Structural Features Promoting Photocatalytic Degradation of Contaminants of Emerging Concern: Insights into Degradation Mechanism Employing QSA/PR Modeling
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Degradation of Selected Organics
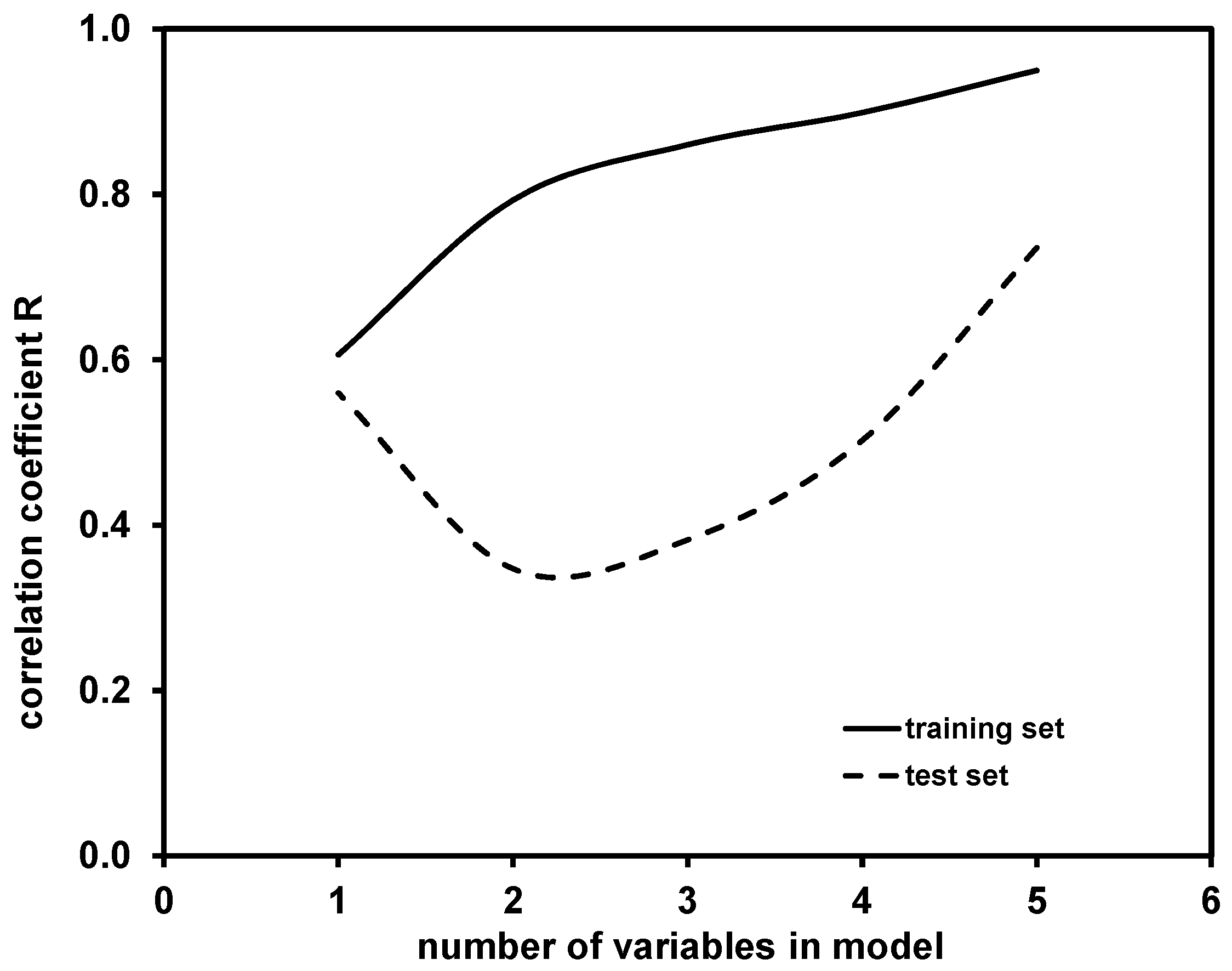
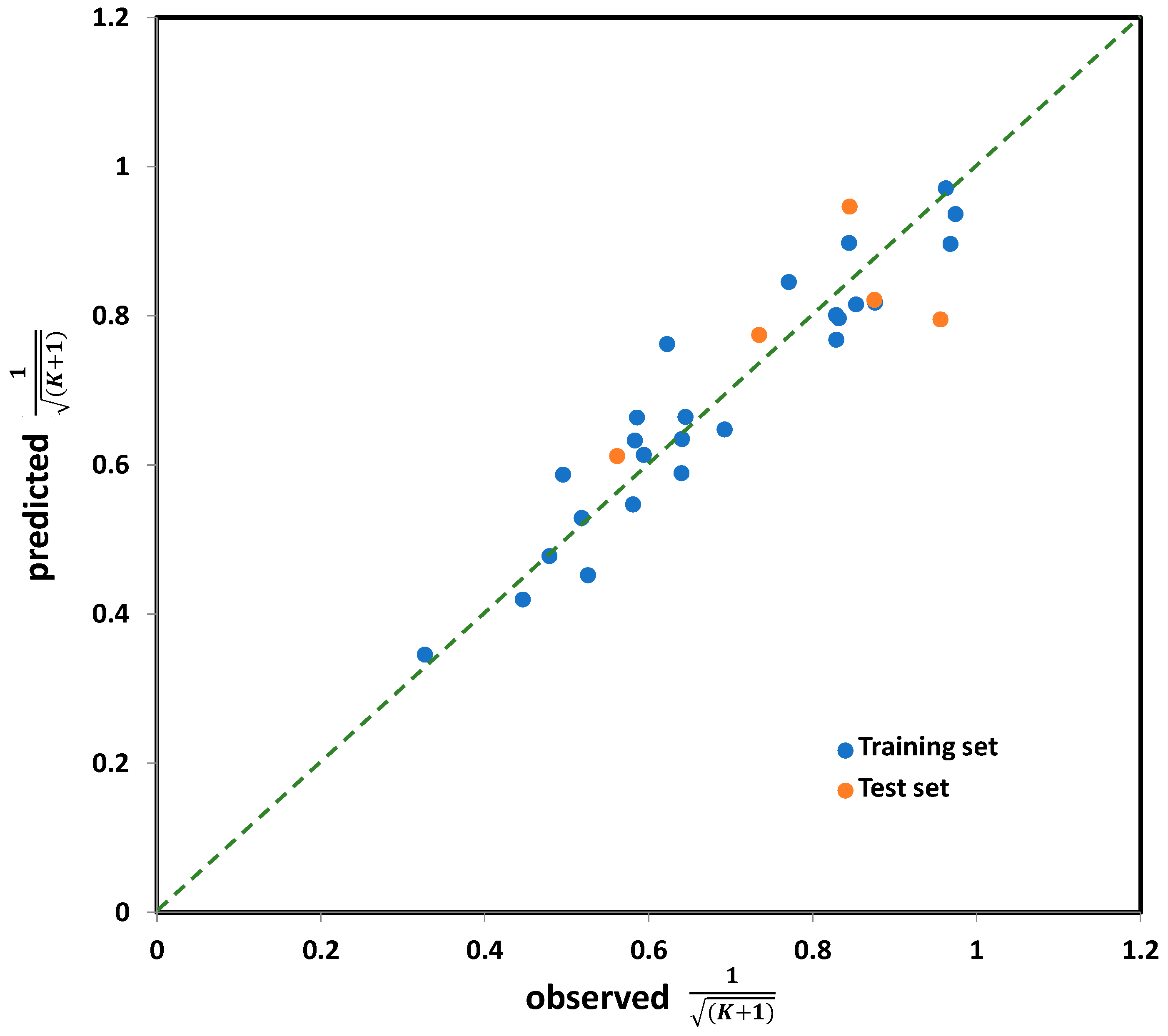
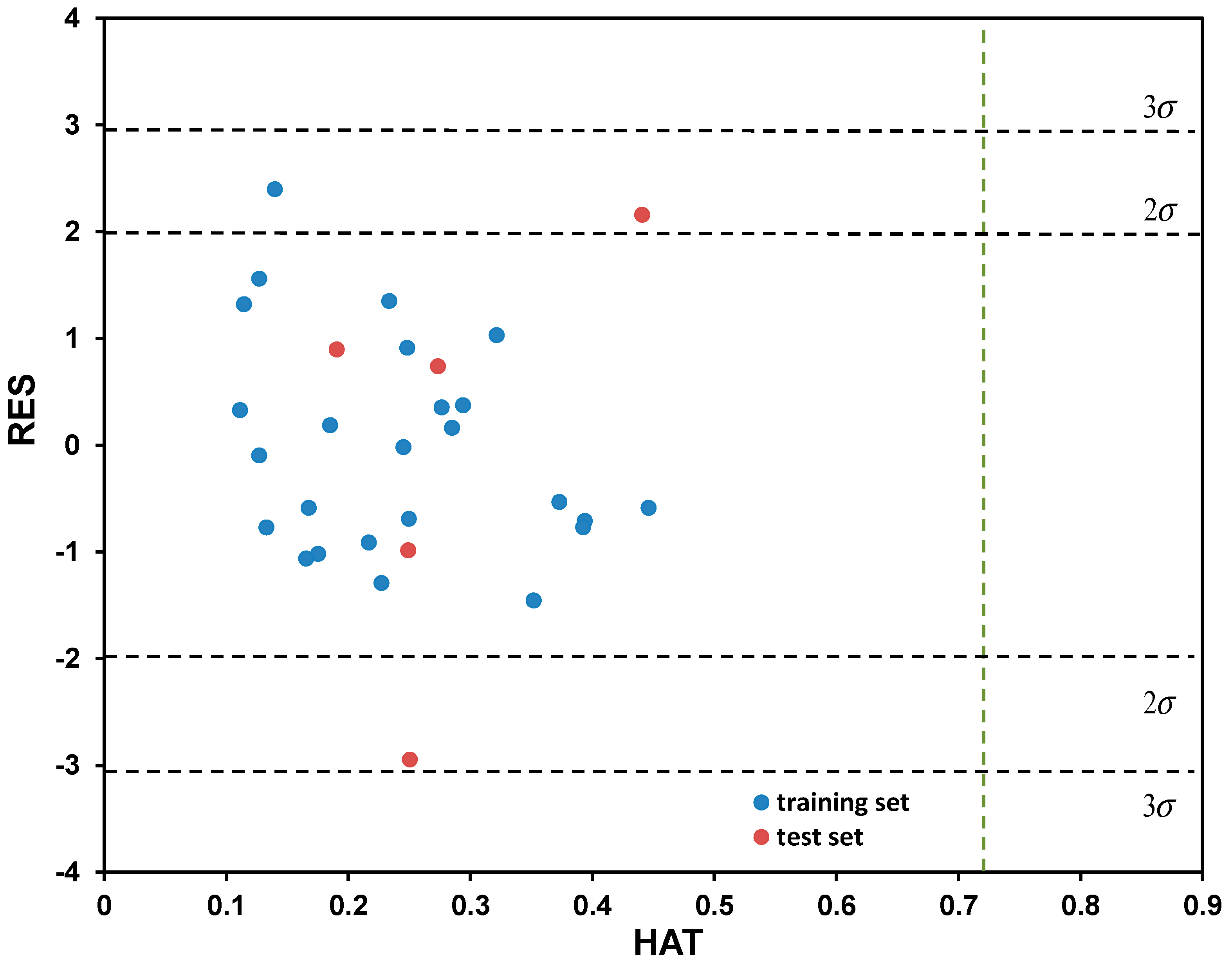
2.2. Modeling of Degradation Mechanisms over K Coefficient Using QSA/PR
2.3. Structural Features Determining Photocatalytic Degradation Mechanisms Occurring in the Bulk
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Procedure
3.3. Analytical Procedure
3.4. Computational Part
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Golovko, O.; Örn, S.; Sörengård, M.; Frieberg, K.; Nassazzi, W.; Lai, F.Y.; Ahrens, L. Occurrence and removal of chemicals of emerging concern in wastewater treatment plants and their impact on receiving water systems. Sci. Total Environ. 2021, 754, 142122. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.F.; Powers, S.M.; Hampton, S.E. An Evidence Synthesis of Pharmaceuticals and Personal Care Products (PPCPs) in the Environment: Imbalances among Compounds, Sewage Treatment Techniques, and Ecosystem Types. Environ. Sci. Technol. 2019, 53, 12961–12973. [Google Scholar] [CrossRef] [Green Version]
- Sörengård, M.; Campos-Pereira, H.; Ullberg, M.; Lai, F.Y.; Golovko, O.; Ahrens, L. Mass loads, source apportionment, and risk estimation of organic micropollutants from hospital and municipal wastewater in recipient catchments. Chemosphere 2019, 234, 931–941. [Google Scholar] [CrossRef] [PubMed]
- Gago-Ferrero, P.; Gros, M.; Ahrens, L.; Wiberg, K. Impact of on-site, small and large scale wastewater treatment facilities on levels and fate of pharmaceuticals, personal care products, artificial sweeteners, pesticides, and perfluoroalkyl substances in recipient waters. Sci. Total Environ. 2017, 601–602, 1289–1297. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathi, B.S.; Kumar, P.S.; Show, P.L. A review on effective removal of emerging contaminants from aquatic systems: Current trends and scope for further research. J. Hazard. Mater. 2021, 409, 124413. [Google Scholar] [CrossRef]
