Hydrothermal Synthesis of Nickel Oxide and Its Application in the Additive Manufacturing of Planar Nanostructures
Abstract
1. Introduction
2. Results and Discussion
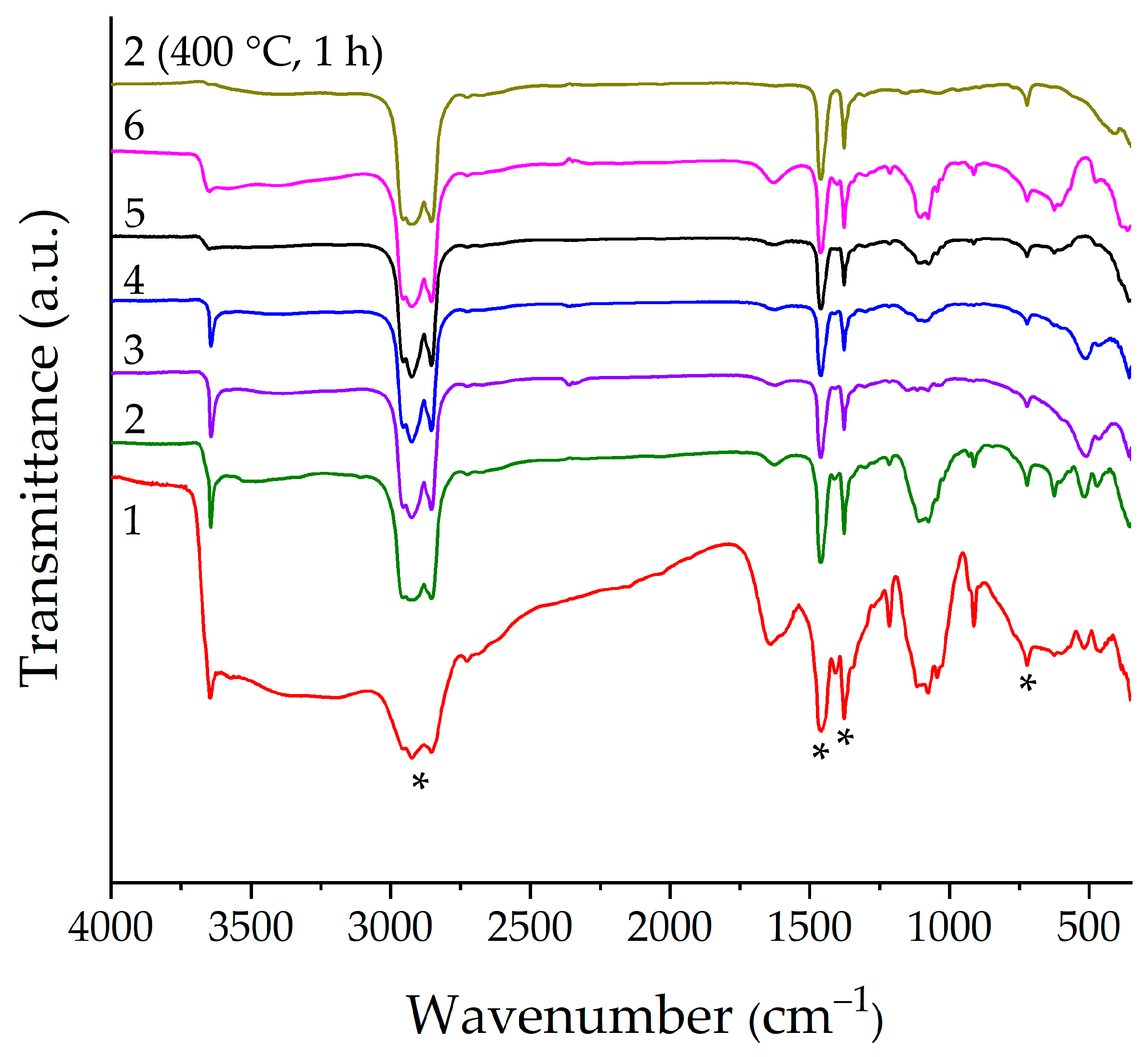
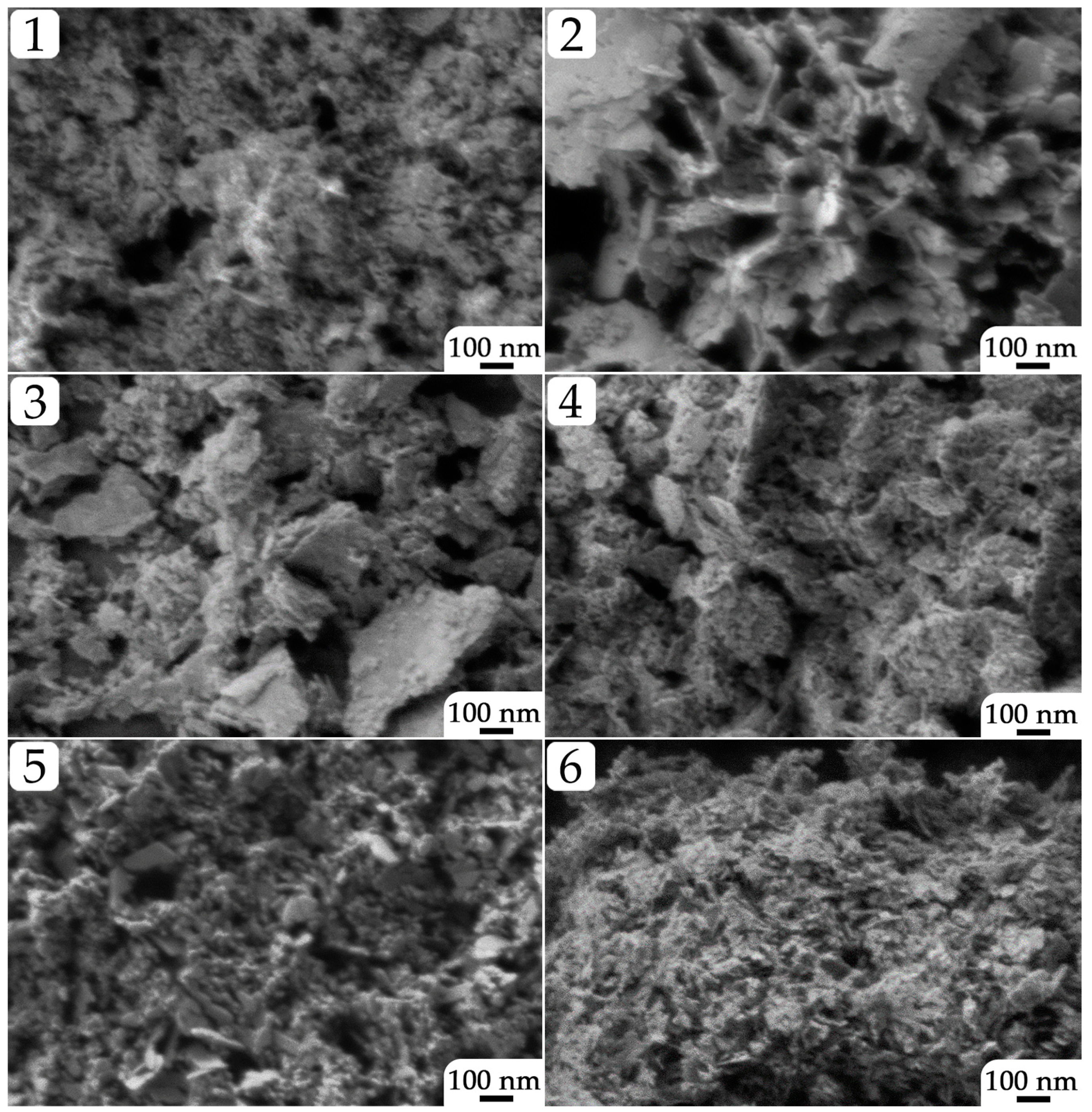
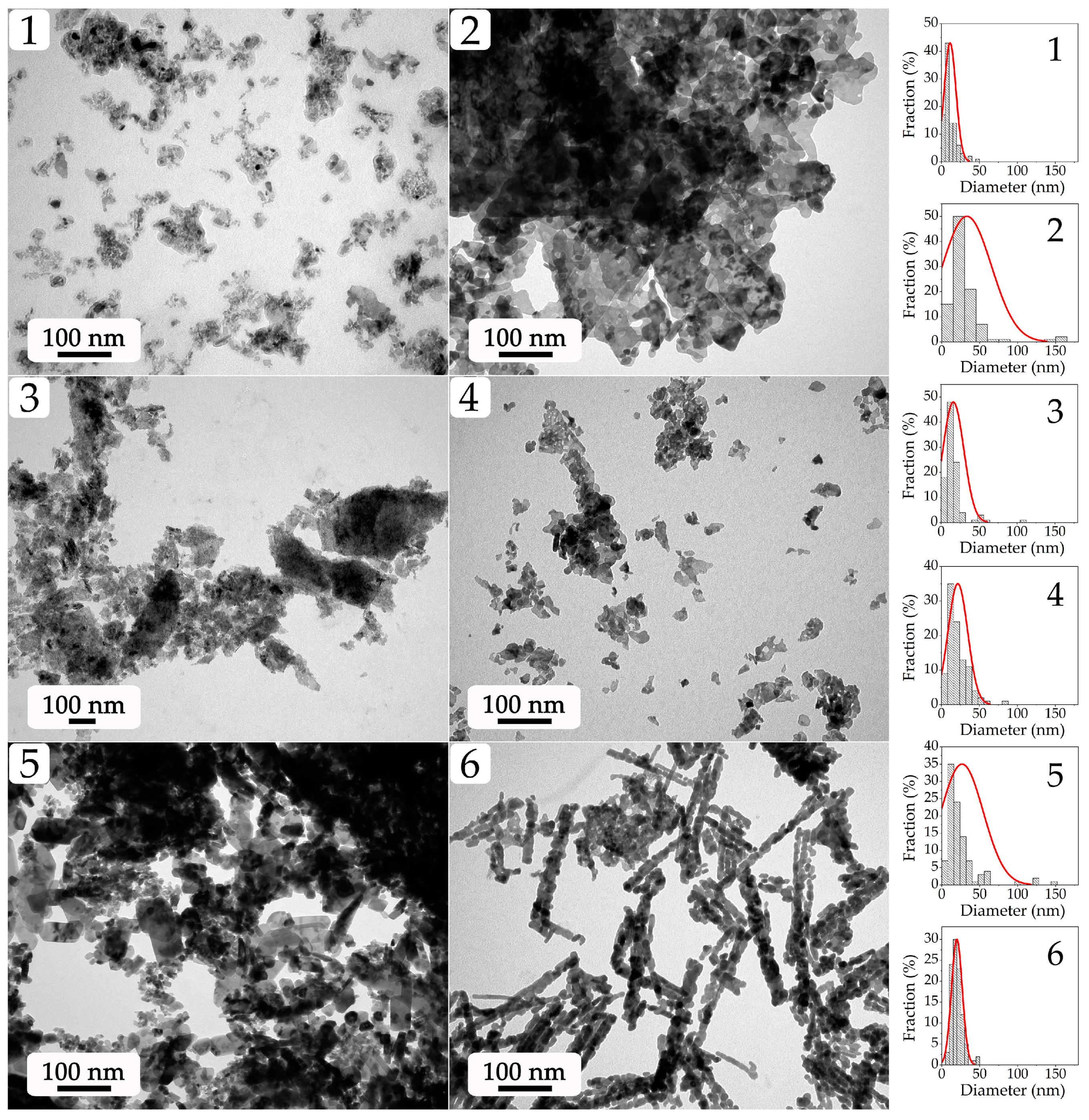
2.1. Examination of Semi-Products and NiO Nanopowders
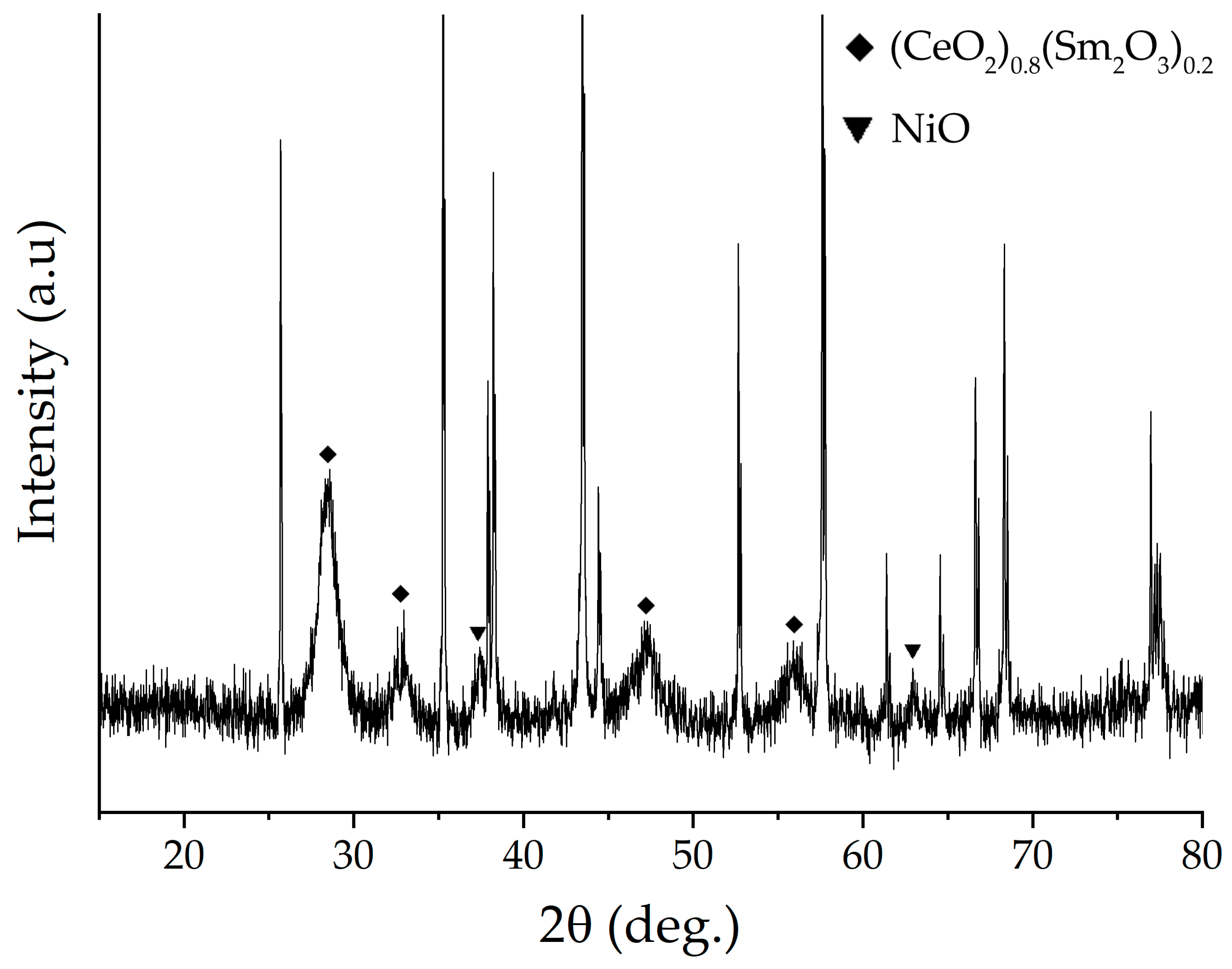
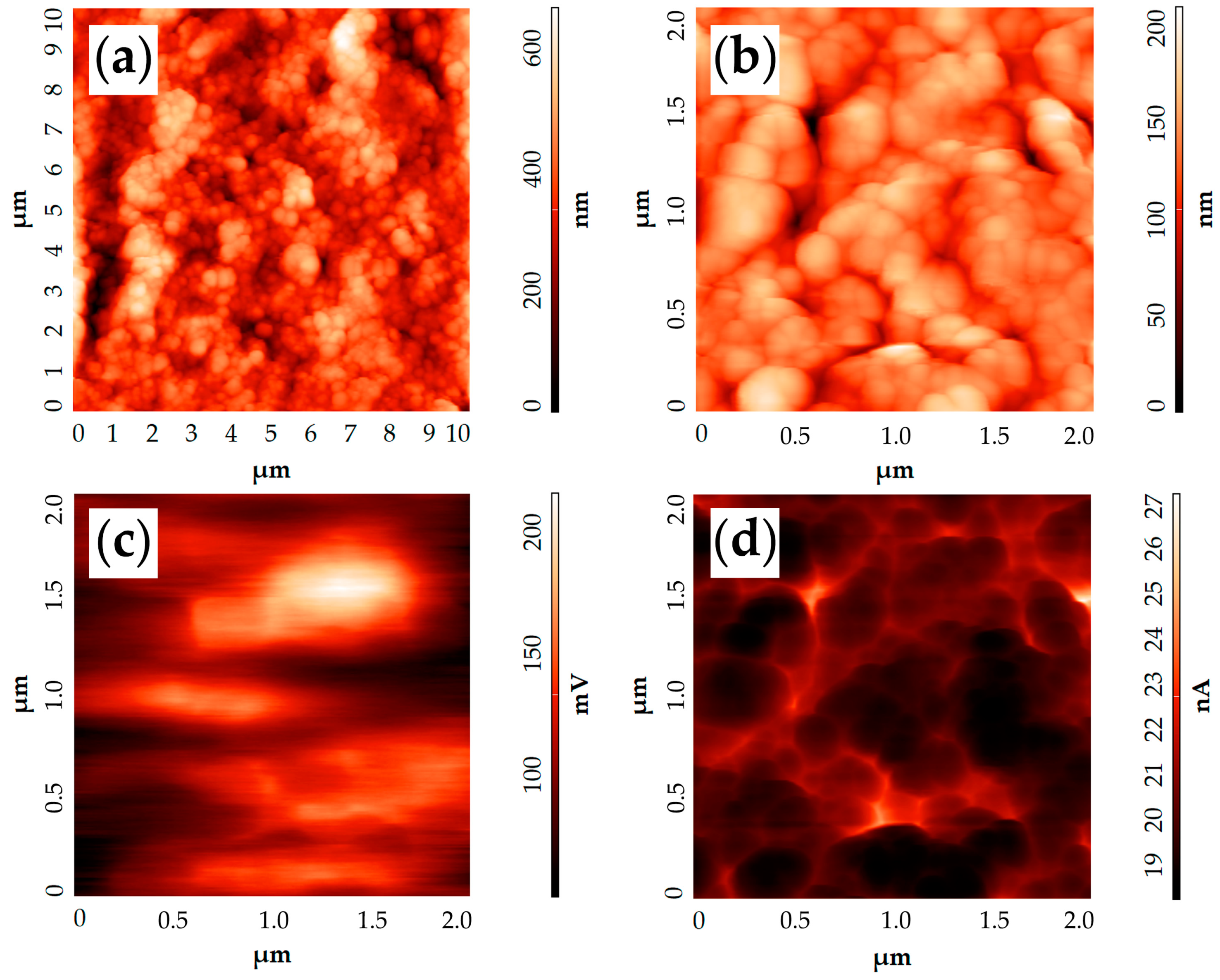
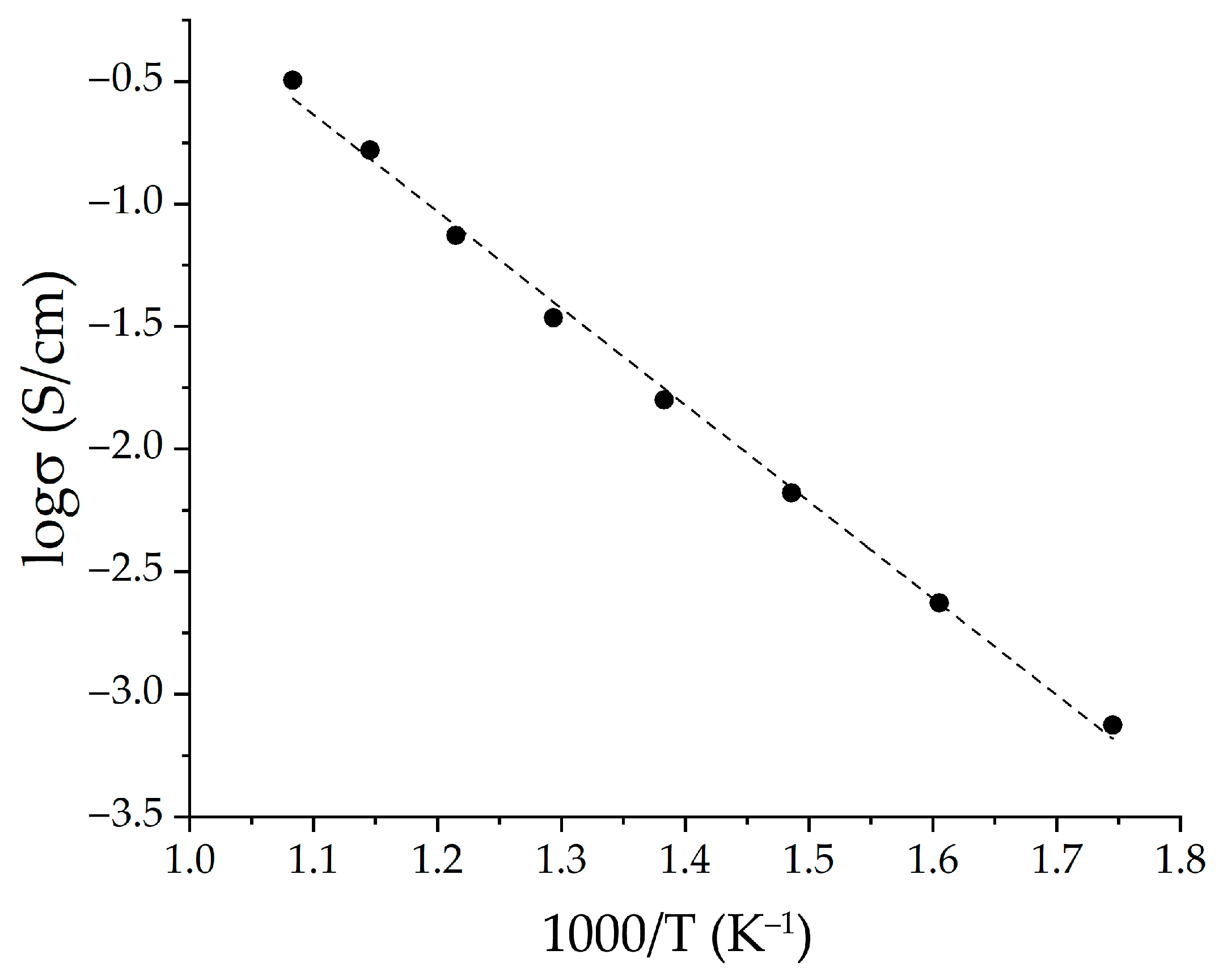
2.2. Characterization of the Printed NiO—SDC Film
3. Materials and Methods
3.1. Materials
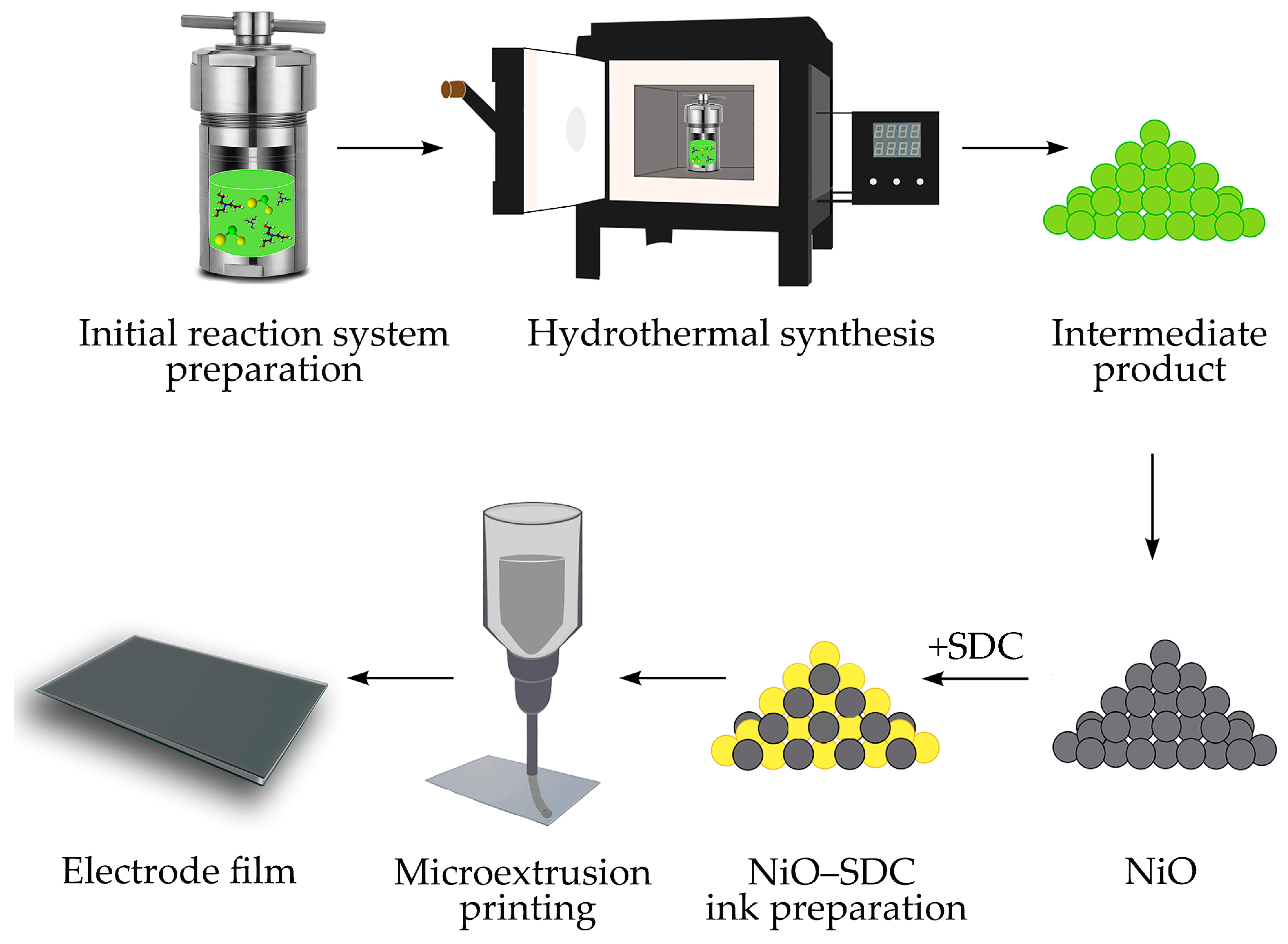
3.2. Hydrothermal Synthesis of NiO Nanopowders
3.3. Microextrusion Printing of NiO—SDC Electrode Film
3.4. Instrumentation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azam, A.; Rafiq, M.; Shafique, M.; Yuan, J. Towards Achieving Environmental Sustainability: The Role of Nuclear Energy, Renewable Energy, and ICT in the Top-Five Carbon Emitting Countries. Front. Energy Res. 2022, 9, 804706. [Google Scholar] [CrossRef]
- Armaroli, N.; Balzani, V. The Future of Energy Supply: Challenges and Opportunities. Angew. Chem. Int. Ed. 2007, 46, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Gielen, D.; Gorini, R.; Wagner, N.; Leme, R.; Gutierrez, L.; Prakash, G.; Asmelash, E.; Janeiro, L.; Gallina, G.; Vale, G.; et al. IRENA Global Energy Transformation: A Roadmap to 2050; Institution of Gas Engineers & Managers (IGEM): Derby, UK, 2019; ISBN 978-92-9260-121-8. [Google Scholar]
- Simonenko, T.L.; Kalinina, M.V.; Simonenko, N.P.; Simonenko, E.P.; Glumov, O.V.; Mel’nikova, N.A.; Murin, I.V.; Shichalin, O.O.; Papynov, E.K.; Shilova, O.A.; et al. Synthesis of BaCe0.9-xZrxY0.1O3-δ nanopowders and the study of proton conductors fabricated on their basis by low-temperature spark plasma sintering. Int. J. Hydrogen Energy 2019, 44, 20345–20354. [Google Scholar] [CrossRef]
- Kuterbekov, K.A.; Nikonov, A.V.; Bekmyrza, K.Z.; Pavzderin, N.B.; Kabyshev, A.M.; Kubenova, M.M.; Kabdrakhimova, G.D.; Aidarbekov, N. Classification of Solid Oxide Fuel Cells. Nanomaterials 2022, 12, 1059. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.; Jia, L.; Yan, D.; Li, J. Liquid biofuels for solid oxide fuel cells: A review. J. Power Sources 2023, 556, 232437. [Google Scholar] [CrossRef]
- Liu, F.; Diercks, D.; Hussain, A.M.; Dale, N.; Furuya, Y.; Miura, Y.; Fukuyama, Y.; Duan, C. Nanocomposite Catalyst for High-Performance and Durable Intermediate-Temperature Methane-Fueled Metal-Supported Solid Oxide Fuel Cells. ACS Appl. Mater. Interfaces 2022, 14, 53840–53849. [Google Scholar] [CrossRef]
- Liu, F.; Duan, C. Direct-Hydrocarbon Proton-Conducting Solid Oxide Fuel Cells. Sustainability 2021, 13, 4736. [Google Scholar] [CrossRef]
- Dicks, A.L.; Rand, D.A.J. Fuel Cell Systems Explained; Wiley: Hoboken, NJ, USA, 2018; ISBN 9781118613528. [Google Scholar]
