Recycling of Plastics from E-Waste via Photodegradation in a Low-Pressure Reactor: The Case of Decabromodiphenyl Ether Dispersed in Poly(acrylonitrile-butadiene-styrene) and Poly(carbonate)
Abstract
1. Introduction
2. Materials and Methods
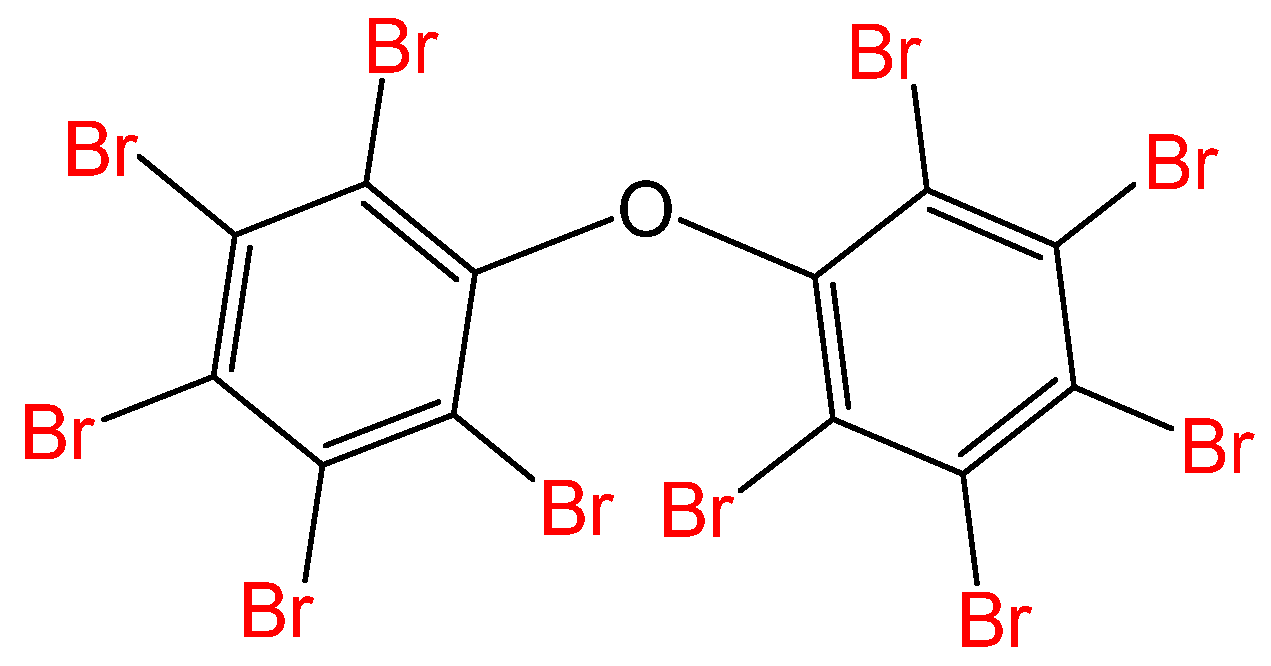
2.1. Chemicals
2.2. Sample Preparation
2.3. Irradiation Experiments
2.4. Ultrasonic-Assisted Extraction and Purification
2.5. Instrumentation
3. Results and Discussion
3.1. Photodegradation Efficiency
3.1.1. Fourier-Transform Infrared Spectroscopy
3.1.2. Gas Chromatography–Mass Spectrometry
3.2. Polymer Properties after UV-Visible Light Exposure
3.2.1. ATR-FTIR Analysis
3.2.2. Thermal Properties
3.2.3. Mechanical Properties
3.2.4. Molecular Weights and Polydispersities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hennebert, P.; Filella, M. WEEE Plastic Sorting for Bromine Essential to Enforce EU Regulation. Waste Manag. 2018, 71, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M. An Overview of Commercially Used Brominated Flame Retardants, Their Applications, Their Use Patterns in Different Countries/Regions and Possible Modes of Release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Kemmlein, S.; Herzke, D.; Law, R.J. Brominated Flame Retardants in the European Chemicals Policy of REACH—Regulation and Determination in Materials. J. Chromatogr. A 2009, 1216, 320–333. [Google Scholar] [CrossRef] [PubMed]
- Dodson, R.E.; Perovich, L.J.; Covaci, A.; Van den Eede, N.; Ionas, A.C.; Dirtu, A.C.; Brody, J.G.; Rudel, R.A. After the PBDE Phase-Out: A Broad Suite of Flame Retardants in Repeat House Dust Samples from California. Environ. Sci. Technol. 2012, 46, 13056–13066. [Google Scholar] [CrossRef] [PubMed]
- Jinhui, L.; Yuan, C.; Wenjing, X. Polybrominated Diphenyl Ethers in Articles: A Review of Its Applications and Legislation. Environ. Sci. Pollut. Res. 2017, 24, 4312–4321. [Google Scholar] [CrossRef]
- Klinčić, D.; Dvoršćak, M.; Jagić, K.; Mendaš, G.; Herceg Romanić, S. Levels and Distribution of Polybrominated Diphenyl Ethers in Humans and Environmental Compartments: A Comprehensive Review of the Last Five Years of Research. Environ. Sci. Pollut. Res. 2020, 27, 5744–5758. [Google Scholar] [CrossRef]
- Darnerud, P. Toxic Effects of Brominated Flame Retardants in Man and in Wildlife. Environ. Int. 2003, 29, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Sharkey, M.; Harrad, S.; Abou-Elwafa Abdallah, M.; Drage, D.S.; Berresheim, H. Phasing-out of Legacy Brominated Flame Retardants: The UNEP Stockholm Convention and Other Legislative Action Worldwide. Environ. Int. 2020, 144, 106041. [Google Scholar] [CrossRef]
