A Superior Corrosion Protection of Mg Alloy via Smart Nontoxic Hybrid Inhibitor-Containing Coatings
Abstract
:1. Introduction
2. Results and Discussion
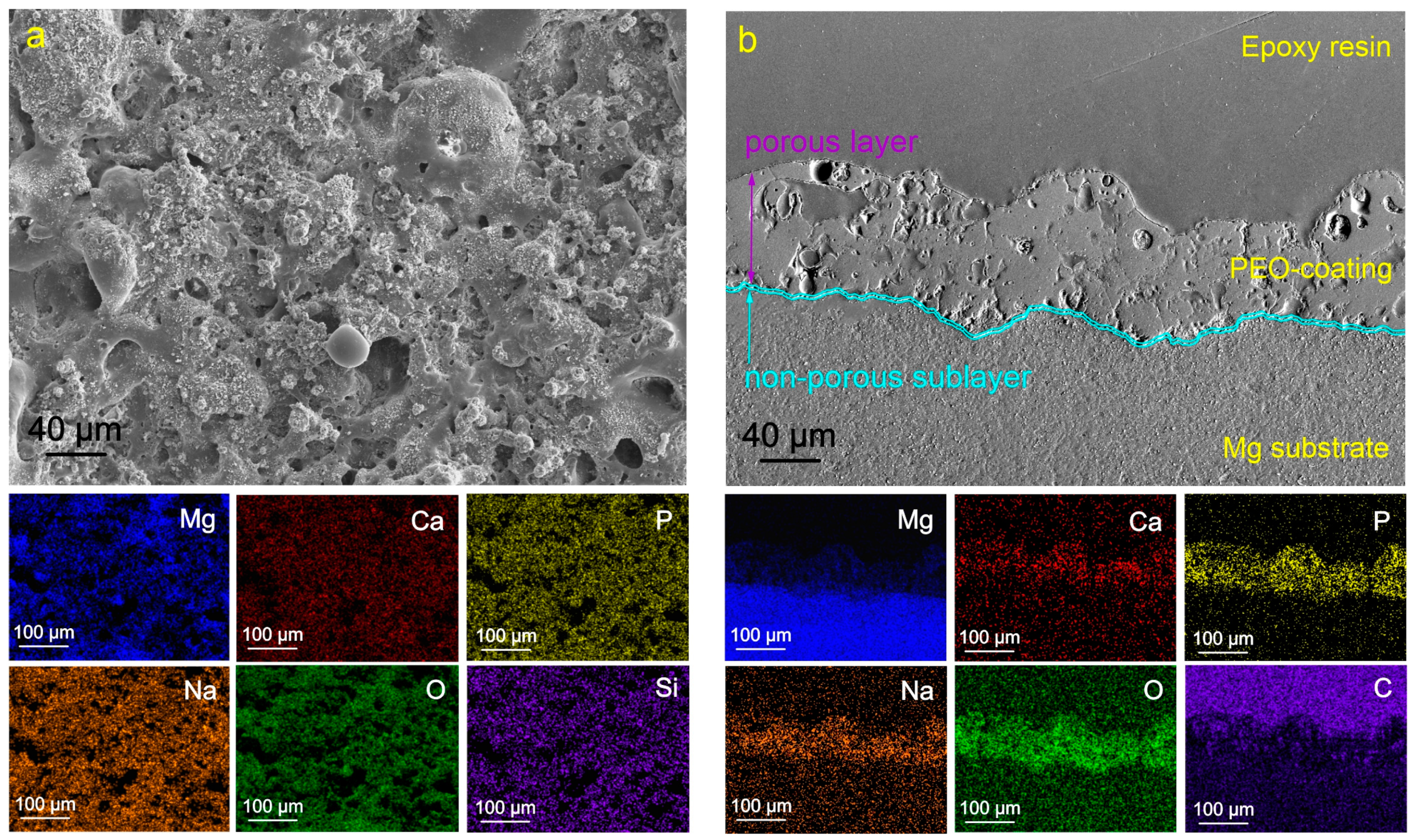
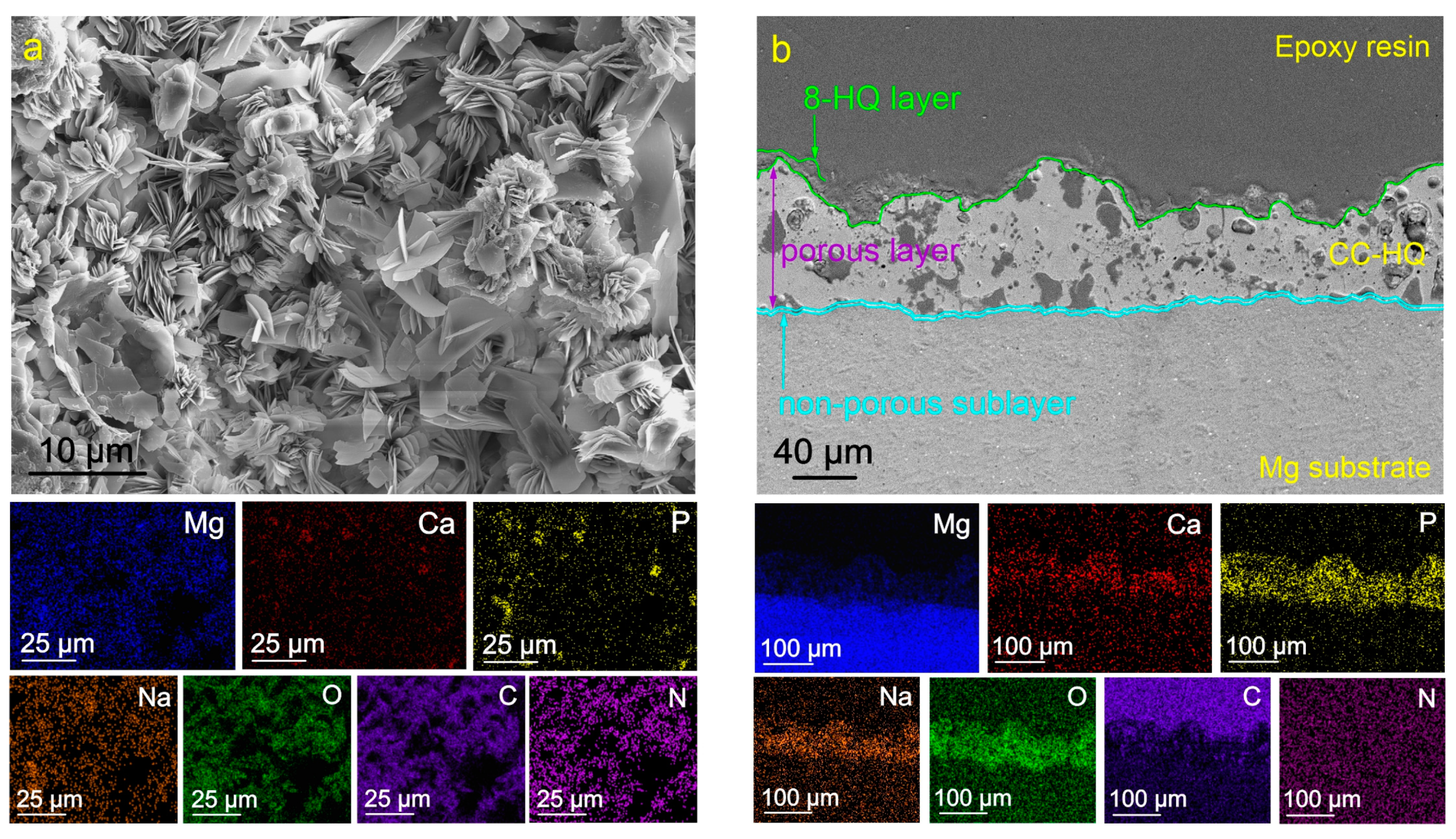
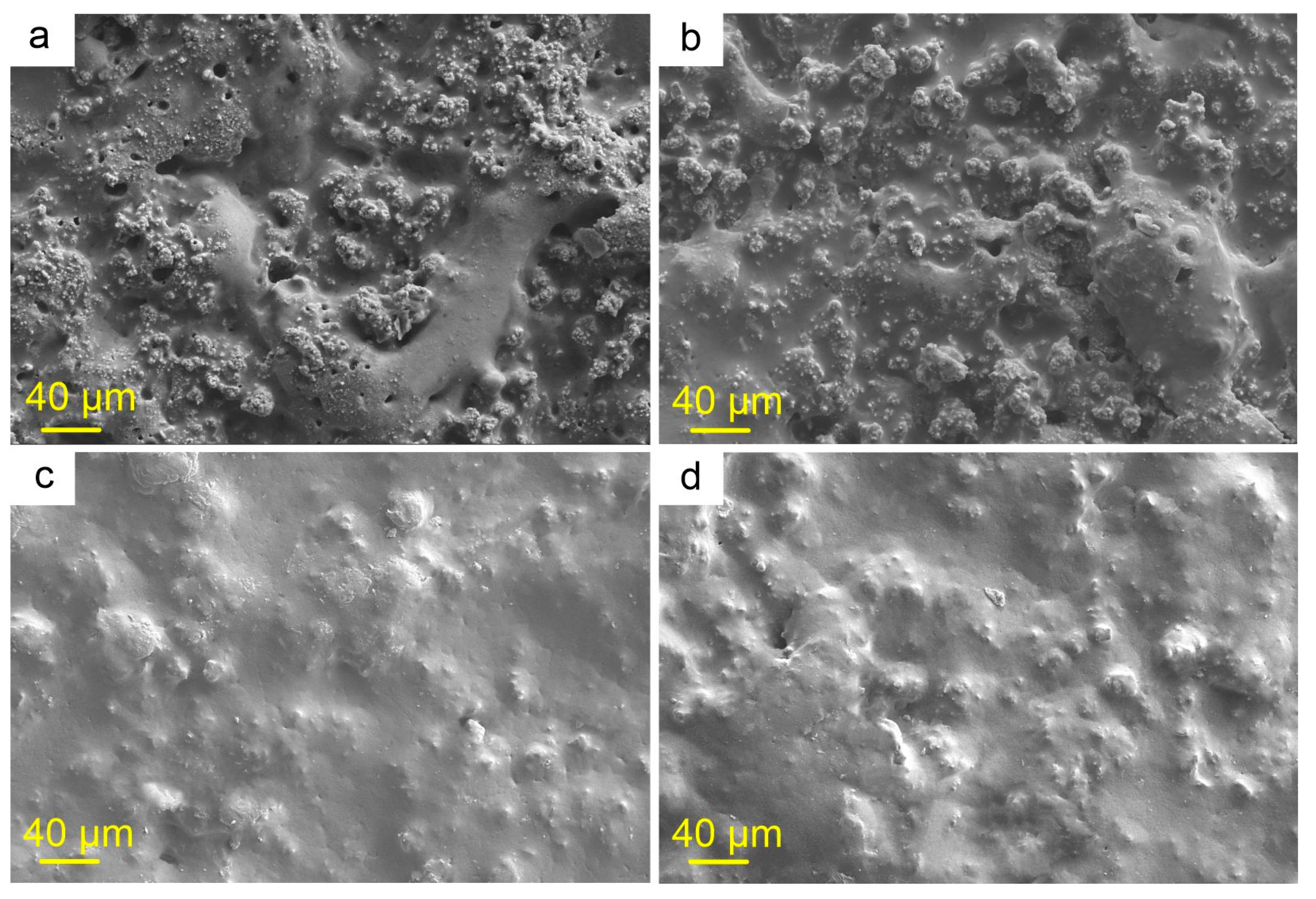
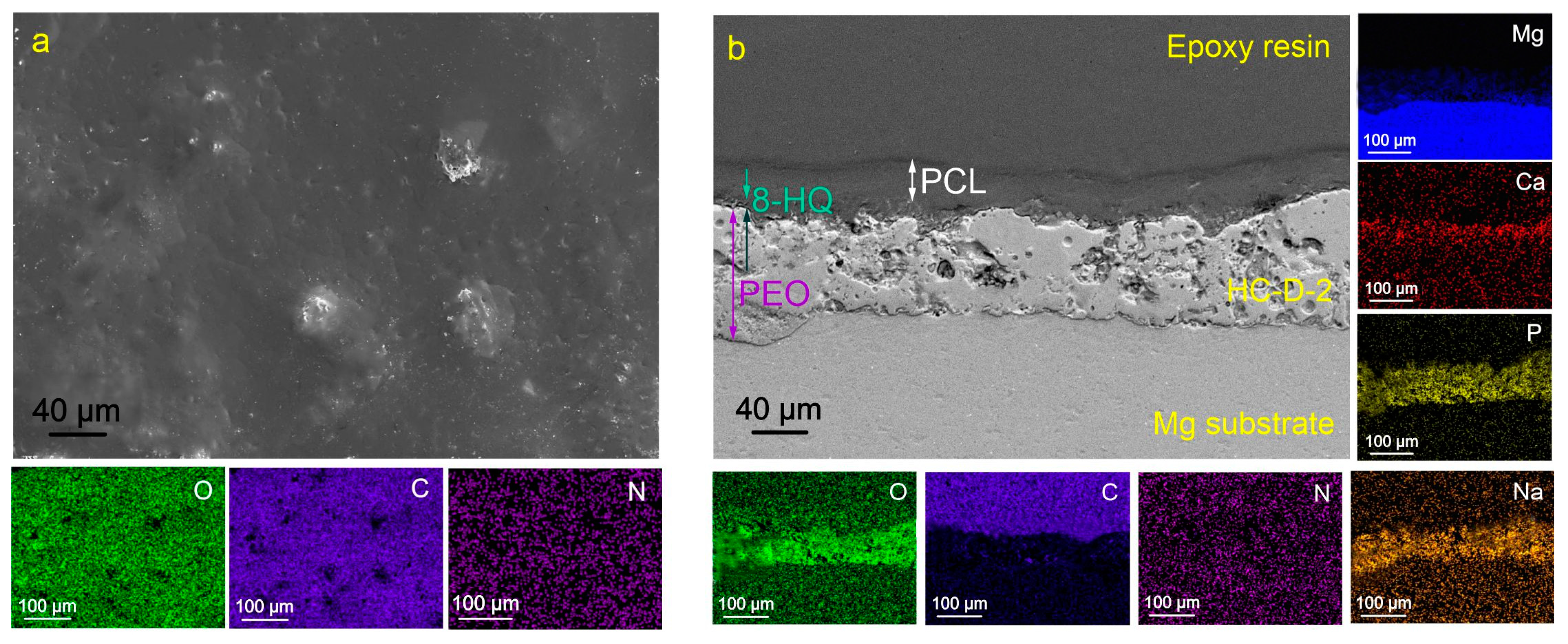
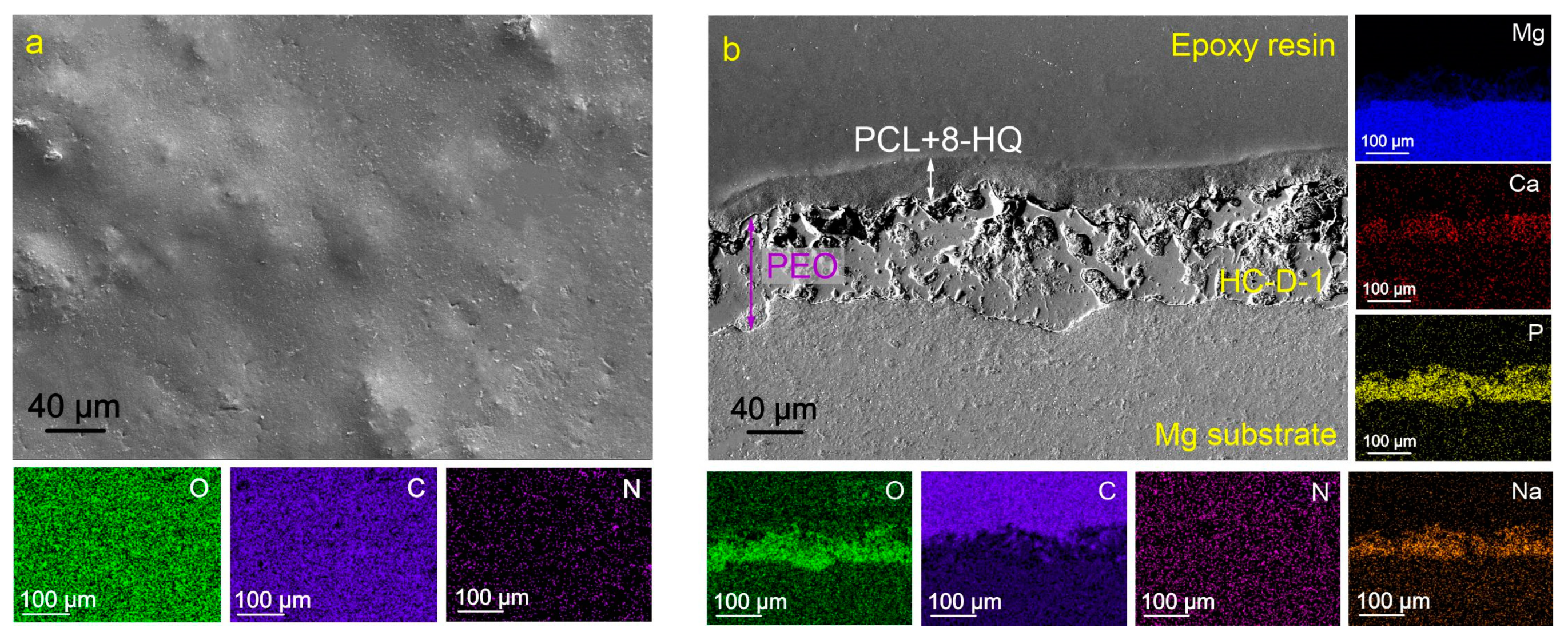
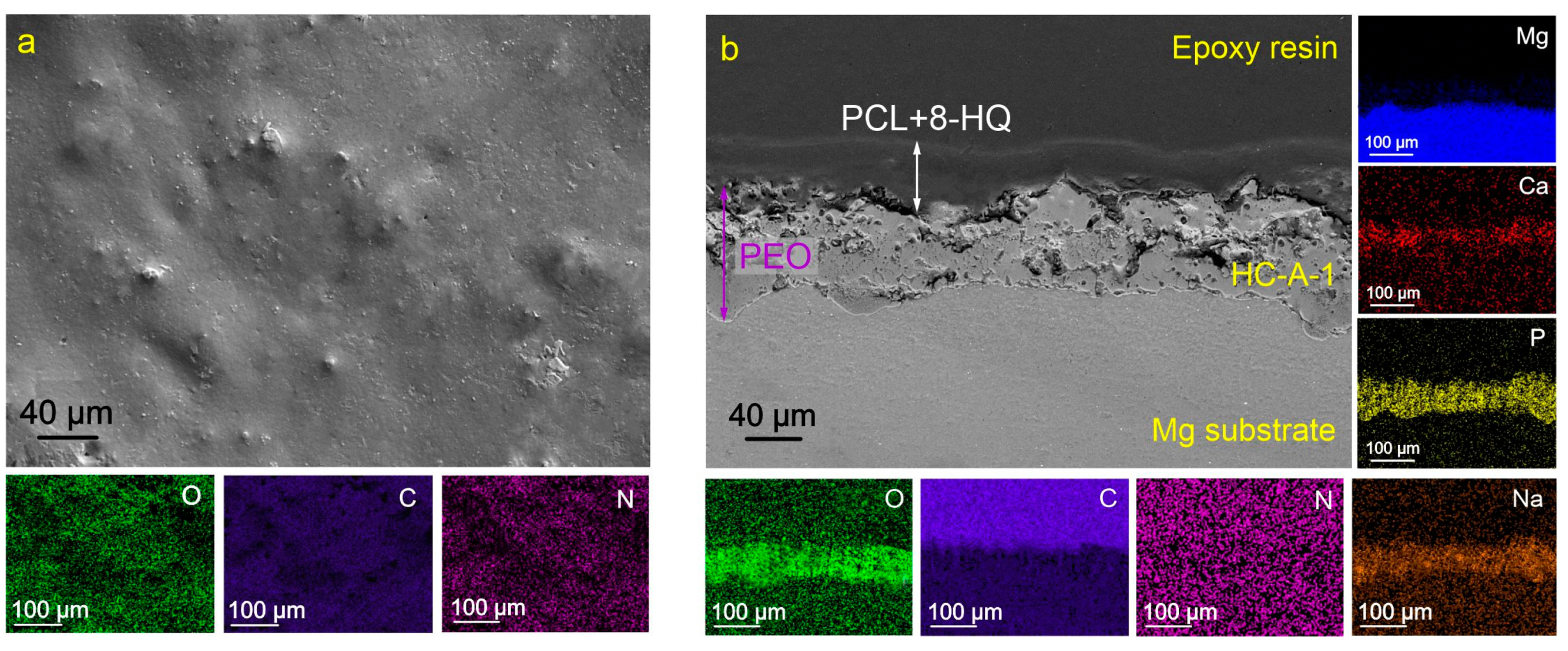
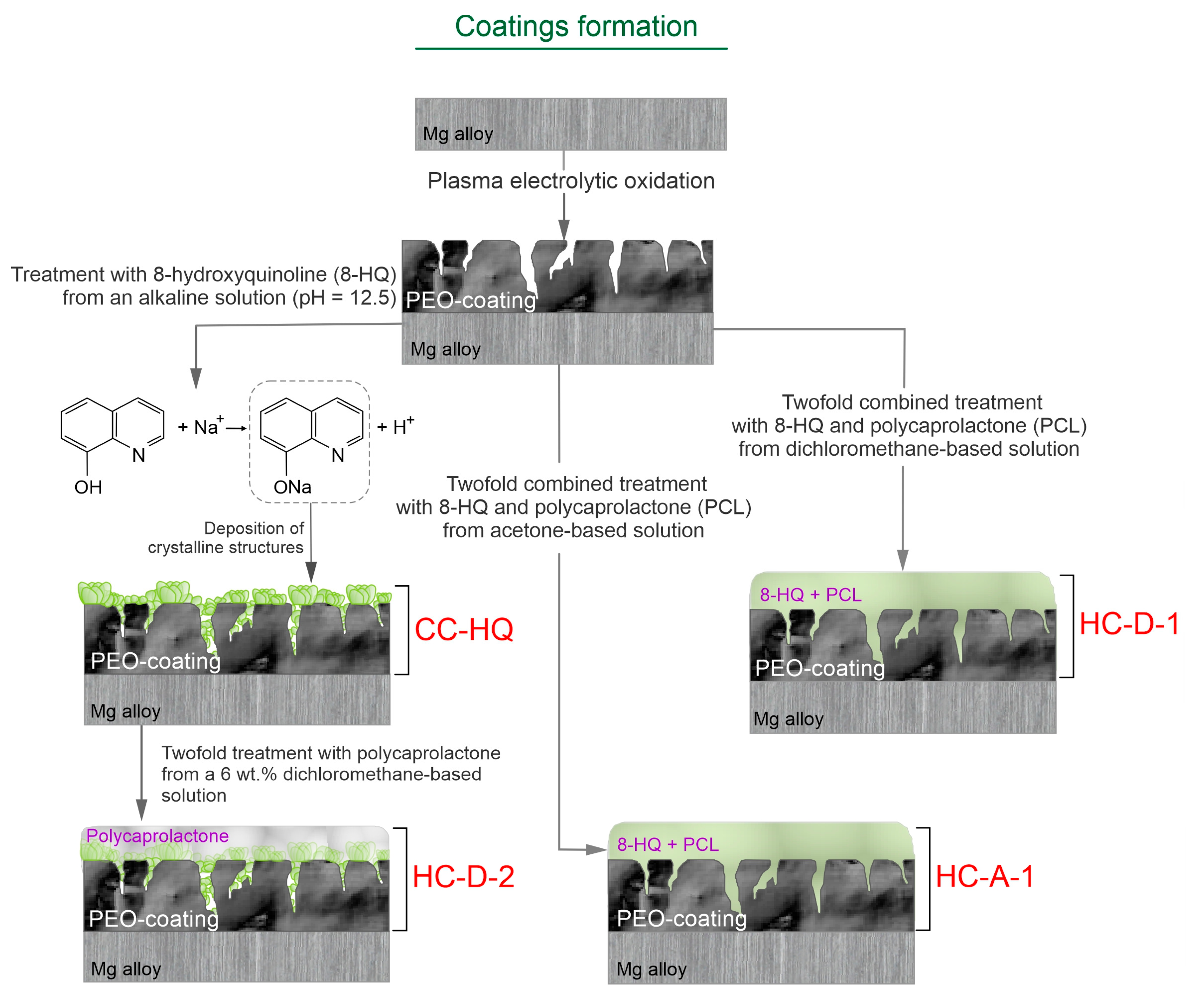
2.1. Coatings’ Structure and Composition
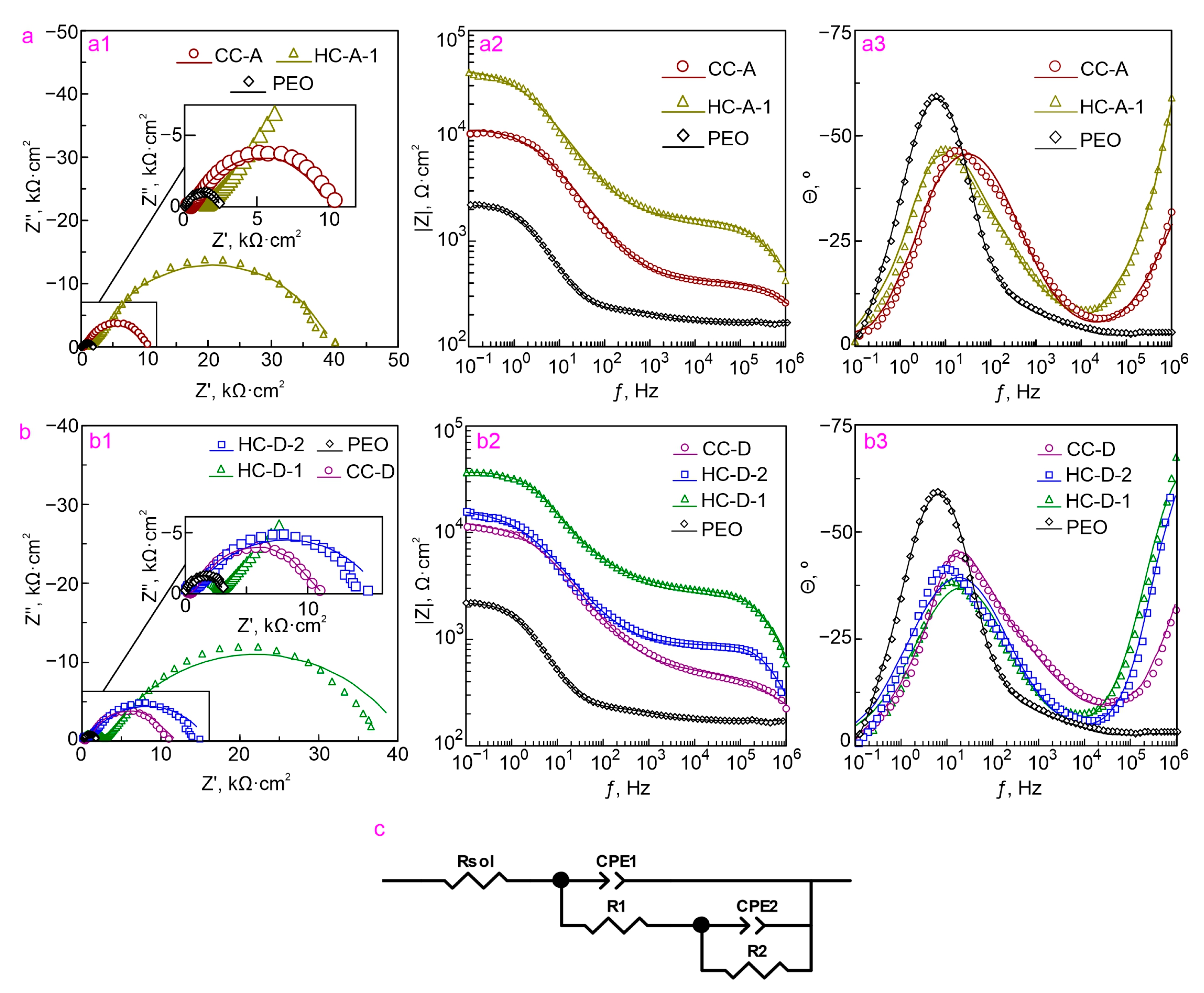
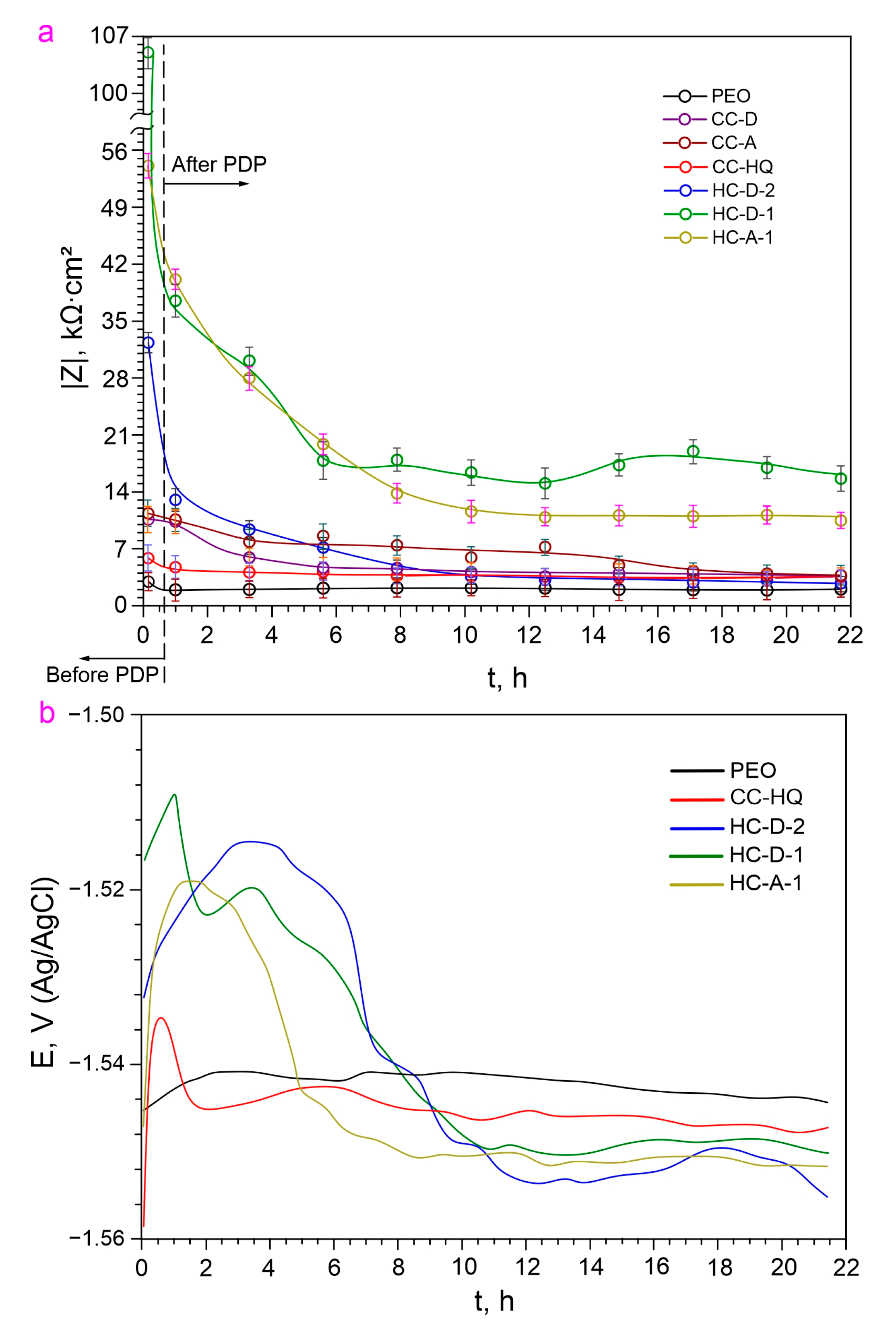
2.2. Electrochemical Properties
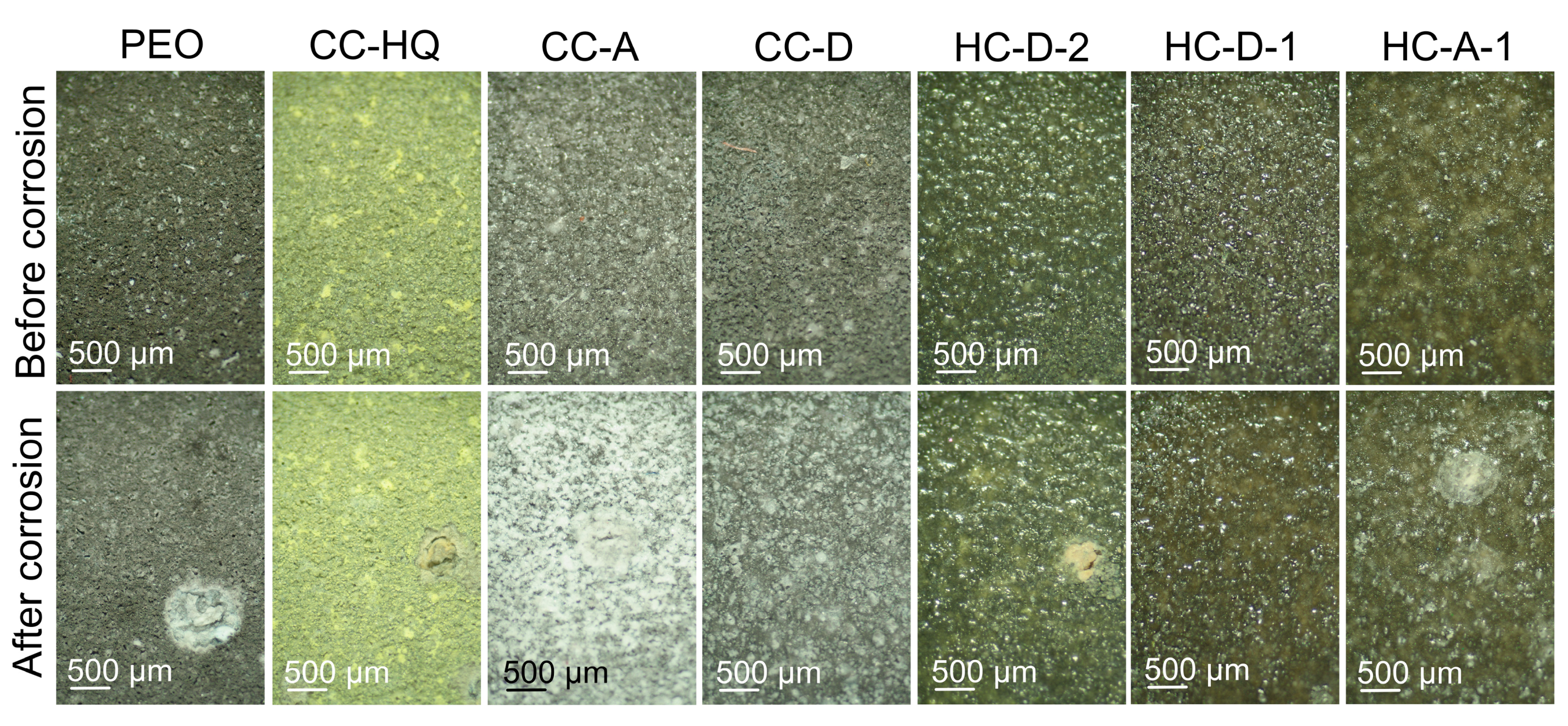
2.3. Inhibitor Efficiency Estimation
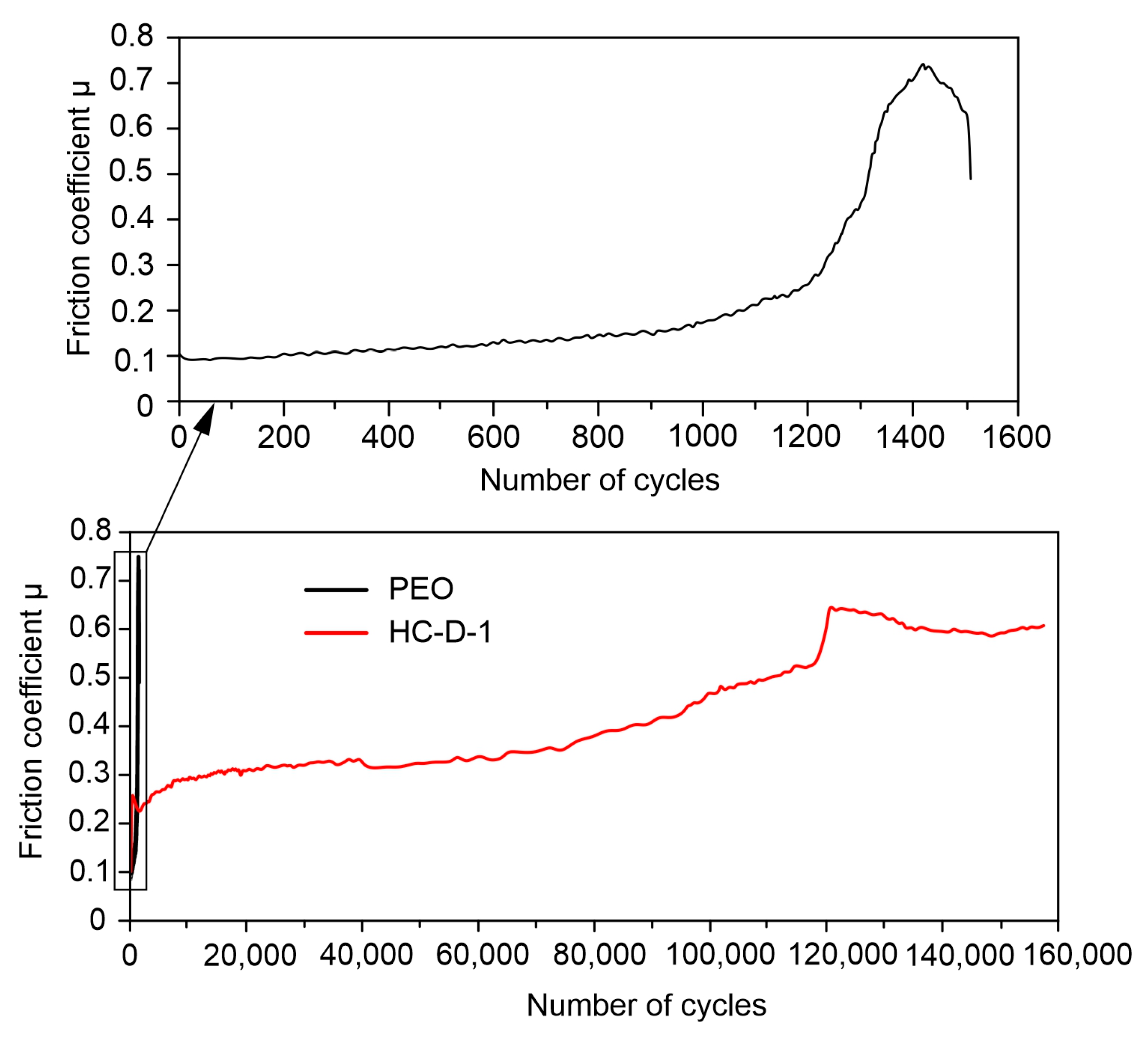
2.4. Wear Resistance
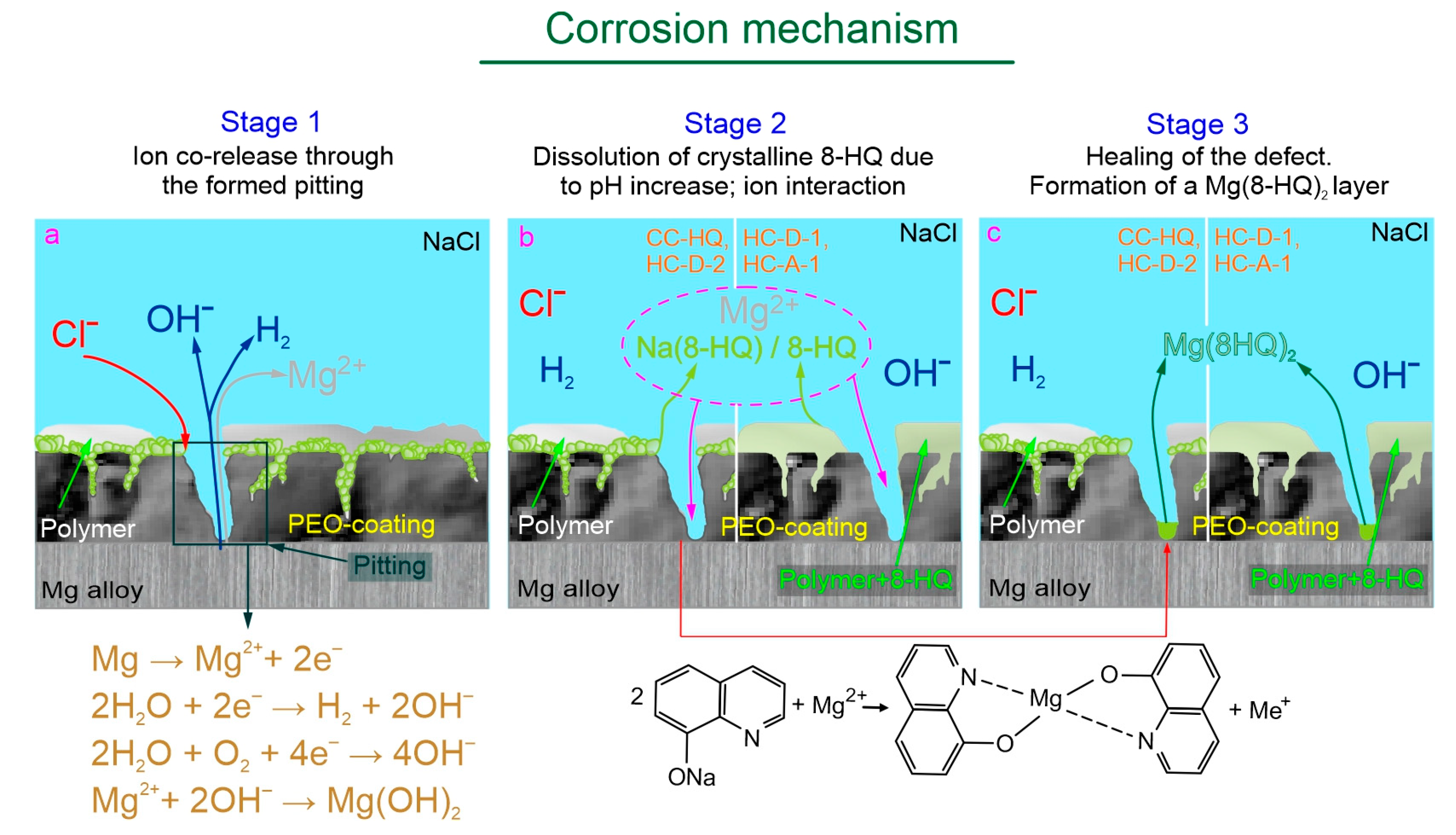
2.5. Self-Healing Mechanism
3. Materials and Methods
3.1. Substrate Characterization and Pretreatment
3.2. PEO Coating Formation
3.3. Inhibitor and Polymer Treatment
3.4. SEM/EDX Analysis
3.5. Electrochemical Studies (EIS, PDP)
3.6. Inhibitor Efficiency and Corrosion Rate Evaluation
3.7. Immersion Test
3.8. Tribological Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chalisgaonkar, R. Insight in Applications, Manufacturing and Corrosion Behaviour of Magnesium and Its Alloys—A Review. Mater. Today Proc. 2020, 26, 1060–1071. [Google Scholar] [CrossRef]
- Sezer, N.; Evis, Z.; Kayhan, S.M.; Tahmasebifar, A.; Koç, M. Review of Magnesium-Based Biomaterials and Their Applications. J. Magnes. Alloy. 2018, 6, 23–43. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent Advances on the Development of Magnesium Alloys for Biodegradable Implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V. Calcium Orthophosphate Coatings on Magnesium and Its Biodegradable Alloys. Acta Biomater. 2014, 10, 2919–2934. [Google Scholar] [CrossRef] [PubMed]
- Bommala, V.K.; Krishna, M.G.; Rao, C.T. Magnesium Matrix Composites for Biomedical Applications: A Review. J. Magnes. Alloy. 2019, 7, 72–79. [Google Scholar] [CrossRef]
- Boyle, C.; Kim, I.Y. Three-Dimensional Micro-Level Computational Study of Wolff’s Law via Trabecular Bone Remodeling in the Human Proximal Femur Using Design Space Topology Optimization. J. Biomech. 2011, 44, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Jang, I.G.; Kim, I.Y. Computational Study of Wolff’s Law with Trabecular Architecture in the Human Proximal Femur Using Topology Optimization. J. Biomech. 2008, 41, 2353–2361. [Google Scholar] [CrossRef]
- Tsubota, K.-i.; Suzuki, Y.; Yamada, T.; Hojo, M.; Makinouchi, A.; Adachi, T. Computer Simulation of Trabecular Remodeling in Human Proximal Femur Using Large-Scale Voxel FE Models: Approach to Understanding Wolff’s Law. J. Biomech. 2009, 42, 1088–1094. [Google Scholar] [CrossRef]
- Barak, M.M.; Lieberman, D.E.; Hublin, J.-J. A Wolff in Sheep’s Clothing: Trabecular Bone Adaptation in Response to Changes in Joint Loading Orientation. Bone 2011, 49, 1141–1151. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Egorkin, V.S.; Ustinov, A.Y.; Sergienko, V.I.; Gnedenkov, S.V. The Detailed Corrosion Performance of Bioresorbable Mg-0.8Ca Alloy in Physiological Solutions. J. Magnes. Alloy. 2022, 10, 1326–1350. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Features of the Magnesium Alloys Corrosion in the Chloride-Containing Media. Solid State Phenom. 2014, 213, 143–148. [Google Scholar] [CrossRef]
- Bertolini, R.; Bruschi, S.; Ghiotti, A. Large Strain Extrusion Machining under Cryogenic Cooling to Enhance Corrosion Resistance of Magnesium Alloys for Biomedical Applications. Procedia Manuf. 2018, 26, 217–227. [Google Scholar] [CrossRef]