- Grigor’yants, R.R.; Zalkind, V.I.; Ivanov, P.P.; Lyalin, D.A.; Miroshnichenko, V.I. Thermodynamic model and analysis of hybrid power installations built around solid-oxide fuel cells and gas-turbine units. Therm. Eng. 2008, 55, 790–794. [Google Scholar] [CrossRef]
- Henke, M.; Willich, C.; Steilen, M.; Kallo, J.; Friedrich, K.A. Solid Oxide Fuel Cell—Gas Turbine Hybrid Power Plant. ECS Trans. 2013, 57, 67–72. [Google Scholar] [CrossRef]
- Dwivedi, S. Solid oxide fuel cell: Materials for anode, cathode and electrolyte. Int. J. Hydrogen Energy 2020, 45, 23988–24013. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Tian, D.; Liu, W.; Chen, Y.; Yu, W.; Yu, L.; Lin, B. A robust NiO–Sm0.2Ce0.8O1.9 anode for direct-methane solid oxide fuel cell. Mater. Res. Bull. 2015, 71, 1–6. [Google Scholar] [CrossRef]
- Namioka, T.; Naruse, T.; Yamane, R. Behavior and mechanisms of Ni/ScSZ cermet anode deterioration by trace tar in wood gas in a solid oxide fuel cell. Int. J. Hydrogen Energy 2011, 36, 5581–5588. [Google Scholar] [CrossRef]
- Fashalameh, K.M.; Sadeghian, Z.; Ebrahimi, R. A high-performance planar anode-supported solid oxide fuel cell with hierarchical porous structure through slurry-based three-dimensional printing. J. Alloys Compd. 2022, 916, 165406. [Google Scholar] [CrossRef]
- Rafique, M.; Nawaz, H.; Shahid Rafique, M.; Bilal Tahir, M.; Nabi, G.; Khalid, N.R. Material and method selection for efficient solid oxide fuel cell anode: Recent advancements and reviews. Int. J. Energy Res. 2019, 43, 2423–2446. [Google Scholar] [CrossRef]
- Shabri, H.A.; Othman, M.H.D.; Mohamed, M.A.; Kurniawan, T.A.; Jamil, S.M. Recent progress in metal-ceramic anode of solid oxide fuel cell for direct hydrocarbon fuel utilization: A review. Fuel Process. Technol. 2021, 212, 106626. [Google Scholar] [CrossRef]
- Toscani, L.M.; Bellora, M.S.; Huck-Iriart, C.; Soldati, A.L.; Sacanell, J.; Martins, T.S.; Craievich, A.F.; Fantini, M.C.A.; Larrondo, S.A.; Lamas, D.G. NiO/CeO2-Sm2O3 nanocomposites for partial oxidation of methane: In-situ experiments by dispersive X-ray absorption spectroscopy. Appl. Catal. A Gen. 2021, 626, 118357. [Google Scholar] [CrossRef]
- Genoveva Zimicz, M.; Reznik, B.A.; Larrondo, S.A. Conversion of biogas to synthesis gas over NiO/CeO2–Sm2O3 catalysts. Fuel 2015, 149, 95–99. [Google Scholar] [CrossRef]
- Gan, T.; Song, H.; Fan, X.; Liu, Y.; Liu, S.; Zhao, Y.; Li, Y. A rational design of highly active and coke-resistant anode for methanol-fueled solid oxide fuel cells with Sn doped Ni-Ce0.8Sm0.2O2−δ. Chem. Eng. J. 2023, 455, 140692. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Applications of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation, and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Gao, L. Facile Synthesis and Hierarchical Assembly of Hollow Nickel Oxide Architectures Bearing Enhanced Photocatalytic Properties. J. Phys. Chem. C 2008, 112, 15299–15305. [Google Scholar] [CrossRef]
