Differential Lipid Accumulation on HepG2 Cells Triggered by Palmitic and Linoleic Fatty Acids Exposure
Abstract
1. Introduction
2. Results
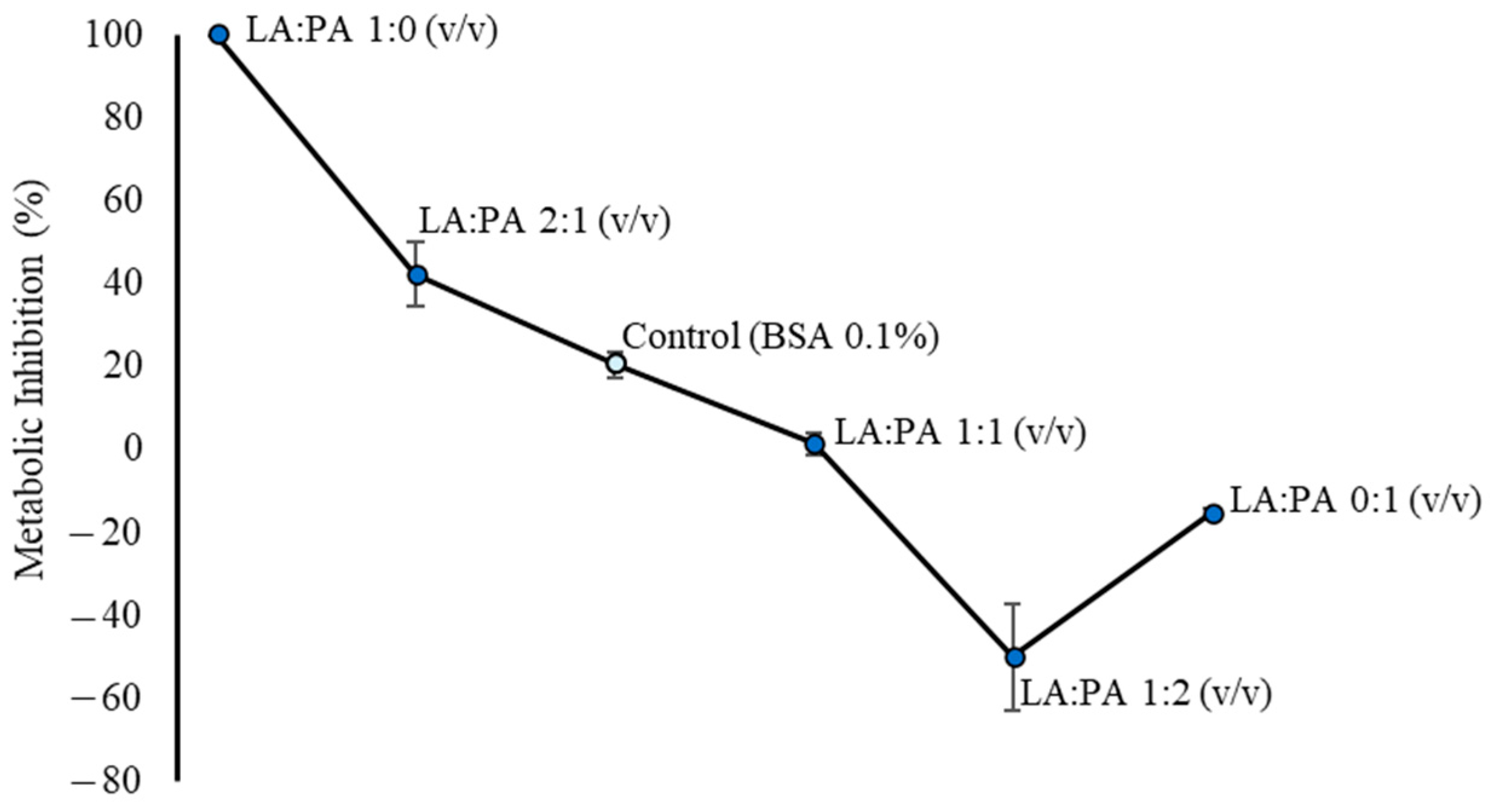
2.1. Metabolic Inhibition of LA and PA on HepG2 Cells
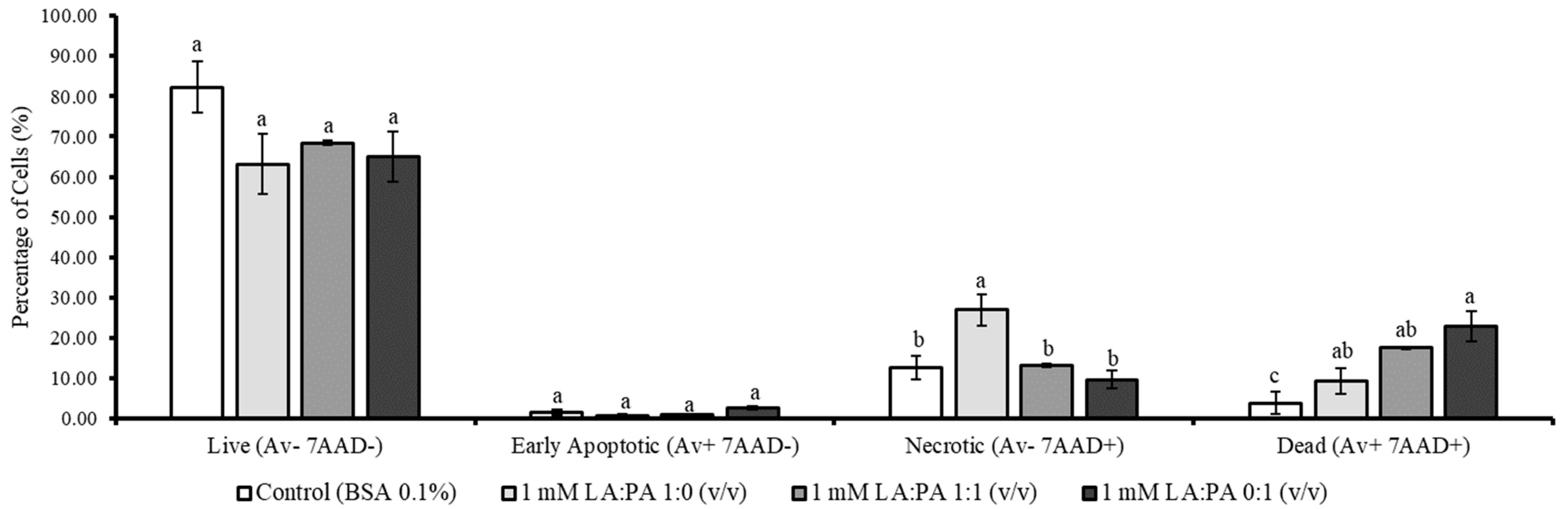
2.2. Flow Cytometric Analysis of LA and PA Effect on Apoptotic Stages
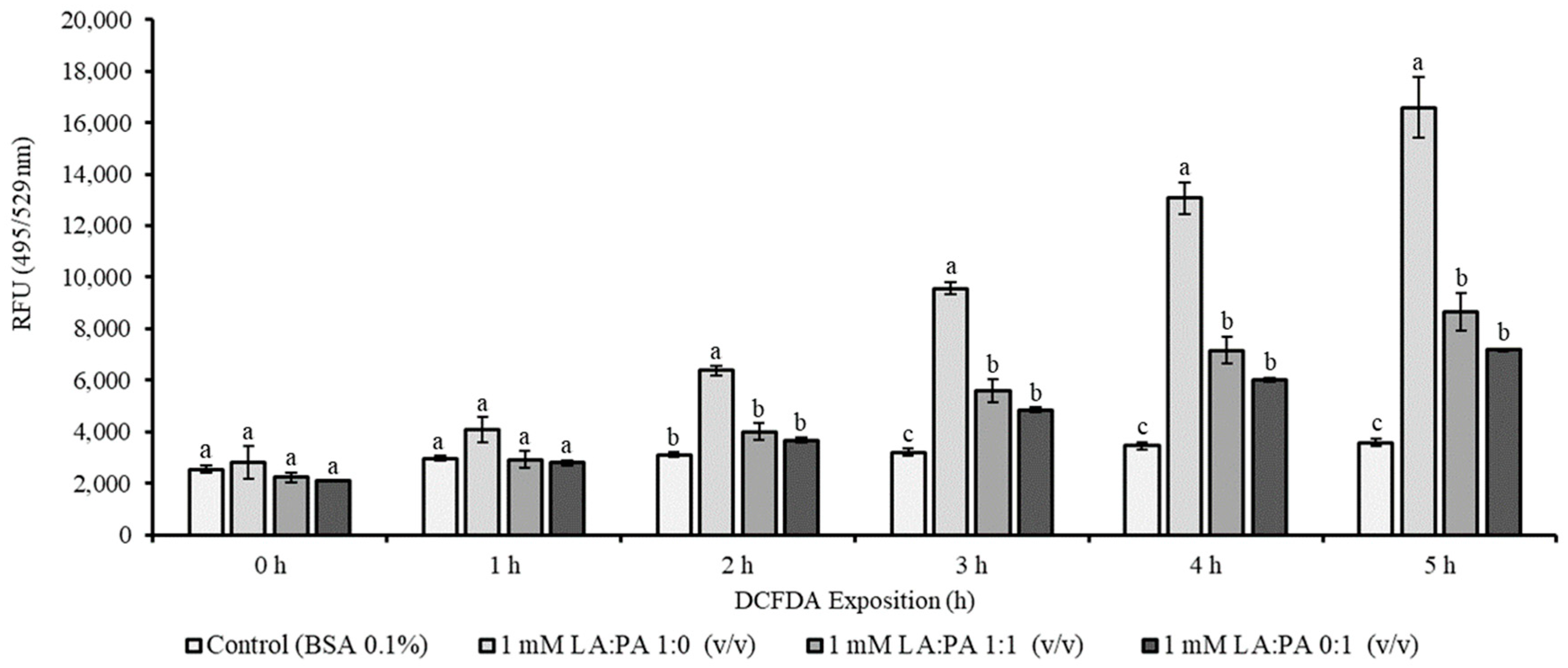
2.3. Assessment of Intracellular Reactive Oxygen Species (ROS)
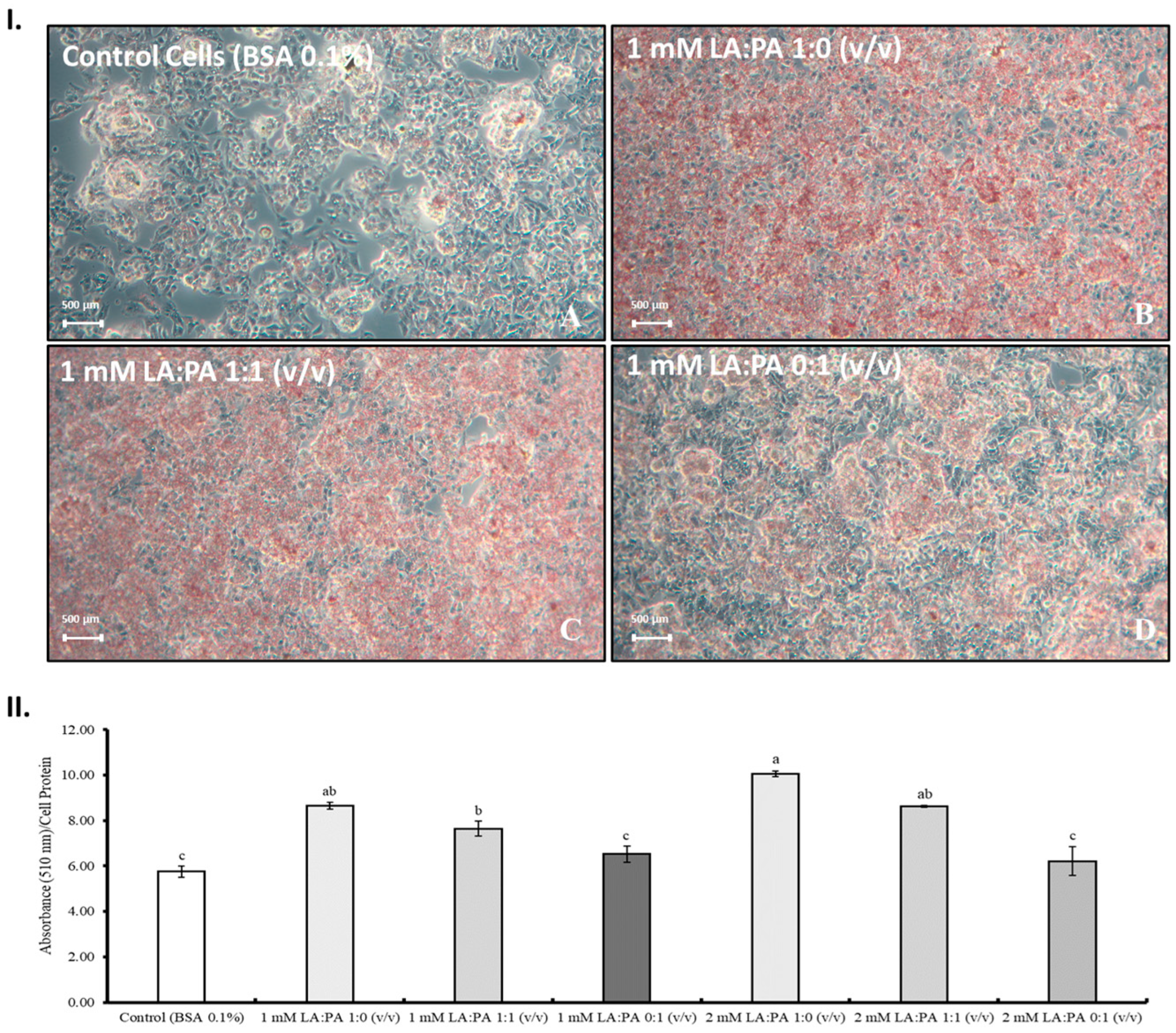
2.4. Lipophilic Dye Oil Red O (ORO)
2.5. Total Fatty Acids Profile
2.6. Free Fatty Acids, Cholesterol and Triglycerides Analysis by Gas Chromatography—Mass Spectrometry (GC-MS)
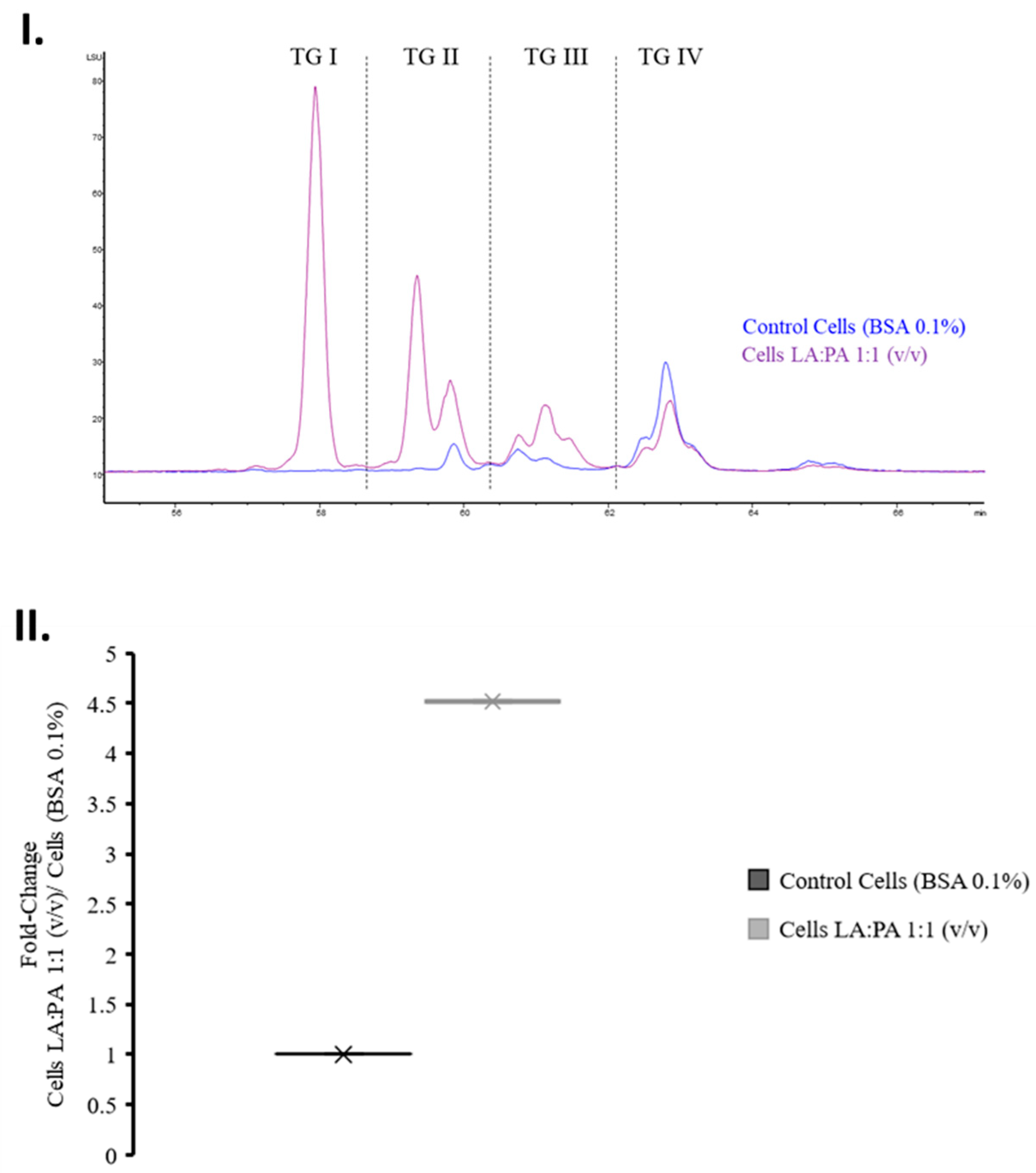
2.7. Triglycerides Analysis by High Performance Liquid Chromatography—Evaporative Light Scattering Detector (HPLC-ELSD)
2.8. Triglyceride Analysis by Liquid Chromatography Coupled with Electrospray Ionization—Quadrupole—Time of Flight (LC-ESI-qTOF) Mass Spectrometry
3. Discussion
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Linoleic Acid (LA) and Palmitic Acid (PA) Preparation
4.3. Cell Culture
4.3.1. Presto-Blue
4.3.2. Flow Cytometric Analysis of LA and PA Effect on Apoptotic Stages
4.3.3. Assessment of Intracellular Reactive Oxygen Species (ROS)
4.4. Determination of Total Lipid Accumulation by Oil Red O Staining (ORO)
