Deep Eutectic Liquids as a Topical Vehicle for Tadalafil: Characterisation and Potential Wound Healing and Antimicrobial Activity
Abstract
:1. Introduction
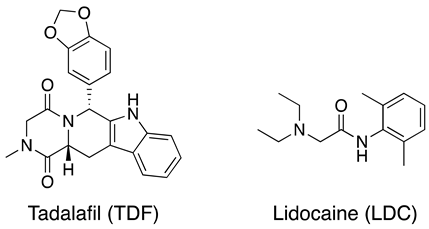
2. Results and Discussion
2.1. Preparation of DESs and Drug-Loaded DES Formulations
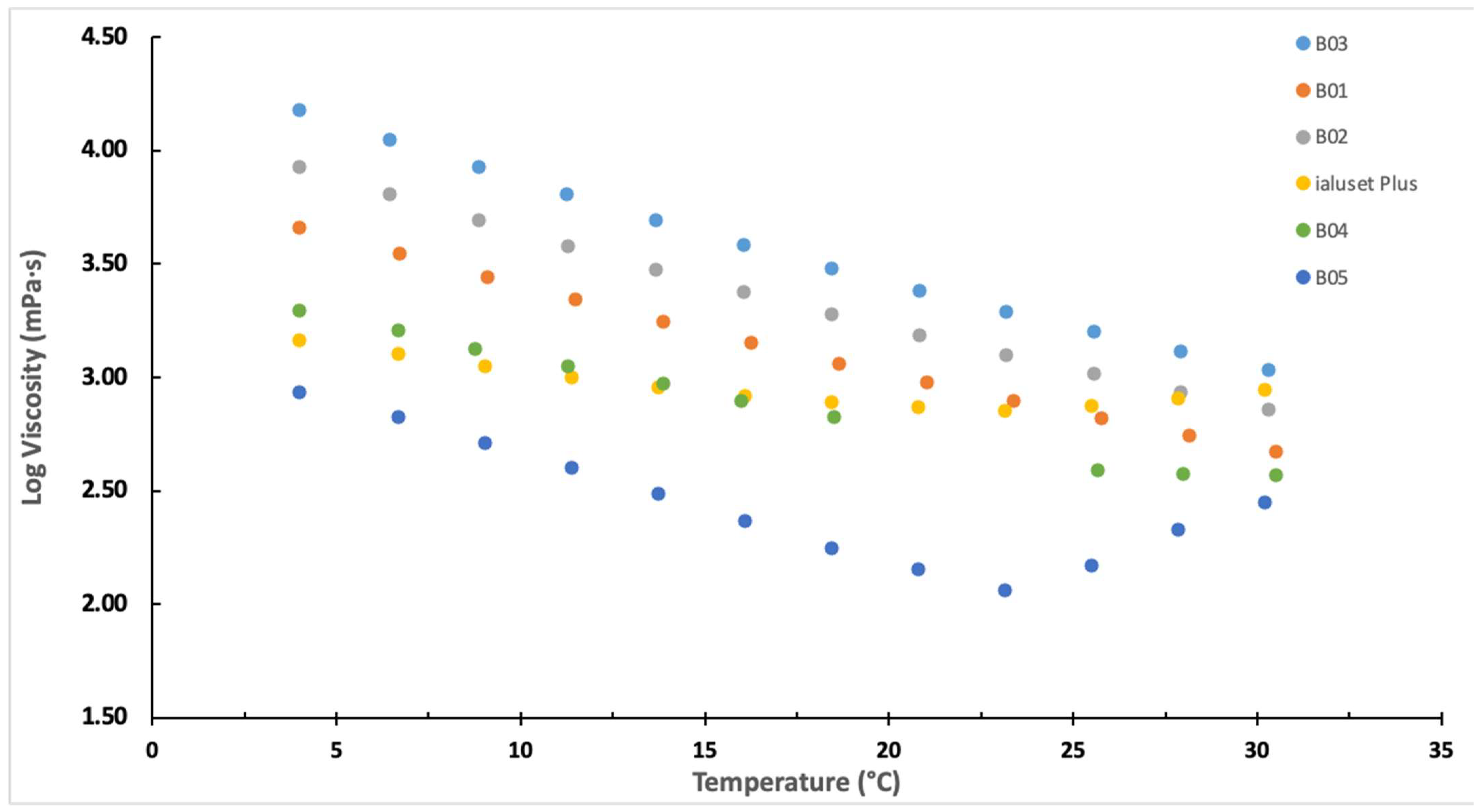
2.2. Characterisation of DES Formulations
2.2.1. Nuclear Magnetic Resonance (NMR)
2.2.2. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
2.2.3. Differential Scanning Calorimetry (DSC)
2.3. Stability Evaluation of the TDF Formulations
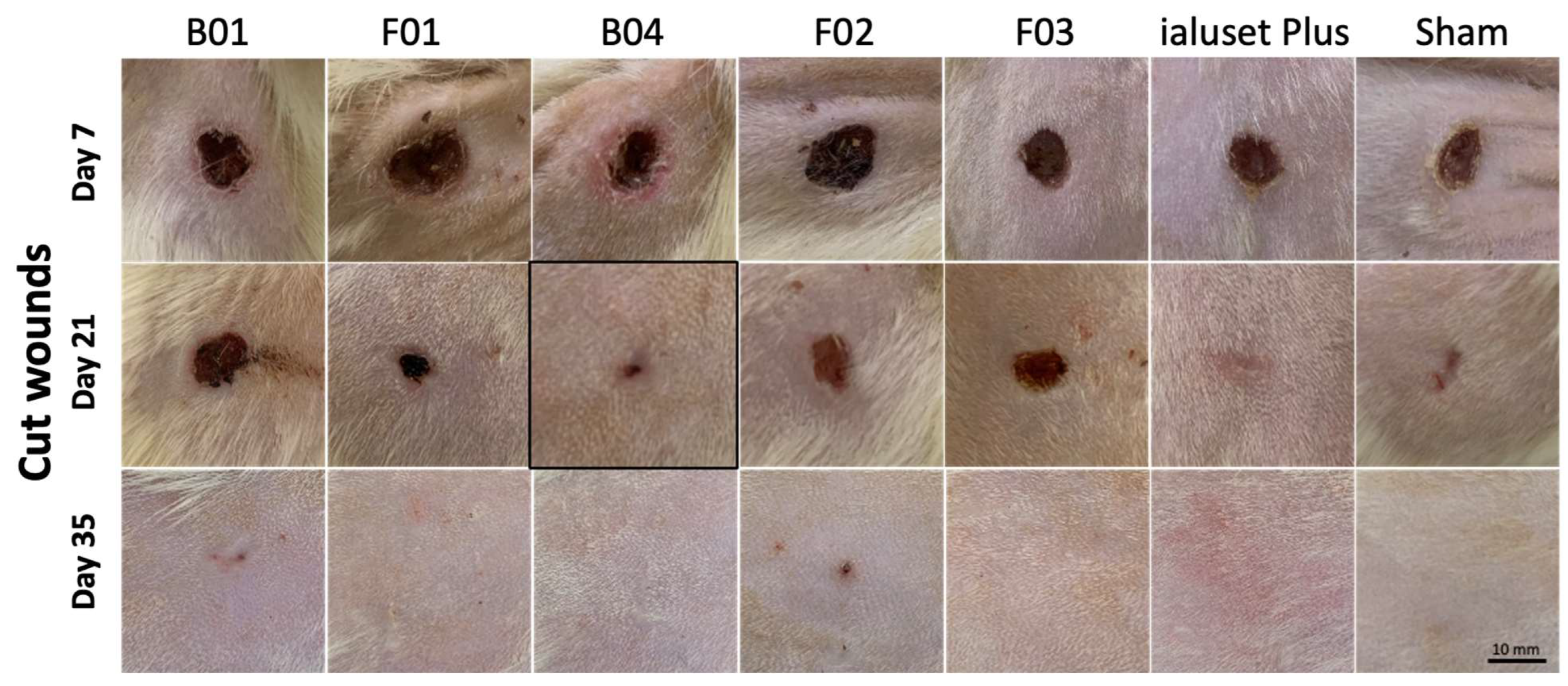
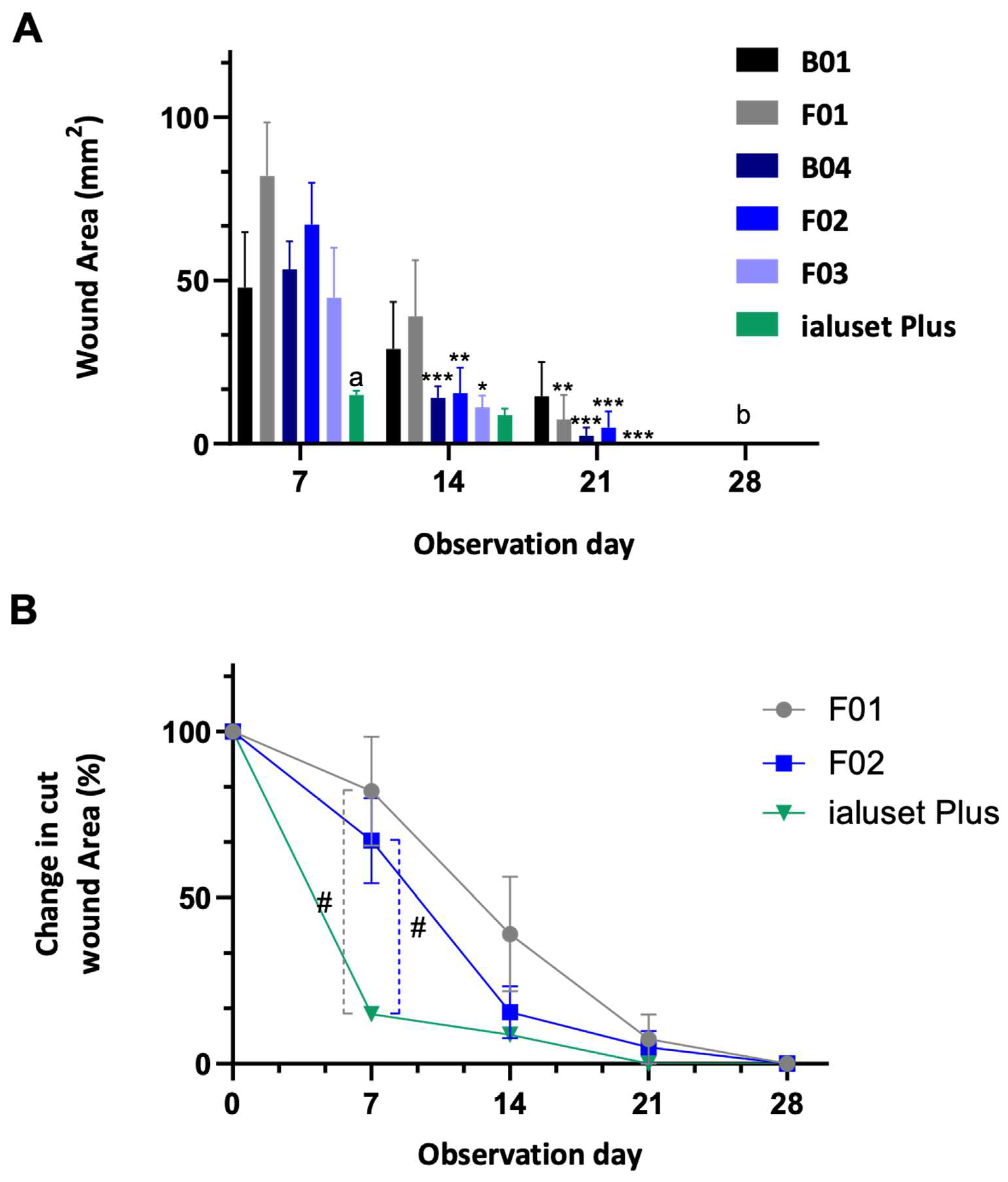
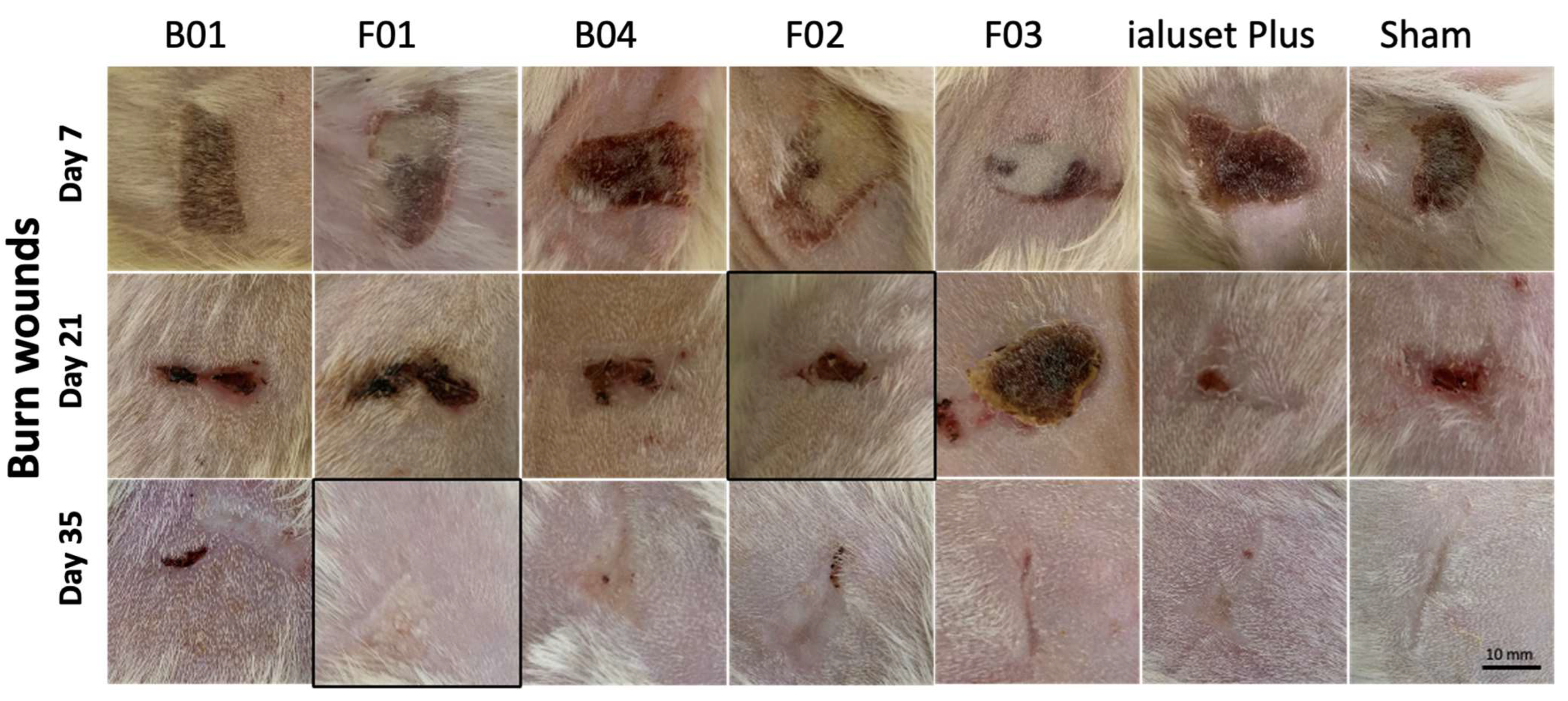
2.4. Effect of DES Formulations on Cut Wound Healing Process
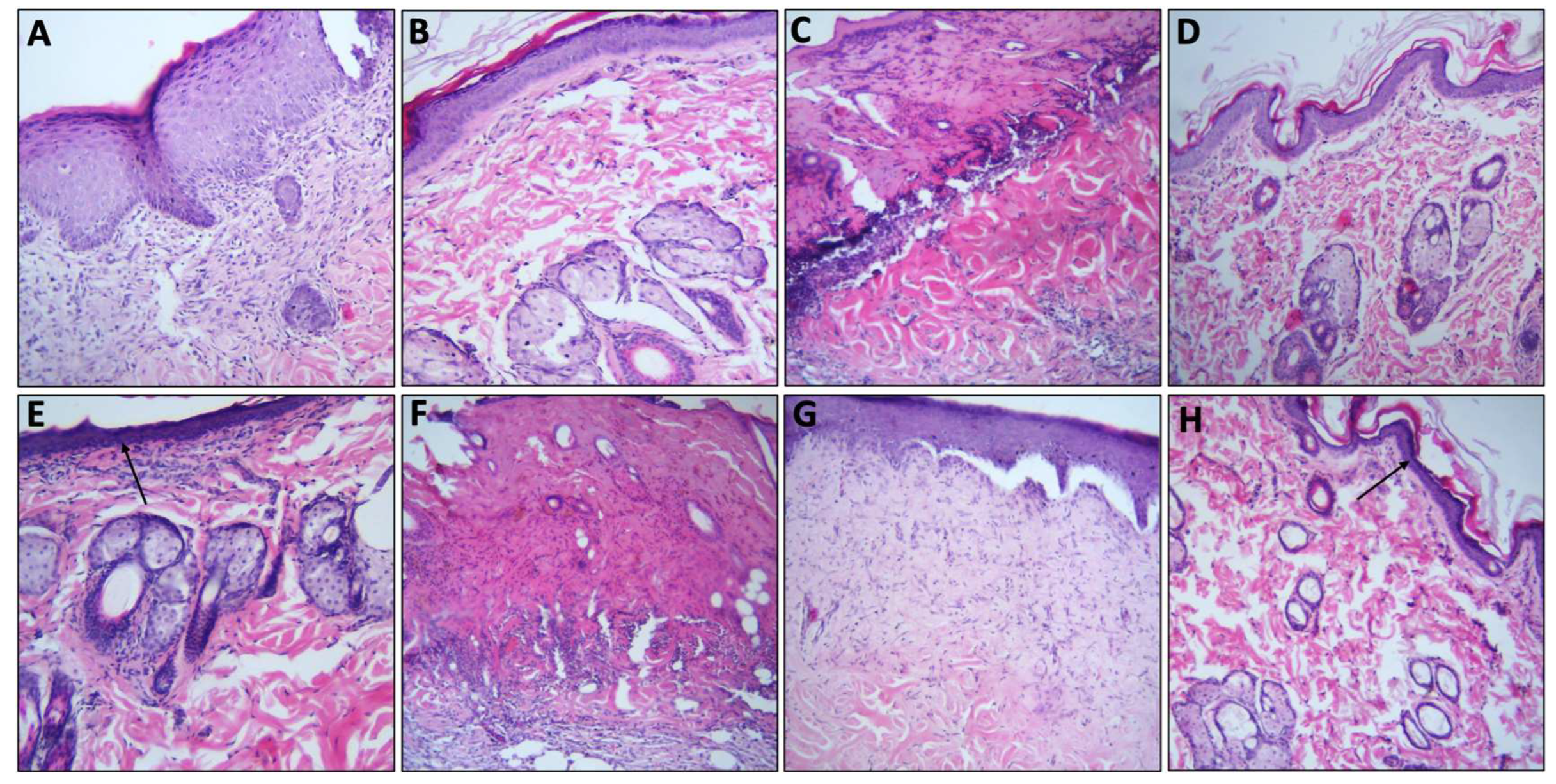
2.5. Effect of DES Formulations on Burn Wound Healing Process
2.6. Antimicrobial Activity Testing (In Vitro)
3. Materials and Methods
3.1. Materials
3.2. HPLC Analysis
3.3. Choline Chloride–Malonic Acid (Blank Formulations) DES Preparation
3.4. Characterisation of Blank DESs
3.4.1. Rheology Study
3.4.2. Contact Angle Measurements
3.4.3. Spreadability
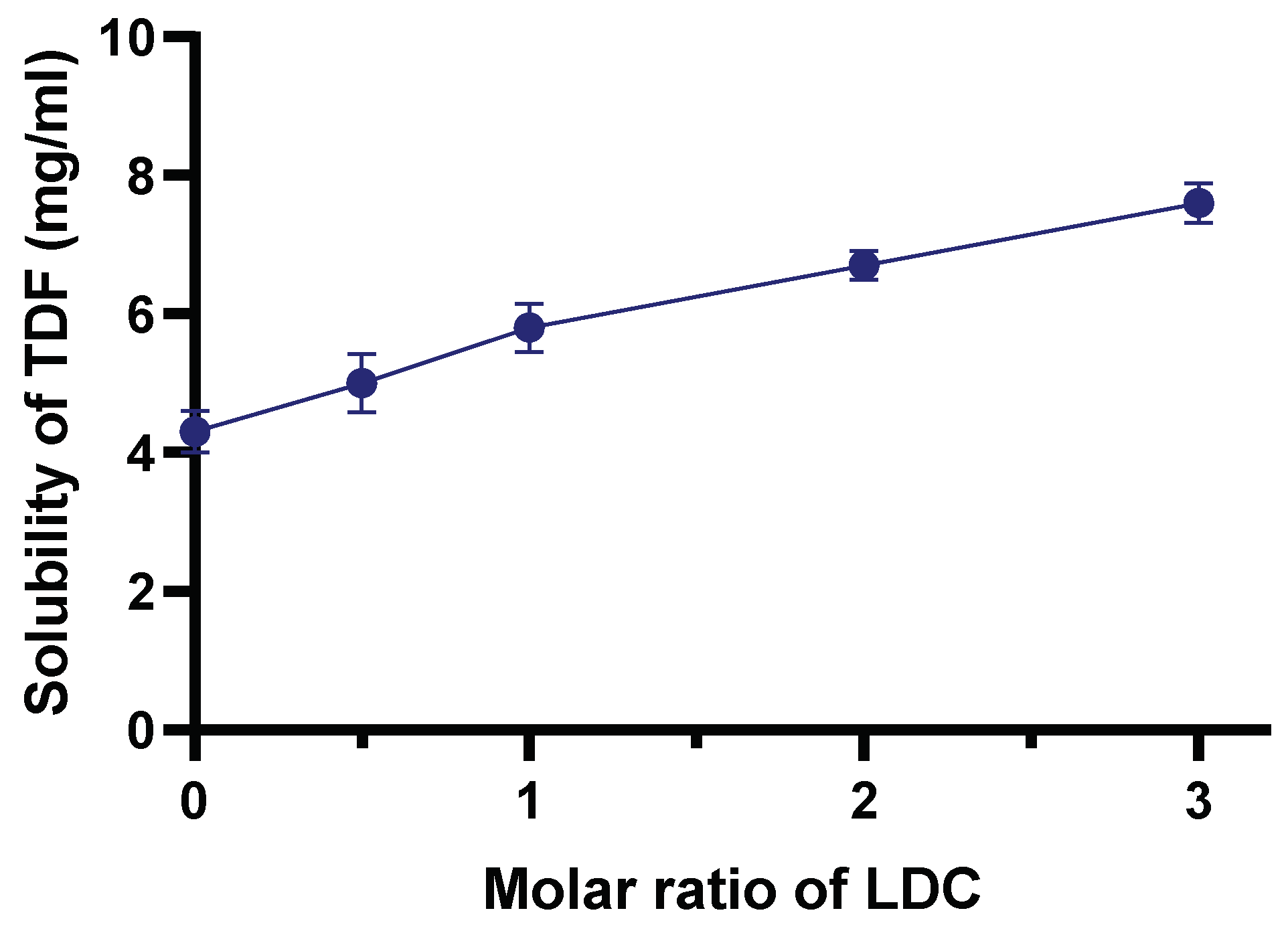
3.5. Determination of TDF Solubility in DESs (Shake-Flask Technique)
3.6. Enhancing TDF Solubility Using LDC
3.7. Stability Study of TDF Formulations
3.8. Nuclear Magnetic Resonance (NMR)
3.9. Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy (ATR-FTIR)
3.10. Differential Scanning Calorimetry (DSC)
3.11. Wound Healing Model (In Vivo)
3.12. Statistical Analysis
3.13. Antimicrobial Activity Testing (In Vitro)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated Total Reflectance—Fourier Transform Infrared Spectroscopy |
| CC | choline chloride |
| cGMP | cyclic guanosine monophosphate |
| DESs | deep eutectic solvents |
| DSC | differential scanning calorimetry |
| IL | ionic liquids |
| LDC | Lidocaine |