- Ratna Sunil, B.; Sampath Kumar, T.S.; Chakkingal, U.; Nandakumar, V.; Doble, M.; Devi Prasad, V.; Raghunath, M. In Vitro and in Vivo Studies of Biodegradable Fine Grained AZ31 Magnesium Alloy Produced by Equal Channel Angular Pressing. Mater. Sci. Eng. C 2016, 59, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Denkena, B.; Lucas, A. Biocompatible Magnesium Alloys as Absorbable Implant Materials—Adjusted Surface and Subsurface Properties by Machining Processes. CIRP Ann. 2007, 56, 113–116. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Zhu, S.J.; Wang, L.G.; Guo, R.M.; Yue, G.C.; Guan, S.K. Microstructures and Degradation Mechanism in Simulated Body Fluid of Biomedical Mg–Zn–Ca Alloy Processed by High Pressure Torsion. Mater. Des. 2016, 96, 54–62. [Google Scholar] [CrossRef] [Green Version]
- Bazhenov, V.; Li, A.; Iliasov, A.; Bautin, V.; Plegunova, S.; Koltygin, A.; Komissarov, A.; Abakumov, M.; Redko, N.; Shin, K.S. Corrosion Behavior and Biocompatibility of Hot-Extruded Mg–Zn–Ga–(Y) Biodegradable Alloys. J. Funct. Biomater. 2022, 13, 294. [Google Scholar] [CrossRef]
- Salahshoor, M.; Guo, Y.B. Surface Integrity of Magnesium-Calcium Implants Processed by Synergistic Dry Cutting-Finish Burnishing. Procedia Eng. 2011, 19, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zheng, Y. Novel Magnesium Alloys Developed for Biomedical Application: A Review. J. Mater. Sci. Technol. 2013, 29, 489–502. [Google Scholar] [CrossRef]
- Hong, K.; Park, H.; Kim, Y.; Knapek, M.; Minárik, P.; Máthis, K.; Yamamoto, A.; Choe, H. Mechanical and Biocorrosive Properties of Magnesium-Aluminum Alloy Scaffold for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2019, 98, 213–224. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, X.; Zhao, C.; Li, J.; Song, Y.; Xie, C.; Tao, H.; Zhang, Y.; He, Y.; Jiang, Y.; et al. Research on an Mg-Zn Alloy as a Degradable Biomaterial. Acta Biomater. 2010, 6, 626–640. [Google Scholar] [CrossRef]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable Magnesium Alloys Developed as Bone Repair Materials: A Review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, R.; Cipriano, A.F.; Zhao, Z.; Lock, J.; Tie, D.; Zhao, T.; Cui, T.; Liu, H. Development and Evaluation of a Magnesium–Zinc–Strontium Alloy for Biomedical Applications—Alloy Processing, Microstructure, Mechanical Properties, and Biodegradation. Mater. Sci. Eng. C 2013, 33, 3661–3669. [Google Scholar] [CrossRef] [PubMed]
- Feyerabend, F.; Fischer, J.; Holtz, J.; Witte, F.; Willumeit, R.; Drücker, H.; Vogt, C.; Hort, N. Evaluation of Short-Term Effects of Rare Earth and Other Elements Used in Magnesium Alloys on Primary Cells and Cell Lines☆. Acta Biomater. 2010, 6, 1834–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catauro, M.; Papale, F.; Sapio, L.; Naviglio, S. Biological Influence of Ca/P Ratio on Calcium Phosphate Coatings by Sol-Gel Processing. Mater. Sci. Eng. C 2016, 65, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Li, Y.; Wen, C. Realization and Characterization of Double-Layer Ca-P Coating on WE43 Mg Alloy for Biomedical Applications. Surf. Coatings Technol. 2020, 398, 126091. [Google Scholar] [CrossRef]
- Cui, L.-Y.; Cheng, S.-C.; Liang, L.-X.; Zhang, J.-C.; Li, S.-Q.; Wang, Z.-L.; Zeng, R.-C. In Vitro Corrosion Resistance of Layer-by-Layer Assembled Polyacrylic Acid Multilayers Induced Ca–P Coating on Magnesium Alloy AZ31. Bioact. Mater. 2020, 5, 153–163. [Google Scholar] [CrossRef]