- Yuan, Y.F.; Xia, X.H.; Wu, J.B.; Yang, J.L.; Chen, Y.B.; Guo, S.Y. Hierarchically ordered porous nickel oxide array film with enhanced electrochemical properties for lithium ion batteries. Electrochem. Commun. 2010, 12, 890–893. [Google Scholar] [CrossRef]
- Yao, M.; Hu, Z.; Xu, Z.; Liu, Y.; Liu, P.; Zhang, Q. High-performance electrode materials of hierarchical mesoporous nickel oxide ultrathin nanosheets derived from self-assembled scroll-like α-nickel hydroxide. J. Power Sources 2015, 273, 914–922. [Google Scholar] [CrossRef]
- Feng, X.; Huang, Y.; Li, C.; Li, Y.; Chen, C.; Liu, P. Raspberry-like Ni/NiO/CoO/Mn3O4 hierarchical structures as novel electrode material for high-performance all-solid-state asymmetric supercapacitors. Ceram. Int. 2019, 45, 18273–18280. [Google Scholar] [CrossRef]
- Cao, C.Y.; Guo, W.; Cui, Z.M.; Song, W.G.; Cai, W. Microwave-assisted gas/liquid interfacial synthesis of flowerlike NiO hollow nanosphere precursors and their application as supercapacitor electrodes. J. Mater. Chem. 2011, 21, 3204–3209. [Google Scholar] [CrossRef]
- Wahyuono, R.A.; Dellith, A.; Schmidt, C.; Dellith, J.; Ignaszak, A.; Seyring, M.; Rettenmayr, M.; Fize, J.; Artero, V.; Chavarot-Kerlidou, M.; et al. Structure of Ni(OH)2 intermediates determines the efficiency of NiO-based photocathodes-a case study using novel mesoporous NiO nanostars. RSC Adv. 2019, 9, 39422–39433. [Google Scholar] [CrossRef]
- Sheokand, S.; Kumar, P.; Jabeen, S.; Samra, K.S. 3D highly porous microspherical morphology of NiO nanoparticles for supercapacitor application. J. Solid State Electrochem. 2023, 27, 727–738. [Google Scholar] [CrossRef]
- Zhang, W.X.; Zhang, J.H.; Guo, J.Q.; He, C.; Wen, J.R. NiS2 nanospheres coated by nitrogen-doped carbon for enhanced sodium storage performance. J. Alloys Compd. 2023, 937, 168379. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Bocharova, V.A.; Gorobtsov, P.Y.; Simonenko, N.P.; Muradova, A.G.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Formation of Hierarchical NiO Coatings on the Surface of Al2O3 Substrates under Hydrothermal Conditions. Russ. J. Inorg. Chem. 2020, 65, 1292–1297. [Google Scholar] [CrossRef]
- Fan, J.; Lu, J.; Sha, Z.; Zuo, W.; Fei, X.; Zhu, M. Conformally anchoring nanocatalyst onto quartz fibers enables versatile microreactor platforms for continuous-flow catalysis. Sci. China Chem. 2021, 64, 1596–1604. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Kang, X.; Chen, G.; Li, Y. Synthesis the flower-like N-C/NiO nanocomposites by one-pot hydrothermal method as efficient electrocatalyst for methanol oxidation in alkaline electrolyte. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127466. [Google Scholar] [CrossRef]