- de la Torre, A.; Navarro, I.; Sanz, P.; de los Ángeles Martínez, M. Organophosphate Compounds, Polybrominated Diphenyl Ethers and Novel Brominated Flame Retardants in European Indoor House Dust: Use, Evidence for Replacements and Assessment of Human Exposure. J. Hazard. Mater. 2020, 382, 121009. [Google Scholar] [CrossRef]
- Abbasi, G.; Li, L.; Breivik, K. Global Historical Stocks and Emissions of PBDEs. Environ. Sci. Technol. 2019, 53, 6330–6340. [Google Scholar] [CrossRef]
- Wäger, P.A.; Schluep, M.; Müller, E.; Gloor, R. RoHS Regulated Substances in Mixed Plastics from Waste Electrical and Electronic Equipment. Environ. Sci. Technol. 2012, 46, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.R.; Vanegas, P.; Kellens, K.; Wang, F.; Huisman, J.; Dewulf, W.; Duflou, J.R. Forecasting Waste Compositions: A Case Study on Plastic Waste of Electronic Display Housings. Waste Manag. 2015, 46, 28–39. [Google Scholar] [CrossRef] [PubMed]
- McGrath, T.J.; Morrison, P.D.; Ball, A.S.; Clarke, B.O. Spatial Distribution of Novel and Legacy Brominated Flame Retardants in Soils Surrounding Two Australian Electronic Waste Recycling Facilities. Environ. Sci. Technol. 2018, 52, 8194–8204. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, K.; Ma, L.-X.; Sun, S.-J.; Jia, L.-R.; Yuan, A.-N.; Shen, J.-M.; Qi, H.; Zhang, A.-P. Deca-BDE and Alternative Halogenated Flame Retardants in a Wastewater Treatment Plant in Harbin (2009–2016): Occurrence, Temporal Trends, Seasonal Variation, and Fate. Sci. Total Environ. 2018, 625, 1156–1163. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, R.; Xu, Z. PBDEs Emission from Waste Printed Wiring Boards during Thermal Process. Environ. Sci. Technol. 2015, 49, 2716–2723. [Google Scholar] [CrossRef]
- Ma, Y.; Stubbings, W.A.; Cline-Cole, R.; Harrad, S. Human Exposure to Halogenated and Organophosphate Flame Retardants through Informal E-Waste Handling Activities—A Critical Review. Environ. Pollut. 2021, 268, 115727. [Google Scholar] [CrossRef]
- Fatunsin, O.T.; Oluseyi, T.O.; Drage, D.; Abdallah, M.A.-E.; Turner, A.; Harrad, S. Children’s Exposure to Hazardous Brominated Flame Retardants in Plastic Toys. Sci. Total Environ. 2020, 720, 137623. [Google Scholar] [CrossRef]
- Guzzonato, A.; Puype, F.; Harrad, S.J. Evidence of Bad Recycling Practices: BFRs in Children’s Toys and Food-Contact Articles. Environ. Sci. Process. Impacts 2017, 19, 956–963. [Google Scholar] [CrossRef]
- EU. Regulation (EU) 2019/1021 of the European Parliament and of the Council of 20 June 2019 on Persistent Organic Pollutants (Recast). Off. J. Eur. Union 2019, 169, 33. [Google Scholar]
- Jonkers, N.; Krop, H.; van Ewijk, H.; Leonards, P.E.G. Life Cycle Assessment of Flame Retardants in an Electronics Application. Int. J. Life Cycle Assess. 2016, 21, 146–161. [Google Scholar] [CrossRef]