- Abdelkebir, K.; Morin-Grognet, S.; Gaudière, F.; Coquerel, G.; Labat, B.; Atmani, H.; Ladam, G. Biomimetic Layer-by-Layer Templates for Calcium Phosphate Biomineralization. Acta Biomater. 2012, 8, 3419–3428. [Google Scholar] [CrossRef]
- Pan, H.; Yang, H.; Tang, X.; Niu, J.; Xiang, Z.; Song, Y.; Lu, W. Effect of Ca/P Ratio on the Structural and Corrosion Properties of Biomimetic Ca[Sbnd]P Coatings on ZK60 Magnesium Alloy. Mater. Sci. Eng. C 2017, 72, 676–681. [Google Scholar] [CrossRef]
- Chunyan, Z.; Shiyu, Z.; Xinpeng, L.; Hongchuan, H. Microstructure and Corrosion Properties of Calcium Phosphate Coating on Magnesium Alloy Prepared by Hydrothermal Treatment at Various PH Values. Rare Met. Mater. Eng. 2018, 47, 2993–2999. [Google Scholar] [CrossRef]
- Su, Y.; Li, K.; Wang, J. Microstructures, Mechanical Properties and Corrosion Resistance of Sprayed Ca–P Coating for Micropatterning Carbon/Carbon Substrate Surface. Ceram. Int. 2020, 46, 7374–7387. [Google Scholar] [CrossRef]
- Su, Y.; Li, K.; Zhu, X.; Wang, C.; Zhang, Y. Microwave-Hydrothermal Method Post-Treatment of Sprayed Ca-P Coating. Ceram. Int. 2019, 45, 874–884. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Li, L.; Bai, Y.; Yang, B. The Controllable Lanthanum Ion Release from Ca-P Coating Fabricated by Laser Cladding and Its Effect on Osteoclast Precursors. Mater. Sci. Eng. C 2018, 93, 1027–1035. [Google Scholar] [CrossRef] [PubMed]
- Etminanfar, M.R.; Khalil-Allafi, J.; Montaseri, A.; Vatankhah-Barenji, R. Endothelialization and the Bioactivity of Ca-P Coatings of Different Ca/P Stoichiometry Electrodeposited on the Nitinol Superelastic Alloy. Mater. Sci. Eng. C 2016, 62, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Zhang, B.; Lu, J.; Chen, J.; Zhang, X.; Gu, Z. Biomimetic Ca-P Coating on Pre-Calcified Ti Plates by Electrodeposition Method. Appl. Surf. Sci. 2010, 256, 2700–2704. [Google Scholar] [CrossRef]
- LeGeros, J.P.; Lin, S.J.; Mijares, D.; Dimaano, F.; LeGeros, R.Z. Electrochemically Deposited Calcium Phosphate Coating on Titanium Alloy Substrates. Key Eng. Mater. 2005, 284–286, 247–250. [Google Scholar] [CrossRef]
- Maqsood, M.F.; Raza, M.A.; Rehman, Z.U.; Tayyeb, A.; Makhdoom, M.A.; Ghafoor, F.; Latif, U.; Khan, M.F. Role of Solvent Used in Development of Graphene Oxide Coating on AZ31B Magnesium Alloy: Corrosion Behavior and Biocompatibility Analysis. Nanomaterials 2022, 12, 3745. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.L.; Wang, Q.; Geng, F.; Xi, X.S.; Qiu, J.H.; Yang, K. Preparation and Characterization of Ca-P Coating on AZ31 Magnesium Alloy. Trans. Nonferrous Met. Soc. China Engl. Ed. 2010, 20, s648–s654. [Google Scholar] [CrossRef]
- Wan, P.; Tan, L.; Yang, K. Surface Modification on Biodegradable Magnesium Alloys as Orthopedic Implant Materials to Improve the Bio-Adaptability: A Review. J. Mater. Sci. Technol. 2016, 32, 827–834. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium Phosphate Coatings on Magnesium Alloys for Biomedical Applications: A Review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Nomerovskii, A.D.; Tsvetnikov, A.K.; Sinebryukhov, S.L.; Gnedenkov, S.V. Cold-Sprayed Composite Metal-Fluoropolymer Coatings for Alloy Protection against Corrosion and Wear. Materials 2023, 16, 918. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Gnedenkov, S.V. Hydroxyapatite-Containing PEO-Coating Design for Biodegradable Mg-0.8Ca Alloy: Formation and Corrosion Behaviour. J. Magnes. Alloy. 