4.5. Lipid Extraction of the Cellular Content
4.6. LC-ESI-qTOF
4.7. GC-MS
4.8. GC-FID
4.9. HPLC-ELSD
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wu, W.; Feng, A.; Ma, W.; Li, D.; Zheng, S.; Xu, F.; Han, D.; Lyu, J. Worldwide long-term trends in the incidence of nonalcoholic fatty liver disease during 1990–2019: A joinpoint and age-period-cohort analysis. Front. Cardiovasc. Med. 2022, 9, 891963. [Google Scholar] [CrossRef] [PubMed]
- Feldstein, A.E.; Lopez, R.; Tamimi, T.A.R.; Yerian, L.; Chung, Y.M.; Berk, M.; Zhang, R.; McIntyre, T.M.; Hazen, S.L. Mass spectrometric profiling of oxidized lipid products in human nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Lipid Res. 2010, 51, 3046–3054. [Google Scholar] [CrossRef]
- Horn, C.L.; Morales, A.L.; Savard, C.; Farrell, G.C.; Ioannou, G.N. Role of Cholesterol-Associated Steatohepatitis in the Development of NASH. Hepatol. Commun. 2022, 6, 12–35. [Google Scholar] [CrossRef]
- Janorkar, A.V.; King, K.R.; Megeed, Z.; Yarmush, M.L. Development of an in vitro cell culture model of hepatic steatosis using hepatocyte-derived reporter cells. Biotechnol. Bioeng. 2009, 102, 1466–1474. [Google Scholar] [CrossRef]
- Cui, W.; Chen, S.L.; Hu, K.Q. Quantification and mechanisms of oleic acid-induced steatosis in HepG2 cells. Am. J. Transl. Res. 2010, 2, 95–104. [Google Scholar]