- Altarawneh, M.; Saeed, A.; Al-Harahsheh, M.; Dlugogorski, B.Z. Thermal Decomposition of Brominated Flame Retardants (BFRs): Products and Mechanisms. Prog. Energy Combust. Sci. 2019, 70, 212–259. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. Contribution of Polybrominated Dibenzo- p -Dioxins and Dibenzofurans (PBDD/Fs) to the Toxic Equivalency of Dioxin-like Compounds in Archived Biosolids from the U.S. EPA’s 2001 National Sewage Sludge Survey. Environ. Sci. Technol. 2014, 48, 10843–10849. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Tsang, D.C.W.; Wang, Y.; Li, Y.; Yang, X. The Photodegradation of Polybrominated Diphenyl Ethers (PBDEs) in Various Environmental Matrices: Kinetics and Mechanisms. Chem. Eng. J. 2016, 297, 74–96. [Google Scholar] [CrossRef]
- Söderström, G.; Sellström, U.; de Wit, C.A.; Tysklind, M. Photolytic Debromination of Decabromodiphenyl Ether (BDE 209). Environ. Sci. Technol. 2004, 38, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Lyche, J.L.; Rosseland, C.; Berge, G.; Polder, A. Human Health Risk Associated with Brominated Flame-Retardants (BFRs). Environ. Int. 2015, 74, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Yao, B.; Luo, Z.; Zhi, D.; Hou, D.; Luo, L.; Du, S.; Zhou, Y. Current Progress in Degradation and Removal Methods of Polybrominated Diphenyl Ethers from Water and Soil: A Review. J. Hazard. Mater. 2021, 403, 123674. [Google Scholar] [CrossRef] [PubMed]
- Raff, J.D.; Hites, R.A. Gas-Phase Reactions of Brominated Diphenyl Ethers with OH Radicals. J. Phys. Chem. A 2006, 110, 10783–10792. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, I.; Tatsukawa, R. Formation of Brominated Dibenzofurans from the Photolysis of Flame Retardant Decabromobiphenyl Ether in Hexane Solution by UV and Sun Light. Bull. Environ. Contam. Toxicol. 1987, 39, 953–959. [Google Scholar] [CrossRef] [PubMed]
- Bezares-Cruz, J.; Jafvert, C.T.; Hua, I. Solar Photodecomposition of Decabromodiphenyl Ether: Products and Quantum Yield. Environ. Sci. Technol. 2004, 38, 4149–4156. [Google Scholar] [CrossRef]
- Christiansson, A.; Eriksson, J.; Teclechiel, D.; Bergman, Å. Identification and Quantification of Products Formed via Photolysis of Decabromodiphenyl Ether. Environ. Sci. Pollut. Res. 2009, 16, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Leal, J.F.; Esteves, V.I.; Santos, E.B.H. BDE-209: Kinetic Studies and Effect of Humic Substances on Photodegradation in Water. Environ. Sci. Technol. 2013, 47, 14010–14017. [Google Scholar] [CrossRef] [PubMed]