- Coimbra, R.N.; Escapa, C.; Otero, M. Removal of pharmaceuticals from water: Conventional and alternative treatments. Water Switz. 2021, 13, 487. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemöller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef]
- Kovacic, M.; Kusic, H.; Loncaric Bozic, A.; Dionysiou, D.D. Advanced Oxidation Processes. In Encyclopedia of Water: Science, Technology, and Society; Wiley: Hoboken, NJ, USA, 2020; pp. 1925–1940. [Google Scholar]
- Chen, D.; Cheng, Y.; Zhou, N.; Chen, P.; Wang, Y.; Li, K.; Huo, S.; Cheng, P.; Peng, P.; Zhang, R.; et al. Photocatalytic degradation of organic pollutants using TiO2-based photocatalysts: A review. J. Clean. Prod. 2020, 268, 121725. [Google Scholar] [CrossRef]
- Lee, C.M.; Palaniandy, P.; Dahlan, I. Pharmaceutical residues in aquatic environment and water remediation by TiO2 heterogeneous photocatalysis: A review. Environ. Earth Sci. 2017, 76, 611. [Google Scholar] [CrossRef]
- Snowdon, M.; Liang, R.; Van Leeuwen, J.C.; Schneider, O.; Khan, A.; Li, L.C.M.; Fong, C.; Zhou, N.Y.; Servos, M.R. Pharmaceutical Micropollutant Treatment with UV-LED/TiO2 Photocatalysis under Various Lighting and Matrix Conditions. Photochem 2022, 2, 503–514. [Google Scholar] [CrossRef]
- Andronic, L.; Isac, L.; Miralles-Cuevas, S.; Visa, M.; Oller, I.; Duta, A.; Malato, S. Pilot-plant evaluation of TiO2 and TiO2-based hybrid photocatalysts for solar treatment of polluted water. J. Hazard. Mater. 2016, 320, 469–478. [Google Scholar] [CrossRef]
- Luna-Sanguino, G.; Ruíz-Delgado, A.; Tolosana-Moranchel, A.; Pascual, L.; Malato, S.; Bahamonde, A.; Faraldos, M. Solar photocatalytic degradation of pesticides over TiO2-rGO nanocomposites at pilot plant scale. Sci. Total Environ. 2020, 737, 140286. [Google Scholar] [CrossRef] [PubMed]