- ten Hove, M.; Pater, L.; Storm, G.; Weiskirchen, S.; Weiskirchen, R.; Lammers, T.; Bansal, R. The hepatic lipidome: From basic science to clinical translation. Adv. Drug Deliv. Rev. 2020, 159, 180–197. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Xu, C.F.; Yu, C.H.; Chen, W.X.; Li, Y.M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014, 20, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Held, P. Lipid Accumulation in HepG2 Cells Exposed to Free Fatty Acids Image-Based Assay to Model Non-Alcoholic Steatohepatitis (NASH). In Proceedings of the BioTek Application Note; Biotek: Winooski, VT, USA, 2017. [Google Scholar]
- Gregor, M.F.; Yang, L.; Fabbrini, E.; Mohammed, B.S.; Eagon, J.C.; Hotamisligil, G.S.; Klein, S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 2009, 58, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, Y.; Nakao, K. To die or not to die: Death signaling in nonalcoholic fatty liver disease. J. Gastroenterol. 2018, 53, 893–906. [Google Scholar] [CrossRef]
- Sobczak, A.; Blindauer, C.; Stewart, A. Changes in Plasma Free Fatty Acids Associated with Type-2 Diabetes. Nutrients 2019, 11, 2022. [Google Scholar] [CrossRef]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Almeida, I.T.D.E.; Fidalgo, G.; Rodrigues, D.; Camilo, M.E. Plasma total and free fatty acids composition in human non-alcoholic steatohepatitis. Clin. Nutr. 2002, 21, 219–223. [Google Scholar] [CrossRef]
