Salam, A.; Horby, P.; Hayden, F.G.; Chen, C.; Pan, J.; Zheng, J.; Lu, B.; Guo, L.; et al. Comparative Effectiveness of Combined Favipiravir and Oseltamivir Therapy Versus Oseltamivir Monotherapy in Critically Ill Patients With Influenza Virus Infection. J. Infect. Dis. 2020, 221, 1688–1698. [Google Scholar] [CrossRef] [PubMed]
- Bureau, E.N. Russian Drug Avifavir Effective against Corona Virus Variants: ChemRar Group. Express Pharma 2021.
- Pilkington, V.; Pepperrell, T.; Hill, A. A Review of the Safety of Favipiravir—A Potential Treatment in the COVID-19 Pandemic? J. Virus Erad. 2020, 6, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Raez, L.E.; Papadopoulos, K.; Ricart, A.D.; Chiorean, E.G.; Dipaola, R.S.; Stein, M.N.; Rocha Lima, C.M.; Schlesselman, J.J.; Tolba, K.; Langmuir, V.K.; et al. A Phase I Dose-Escalation Trial of 2-Deoxy-D-Glucose Alone or Combined with Docetaxel in Patients with Advanced Solid Tumors. Cancer Chemother. Pharmacol. 2013, 71, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Samal, K.C.; Panda, B.; Behera, L. Anti-Covid Drug: 2-Deoxy-D-Glucose and Its Mechanism of Action. Biot. Res. Today 2021, 3, 345–347. [Google Scholar]
- Kalyanaraman, B. Reactive Oxygen Species, Proinflammatory and Immunosuppressive Mediators Induced in COVID-19: Overlapping Biology with Cancer. RSC Chem. Biol. 2021, 2, 1402–1414. [Google Scholar] [CrossRef]
- Bhatt, A.N.; Kumar, A.; Rai, Y.; Kumari, N.; Vedagiri, D.; Harshan, K.H.; Chinnadurai, V.; Chandna, S. Glycolytic Inhibitor 2-Deoxy-d-Glucose Attenuates SARS-CoV-2 Multiplication in Host Cells and Weakens the Infective Potential of Progeny Virions. Life Sci. 2022, 295, 120411. [Google Scholar] [CrossRef] [PubMed]
- Ogando, N.S.; Dalebout, T.J.; Zevenhoven-Dobbe, J.C.; Limpens, R.W.A.L.; van der Meer, Y.; Caly, L.; Druce, J.; de Vries, J.J.C.; Kikkert, M.; Bárcena, M.; et al. SARS-Coronavirus-2 Replication in Vero E6 Cells: Replication Kinetics, Rapid Adaptation and Cytopathology. J. Gen. Virol. 2020, 101, 925–940. [Google Scholar] [CrossRef]
- Balkrishna, A.; Thakur, P.; Singh, S.; Dev, S.; Jain, V.; Varshney, A.; Sharma, R. Glucose Antimetabolite 2-Deoxy-D-Glucose and Its Derivative as Promising Candidates for Tackling COVID-19: Insights Derived from in Silico Docking and Molecular Simulations. Authorea 2021. Preprint. [Google Scholar] [CrossRef]
- Samui, P.; Mondal, J.; Ahmad, B.; Chatterjee, A.N. Clinical Effects of 2-DG Drug Restraining SARS-CoV-2 Infection: A Fractional Order Optimal Control Study. J. Biol. Phys. 2022, 48, 415–438. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Adhikary, A.; Woloschak, G.; Dwarakanath, B.S.; Papineni, R.V.L. A Combinatorial Approach of a Polypharmacological Adjuvant 2-Deoxy-D-Glucose with Low Dose Radiation Therapy to Quell the Cytokine Storm in COVID-19 Management. Int. J. Radiat. Biol. 2020, 96, 1323–1328. [Google Scholar] [CrossRef] [PubMed]
- www.ETHealthworld.com. Indian Drug 2DG Can Reduce Heart Damage by Coronavirus, Find US Researchers—ET HealthWorld. Available online: https://health.economictimes.indiatimes.com/news/pharma/indian-drug-2dg-can-reduce-heart-damage-by-coronavirus-find-us-researchers/95382862 (accessed on 9 December 2022).