- Pichat, P. Photocatalysis and Water Purification; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- dela Rosa, F.; Popović, M.; Papac Zjačić, J.; Radić, G.; Kraljić Roković, M.; Kovačić, M.; José Farré, M.; Genorio, B.; Lavrenčič Štangar, U.; Kušić, H.; et al. A Visible light activation of persulfate or H2O2 by TiO2/Fe2O3 immobilized on glass support for photocatalytic removal of amoxicillin: Mechanism, transformation products and toxicity assessment. Nanomaterials 2022, 12, 4328. [Google Scholar] [CrossRef]
- Matoh, L.; Zener, B.; Kovacic, M.; Kusic, H.; Arcon, I.; Levstek, M.; Lavrencic Stangar, U. Photocatalytic sol-gel/P25 TiO2 coatings for water treatment: Degradation of 7 selected pharmaceuticals. Ceram. Inter. 2022, in press. [Google Scholar] [CrossRef]
- Sruthi, L.; Janani, B.; Sudheer Khan, S. Ibuprofen removal from aqueous solution via light-harvesting photocatalysis by nano-heterojunctions: A. review. Sep. Pur. Technol. 2021, 27915, 119709. [Google Scholar] [CrossRef]
- Chen, Q.; Song, J.M.; Pan, F.; Xia, F.L.; Yuan, J.Y. The kinetics of photocatalytic degradation of aliphatic carboxylic acids in an UV/TiO2 suspension system. Environ. Technol. 2009, 30, 1103–1109. [Google Scholar] [CrossRef]
- Yin, R.; Ling, L.; Lu, S.; Li, H.; Li, C.; Shang, C. Degradation of aliphatic halogenated contaminants in water by UVA/Cu–TiO2 and UVA/TiO2 photocatalytic processes: Structure-activity relationship and role of reactive species. Chemosphere 2020, 260, 127644. [Google Scholar] [CrossRef]
- Huang, X.; Feng, Y.; Hu, C.; Xiao, X.; Yu, D.; Zou, D. Mechanistic QSAR models for interpreting degradation rates of sulfonamides in UV-photocatalysis systems. Chemosphere 2015, 138, 183–189. [Google Scholar] [CrossRef]
- Kušić, H.; Rasulev, B.; Leszczynska, D.; Leszczynski, J.; Koprivanac, N. Prediction of rate constants for radical degradation of aromatic pollutants in water matrix: A QSAR study. Chemosphere 2009, 75, 1128–1134. [Google Scholar] [CrossRef]
- Cvetnić, M.; Kovacic, M.; Novak Stankov, M.; Ukić, Š.; Bolanča, T.; Kušić, H.; Rasulev, B.; Dionysiou, D.D.; Lončarić Božić, A. Key structural features promoting radical driven degradation of emerging contaminants in water. Environ. Inter. 2019, 124, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Güsten, H. Predicting the abiotic degradability of organic pollutants in the troposphere. Chemosphere 1999, 38, 1361–1370. [Google Scholar] [CrossRef]