2022. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Imshinetskiy, I.M.; Zheludkevich, M.L.; Gnedenkov, S.V. Control of the Mg Alloy Biodegradation via PEO and Polymer-Containing Coatings. Corros. Sci. 2021, 182, 109254. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 2: Coating Protection. Materials 2018, 11, 2177. [Google Scholar] [CrossRef] [PubMed]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Imshinetskiy, I.M.; Vyaliy, I.E.; Gnedenkov, S.V. Effect of Microstructure on the Corrosion Resistance of TIG Welded 1579 Alloy. Materials 2019, 12, 2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnedenkov, S.V.; Khrisanfova, O.A.; Sinebryukhov, S.L.; Puz, A.V.; Gnedenkov, A.S. Composite Protective Coatings on Nitinol Surface. Mater. Manuf. Process. 2008, 23, 879–883. [Google Scholar] [CrossRef]
- Sinebryukhov, S.L.; Gnedenkov, A.S.; Khrisanfova, O.A.; Gnedenkov, S.V. Influence of Plasma Electrolytic Oxidation on Mechanical Characteristics of NiTi Alloy. Surf. Eng. 2009, 25, 565–569. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, C.; Bandopadhyay, S.; Ning, C.; Zhang, Y.; Guo, Y. Corrosion Mechanism and Model of Pulsed DC Microarc Oxidation Treated AZ31 Alloy in Simulated Body Fluid. Appl. Surf. Sci. 2012, 258, 6116–6126. [Google Scholar] [CrossRef]
- Gao, Y.; Yerokhin, A.; Matthews, A. Effect of Current Mode on PEO Treatment of Magnesium in Ca- and P-Containing Electrolyte and Resulting Coatings. Appl. Surf. Sci. 2014, 316, 558–567. [Google Scholar] [CrossRef]
- Chandra, G.; Pandey, A. Preparation Strategies for Mg-Alloys for Biodegradable Orthopaedic Implants and Other Biomedical Applications: A Review. Irbm 2022, 43, 229–249. [Google Scholar] [CrossRef]
- Pan, Y.K.; Chen, C.Z.; Wang, D.G.; Zhao, T.G. Improvement of Corrosion and Biological Properties of Microarc Oxidized Coatings on Mg-Zn-Zr Alloy by Optimizing Negative Power Density Parameters. Colloids Surfaces B Biointerfaces 2014, 113, 421–428. [Google Scholar] [CrossRef]
- Pan, Y.K.; Wang, D.G.; Chen, C.Z. Effect of Negative Voltage on the Microstructure, Degradability and in Vitro Bioactivity of Microarc Oxidized Coatings on ZK60 Magnesium Alloy. Mater. Lett. 2014, 119, 127–130. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Plekhova, N.G.; Gnedenkov, S.V. Smart Composite Antibacterial Coatings with Active Corrosion Protection of Magnesium Alloys. J. Magnes. Alloy. 2022, 10, 3589–3611. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Inhibitor-Containing Composite Coatings on Mg Alloys: Corrosion Mechanism and Self-Healing Protection. Solid State Phenom. 2016, 245, 89–96. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Gnedenkov, S.V. Protective Properties of Inhibitor-Containing Composite Coatings on a Mg Alloy. Corros. Sci. 2016, 102, 348–354. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. Formation of Flower-like Structures for Optimizing the Corrosion Resistance of Mg Alloy. Mater. Lett. 2018, 221, 196–200. [Google Scholar] [CrossRef]
- Snihirova, D.; Lamaka, S.V.; Taryba, M.; Salak, A.N.; Kallip, S.; Zheludkevich, M.L.; Ferreira, M.G.S.; Montemor, M.F. Hydroxyapatite Microparticles as Feedback-Active Reservoirs of Corrosion Inhibitors. ACS Appl. Mater. Interfaces 2010, 2, 3011–3022. [Google Scholar] [CrossRef] [PubMed]