| MA | malonic acid |
| NO | nitric oxide |
| NMR | nuclear magnetic resonance |
| PDF-5 | phosphodiesterase type 5 |
| PG | propylene glycol |
| TDF | Tadalafil |
References
- Al-Akayleh, F.; Khalid, R.M.; Hawash, D.; Al-Kaissi, E.; Al-Adham, I.S.; Al-Muhtaseb, N.; Collier, P.J. Antimicrobial potential of natural deep eutectic solvents. Lett. Appl. Microbiol. 2022, 75, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Savoy, A.W. Ionic Liquids Synthesis and Applications: An Overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Clarke, C.J.; Tu, W.C.; Levers, O.; Bröhl, A.; Hallett, J.P. Green and Sustainable Solvents in Chemical Processes. Chem. Rev. 2018, 118, 747–800. [Google Scholar] [CrossRef] [PubMed]
- Hallett, J.P.; Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [Green Version]
- Ünlü, A.E.; Arlkaya, A.; Takaç, S. Use of Deep Eutectic Solvents as Catalyst: A Mini-Review. Green Process. Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Carriazo, D.; Serrano, M.C.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Deep-Eutectic Solvents Playing Multiple Roles in the Synthesis of Polymers and Related Materials. Chem. Soc. Rev. 2012, 41, 4996–5014. [Google Scholar] [CrossRef]
- Deetlefs, M.; Seddon, K.R. Assessing the Greenness of Some Typical Laboratory Ionic Liquid Preparations. Green Chem. 2010, 12, 17–30. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R.K. Deep Eutectic Solvents Formed between Choline Chloride and Carboxylic Acids: Versatile Alternatives to Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef]
- Abbott, A.P.; Bell, T.J.; Handa, S.; Stoddart, B. Cationic Functionalisation of Cellulose Using a Choline Based Ionic Liquid Analogue. Green Chem. 2006, 8, 784–786. [Google Scholar] [CrossRef] [Green Version]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline Chloride-Based Deep Eutectic Solvents as Green