- Ress, C.; Kaser, S. Mechanisms of intrahepatic triglyceride accumulation. World J. Gastroenterol. 2016, 22, 1664–1673. [Google Scholar] [CrossRef] [PubMed]
- Activate, A.; Heden, T.D.; Parker, L.; Chow, L.S.; Mashek, D.G.; Najt, C.P.; Khan, S.A.; Heden, T.D.; Witthuhn, B.A.; Perez, M.; et al. Lipid Droplet-Derived Monounsaturated Fatty Acids Article Lipid Droplet-Derived Monounsaturated Fatty Acids Traffic via PLIN5 to Allosterically Activate SIRT1. Mol. Cell 2020, 77, 810–824. [Google Scholar]
- Gómez-Lechón, M.J.; Donato, M.T.; Martínez-Romero, A.; Jiménez, N.; Castell, J.V.; O’Connor, J.E. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 2007, 165, 106–116. [Google Scholar] [CrossRef]
- Schuster, S.; Johnson, C.D.; Hennebelle, M.; Holtmann, T.; Taha, A.Y.; Kirpich, I.A.; Eguchi, A.; Ramsden, C.E.; Papouchado, B.G.; McClain, C.J.; et al. Oxidized linoleic acid metabolites induce liver mitochondrial dysfunction, apoptosis, and NLRP3 activation in mice. J. Lipid Res. 2018, 59, 1597–1609. [Google Scholar] [CrossRef]