- Wan, X.; Yuan, M.; Tie, S.; Lan, S. Effects of catalyst characters on the photocatalytic activity and process of NiO nanoparticles in the degradation of methylene blue. Appl. Surf. Sci. 2013, 277, 40–46. [Google Scholar] [CrossRef]
- Madhura, T.R.; Kumar, G.G.; Ramaraj, R. Reduced graphene oxide supported 2D-NiO nanosheets modified electrode for urea detection. J. Solid State Electrochem. 2020, 24, 3073–3081. [Google Scholar] [CrossRef]
- Manibalan, G.; Govindaraj, Y.; Yesuraj, J.; Kuppusami, P.; Murugadoss, G.; Murugavel, R.; Rajesh Kumar, M. Facile synthesis of NiO@Ni(OH)2-α-MoO3 nanocomposite for enhanced solid-state symmetric supercapacitor application. J. Colloid Interface Sci. 2021, 585, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Jian, J.; Kou, X.; Wang, H.; Chang, L.; Zhang, L.; Gao, S.; Xu, Y.; Yuan, H. Fascinating Tin Effects on the Enhanced and Large-Current-Density Water Splitting Performance of Sn–Ni(OH)2. ACS Appl. Mater. Interfaces 2021, 13, 42861–42869. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Lee, S.; Kim, S.; Singh, S.P.; Kim, J.; Rai, A.K. Fabrication of 1D mesoporous NiO nano-rods as high capacity and long-life anode material for lithium ion batteries. J. Alloys Compd. 2021, 850, 156755. [Google Scholar] [CrossRef]
- Chung, Y.; Park, H.; Lee, E.; Kim, S.-H.; Kim, D.-J. Communication—Gas Sensing Behaviors of Electrophoretically Deposited Nickel Oxide Films from Morphologically Tailored Particles. J. Electrochem. Soc. 2016, 163, B624–B626. [Google Scholar] [CrossRef]
- Baharuddin, N.A.; Abdul Rahman, N.F.; Abdul Rahman, H.; Somalu, M.R.; Azmi, M.A.; Raharjo, J. Fabrication of high-quality electrode films for solid oxide fuel cell by screen printing: A review on important processing parameters. Int. J. Energy Res. 2020, 44, 8296–8313. [Google Scholar] [CrossRef]
- Hong, G.; Kim, T.W.; Kwak, M.J.; Song, J.; Choi, Y.; Woo, S.-K.; Han, M.H.; Cho, C.H.; Kim, S.-D. Composite electrodes of Ti-doped SrFeO3-δ and LSGMZ electrolytes as both the anode and cathode in symmetric solid oxide fuel cells. J. Alloys Compd. 2020, 846, 156154. [Google Scholar] [CrossRef]
- Hanif, M.B.; Gao, J.-T.; Shaheen, K.; Wang, Y.-P.; Yasir, M.; Zhang, S.-L.; Li, C.-J.; Li, C.-X. Performance evaluation of highly active and novel La0.7Sr0.3Ti0.1Fe0.6Ni0.3O3-δ material both as cathode and anode for intermediate-temperature symmetrical solid oxide fuel cell. J. Power Sources 2020, 472, 228498. [Google Scholar] [CrossRef]
- Lin, J.; Li, H.; Wang, W.; Qiu, P.; Tao, G.; Huang, K.; Chen, F. Atmospheric plasma spraying to fabricate metal-supported solid oxide fuel cells with open-channel porous metal support. J. Am. Ceram. Soc. 2023, 106, 68–78. [Google Scholar] [CrossRef]