Extractants for the Isolation of Phenolic Compounds from Biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Lomba, L.; García, C.B.; Ribate, M.P.; Giner, B.; Zuriaga, E. Applications of Deep Eutectic Solvents Related to Health, Synthesis, and Extraction of Natural Based Chemicals. Appl. Sci. 2021, 11, 10156. [Google Scholar] [CrossRef]
- Amoroso, R.; Hollmann, F.; Maccallini, C. Choline Chloride-Based DES as Solvents/Catalysts/Chemical Donors in Pharmaceutical Synthesis. Molecules 2021, 26, 6286. [Google Scholar] [CrossRef] [PubMed]
- Riaz, M.; Akhlaq, M.; Naz, S.; Uroos, M. An Overview of Biomedical Applications of Choline Geranate (CAGE): A Major Breakthrough in Drug Delivery. RSC Adv. 2022, 12, 25977–25991. [Google Scholar] [CrossRef]
- Vaidya, A.; Mitragotri, S. Ionic Liquid-Mediated Delivery of Insulin to Buccal Mucosa. J. Control. Release 2020, 327, 26–34. [Google Scholar] [CrossRef]
- Saha, M.; Saha, M.; Rahman, M.S.; Hossain, M.N.; Raynie, D.E.; Halim, M.A. Molecular and Spectroscopic Insights of a Choline Chloride Based Therapeutic Deep Eutectic Solvent. J. Phys. Chem. A 2020, 124, 4690–4699. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.M.; Branco, L.C. Ionic Liquids and Deep Eutectic Solvents for Application in Pharmaceutics. Pharmaceutics 2020, 12, 909. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, Y.; Su, J.; Zhang, X.; Wang, J.; Tu, Q. Preparation of a Multifunctional Wound Dressing Based on a Natural Deep Eutectic Solvent. ACS Sustain. Chem. Eng. 2020, 8, 14243–14252. [Google Scholar] [CrossRef]
- Zakrewsky, M.; Lovejoy, K.S.; Kern, T.L.; Miller, T.E.; Le, V.; Nagy, A.; Goumas, A.M.; Iyer, R.S.; DelSesto, R.E.; Koppisch, A.T.; et al. Ionic Liquids as a Class of Materials for Transdermal Delivery and Pathogen Neutralization. Proc. Natl. Acad. Sci. USA 2014, 111, 13313–13318. [Google Scholar] [CrossRef] [Green Version]