- Jeyapal, S.; Kona, S.R.; Mullapudi, S.V.; Putcha, U.K.; Gurumurthy, P.; Ibrahim, A. Substitution of linoleic acid with α-linolenic acid or long chain n-3 polyunsaturated fatty acid prevents Western diet induced nonalcoholic steatohepatitis. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Murru, E.; Manca, C.; Carta, G.; Banni, S. Impact of Dietary Palmitic Acid on Lipid Metabolism. Front. Nutr. 2022, 9, 1–9. [Google Scholar] [CrossRef]
- Prasad, P.; Anjali, P.; Sreedhar, R.V. Plant-based stearidonic acid as sustainable source of omega-3 fatty acid with functional outcomes on human health. Crit. Rev. Food Sci. Nutr. 2021, 61, 1725–1737. [Google Scholar] [CrossRef]
- Zhu, T.; Lu, X.; Liu, Z. Dietary linoleic acid and the ratio of unsaturated to saturated fatty acids are inversely associated with significant liver fibrosis risk: A nationwide survey. Front. Nutr. 2022, 9, 938645. [Google Scholar] [CrossRef]
- Green, C.J.; Johnson, D.; Amin, H.D.; Sivathondan, P.; Silva, M.A.; Wang, L.M.; Stevanato, L.; McNeil, C.A.; Miljan, E.A.; Sinden, J.D.; et al. Characterization of lipid metabolism in a novel immortalized human hepatocyte cell line. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E511–E522. [Google Scholar] [CrossRef]
- Dufour, J.F.; Anstee, Q.M.; Bugianesi, E.; Harrison, S.; Loomba, R.; Paradis, V.; Tilg, H.; Wong, V.W.S.; Zelber-Sagi, S. Current therapies and new developments in NASH. Gut 2022, 71, 2123–2134. [Google Scholar] [CrossRef]