- Chirkunov, A.A.; Rakoch, A.G.; Monakhova, E.V.; Gladkova, A.A.; Khabibullina, Z.V.; Ogorodnikova, V.A.; Serdechnova, M.; Blawert, C.; Kuznetsov, Y.I.; Zheludkevich, M.L. Corrosion Protection of Magnesium Alloy by PEO-Coatings Containing Sodium Oleate. Int. J. Corros. Scale Inhib. 2019, 8, 1170–1188. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Xie, Z.-H.; Yu, G. Duplex Coating Combining Layered Double Hydroxide and 8-Quinolinol Layers on Mg Alloy for Corrosion Protection. Electrochim. Acta 2018, 283, 1845–1857. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Ko, Y.G. Self-Assembly of Hierarchical N-Heterocycles-Inorganic Materials into Three-Dimensional Structure for Superior Corrosion Protection. Chem. Eng. J. 2019, 356, 850–856. [Google Scholar] [CrossRef]
- Al Zoubi, W.; Kim, M.J.; Yoon, D.K.; Salih Al-Hamdani, A.A.; Kim, Y.G.; Ko, Y.G. Effect of Organic Compounds and Rough Inorganic Layer Formed by Plasma Electrolytic Oxidation on Photocatalytic Performance. J. Alloy. Compd. 2020, 823, 153787. [Google Scholar] [CrossRef]
- Anand, R.; Gill, K.D.; Mahdi, A.A. Therapeutics of Alzheimer’s Disease: Past, Present and Future. Neuropharmacology 2014, 76, 27–50. [Google Scholar] [CrossRef]
- Martirosyan, A.; Leonard, S.; Shi, X.; Griffith, B.; Gannett, P.; Strobl, J. Actions of a Histone Deacetylase Inhibitor NSC3852 (5-Nitroso-8-Quinolinol) Link Reactive Oxygen Species to Cell Differentiation and Apoptosis in MCF-7 Human Mammary Tumor Cells. J. Pharmacol. Exp. Ther. 2006, 317, 546–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loenarz, C.; Schofield, C.J. Expanding Chemical Biology of 2-Oxoglutarate Oxygenases. Nat. Chem. Biol. 2008, 4, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, J.A.; Fullagar, J.L.; Miller, M.T.; Cohen, S.M. Identifying Chelators for Metalloprotein Inhibitors Using a Fragment-Based Approach. J. Med. Chem. 2011, 54, 591–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekouar, K.; Mouscadet, J.-F.; Desmaële, D.; Subra, F.; Leh, H.; Savouré, D.; Auclair, C.; D’Angelo, J. Styrylquinoline Derivatives: A New Class of Potent HIV-1 Integrase Inhibitors That Block HIV-1 Replication in CEM Cells. J. Med. Chem. 1998, 41, 2846–2857. [Google Scholar] [CrossRef]
- Musiol, R.; Jampilek, J.; Buchta, V.; Silva, L.; Niedbala, H.; Podeszwa, B.; Palka, A.; Majerz-Maniecka, K.; Oleksyn, B.; Polanski, J. Antifungal Properties of New Series of Quinoline Derivatives. Bioorg. Med. Chem. 2006, 14, 3592–3598. [Google Scholar] [CrossRef]
- Eweas, A.F.; Allam, G.; Abuelsaad, A.S.A.; Alghamdi, A.H.; Maghrabi, I.A. Design, Synthesis, Anti-Schistosomal Activity and Molecular Docking of Novel 8-Hydroxyquinoline-5-Sufonyl 1,4-Diazepine Derivatives. Bioorg. Chem. 2013, 46, 17–25. [Google Scholar] [CrossRef]
- Pandey, A.K.; Sharma, R.; Shivahare, R.; Arora, A.; Rastogi, N.; Gupta, S.; Chauhan, P.M.S. Synthesis of Perspicamide A and Related Diverse Analogues: Their Bioevaluation as Potent Antileishmanial Agents. J. Org. Chem. 2013, 78, 1534–1546. [Google Scholar] [CrossRef]
- Capodagli, G.C.; Sedhom, W.G.; Jackson, M.; Ahrendt, K.A.; Pegan, S.D. A Oncompetitive Inhibitor for Mycobacterium Tuberculosis ’s Class Iia Fructose 1,6-Bisphosphate Aldolase. Biochemistry 2014, 53, 202–213. [Google Scholar] [CrossRef] [Green Version]
- Caglič, D.; Krutein, M.C.; Bompiani, K.M.; Barlow, D.J.; Benoni, G.; Pelletier, J.C.; Reitz, A.B.; Lairson, L.L.; Houseknecht, K.L.; Smith, G.R.; et al. Identification of Clinically Viable Quinolinol Inhibitors of Botulinum Neurotoxin A Light Chain. J. Med. Chem. 2014, 57, 669–676. [Google Scholar] [CrossRef]
- Song, Y.; Xu, H.; Chen, W.; Zhan, P.; Liu, X. 8-Hydroxyquinoline: A Privileged Structure with a Broad-Ranging Pharmacological Potential. Medchemcomm 2015, 6, 61–74. [Google Scholar] [CrossRef]