- Dong, R.; Guo, B. Smart Wound Dressings for Wound Healing. Nano Today 2021, 41, 101290. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc. Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef] [PubMed]
- Gosain, A.; DiPietro, L.A. Aging and Wound Healing. World J. Surg. 2004, 28, 321–326. [Google Scholar] [CrossRef]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219. [Google Scholar] [CrossRef] [PubMed]
- Bishop, A. Role of Oxygen in Wound Healing. J. Wound Care 2008, 17, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Abu Dayyih, W.; Abu Rayyan, W.; Al-Matubsi, H.Y. Impact of Sildenafil-Containing Ointment on Wound Healing in Healthy and Experimental Diabetic Rats. Acta Diabetol. 2020, 57, 1351–1358. [Google Scholar] [CrossRef] [PubMed]
- Al-Akayleh, F.; Jaber, N.; Al-Remawi, M.; Al Odwan, G.; Qinna, N. Chitosan-biotin topical film: Preparation and evaluation of burn wound healing activity. Pharm. Devel. Technol. 2022, 27, 479–489. [Google Scholar] [CrossRef]
- Alwattar, J.K.; Chouaib, R.; Khalil, A.; Mehanna, M.M. A Novel Multifaceted Approach for Wound Healing: Optimization and in Vivo Evaluation of Spray Dried Tadalafil Loaded pro-Nanoliposomal Powder. Int. J. Pharm. 2020, 587, 119647. [Google Scholar] [CrossRef]
- Mahmoud, N.N.; Hikmat, S.; Ghith, D.A.; Hajeer, M.; Hamadneh, L.; Qattan, D.; Khalil, E.A. Gold nanoparticles loaded into polymeric hydrogel for wound healing in rats: Effect of nanoparticles’ shape and surface modification. Inter. J. Pharm. 2019, 565, 174–186. [Google Scholar]
- Brock, G.B.; McMahon, C.G.; Chen, K.K.; Costigan, T.; Shen, W.; Watkins, V.; Anglin, G.; Whitaker, S. Efficacy and Safety of Tadalafil for the Treatment of Erectile Dysfunction: Results of Integrated Analyses. J. Urol. 2002, 168, 1332–1336. [Google Scholar] [CrossRef]