- Kajiwara, N.; Noma, Y.; Takigami, H. Photolysis Studies of Technical Decabromodiphenyl Ether (DecaBDE) and Ethane (DeBDethane) in Plastics under Natural Sunlight. Environ. Sci. Technol. 2008, 42, 4404–4409. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, H.M.; Dodder, N.G. Photodegradation of Decabromodiphenyl Ether in House Dust by Natural Sunlight. Environ. Toxicol. Chem. 2008, 27, 306. [Google Scholar] [CrossRef] [PubMed]
- Khaled, A.; Richard, C.; Rivaton, A.; Jaber, F.; Sleiman, M. Photodegradation of Brominated Flame Retardants in Polystyrene: Quantum Yields, Products and Influencing Factors. Chemosphere 2018, 211, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Bahadur, N.; Dumée, L.F. Photo-Catalytic Membrane Reactors for the Remediation of Persistent Organic Pollutants—A Review. Sep. Purif. Technol. 2020, 230, 115878. [Google Scholar] [CrossRef]
- Khaled, A.; Rivaton, A.; Richard, C.; Jaber, F.; Sleiman, M. Phototransformation of Plastic Containing Brominated Flame Retardants: Enhanced Fragmentation and Release of Photoproducts to Water and Air. Environ. Sci. Technol. 2018, 52, 11123–11131. [Google Scholar] [CrossRef] [PubMed]
- Celina, M.; Linde, E.; Brunson, D.; Quintana, A.; Giron, N. Overview of Accelerated Aging and Polymer Degradation Kinetics for Combined Radiation-Thermal Environments. Polym. Degrad. Stab. 2019, 166, 353–378. [Google Scholar] [CrossRef]
- Jandric, A.; Part, F.; Fink, N.; Huber-Humer, M.; Salhofer, S.; Zafiu, C. Bromierte Flammschutzmittel in Elektroaltgeräten: Untersuchung der Brom-Konzentration nach Kunststofftypen und Gerätekategorien mittels Röntgenfluoreszenzanalyse. Osterr. Wasser Abfallwirtsch. 2020, 72, 68–76. [Google Scholar] [CrossRef]
- Maris, E.; Botané, P.; Wavrer, P.; Froelich, D. Characterizing Plastics Originating from WEEE: A Case Study in France. Miner. Eng. 2015, 76, 28–37. [Google Scholar] [CrossRef]
- Farzadfar, A.; Khorasani, S.N.; Khalili, S. Blends of Recycled Polycarbonate and Acrylonitrile-Butadiene-Styrene: Comparing the Effect of Reactive Compatibilizers on Mechanical and Morphological Properties: Compatibilized Blends of Recycled PC and ABS. Polym. Int. 2014, 63, 145–150. [Google Scholar] [CrossRef]
- Rånby, B. Photodegradation and Photo-Oxidation of Synthetic Polymers. J. Anal. Appl. Pyrolysis 1989, 15, 237–247. [Google Scholar] [CrossRef]
- Duh, Y.-S.; Ho, T.-C.; Chen, J.-R.; Kao, C.-S. Study on Exothermic Oxidation of Acrylonitrile-Butadiene-Styrene (ABS) Resin Powder with Application to ABS Processing Safety. Polymers 2010, 2, 174–187. [Google Scholar] [CrossRef]
- Maschke, U.; Poutch, F.; Khelifi, S.; Debert, J.-M.; Agguine, Y.; Laouedj, N.; Khouba, Z.; Bouberka, Z.; Nadim, A.; Gobert, P.; et al. Method for Deactivating Brominated Derivatives Contained in Plastic Materials. Patent EP2839863A1, 25 February 2015. Available online: https://patents.google.com/patent/EP2839863A1/en (accessed on 23 December 2022).