- Duarte, C.; Barreiro, E.; Fraga, C. Privileged Structures: A Useful Concept for the Rational Design of New Lead Drug Candidates. Mini-Rev. Med. Chem. 2007, 7, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Zhan, P.; Li, D.; Li, J.; Chen, X.; Liu, X. Benzimidazole Heterocycle as a Privileged Scaffold in Antiviral Agents. Mini. Rev. Org. Chem. 2012, 9, 397–410. [Google Scholar] [CrossRef]
- Joaquim, A.R.; Gionbelli, M.P.; Gosmann, G.; Fuentefria, A.M.; Lopes, M.S.; Fernandes de Andrade, S. Novel Antimicrobial 8-Hydroxyquinoline-Based Agents: Current Development, Structure–Activity Relationships, and Perspectives. J. Med. Chem. 2021, 64, 16349–16379. [Google Scholar] [CrossRef]
- Saadeh, H.; Sweidan, K.; Mubarak, M. Recent Advances in the Synthesis and Biological Activity of 8-Hydroxyquinolines. Molecules 2020, 25, 4321. [Google Scholar] [CrossRef]
- Li, L.-Y.; Cui, L.-Y.; Zeng, R.-C.; Li, S.-Q.; Chen, X.-B.; Zheng, Y.; Kannan, M.B. Advances in Functionalized Polymer Coatings on Biodegradable Magnesium Alloys—A Review. Acta Biomater. 2018, 79, 23–36. [Google Scholar] [CrossRef]
- Rezwan, K.; Chen, Q.Z.; Blaker, J.J.; Boccaccini, A.R. Biodegradable and Bioactive Porous Polymer/Inorganic Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2006, 27, 3413–3431. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, Y.; Meng, L.; Chen, P.; Shi, T.; Liu, X.; Zheng, Y. Electrophoretic Deposition of Colloidal Particles on Mg with Cytocompatibility, Antibacterial Performance, and Corrosion Resistance. Acta Biomater. 2016, 45, 387–398. [Google Scholar] [CrossRef]
- Blacklock, J.; Sievers, T.K.; Handa, H.; You, Y.Z.; Oupický, D.; Mao, G.; Möhwald, H. Cross-Linked Bioreducible Layer-by-Layer Films for Increased Cell Adhesion and Transgene Expression. J. Phys. Chem. B 2010, 114, 5283–5291. [Google Scholar] [CrossRef] [Green Version]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Filonina, V.S.; Ustinov, A.Y.; Sukhoverkhov, S.V.; Gnedenkov, S.V. New Polycaprolactone-Containing Self-Healing Coating Design for Enhance Corrosion Resistance of the Magnesium and Its Alloys. Polymers 2023, 15, 202. [Google Scholar] [CrossRef]
- Vandrovcova, M.; Douglas, T.E.L.; Mróz, W.; Musial, O.; Schaubroeck, D.; Budner, B.; Syroka, R.; Dubruel, P.; Bacakova, L. Pulsed Laser Deposition of Magnesium-Doped Calcium Phosphate Coatings on Porous Polycaprolactone Scaffolds Produced by Rapid Prototyping. Mater. Lett. 2015, 148, 178–183. [Google Scholar] [CrossRef]
- Wong, H.M.; Zhao, Y.; Leung, F.K.L.; Xi, T.; Zhang, Z.; Zheng, Y.; Wu, S.; Luk, K.D.K.; Cheung, K.M.C.; Chu, P.K.; et al. Functionalized Polymeric Membrane with Enhanced Mechanical and Biological Properties to Control the Degradation of Magnesium Alloy. Adv. Healthc. Mater. 2017, 6, 1601269. [Google Scholar] [CrossRef] [PubMed]
- Domingos, M.; Intranuovo, F.; Gloria, A.; Gristina, R.; Ambrosio, L.; Bártolo, P.J.; Favia, P. Improved Osteoblast Cell Affinity on Plasma-Modified 3-D Extruded PCL Scaffolds. Acta Biomater. 2013, 9, 5997–6005. [Google Scholar] [CrossRef] [PubMed]
- Dash, T.K.; Konkimalla, V.B. Poly-ε-Caprolactone Based Formulations for Drug Delivery and Tissue Engineering: A Review. J. Control. Release 2012, 158, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Li, L.H.; Sankara Narayanan, T.S.N.; Kim, Y.K.; Kong, Y.M.; Park, I.S.; Bae, T.S.; Lee, M.H. Deposition of Microarc Oxidation-Polycaprolactone Duplex Coating to Improve the Corrosion Resistance of Magnesium for Biodegradable Implants. Thin Solid Films 2014, 562, 561–567. [Google Scholar] [CrossRef]