- Goldsmith, K.; Goradia, E.; McClain, S.A.; Sandoval, S.; Singer, A.J. The Effect of Tadalafil on Reepithelialization and Scarring of Partial Thickness Porcine Burns. Wound Repair Regen. 2020, 28, 26–32. [Google Scholar] [CrossRef]
- Souza, R.A.C.; Martinelli-Kläy, C.P.; d’Acampora, A.J.; Bernardes, G.J.S.; Sgrott, S.M.; Souza, L.A.C.; Lombardi, T.; Sudbrack, T.R. Effects of Sildenafil and Tadalafil on Skin Flap Viability. Arch. Dermatol. Res. 2022, 314, 151–157. [Google Scholar] [CrossRef]
- Galiè, N.; Brundage, B.H.; Ghofrani, H.A.; Oudiz, R.J.; Simonneau, G.; Safdar, Z.; Shapiro, S.; White, R.J.; Chan, M.; Beardsworth, A.; et al. Tadalafil Therapy for Pulmonary Arterial Hypertension. Circulation 2009, 119, 2894–2903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daadoue, S.; Al-Remawi, M.; Al-Mawla, L.; Idkaidek, N.; Khalid, R.M.; Al-Akayleh, F. Deep Eutectic Liquid as Transdermal Delivery Vehicle of Risperidone. J. Mol. Liq. 2022, 345, 117347. [Google Scholar] [CrossRef]
- Al-Akayleh, F.; Adwan, S.; Khanfer, M.; Idkaidek, N.; Al-Remawi, M. A Novel Eutectic-Based Transdermal Delivery System for Risperidone. AAPS PharmSciTech 2021, 22, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Calatayud, J.; González, Á. History of the Development and Evolution of Local Anesthesia Since the Coca Leaf. Anesthesiology 2003, 98, 1503–1508. [Google Scholar] [CrossRef]
- Dave, R.A.; Morris, M.E. Novel High/Low Solubility Classification Methods for New Molecular Entities. Int. J. Pharm. 2016, 511, 111. [Google Scholar] [CrossRef] [Green Version]
- Al-Akayleh, F.; Mohammed Ali, H.H.; Ghareeb, M.M.; Al-Remawi, M. Therapeutic Deep Eutectic System of Capric Acid and Menthol: Characterization and Pharmaceutical Application. J. Drug Deliv. Sci. Technol. 2019, 53, 101159. [Google Scholar] [CrossRef]
- Rawat, A.; Gupta, S.S.; Kalluri, H.; Lowenborg, M.; Bhatia, K.; Warner, K. Rheological Characterization in the Development of Topical Drug Products. AAPS Adv. Pharm. Sci. Ser. 2019, 36, 3–45. [Google Scholar] [CrossRef]