- Aldoori, H.; Bouberka, Z.; Nadim, A.; Agguine, Y.; Eddarir, S.; Supiot, P.; Foissac, C.; Maschke, U. Photodegradation of Decabromo Diphenyl Ether Flame Retardant in Poly (Acrylonitrile Butadiene Styrene) (ABS). J. Macromol. Sci. Part B 2020, 59, 609–620. [Google Scholar] [CrossRef]
- Adams, M.R.; Garton, A. Surface Modification of Bisphenoi-A- Polycarbonate by Far-UV Radiation. Part I: In Vacuum. Polym. Degrad. Stab. 1993, 41, 265–273. [Google Scholar] [CrossRef]
- Yousif, E.; Haddad, R. Photodegradation and Photostabilization of Polymers, Especially Polystyrene: Review. SpringerPlus 2013, 2, 398. [Google Scholar] [CrossRef] [PubMed]
- Blom, H.; Yeh, R.; Wojnarowski, R.; Ling, M. Detection of Degradation of ABS Materials via DSC. J. Therm. Anal. Calorim. 2006, 83, 113–115. [Google Scholar] [CrossRef]
- Torikai, A.; Kato, H.; Fueki, K.; Suzuki, Y.; Okisaki, F.; Nagata, M. Photodegradation of Polymer Materials Containing Flame-Cut Agents. J. Appl. Polym. Sci. 1993, 50, 2185–2190. [Google Scholar] [CrossRef]








| Sample | Young’s Modulus (MPa) | Stress at Yield (MPa) | Modulus of Resilience (J/m3) | Strain at Break (%) | Stress at Break (MPa) | Modulus of Toughness (J/m3) |
|---|---|---|---|---|---|---|
| Virgin ABS | 1454 ± 70 | 40 ± 0.5 | 5 × 105 | 18 ± 2 | 31 ± 2 | 5.3 × 106 |
| 90 wt-% virgin ABS + 10 wt-% of the irradiated (90 wt-% ABS/10 wt-% DBDE) blend | 1755 ± 45 | 43 ± 2 | 6.7 × 105 | 16.8 ± 2.5 | 32.6 ± 1.3 | 5.23 × 106 |
| Virgin PC | 1940 ± 50 | 68 ± 0.5 | 1.32 × 106 | 44.7 ± 5.4 | 56.0 ± 4.5 | 2.2 × 107 |
| 90 wt-% virgin PC + 10 wt-% of the irradiated (90 wt-% PC/10 wt-% DBDE) blend | 1880 ± 35 | 67 ± 1 | 1.38 × 106 | 32.7 ± 2.5 | 54.0 ± 4.4 | 2.0 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldoori, H.; Bouberka, Z.; Feuchter, H.; Khelifi, S.; Poutch, F.; Brison, L.; Laoutid, F.; Steuperaert, S.; Foissac, C.; Supiot, P.; et al. Recycling of Plastics from E-Waste via Photodegradation in a Low-Pressure Reactor: The Case of Decabromodiphenyl Ether Dispersed in Poly(acrylonitrile-butadiene-styrene) and Poly(carbonate). Molecules 2023, 28, 2491. https://doi.org/10.3390/molecules28062491
Aldoori H, Bouberka Z, Feuchter H, Khelifi S, Poutch F, Brison L, Laoutid F, Steuperaert S, Foissac C, Supiot P, et al. Recycling of Plastics from E-Waste via Photodegradation in a Low-Pressure Reactor: The Case of Decabromodiphenyl Ether Dispersed in Poly(acrylonitrile-butadiene-styrene) and Poly(carbonate). Molecules. 2023; 28(6):2491. https://doi.org/10.3390/molecules28062491
Chicago/Turabian StyleAldoori, Hussam, Zohra Bouberka, Hervé Feuchter, Skander Khelifi, Franck Poutch, Loic Brison, Fouad Laoutid, Stijn Steuperaert, Corinne Foissac, Philippe Supiot, and et al. 2023. "Recycling of Plastics from E-Waste via Photodegradation in a Low-Pressure Reactor: The Case of Decabromodiphenyl Ether Dispersed in Poly(acrylonitrile-butadiene-styrene) and Poly(carbonate)" Molecules 28, no. 6: 2491. https://doi.org/10.3390/molecules28062491
APA StyleAldoori, H., Bouberka, Z., Feuchter, H., Khelifi, S., Poutch, F., Brison, L., Laoutid, F., Steuperaert, S., Foissac, C., Supiot, P., Malas, C., & Maschke, U. (2023). Recycling of Plastics from E-Waste via Photodegradation in a Low-Pressure Reactor: The Case of Decabromodiphenyl Ether Dispersed in Poly(acrylonitrile-butadiene-styrene) and Poly(carbonate). Molecules, 28(6), 2491. https://doi.org/10.3390/molecules28062491