- Zomorodian, A.; Garcia, M.P.; Moura e Silva, T.; Fernandes, J.C.S.; Fernandes, M.H.; Montemor, M.F. Biofunctional Composite Coating Architectures Based on Polycaprolactone and Nanohydroxyapatite for Controlled Corrosion Activity and Enhanced Biocompatibility of Magnesium AZ31 Alloy. Mater. Sci. Eng. C 2015, 48, 434–443. [Google Scholar] [CrossRef]
- Mavis, B.; Demirtaş, T.T.; Gümüşderelioǧlu, M.; Gündüz, G.; Çolak, Ü. Synthesis, Characterization and Osteoblastic Activity of Polycaprolactone Nanofibers Coated with Biomimetic Calcium Phosphate. Acta Biomater. 2009, 5, 3098–3111. [Google Scholar] [CrossRef]
- Xu, W.; Yagoshi, K.; Koga, Y.; Sasaki, M.; Niidome, T. Optimized Polymer Coating for Magnesium Alloy-Based Bioresorbable Scaffolds for Long-Lasting Drug Release and Corrosion Resistance. Colloids Surf. B Biointerfaces 2018, 163, 100–106. [Google Scholar] [CrossRef]
- Wong, H.M.; Yeung, K.W.K.; Lam, K.O.; Tam, V.; Chu, P.K.; Luk, K.D.K.; Cheung, K.M.C. A Biodegradable Polymer-Based Coating to Control the Performance of Magnesium Alloy Orthopaedic Implants. Biomaterials 2010, 31, 2084–2096. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yamamoto, A. Characteristics and Cytocompatibility of Biodegradable Polymer Film on Magnesium by Spin Coating. Colloids Surf. B Biointerfaces 2012, 93, 67–74. [Google Scholar] [CrossRef]
- Abdal-hay, A.; Dewidar, M.; Lim, J.K. Biocorrosion Behavior and Cell Viability of Adhesive Polymer Coated Magnesium Based Alloys for Medical Implants. Appl. Surf. Sci. 2012, 261, 536–546. [Google Scholar] [CrossRef]
- Vaghefinazari, B.; Lamaka, S.V.; Blawert, C.; Serdechnova, M.; Scharnagl, N.; Karlova, P.; Wieland, D.C.F.; Zheludkevich, M.L. Exploring the Corrosion Inhibition Mechanism of 8-Hydroxyquinoline for a PEO-Coated Magnesium Alloy. Corros. Sci. 2022, 203, 110344. [Google Scholar] [CrossRef]
- Gnedenkov, A.S.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Vyaliy, I.E.; Egorkin, V.S.; Gnedenkov, S.V. Corrosion of the Welded Aluminium Alloy in 0.5 M NaCl Solution. Part 1: Specificity of Development. Materials 2018, 11, 2053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnedenkov, A.S.; Lamaka, S.V.; Sinebryukhov, S.L.; Mashtalyar, D.V.; Egorkin, V.S.; Imshinetskiy, I.M.; Zavidnaya, A.G.; Zheludkevich, M.L.; Gnedenkov, S.V. Electrochemical Behaviour of the MA8 Mg Alloy in Minimum Essential Medium. Corros. Sci. 2020, 168, 108552. [Google Scholar] [CrossRef]
- Shi, Z.; Liu, M.; Atrens, A. Measurement of the Corrosion Rate of Magnesium Alloys Using Tafel Extrapolation. Corros. Sci. 2010, 52, 579–588. [Google Scholar] [CrossRef]


| Coating Type | βa, mV/Decade | −βc, mV/Decade | Ic, A·cm−2 | CR, mm Year−1 | Ec, V (Ag/AgCl) | RP, Ω·cm2 | |Z|f=0.1 Hz, Ω·cm2 |
|---|---|---|---|---|---|---|---|
| PEO after 10 min exposure | 47.06 | 239.89 | 5.61 × 10−6 | 0.128 | −1.53 | 3.05 × 103 | 2913 |
| PEO after 22 h exposure | 75.83 | 225.22 | 3.89 × 10−6 | 0.088 | −1.41 | 6.33 × 103 | 2002 |
| CC-HQ after 10 min exposure | 43.41 | 125.03 | 1.46 × 10−6 | 0.033 | −1.51 | 9.61 × 103 | 5838 |
| CC-HQ after 22 h exposure | 81.33 | 76.78 | 9.56 × 10−7 | 0.022 | −1.38 | 1.80 × 104 | 3642 |
| CC-D after 10 min exposure | 118.76 | 188.07 | 1.52 × 10−6 | 0.035 | −1.49 | 2.08 × 104 | 10,578 |
| CC-D after 22 h exposure | 162.73 | 207.76 | 1.02 × 10−6 | 0.023 | −1.32 | 3.89 × 104 | 3566 |