- Forbes-Hernández, T.Y.; Giampieri, F.; Gasparrini, M.; Afrin, S.; Mazzoni, L.; Cordero, M.D.; Mezzetti, B.; Quiles, J.L.; Battino, M. Lipid accumulation in HepG2 cells is attenuated by strawberry extract through AMPK activation. Nutrients 2017, 9, 621. [Google Scholar] [CrossRef] [PubMed]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-terf-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, M.H.; Gaudin, M.; Lewis, M.R.; Martin, F.; Holmes, E.; Nicholson, J.K.; Dumas, M. Objective Set of Criteria for Optimization of Sample Preparation Procedures for Ultra-High Throughput Untargeted Blood Plasma Lipid Profiling by Ultra Performance Liquid Chromatography–Mass Spectrometry. Anal. Chem. 2014, 86, 5766–5774. [Google Scholar] [CrossRef] [PubMed]
- Calderón, C.; Sanwald, C.; Schlotterbeck, J.; Drotleff, B.; Lämmerhofer, M. Comparison of simple monophasic versus classical biphasic extraction protocols for comprehensive UHPLC-MS/MS lipidomic analysis of Hela cells. Anal. Chim. Acta 2019, 1048, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, F.S.; Pimentel, L.L.; Vidigal, S.S.M.P.; Costa, P.T.; Pintado, M.E.; Rodríguez-Alcalá, L.M. Suitability of Solvent-Assisted Extraction for Recovery of Lipophilic Phytochemicals in Sugarcane Straw and Bagasse. Foods 2022, 11, 2661. [Google Scholar] [CrossRef]