- DCGI Approves Anti-COVID Drug Developed by DRDO for Emergency Use. Available online: https://pib.gov.in/pib.gov.in/Pressreleaseshare.aspx?PRID=1717007 (accessed on 25 November 2022).
- Bhatt, A.N.; Shenoy, S.; Munjal, S.; Chinnadurai, V.; Agarwal, A.; Vinoth Kumar, A.; Shanavas, A.; Kanwar, R.; Chandna, S. 2-Deoxy-d-Glucose as an Adjunct to Standard of Care in the Medical Management of COVID-19: A Proof-of-Concept and Dose-Ranging Randomised Phase II Clinical Trial. BMC Infect. Dis. 2022, 22, 669. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, P.; Petrakis, V.; Trypsianis, G.; Papazoglou, D. Early 3-Day Course of Remdesivir in Vaccinated Outpatients with SARS-CoV-2 Infection. A Success Story. J. Chemother. 2022, 34, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Khoo, S.H.; FitzGerald, R.; Saunders, G.; Middleton, C.; Ahmad, S.; Edwards, C.J.; Hadjiyiannakis, D.; Walker, L.; Lyon, R.; Shaw, V.; et al. Molnupiravir versus Placebo in Unvaccinated and Vaccinated Patients with Early SARS-CoV-2 Infection in the UK (AGILE CST-2): A Randomised, Placebo-Controlled, Double-Blind, Phase 2 Trial. Lancet Infect. Dis. 2023, 23, 183–195. [Google Scholar] [CrossRef] [PubMed]
- Herman, B.; Wong, M.C.; Viwattanakulvanid, P. Vaccination Status, Favipiravir, and Micronutrient Supplementation Roles in Post-COVID Symptoms: A Longitudinal Study. PLoS ONE 2022, 17, e0271385. [Google Scholar] [CrossRef]




| Other ID | Study Type | Study Starts | End of Study | Phase | Age Group | Remarks | NCT No. |
|---|---|---|---|---|---|---|---|
| CAP-China Remdesivir 2 | Interventional | 6 February 2020 | 10 April 2020 | 3 | ≥18 years, |
| NCT04257656 |
| LGH003 | Interventional | 1 June 2020 | 30 November 2020 | Early phase 1 | 15 to 80 years |
| NCT04560231 |
| BEX-06001 | Interventional | 4 September 2020 | 30 April 2021 | 2 | ≥18 years |
| NCT04596839 |
| COVID 19 treatment | Interventional | 16 June 2020 | 1 December 2020 | 2, 3 | Older adults, |
| NCT04345419 |
| REC-H-PhBSU-21001 | Interventional | 1 November 2020 | 1 April 2021 | 4 | 18 to 80 years |
| NCT04738045 |
| UW 20-535 | Interventional | 20 November 2020 | 30 September 2021 | 2 | Above 18 years |
| NCT04647695 |
| M.A.R.M.C.D./2020/1985 | Interventional | 15 August 2020 | 10 February 2021 | 3 | 16 to 80 years |
| NCT04678739 |
| GS-US-553-9020 | Interventional | 14 September 2020 | 22 March 2021 | 1, 2 | Above 18 years |
| NCT04539262 |
| FMASU P56a/2020 | Interventional | 27 July 2020 | 1 March 2021 | 3 | 18 to 80 years |
| NCT04853901 |
| REM-ENY-01 | Interventional | 12 October 2020 | 30 November 2021 | 3 | 12 to 100 years |
| NCT04610541 |
| CAP-China Remdesivir 1 | Interventional | 12 February 2020 | 27 April 2020 | 3 | Above 18 years |
| NCT04252664 |
| DW_DWJ1248302 | Interventional | 2 February 2021 | 30 June 2021 | 3 | 19 years and older |
| NCT04713176 |
| VC-02-01 | Interventional | 16 June 2020 | 1 December 2020 | 2 | 18 years and older |
| NCT04410354 |