- Carrer, V.; Alonso, C.; Pont, M.; Zanuy, M.; Córdoba, M.; Espinosa, S.; Barba, C.; Oliver, M.A.; Martí, M.; Coderch, L. Effect of Propylene Glycol on the Skin Penetration of Drugs. Arch. Dermatolog. Res. 2019, 312, 337–352. [Google Scholar] [CrossRef]
- Kim, S.; Chen, J.; Cheng, T.; Gindulyte, A.; He, J.; He, S.; Li, Q.; Shoemaker, B.A.; Thiessen, P.A.; Yu, B.; et al. PubChem in 2021: New Data Content and Improved Web Interfaces. Nucleic Acids Res. 2021, 49, D1388–D1395. [Google Scholar] [CrossRef]
- Tadalafil. C22H19N3O4. PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Tadalafil (accessed on 13 January 2023).
- Rahman, M.S.; Roy, R.; Jadhav, B.; Hossain, M.N.; Halim, M.A.; Raynie, D.E. Formulation, Structure, and Applications of Therapeutic and Amino Acid-Based Deep Eutectic Solvents: An Overview. J. Mol. Liq. 2021, 321, 114745. [Google Scholar] [CrossRef]
- Marei, H.F.; Arafa, M.F.; Essa, E.A.; El Maghraby, G.M. Lidocaine as Eutectic Forming Drug for Enhanced Transdermal Delivery of Nonsteroidal Anti-Inflammatory Drugs. J. Drug Deliv. Sci. Technol. 2021, 61, 102338. [Google Scholar] [CrossRef]
- Zhang, H.; Lang, J.; Lan, P.; Yang, H.; Lu, J.; Wang, Z. Study on the Dissolution Mechanism of Cellulose by ChCl-Based Deep Eutectic Solvents. Materials 2020, 13, 278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.M. Determination of Minimum Inhibitory Concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. S1), 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheil, M.; Chambers, M.; Polkinghorne, A.; Sharpe, B. Topical Application of Lidocaine and Bupivacaine to Disbudding Wounds in Dairy Calves: Safety, Toxicology and Wound Healing. Animals 2021, 11, 869. [Google Scholar] [CrossRef] [PubMed]