- Gramatica, P.; Pilutti, P.; Papa, E. A tool for the assessment of VOC degradability by tropospheric oxidants starting from chemical structure. Atmos. Environ. 2004, 38, 6167–6175. [Google Scholar] [CrossRef]
- Luo, X.; Yang, X.; Qiao, X.; Wang, Y.; Chen, J.; Wei, X.; Peijnenburg, W.J.G.M. Development of a QSAR model for predicting aqueous reaction rate constants of organic chemicals with hydroxyl radicals. Environ. Sci. Process. Impacts 2017, 19, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Sun, S.; Wu, Y.; Shi, Y.; Wang, Z.-J.; Cao, H.; Xie, Y. The structure-activity relationship of aromatic compounds in advanced oxidation processes: A review. Chemosphere 2022, 296, 134071. [Google Scholar] [CrossRef]
- Sharifi, T.; Crmaric, D.; Kovacic, M.; Popovic, M.; Rokovic, M.K.; Kusic, H.; Jozić, D.; Ambrožić, G.; Kralj, D.; Kontrec, J.; et al. Tailored BiVO4 for enhanced visible-light photocatalytic performance. J. Environ. Chem. Eng. 2021, 9, 106025. [Google Scholar] [CrossRef]
- Buxton, G.V.; Greenstock, C.L.; Helman, W.P.; Ross, A.B. Critical review of rate constants for reactions of hydrated electrons, hydrogen atoms and hydroxyl radicals (•OH/•O−) in aqueous solution. J. Phys. Chem. Ref. Data 1988, 17, 513–586. [Google Scholar] [CrossRef] [Green Version]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-hydroquinone based determination of electron and superoxide radical formed in heterogeneous photocatalytic systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]
- Tomic, A.; Cvetnic, M.; Kovacic, M.; Kusic, H.; Karamanis, P.; Loncaric, A. Structural features promoting adsorption of contaminants of emerging concern onto TiO2 P25: Experimental and computational approaches. Environ. Sci. Pollut. Res. 2022, 28, 1–17. [Google Scholar] [CrossRef]