- Fontes, A.L.; Pimentel, L.; Rodríguez-Alcalá, L.M.; Gomes, A. Effect of Pufa Substrates on Fatty Acid Profile of Bifidobacterium breve Ncimb 702258 and CLA/CLNA Production in Commercial Semi-Skimmed Milk. Sci. Rep. 2018, 8, 15591. [Google Scholar] [CrossRef]
- Plante, M.; Bailey, B.; Acworth, I.; Clark, D. Analysis of Lipids by HPLC-CAD; Dionex Company: Sunnyvale, CA, USA, 2011. [Google Scholar]


| Control (BSA 0.1%) | LA:PA 1:1 (v/v) | p | |||||
|---|---|---|---|---|---|---|---|
| mg FA/g Cell Pellet | |||||||
| Myristic Acid (C14) | 3.87 | ± | 0.01 | 5.61 | ± | 0.01 | ** |
| Palmitic Acid (C16) | 34.81 | ± | 0.03 | 60.77 | ± | 0.12 | ** |
| Palmitoleic Acid (C16:1c9) | 10.85 | ± | 0.01 | 7.56 | ± | 0.02 | ** |
| Stearic Acid (C18) | 9.55 | ± | 0.01 | 17.90 | ± | 0.03 | ** |
| Oleic Acid (C18:1c9) | 26.41 | ± | 0.01 | 22.62 | ± | 0.05 | ** |
| cis-11-Vaccenic Acid (C18:1c11) | 15.67 | ± | 0.03 | 12.15 | ± | 0.02 | ** |
| Linoleic Acid (C18:2c9c12) | 1.12 | ± | 0.01 | 116.75 | ± | 0.26 | ** |
| γ-Linolenic Acid (C18:3c6c9c12) | nd | 1.07 | ± | 0.02 | * | ||
| cis-11-Eicosenoic Acid (C20:1c11) | 1.12 | ± | 0.01 | 0.97 | ± | 0.01 | * |
| cis-11,14-Eicosadienoic Acid (C20:2c11c14) | nd | 3.78 | ± | 0.03 | ** | ||
| cis-8,11,14-Eicosatrienoic Acid (C20:3c8c11c14) | 0.60 | ± | 0.05 | 2.91 | ± | 0.04 | ** |
| Arachidonic Acid (C20:4c5c8c11c14) | 2.77 | ± | 0.01 | 4.57 | ± | 0.03 | ** |