- Leavitt, T.; Hu, M.S.; Marshall, C.D.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Scarless Wound Healing: Finding the Right Cells and Signals. Cell Tissue Res. 2016, 365, 483. [Google Scholar] [CrossRef] [Green Version]
- Dart, A.J.; Cries, L.; Jeffcott, L.B.; Hodgson, D.R.; Rose, R.J. Effects of 25% Propylene Glycol Hydrogel (Solugel) on Second Intention Wound Healing in Horses. Vet. Surg. 2002, 31, 309–313. [Google Scholar] [CrossRef]
- Reddy, B.P.; Reddy, K.A.; Reddy, M.S. Validation and Stability Indicating RP-HPLC Method for the Determination of Tadalafil API in Pharmaceutical Formulations. Res. Pharm. Biotechnol. 2010, 2, 1–006. [Google Scholar]
- Masson-Meyers, D.S.; Andrade, T.A.M.; Caetano, G.F.; Guimaraes, F.R.; Leite, M.N.; Leite, S.N.; Frade, M.A.C. Experimental Models and Methods for Cutaneous Wound Healing Assessment. Int. J. Exp. Pathol. 2020, 101, 21–37. [Google Scholar] [CrossRef]
- Peters, J.E.; Thate, T.E.; Craig, N.L. Definition of the Escherichia Coli MC4100 Genome by Use of a DNA Array. J. Bacteriol. 2003, 185, 2017. [Google Scholar] [CrossRef] [Green Version]
- HOLLOWAY, B.W. Genetic Recombination in Pseudomonas Aeruginosa. J. Gen. Microbiol. 1955, 13, 572–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillum, A.M.; Tsay, E.Y.H.; Kirsch, D.R. Isolation of the Candida Albicans Gene for Orotidine-5’-Phosphate Decarboxylase by Complementation of S. Cerevisiae Ura3 and E. Coli PyrF Mutations. Mol. Gen. Genet. 1984, 198, 179–182. [Google Scholar] [CrossRef] [PubMed]



| Entry | Composition | Ratio | Spreadability Cm (mean ±SD) | Contact Angle (θ) |
|---|---|---|---|---|
| B01 | MA:CC | 1:1 | 3.51 ± 0.13 | NM ** |
| B02 | MA:CC | 1:2 | 2.51 ± 0.13 | NM ** |
| B03 | MA: CC | 2:1 | 1.61 ± 0.13 | NM ** |
| B04 | B01:PG | 1:1 | 6.0 ± 0.12 | 70 ± 2.9 |
| B05 | B01:PG | 1:2 | 7.2 ± 0.11 | 67 ± 2.4 |
| B06 | B02:PG | 1:1 | 4.1 ± 0.13 | 79 ± 3.4 |
| B07 | B02:PG | 1:2 | 4.9 ± 0.16 | 76 ± 2.9 |
| ialuset Plus | NA * | NA * | 6.3 ± 0.11 | NM ** |