- Gramatica, P. Chemometric Methods and Theoretical Molecular Descriptors in Predictive QSAR Modeling of the Environmental Behavior of Organic Pollutants. In Recent Advances in QSAR Studies; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Smidt, M.; Kusic, H.; Juretic, D.; Stankov, M.N.; Ukic, S.; Bolanca, T.; Rogosic, M.; Bozic, A.L. Modeling Photo-oxidative Degradation of Aromatics in Water. Optimization Study Using Response Surface and Structural Relationship Approaches. Ind. Eng. Chem. Res. 2015, 54, 5427–5441. [Google Scholar] [CrossRef]
- Wehrens, R.; Putter, H.; Buydens, L.M.C. The bootstrap: A tutorial. Chemom. Intell. Lab. Syst. 2000, 54, 35–52. [Google Scholar] [CrossRef]
- Rücker, C.; Rücker, G.; Meringer, M. Y-randomization and its variants in QSPR/QSAR. J. Chem. Inf. Model. 2007, 47, 2345–2357. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Papa, E.; Gramatica, P. Evaluation and QSAR modeling on multiple endpoints of estrogen activity based on different bioassays. Chemosphere 2008, 70, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Todeschini, R.; Consonni, V. Handbook of Molecular Descriptors. In Handbook of Chemoinformatics: From Data to Knowledge; Gasteiger, J., Ed.; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2003. [Google Scholar]
- Dreher, J.; Scheiber, J.; Stiefl, N.; Baumann, K. XMaP—An Interpretable Alignment-Free Four-Dimensional Quantitative Structure-Activity Relationship Technique Based on Molecular Surface Properties and Conformer Ensembles. J. Chem. Inf. Model. 2018, 58, 165–181. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, J.; Kaczmarek-Kedziera, A.; Puzyn, T.; Papadopoulos, M.G.; Reis, H.; Shukla, M.K. Handbook of Computational Chemistry; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Xiao, J.; Xie, Y.; Han, Q.; Cao, H.; Wang, Y.; Nawaz, F.; Duan, F. Superoxide radical-mediated photocatalytic oxidation of phenolic compounds over Ag+/TiO2: Influence of electron donating and withdrawing substituents. J. Hazard Mater. 2016, 304, 126–133. [Google Scholar] [CrossRef]
- Kralik, P.; Kusic, H.; Koprivanac, N.; Loncaric Bozic, A. Degradation of chlorinated hydrocarbons by UV/H2O2: The application of experimental design and kinetic modeling approach. Chem. Eng. J. 2010, 158, 154–166. [Google Scholar] [CrossRef]