- Won, B.-R.; Kim, Y.H.; Jo, S.; Myung, J. Highly flexible solid oxide fuel cells using phase-controlled electrolyte support. J. Eur. Ceram. Soc. 2022, 42, 5813–5819. [Google Scholar] [CrossRef]
- Zhu, J.; Pérez, C.R.; Oh, T.-S.; Küngas, R.; Vohs, J.M.; Bonnell, D.A.; Nonnenmann, S.S. Probing local electrochemical activity within yttria-stabilized-zirconia via in situ high-temperature atomic force microscopy. J. Mater. Res. 2015, 30, 357–363. [Google Scholar] [CrossRef]
- Ding, D.; Li, X.; Lai, S.Y.; Gerdes, K.; Liu, M. Enhancing SOFC cathode performance by surface modification through infiltration. Energy Environ. Sci. 2014, 7, 552. [Google Scholar] [CrossRef]
- Liu, L.; Kim, G.-Y.; Chandra, A. Fabrication of solid oxide fuel cell anode electrode by spray pyrolysis. J. Power Sources 2010, 195, 7046–7053. [Google Scholar] [CrossRef]
- dos Santos-Gómez, L.; Zamudio-García, J.; Porras-Vázquez, J.M.; Losilla, E.R.; Marrero-López, D. Recent progress in nanostructured electrodes for solid oxide fuel cells deposited by spray pyrolysis. J. Power Sources 2021, 507, 230277. [Google Scholar] [CrossRef]
- Lee, Y.H.; Ren, H.; Wu, E.A.; Fullerton, E.E.; Meng, Y.S.; Minh, N.Q. All-Sputtered, Superior Power Density Thin-Film Solid Oxide Fuel Cells with a Novel Nanofibrous Ceramic Cathode. Nano Lett. 2020, 20, 2943–2949. [Google Scholar] [CrossRef]
- Rezugina, E.; Thomann, A.L.; Hidalgo, H.; Brault, P.; Dolique, V.; Tessier, Y. Ni-YSZ films deposited by reactive magnetron sputtering for SOFC applications. Surf. Coat. Technol. 2010, 204, 2376–2380. [Google Scholar] [CrossRef]
- Simonenko, E.P.; Mokrushin, A.S.; Simonenko, N.P.; Voronov, V.A.; Kim, V.P.; Tkachev, S.V.; Gubin, S.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Ink-jet printing of a TiO2–10%ZrO2 thin film for oxygen detection using a solution of metal alkoxoacetylacetonates. Thin Solid Films 2019, 670, 46–53. [Google Scholar] [CrossRef]
- Qin, F.; Liu, C.; Wu, W.; Peng, W.; Huo, S.; Ye, J.; Gu, S. Inkjet Printed Quantum Dots Color Conversion Layers for Full-Color Micro-LED Displays. Electron. Mater. Lett. 2023, 19, 19–28. [Google Scholar] [CrossRef]
- Sajedi-Moghaddam, A.; Gholami, M.; Naseri, N. Inkjet Printing of MnO2 Nanoflowers on Surface-Modified A4 Paper for Flexible All-Solid-State Microsupercapacitors. ACS Appl. Mater. Interfaces 2023, 15, 3894–3903. [Google Scholar] [CrossRef] [PubMed]
- Ha, J.; Yoo, H.; Seo, J.; Yoon, J.; Hong, Y. Photoresponse Analysis of All-Inkjet-Printed Single-Walled Carbon Nanotube Thin-Film Transistors for Flexible Light-Insensitive Transparent Circuit Applications. ACS Appl. Mater. Interfaces 2023, 15, 3192–3201. [Google Scholar] [CrossRef] [PubMed]
- Volkov, I.A.; Simonenko, N.P.; Efimov, A.A.; Simonenko, T.L.; Vlasov, I.S.; Borisov, V.I.; Arsenov, P.V.; Lebedinskii, Y.Y.; Markeev, A.M.; Lizunova, A.A.; et al. Platinum Based Nanoparticles Produced by a Pulsed Spark Discharge as a Promising Material for Gas Sensors. Appl. Sci. 2021, 11, 526. [Google Scholar] [CrossRef]