| GS-US-540-5773-2020-000841-15 ISRCTN1587426 | Interventional | 6 March 2020 | 30 June 2020 | 3 | Above 12 years |
| NCT04292899 |
| NEUROSIVIR | Interventional | 15 September 2020 | 31 March 2021 | 1 | 21 to 50 years |
| NCT04480333 |
| GS-US-540-9012 2020-003510-12 | Interventional | 18 September 2020 | 6 May 2021 | 3 | Above 12 years |
| NCT04501952 |
| GS-US-540-5774 2020-000842-32 ISRCTN85762140 | Interventional | 15 March 2020 | 26 June 2020 | 3 | Above 12 years |
| NCT04292730 |
| GS-US-540-5912 2020-005416-22 DOH-27-012022-4779 | Interventional | 31 March 2021 | 24 May 2022 | 3 | Above 12 years |
| NCT04745351 |
| SOL21 | Interventional | 24 July 2021 | 31 December 2023 | 4 | Above 18 years |
| NCT04978259 |
| RECH-PhBSU-21011 | Interventional | 1 October 2020 | 5 April 2021 | 4 | 18 to 88 years |
| NCT04779047 |
| Other ID | Study Type | Study Start Date | Study Completion Date | Phase | Age Group | Remarks | NCT No. |
|---|---|---|---|---|---|---|---|
| EIDD-2801-2003 | Interventional | 19 June 2020 | 21 February 2021 | Phase 2 | 18 years and older |
| NCT04405570 |
| EZ-SS-029 | Interventional | 12 August 2022 | July 2027 | Phase 3 | 50 years and older |
| NCT05459532 |
| MOL-112021 | Interventional | 1 December 2021 | 11 March 2022 | Phase 3 | 18 Years to 80 years |
| NCT05595824 |
| 4482-001 MK-4482-001 PHRR201210-003189 jRCT2031200404 2020-003367-26 | Interventional | 19 October 2020 | 11 August 2021 | Phase 2 Phase 3 | 18 years and older |
| NCT04575584 |
| 4482-002 MK-4482-002 PHRR201209-003186 jRCT2031210148 2020-003368-24 | Interventional | 19 October 2020 | 5 May 2022 | Phase 2/3 | 18 years and older |
| NCT04575597 |
| 4482-013 MK-4482-013 jRCT2031210281 PHRR211007-003980 2021-000904-39 | Interventional | 11 August 2021 | 17 November 2022 | Phase 3 | 18 years and older |
| NCT04939428 |
| EIDD-2801-2004 | Interventional | 16 June 2020 | 21 February 2022 | Phase 2 | 18 years and older |
| NCT04405739 |
| NL78705.018.21 | Observational | 14 December 2021 | 14 June 2024 | - | 18 years and older |
| NCT05195060 |
| UoL001542 | Interventional | 3 July 2020 | 30 April 2024 | Phase 1 Phase 2 | 18 years and older |
| NCT04746183 |
| MOL-05-02-2021 | Interventional | 1 April 2022 | 31 December 2023 | Not Applicable | 18 years to 45 years |
| NCT05412173 |
| NDPHRECOVERY 2020-001113-21 ISRCTN50189673 | Interventional | 19 March 2020 | November 2032 | Phase 2 Phase 3 | 0 years and older |
| NCT04381936 |
| COVIC-19 | Interventional | 1 April 2022 | 10 September 2024 | Phase 3 | Child, adult, older adult |
| NCT05271929 |
| ASC10-102 | Interventional | 28 November 2022 | 9 October 2023 | Phase 1 | 18 years and older |
| NCT05596045 |
| PR005 | Observational | 1 December 2021 | 30 September 2027 | - | 18 years to 50 years |
| NCT05013632 |
| VIR21001 | Interventional | 30 September 2021 | August 2024 | Phase 2 | 18 years to 50 years |
| NCT05041907 |
| EIDD-2801-1001 2020-001407-17 | Interventional | 10 April 2020 | 11 August 2020 | Phase 1 | 18 years to 60 years |