- Lai, F.; Tian, F.X.; Xu, B.; Ye, W.K.; Gao, Y.Q.; Chen, C.; Xing, H.B.; Wang, B.; Xie, M.J.; Hu, X.J. A comparative study on the degradation of phenylurea herbicides by UV/persulfate process: Kinetics, mechanisms, energy demand and toxicity evaluation associated with DBPs. Chem. Eng. J. 2022, 428, 132088. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, Y.; Lim, T.T. UV direct photolysis of halogenated disinfection byproducts: Experimental study and QSAR modeling. Chemosphere 2019, 235, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Belelli, P.G.; Ferullo, R.M.; Branda, M.M.; Castellani, N.J. Theoretical modeling of photocatalytic active species on illuminated TiO2. Appl. Surf. Sci. 2007, 254, 32–35. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, W.; Peng, Y.; Chen, Z.; Chen, J.; Xiao, Z.; Zhao, X.; Qu, Y.; Li, J. Predicting the adsorption of organic pollutants on boron nitride nanosheets: Via in silico techniques: DFT computations and QSAR modeling. Environ. Sci. Nano. 2021, 8, 795–805. [Google Scholar] [CrossRef]
- Gramatica, P. Principles of QSAR Modeling: Comments and Suggestions from Personal Experience. Int. J. Quant. Struct. Relationships 2020, 5, 61–97. [Google Scholar] [CrossRef]
- Gramatica, P.; Cassani, S.; Chirico, N. QSARINS-Chem: Insubria datasets and new QSAR/QSPR models for environmental pollutants in QSARINS. J. Comput. Chem. 2014, 35, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Gramatica, P.; Chirico, N.; Papa, E.; Cassani, S.; Kovarich, S. QSARINS: A new software for the development, analysis, and validation of QSAR MLR models. J. Comput. Chem. 2013, 34, 2121–2132. [Google Scholar] [CrossRef]
- Gramatica, P. Principles of QSAR models validation: Internal and external. QSAR Comb. Sci. 2007, 26, 694–701. [Google Scholar] [CrossRef]
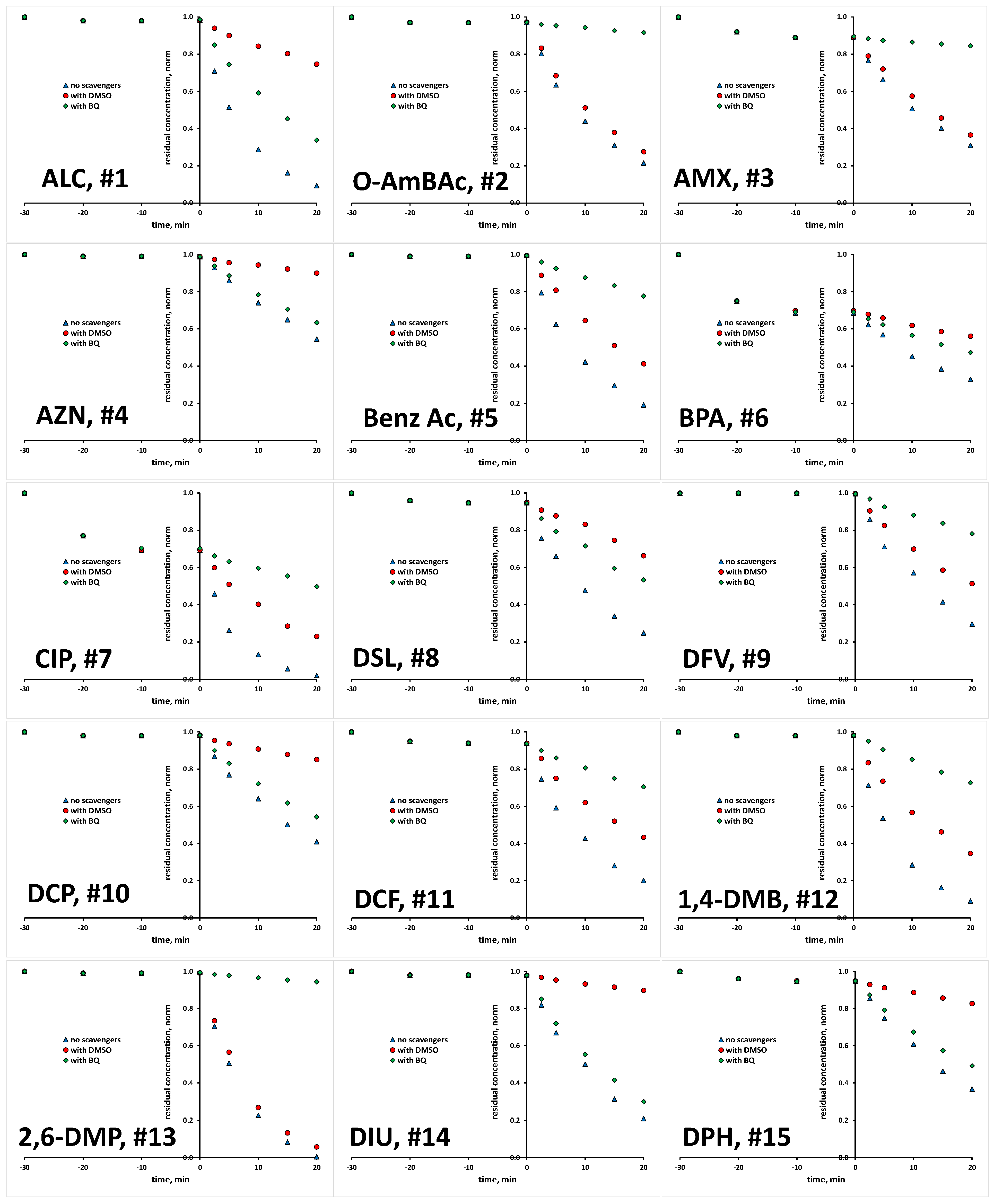
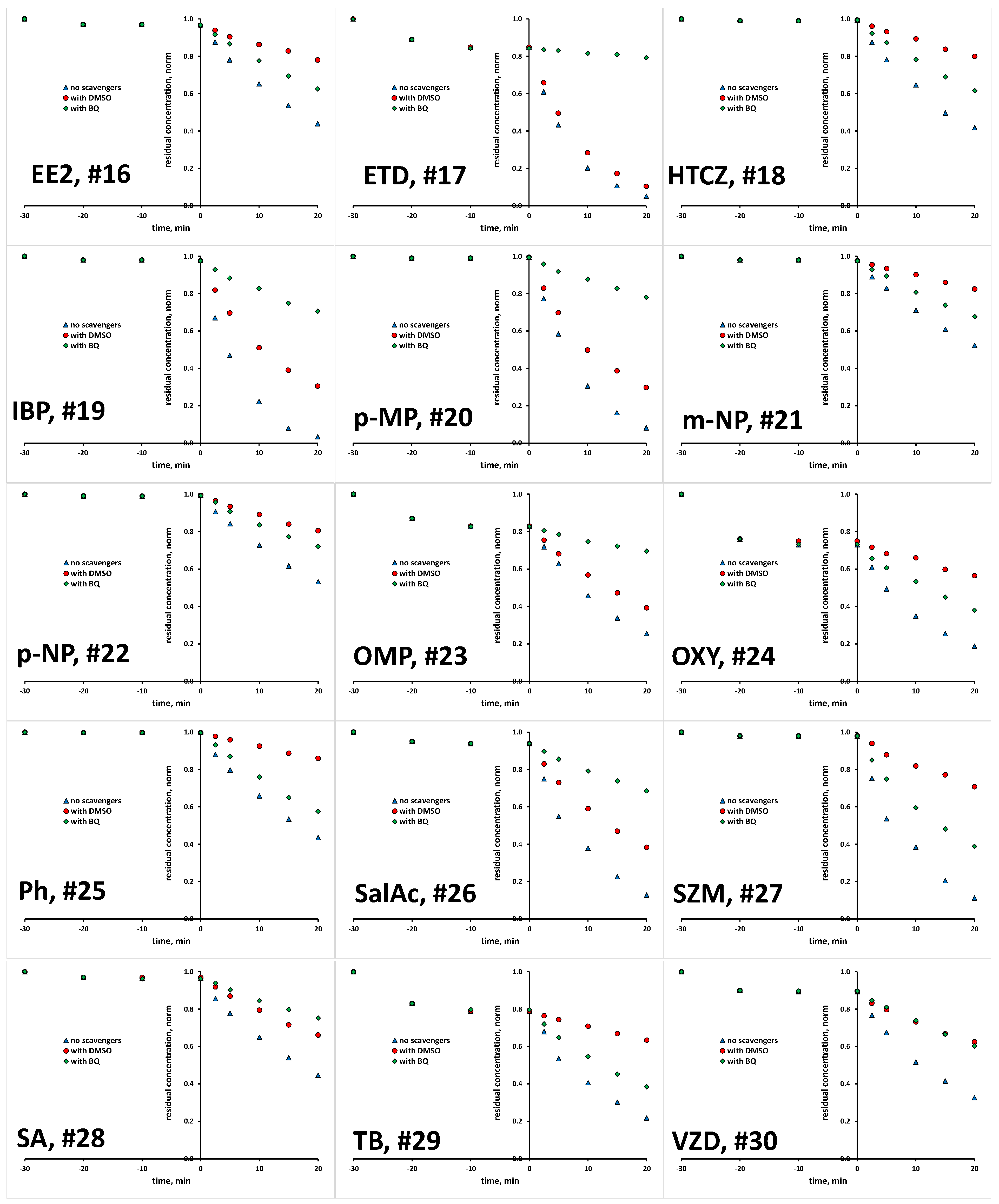
| # | Compound | Abbreviation | CAS | Molecular Formula | K | |
|---|---|---|---|---|---|---|
| 1 | Alachlor | ALC | 15972-60-8 | C14H20ClNO2 | 2.722 | 0.518 |
| 2 | o-Aminobenzoic acid | o-aminoBenzAc | 118-92-3 | C7H7NO2 | 0.079 | 0.963 |