| Total Fatty Acids | 106.78 | ± | 0.11 | 256.66 | ± | 0.54 | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, F.S.; Pimentel, L.L.; Vidigal, S.S.M.P.; Azevedo-Silva, J.; Pintado, M.E.; Rodríguez-Alcalá, L.M. Differential Lipid Accumulation on HepG2 Cells Triggered by Palmitic and Linoleic Fatty Acids Exposure. Molecules 2023, 28, 2367. https://doi.org/10.3390/molecules28052367
Teixeira FS, Pimentel LL, Vidigal SSMP, Azevedo-Silva J, Pintado ME, Rodríguez-Alcalá LM. Differential Lipid Accumulation on HepG2 Cells Triggered by Palmitic and Linoleic Fatty Acids Exposure. Molecules. 2023; 28(5):2367. https://doi.org/10.3390/molecules28052367
Chicago/Turabian StyleTeixeira, Francisca S., Lígia L. Pimentel, Susana S. M. P. Vidigal, João Azevedo-Silva, Manuela E. Pintado, and Luís M. Rodríguez-Alcalá. 2023. "Differential Lipid Accumulation on HepG2 Cells Triggered by Palmitic and Linoleic Fatty Acids Exposure" Molecules 28, no. 5: 2367. https://doi.org/10.3390/molecules28052367
APA StyleTeixeira, F. S., Pimentel, L. L., Vidigal, S. S. M. P., Azevedo-Silva, J., Pintado, M. E., & Rodríguez-Alcalá, L. M. (2023). Differential Lipid Accumulation on HepG2 Cells Triggered by Palmitic and Linoleic Fatty Acids Exposure. Molecules, 28(5), 2367. https://doi.org/10.3390/molecules28052367