| HC-D-2 after 10 min exposure | 127.03 | 147.03 | 4.99 × 10−7 | 0.011 | −1.57 | 5.93 × 104 | 32,338 |
| HC-D-2 after 22 h exposure | 201.40 | 139.23 | 3.52 × 10−7 | 0.008 | −1.37 | 1.02 × 105 | 2750 |
| HC-D-1 after 10 min exposure | 138.58 | 155.99 | 3.02 × 10−7 | 0.007 | −1.48 | 1.06 × 105 | 105,350 |
| HC-D-1 after 22 h exposure | 235.30 | 97.99 | 1.64 × 10−7 | 0.004 | −1.35 | 1.83 × 105 | 15,591 |
| CC-A after 10 min exposure | 100.44 | 168.97 | 1.74 × 10−6 | 0.039 | −1.49 | 1.58 × 104 | 11,318 |
| CC-A after 22 h exposure | 121.60 | 224.10 | 1.77 × 10−6 | 0.040 | −1.37 | 1.94 × 104 | 3708 |
| HC-A-1 after 10 min exposure | 130.11 | 230.26 | 5.20 × 10−7 | 0.012 | −1.48 | 6.95 × 104 | 54,074 |
| HC-A-1 after 22 h exposure | 282.98 | 109.08 | 6.37 × 10−7 | 0.014 | −1.40 | 5.37 × 104 | 10,469 |
| Exposure Time, h | CPE1 | R1, Ω·cm2 | CPE2 | R2, Ω·cm2 | ||
|---|---|---|---|---|---|---|
| Q1, S·cm−2·sn | n1 | Q2, S·cm−2·sn | n2 | |||
| PEO | ||||||
| 0.17 (10 min) | 9.29 × 10−6 | 0.86 | 134.4 | 1.17 × 10−6 | 0.86 | 2527 |
| 21.7 | 1.96 × 10−5 | 0.78 | 17.8 | 4.22 × 10−5 | 0.93 | 1751 |
| CC-HQ | ||||||
| 0.17 (10 min) | 7.92 × 10−6 | 0.47 | 423.4 | 2.90 × 10−6 | 0.87 | 5123 |
| 21.7 | 6.40 × 10−6 | 0.90 | 199.7 | 1.36 × 10−5 | 0.87 | 2584 |
| CC-D | ||||||
| 0.17 (10 min) | 1.66 × 10−9 | 0.88 | 808.5 | 7.03 × 10−6 | 0.67 | 10,360 |
| 21.7 | 1.24 × 10−7 | 0.51 | 165.8 | 1.26 × 10−5 | 0.80 | 3817 |
| HC-D-2 | ||||||
| 0.17 (10 min) | 1.14 × 10−9 | 0.89 | 5360.0 | 3.39 × 10−6 | 0.65 | 29,068 |
| 21.7 | 2.62 × 10−9 | 0.93 | 309.0 | 1.43 × 10−5 | 0.77 | 2701 |
| HC-D-1 | ||||||
| 0.17(10 min) | 5.25 × 10−9 | 0.79 | 3548 | 1.61 × 10−6 | 0.68 | 106,450 |
| 21.7 | 2.78 × 10−9 | 0.85 | 835.5 | 5.53 × 10−6 | 0.78 | 12,995 |
| CC-A | ||||||
| 0.17 (10 min) | 1.63 × 10−9 | 0.88 | 938.0 | 7.04 × 10−6 | 0.73 | 11,114 |
| 21.7 | 1.65 × 10−8 | 0.58 | 113.0 | 1.51 × 10−5 | 0.81 | 3712 |
| HC-A-1 | ||||||
| 0.17 (10 min) | 4.07 × 10−9 | 0.80 | 1610 | 1.34 × 10−6 | 0.73 | 51,048 |
| 21.7 | 4.23 × 10−8 | 0.69 | 581 | 5.99 × 10−6 | 0.80 | 10,167 |
| Coating Type | CC-HQ | HC-D-2 | HC-D-1 | HC-A-1 |
|---|---|---|---|---|
| ηi. % (after 10 min exposure) | 73.9 | 67.2 | 80.1 | 70.1 |
| ηi. % (after 22 h exposure) | 75.4 | 65.4 | 83.9 | 64.0 |
| Sample Type | Number of Cycles | Distance, m | Wear, mm3∙(N∙m)−1 |
|---|---|---|---|
| PEO | 1385 | 48 | (1.34 ± 0.30) × 10−2 |
| HC-D-1 | 157,481 | 5654.7 | (5.28 ± 1.00) × 10−4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnedenkov, A.S.; Filonina, V.S.; Sinebryukhov, S.L.; Gnedenkov, S.V. A Superior Corrosion Protection of Mg Alloy via Smart Nontoxic Hybrid Inhibitor-Containing Coatings. Molecules 2023, 28, 2538. https://doi.org/10.3390/molecules28062538
Gnedenkov AS, Filonina VS, Sinebryukhov SL, Gnedenkov SV. A Superior Corrosion Protection of Mg Alloy via Smart Nontoxic Hybrid Inhibitor-Containing Coatings. Molecules. 2023; 28(6):2538. https://doi.org/10.3390/molecules28062538
Chicago/Turabian StyleGnedenkov, Andrey S., Valeriia S. Filonina, Sergey L. Sinebryukhov, and Sergey V. Gnedenkov. 2023. "A Superior Corrosion Protection of Mg Alloy via Smart Nontoxic Hybrid Inhibitor-Containing Coatings" Molecules 28, no. 6: 2538. https://doi.org/10.3390/molecules28062538
APA StyleGnedenkov, A. S., Filonina, V. S., Sinebryukhov, S. L., & Gnedenkov, S. V. (2023). A Superior Corrosion Protection of Mg Alloy via Smart Nontoxic Hybrid Inhibitor-Containing Coatings. Molecules, 28(6), 2538. https://doi.org/10.3390/molecules28062538