| 3 | Amoxicillin | AMX | 26787-78-0 | C16H19N3O5S | 0.095 | 0.956 |
| 4 | Atrazine | AZN | 1912-24-9 | C8H14ClN5 | 4.017 | 0.446 |
| 5 | Benzoic acid | BenzAc | 65-85-0 | C6H5COOH | 0.374 | 0.853 |
| 6 | Bisphenol A | BPA | 80-05-7 | C15H16O2 | 1.580 | 0.623 |
| 7 | Ciprofloxacin | CIP | 85721-33-1 | C₁₇H₁₈FN₃O₃ | 0.852 | 0.735 |
| 8 | Desloratadine | DSL | 100643-71-8 | C19H19ClN2 | 1.442 | 0.640 |
| 9 | Desvenlafaxine | DVF | 93413-62-8 | C16H25NO2 | 0.445 | 0.832 |
| 10 | 2,4-Dichlorophenol | DCP | 120-83-2 | C6H4Cl2O | 3.358 | 0.479 |
| 11 | Diclofenac | DCF | 15307-79-6 | C14H10Cl2NNaO2 | 0.457 | 0.829 |
| 12 | 1,4-Dimethoxybenzene | 1,4-DMB | 150-78-7 | C6H4(OCH3)2 | 0.400 | 0.845 |
| 13 | 2,6-Dimethoxyphenol | 2,6-DMP | 91-10-1 | (CH3O)2C6H3OH | 0.053 | 0.974 |
| 14 | Diuron | DIU | 330-54-1 | C9H10Cl2N2O | 8.358 | 0.327 |
| 15 | Donepezil HCl | DPH | 120011-70-3 | C24H30ClNO3 | 1.404 | 0.645 |
| 16 | 17α-Ethynylestradiol | EE2 | 57-63-6 | C20H24O2 | 1.833 | 0.594 |
| 17 | Etodolac | ETD | 41340-25-4 | C17H21NO3 | 0.067 | 0.968 |
| 18 | Hydrochlorothiazide | HCTZ | 58-93-5 | C7H8ClN3O4S2 | 1.940 | 0.583 |
| 19 | Ibuprofene | IBP | 15687-27-1 | C13H18O2 | 0.402 | 0.844 |
| 20 | p-Methoxyphenol | p-MP | 150-76-5 | CH3OC6H4OH | 0.305 | 0.875 |
| 21 | m-Nitrophenol | m-NP | 554-84-7 | O2NC6H4OH | 1.965 | 0.581 |
| 22 | p-Nitrophenol | p-NP | 100-02-7 | O2NC6H4OH | 1.437 | 0.641 |
| 23 | Omeprazole HCl | OMP | 73590-58-6 | C17H20ClN3O3S | 0.302 | 0.876 |
| 24 | Oxytetracycline | OXY | 79-57-2 | C22H24N2O9 | 1.916 | 0.586 |
| 25 | Phenol | Ph | 108-95-2 | C6H5OH | 3.069 | 0.496 |
| 26 | Salicylic acid | SalAc | 69-72-7 | C7H6O3 | 0.455 | 0.829 |
| 27 | Simazine | SZM | 122-34-9 | C7H12ClN5 | 2.171 | 0.562 |
| 28 | Sulfanilic acid | SA | 121-57-3 | C6H7NO3S | 0.682 | 0.771 |
| 29 | Tobramycin | TB | 32986-56-4 | C18H37N5O9 | 2.614 | 0.526 |
| 30 | Vilazodone HCl | VZD | 163521-08-2 | C26H27N5O2 | 1.084 | 0.693 |
| Descriptor Name | Descriptor Definition | Descriptor Type |
|---|---|---|