- Gamba, L.; Johnson, Z.T.; Atterberg, J.; Diaz-Arauzo, S.; Downing, J.R.; Claussen, J.C.; Hersam, M.C.; Secor, E.B. Systematic Design of a Graphene Ink Formulation for Aerosol Jet Printing. ACS Appl. Mater. Interfaces 2023, 15, 3325–3335. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L.; Wang, Y.; Zhao, S.; Zhou, Y.; Guo, Y.; Wang, Y.; Liang, J.; Chen, H. Aerosol Jet Printing of Multi-Dimensional OECT Force Sensor with High Sensitivity and Large Measuring Range. Adv. Mater. Technol. 2023, 2201272. [Google Scholar] [CrossRef]
- Zuo, Y.; Yu, Y.; Feng, J.; Zuo, C. Ultrathin Al–air batteries by reducing the thickness of solid electrolyte using aerosol jet printing. Sci. Rep. 2022, 12, 9801. [Google Scholar] [CrossRef]
- Zang, Z.; Tang, X.; Liu, X.; Lei, X.; Chen, W. Fabrication of high quality and low cost microlenses on a glass substrate by direct printing technique. Appl. Opt. 2014, 53, 7868. [Google Scholar] [CrossRef]
- Fragua, D.M.; Abargues, R.; Rodriguez-Canto, P.J.; Sanchez-Royo, J.F.; Agouram, S.; Martinez-Pastor, J.P. Au-ZnO Nanocomposite Films for Plasmonic Photocatalysis. Adv. Mater. Interfaces 2015, 2, 1500156. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Topalova, Y.P.; Gorobtsov, P.Y.; Simonenko, E.P.; Kuznetsov, N.T. Synthesis of Nanoscale Co3O4 Spinel and Its Application to Form Miniature Planar Structures by Microplotter Printing. Russ. J. Inorg. Chem. 2022, 67, 1939–1947. [Google Scholar] [CrossRef]
- Seo, H.; Nishi, T.; Kishimoto, M.; Ding, C.; Iwai, H.; Saito, M.; Yoshida, H. Study of Microextrusion Printing for Enlarging Electrode–Electrolyte Interfacial Area in Anode-Supported SOFCs. ECS Trans. 2019, 91, 1923–1931. [Google Scholar] [CrossRef]
- Mokrushin, A.S.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Bocharova, V.A.; Kozodaev, M.G.; Markeev, A.M.; Lizunova, A.A.; Volkov, I.A.; Simonenko, E.P.; et al. Microextrusion printing of gas-sensitive planar anisotropic NiO nanostructures and their surface modification in an H2S atmosphere. Appl. Surf. Sci. 2022, 578, 151984. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Klyuev, A.L.; Grafov, O.Y.; Ivanova, T.M.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Hydrothermally synthesized hierarchical Ce1-xSmxO2-δ oxides for additive manufacturing of planar solid electrolytes. Ceram. Int. 2022, 48, 22401–22410. [Google Scholar] [CrossRef]
- Liu, S.; Cao, R.; Wu, J.; Guan, L.; Li, M.; Liu, J.; Tian, J. Directly writing barrier-free patterned biosensors and bioassays on paper for low-cost diagnostics. Sens. Actuators B Chem. 2019, 285, 529–535. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Pozharnitskaya, V.M.; Simonenko, E.P.; Glumov, O.V.; Melnikova, N.A.; Sevastyanov, V.G.; Kuznetsov, N.T. Pen Plotter Printing of MnOx Thin Films Using Manganese Alkoxoacetylacetonate. Russ. J. Inorg. Chem. 2021, 66, 1416–1424. [Google Scholar] [CrossRef]