| NCT04392219 |
| Other ID | Study Type | Study Start Date | Study Complication Date | Phase | Age Group | Remark | NCT Number |
|---|---|---|---|---|---|---|---|
| Favipiravir-A | Interventional | 16 February 2021 | 13 July 2021 | Phase 3 | 50 years and older |
| NCT04818320 |
| CONTROL-COVID-Favipiravir-1 | Interventional | 16 October 2020 | November 2021 | Phase 2 | 65 years and older |
| NCT04448119 |
| FMASU P14/2020 | Interventional | 18 April 2020 | 20 June 2020 | Phase 3 | 18 to 80 years |
| NCT04349241 |
| RC 20/220/R | Interventional | 23 July 2020 | 4 August 2021 | phase 2 and 3 | 18 years and older |
| NCT04464408 |
| FAV-052021 | Interventional | 11 August 2021 | 30 December 2021 | phase 3 | 18 years to 80 years |
| NCT05185284 |
| FAV052020 | Interventional | 21 May 2020 | 20 August 2020 | phase 3 | 18 years to 80 years |
| NCT04542694 |
| FAVI-COV-US201 | Interventional | 17 April 2020 | 30 October 2020 | Phase 2 | 18 years to 80 years |
| NCT04358549 |
| CVD-04-CD-001 | Interventional | 22 August 2020 | 27 January 2021 | Phase 3 | 21 to 70 years |
| NCT04529499 |
| COVID-19-FAV-HQ | Interventional | 16 November 2020 | 16 February 2021 | Phase 3 | 18 to 59 years |
| NCT04981379 |
| Favipiravir covid | Interventional | 20 April 2020 | 28 September 2020 | Phase 2 and phase 3 | 18 to 80 years |
| NCT04351295 |
| Other ID | Study Type | Study Start Date | Study Complication Date | Phase | Age Group | Remarks | NCT Number |
|---|---|---|---|---|---|---|---|
| COMPOSIT Study | Interventional | 4 January 2021 | 4 January 2022 | Not applicable | 40 years and older | 50 participants This study was conducted on 220 COVID patients; 42% of patients improved symptomatically and became free from depending on oxygen supplementation. | NCT05009563 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Teli, D.; Balar, P.C.; Vaghela, D.; Solanki, H.K.; Vaishnav, A.; Vora, L. Potential Anti-SARS-CoV-2 Prodrugs Activated by Phosphorylation and Their Role in the Aged Population. Molecules 2023, 28, 2332. https://doi.org/10.3390/molecules28052332
Chavda VP, Teli D, Balar PC, Vaghela D, Solanki HK, Vaishnav A, Vora L. Potential Anti-SARS-CoV-2 Prodrugs Activated by Phosphorylation and Their Role in the Aged Population. Molecules. 2023; 28(5):2332. https://doi.org/10.3390/molecules28052332
Chicago/Turabian StyleChavda, Vivek P., Divya Teli, Pankti C. Balar, Dixa Vaghela, Hetvi K. Solanki, Akta Vaishnav, and Lalitkumar Vora. 2023. "Potential Anti-SARS-CoV-2 Prodrugs Activated by Phosphorylation and Their Role in the Aged Population" Molecules 28, no. 5: 2332. https://doi.org/10.3390/molecules28052332
APA StyleChavda, V. P., Teli, D., Balar, P. C., Vaghela, D., Solanki, H. K., Vaishnav, A., & Vora, L. (2023). Potential Anti-SARS-CoV-2 Prodrugs Activated by Phosphorylation and Their Role in the Aged Population. Molecules, 28(5), 2332. https://doi.org/10.3390/molecules28052332