| MATS4v | Moran autocorrelation of lag 4 weighted by van der Waals volume | 2D autocorrelations |
| Mor10u | signal 10/unweighted | 3D-MoRSE |
| CATS2D_01_DN | CATS2D Donor-Negative at lag 01 | CATS 2D |
| B04[C − Cl] | Presence/absence of C − Cl at topological distance 4 | 2D Atom Pairs |
| B08[C − O] | Presence/absence of C O at topological distance 8 | 2D Atom Pairs |
| # | Symbol | Definition |
|---|---|---|
| 1 | R | the correlation coefficient of regression |
| 2 | R2 | the model explained variance |
| 3 | Q2 | the leave-one-out cross-validation coefficient |
| 4 | F | F-ratio between variances of observed and calculated values |
| 5 | p | probability value for calculated F |
| 6 | s | standard error |
| 7 | SPRESS | standard error of the predictive residue of sum of squares |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomic, A.; Kovacic, M.; Kusic, H.; Karamanis, P.; Rasulev, B.; Loncaric Bozic, A. Structural Features Promoting Photocatalytic Degradation of Contaminants of Emerging Concern: Insights into Degradation Mechanism Employing QSA/PR Modeling. Molecules 2023, 28, 2443. https://doi.org/10.3390/molecules28062443
Tomic A, Kovacic M, Kusic H, Karamanis P, Rasulev B, Loncaric Bozic A. Structural Features Promoting Photocatalytic Degradation of Contaminants of Emerging Concern: Insights into Degradation Mechanism Employing QSA/PR Modeling. Molecules. 2023; 28(6):2443. https://doi.org/10.3390/molecules28062443
Chicago/Turabian StyleTomic, Antonija, Marin Kovacic, Hrvoje Kusic, Panaghiotis Karamanis, Bakhtiyor Rasulev, and Ana Loncaric Bozic. 2023. "Structural Features Promoting Photocatalytic Degradation of Contaminants of Emerging Concern: Insights into Degradation Mechanism Employing QSA/PR Modeling" Molecules 28, no. 6: 2443. https://doi.org/10.3390/molecules28062443
APA StyleTomic, A., Kovacic, M., Kusic, H., Karamanis, P., Rasulev, B., & Loncaric Bozic, A. (2023). Structural Features Promoting Photocatalytic Degradation of Contaminants of Emerging Concern: Insights into Degradation Mechanism Employing QSA/PR Modeling. Molecules, 28(6), 2443. https://doi.org/10.3390/molecules28062443