- Liang, Z.-H.; Zhu, Y.-J.; Hu, X.-L. β-Nickel Hydroxide Nanosheets and Their Thermal Decomposition to Nickel Oxide Nanosheets. J. Phys. Chem. B 2004, 108, 3488–3491. [Google Scholar] [CrossRef]
- Rana, P.; Jeevanandam, P. Synthesis of NiO Nanoparticles via Calcination of Surfactant Intercalated Layered Nickel Hydroxides and their Application as Adsorbent. J. Clust. Sci. 2022, 34, 517–533. [Google Scholar] [CrossRef]
- Xu, L.; Ding, Y.-S.; Chen, C.-H.; Zhao, L.; Rimkus, C.; Joesten, R.; Suib, S.L. 3D Flowerlike α-Nickel Hydroxide with Enhanced Electrochemical Activity Synthesized by Microwave-Assisted Hydrothermal Method. Chem. Mater. 2008, 20, 308–316. [Google Scholar] [CrossRef]
- Lee, D.; Shinde, N.; Ding, J.C.; Fu, J.; Sahoo, R.K.; Lee, H.W.; Yun, J.M.; Shin, H.-C.; Kim, K.H. Improvement of electrical performance by surface structure of Ni-material as a high-performance asymmetric supercapacitor electrode. Ceram. Int. 2020, 46, 11189–11197. [Google Scholar] [CrossRef]
- He, W.; Li, X.; An, S.; Li, T.; Zhang, Y.; Cui, J. 3D β-Ni(OH)2 nanowires/RGO composite prepared by phase transformation method for superior electrochemical performance. Sci. Rep. 2019, 9, 10838. [Google Scholar] [CrossRef]
- Fazlali, F.; reza Mahjoub, A.; Abazari, R. A new route for synthesis of spherical NiO nanoparticles via emulsion nano-reactors with enhanced photocatalytic activity. Solid State Sci. 2015, 48, 263–269. [Google Scholar] [CrossRef]
- Cui, H.; Xue, J.; Ren, W.; Wang, M. Ultra-high specific capacitance of β-Ni(OH)2 monolayer nanosheets synthesized by an exfoliation-free sol–gel route. J. Nanopart. Res. 2014, 16, 2601. [Google Scholar] [CrossRef]
- Baraldi, P.; Davolio, G. An electrochemical and spectral study of the nickel oxide electrode. Mater. Chem. Phys. 1989, 21, 143–154. [Google Scholar] [CrossRef]
- Zhou, T.; Gao, W.; Wang, Q.; Umar, A. Effect of Fluoride on the Morphology and Electrochemical Property of Co3O4 Nanostructures for Hydrazine Detection. Materials 2018, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Khairnar, S.D.; Shrivastava, V.S. Facile synthesis of nickel oxide nanoparticles for the degradation of Methylene blue and Rhodamine B dye: A comparative study. J. Taibah Univ. Sci. 2019, 13, 1108–1118. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Dudorova, D.A.; Simonenko, E.P.; Sevastyanov, V.G.; Kuznetsov, N.T. Triethanolamine-Assisted Hydrothermal Synthesis of Hierarchically Organized Nickel Oxide Particles. Russ. J. Inorg. Chem. 2022, 67, 622–627. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S.; Li, J.; Bai, J.; Qian, Y. Mesoporous NiO ultrathin nanowire networks topotactically transformed from α-Ni(OH) 2 hierarchical microspheres and their superior electrochemical capacitance properties and excellent capability for water treatment. J. Mater. Chem. 2012, 22, 14276–14283. [Google Scholar] [CrossRef]