| DES Formulation | Composition | Molar Ratio of Drugs | Vehicle |
|---|---|---|---|
| F01 | TDF and LDC | 1:3 | B01 |
| F02 | TDF and LDC | 1:3 | B04 |
| F03 | TDF | - | B04 |
| MIC in Liquid Broth (µL/mL) | ||
|---|---|---|
| Strain | B01 | F01 |
| Enterococcus faecalis ATCC19422 | 5 | 5 |
| Staphylococcus aureus NCTC 6571 | 5 | 5 |
| Escherichia coli MC4100 | 5 | 5 |
| Pseudomonas aeruginosa PAO1 | 2.5 | 5 |
| Candida albicans SC5314 | >10* | >10 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alkhawaja, B.; Al-Akayleh, F.; Al-Khateeb, A.; Nasereddin, J.; Ghanim, B.Y.; Bolhuis, A.; Jaber, N.; Al-Remawi, M.; Qinna, N.A. Deep Eutectic Liquids as a Topical Vehicle for Tadalafil: Characterisation and Potential Wound Healing and Antimicrobial Activity. Molecules 2023, 28, 2402. https://doi.org/10.3390/molecules28052402
Alkhawaja B, Al-Akayleh F, Al-Khateeb A, Nasereddin J, Ghanim BY, Bolhuis A, Jaber N, Al-Remawi M, Qinna NA. Deep Eutectic Liquids as a Topical Vehicle for Tadalafil: Characterisation and Potential Wound Healing and Antimicrobial Activity. Molecules. 2023; 28(5):2402. https://doi.org/10.3390/molecules28052402
Chicago/Turabian StyleAlkhawaja, Bayan, Faisal Al-Akayleh, Ashraf Al-Khateeb, Jehad Nasereddin, Bayan Y. Ghanim, Albert Bolhuis, Nisrein Jaber, Mayyas Al-Remawi, and Nidal A. Qinna. 2023. "Deep Eutectic Liquids as a Topical Vehicle for Tadalafil: Characterisation and Potential Wound Healing and Antimicrobial Activity" Molecules 28, no. 5: 2402. https://doi.org/10.3390/molecules28052402
APA StyleAlkhawaja, B., Al-Akayleh, F., Al-Khateeb, A., Nasereddin, J., Ghanim, B. Y., Bolhuis, A., Jaber, N., Al-Remawi, M., & Qinna, N. A. (2023). Deep Eutectic Liquids as a Topical Vehicle for Tadalafil: Characterisation and Potential Wound Healing and Antimicrobial Activity. Molecules, 28(5), 2402. https://doi.org/10.3390/molecules28052402