- Chavan, A.U.; Jadhav, L.D.; Jamale, A.P.; Patil, S.P.; Bhosale, C.H.; Bharadwaj, S.R.; Patil, P.S. Effect of variation of NiO on properties of NiO/GDC (gadolinium doped ceria) nano-composites. Ceram. Int. 2012, 38, 3191–3196. [Google Scholar] [CrossRef]
- Yao, X.; Li, P.; Yu, B.; Yang, F.; Li, J.; Zhao, Y.; Li, Y. Hydrothermally synthesized NiO-samarium doped ceria nano-composite as an anode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2017, 42, 22192–22200. [Google Scholar] [CrossRef]
- Kishimoto, H.; Yamaji, K.; Horita, T.; Xiong, Y.-P.; Brito, M.E.; Yoshinaga, M.; Yokokawa, H. Reaction Process in the Ni-SDC Cermet Anode. Electrochemistry 2009, 77, 190–194. [Google Scholar] [CrossRef]
- Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Grafov, O.Y.; Simonenko, E.P.; Kuznetsov, N.T. Synthesis of ((CeO2)0.8(Sm2O3)0.2)@NiO Core-Shell Type Nanostructures and Microextrusion Printing of a Composite Anode Based on Them. Materials 2022, 15, 8918. [Google Scholar] [CrossRef] [PubMed]



| Sample | c(Ni2+), mol/L | c(TEA), mol/L | c(TEA)/c(Ni2+) |
|---|---|---|---|
| 1 | 0.005 | 0.400 | 80 |
| 2 | 0.100 | 0.200 | 2 |
| 3 | 0.005 | 0.010 | 2 |
| 4 | 0.025 | 0.050 | 2 |
| 5 | 0.100 | 0.400 | 4 |
| 6 | 0.025 | 0.400 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudorova, D.A.; Simonenko, T.L.; Simonenko, N.P.; Gorobtsov, P.Y.; Volkov, I.A.; Simonenko, E.P.; Kuznetsov, N.T. Hydrothermal Synthesis of Nickel Oxide and Its Application in the Additive Manufacturing of Planar Nanostructures. Molecules 2023, 28, 2515. https://doi.org/10.3390/molecules28062515
Dudorova DA, Simonenko TL, Simonenko NP, Gorobtsov PY, Volkov IA, Simonenko EP, Kuznetsov NT. Hydrothermal Synthesis of Nickel Oxide and Its Application in the Additive Manufacturing of Planar Nanostructures. Molecules. 2023; 28(6):2515. https://doi.org/10.3390/molecules28062515
Chicago/Turabian StyleDudorova, Darya A., Tatiana L. Simonenko, Nikolay P. Simonenko, Philipp Yu. Gorobtsov, Ivan A. Volkov, Elizaveta P. Simonenko, and Nikolay T. Kuznetsov. 2023. "Hydrothermal Synthesis of Nickel Oxide and Its Application in the Additive Manufacturing of Planar Nanostructures" Molecules 28, no. 6: 2515. https://doi.org/10.3390/molecules28062515
APA StyleDudorova, D. A., Simonenko, T. L., Simonenko, N. P., Gorobtsov, P. Y., Volkov, I. A., Simonenko, E. P., & Kuznetsov, N. T. (2023). Hydrothermal Synthesis of Nickel Oxide and Its Application in the Additive Manufacturing of Planar Nanostructures. Molecules, 28(6), 2515. https://doi.org/10.3390/molecules28062515







