Abstract
The CO2 aqueous foams stabilized by bioresource-derived ultra-long chain surfactants have demonstrated considerable promising application potential owing to their remarkable longevity. Nevertheless, existing research is still inadequate to establish the relationships among surfactant architecture, environmental factors, and foam properties. Herein, two cases of ultra-long chain tertiary amines with different tail lengths, N-erucamidopropyl-N,N-dimethylamine (UC22AMPM) and N-oleicamidopropyl-N,N-dimethylamine (UC18AMPM), were employed to fabricate CO2 foams. The effect of temperature, pressure and salinity on the properties of two foam systems (i.e., foamability and foam stability) was compared using a high-temperature, high-pressure visualization foam meter. The continuous phase viscosity and liquid content for both samples were characterized using rheometry and FoamScan. The results showed that the increased concentrations or pressure enhanced the properties of both foam samples, but the increased scope for UC22AMPM was more pronounced. By contrast, the foam stability for both cases was impaired with increasing salinity or temperature, but the UC18AMPM sample is more sensitive to temperature and salinity, indicating the salt and temperature resistance of UC18AMPM-CO2 foams is weaker than those of the UC22AMPM counterpart. These differences are associated with the longer hydrophobic chain of UC22AMPM, which imparts a higher viscosity and lower surface tension to foams, resisting the adverse effects of temperature and salinity.
1. Introduction
Carbon dioxide (CO2) aqueous foams are colloidal dispersions composed of CO2 bubbles dispersed in a continuous aqueous phase [1]. Due to their relatively low density, larger surface area and excellent fluidity, CO2 aqueous foams have been widely used in many industrial processes and applications including the petroleum industry [2], ore flotation [3] and firefights [4]. The traditional CO2 aqueous foams were obtained using anionic surfactants such as sodium dodecyl sulfate (SDS) [5] and alpha olefin sulfonate (AOS) [6] as foaming agents by decreasing the CO2–water interfacial tension (C–W IFT) and capillary forces (Pc). Unfortunately, such CO2 aqueous foams rapidly destabilized through a combination of drainage [7,8], coalescence [7,8], and Ostwald ripening [9]. As a result, the lifetime CO2 aqueous foams made of common surfactants do not exceed a few tens of minutes [10,11] and fail to satisfy the practical requirements. To improve foam lifetime, various foam stabilizers, including polymer [12,13], protein [14,15,16], and nanoparticles [17,18], are introduced into the aqueous foam systems against foam destabilization. In some cases, there is a demand for both the stable foam formed and controlled foam destruction. Taking cleaning processes as an example, the stable foam needs to be destabilized rapidly in a controlled way at the end of cleaning to obtain only a small volume of contaminated liquid that is easier to handle compared with foam [19]. Therefore, switchable or stimuli-responsive foams with tunable stability have been paid much attention in recent years.
During the past decade, the use of bioresource-derived ultra-long-chain surfactants (the hydrophobic chains ≥ C18) as stabilizers to prepare long-lasting aqueous foams has been widely reported and attracted significant interest. Johnston et al. [20] pioneered the utilization of erucylamidopropyldimethyl betaine (EAPB) to prepare long-lived CO2 foams. The CO2 foams stabilized by EAPB are intact at temperatures up to 120 °C and CO2 volumetric fractions up to 0.98. Likewise, our laboratory used N-erucamidopropyl-N,N-dimethylamine (UC22AMPM) to develop a CO2 aqueous foam with a lifetime of up to 6 h in 120 °C and 10 MPa [21]. The mechanism behind the foams stabilization by ultra-long-chain surfactants can be summarized as follows: (i) ultra-long-chain surfactants adsorb at CO2–water interfaces to form a dense surfactants layer in foam film, resisting coarsening and coalescence of bubbles [22]; (ii) the ultra-long-chain surfactants can assemble into viscoelastic aggregates, enhancing the solution viscosity and thus suppressing the liquid drains within the foam film [23,24]. In addition to their excellent foam stabilization capability, another merit of ultra-long-chain surfactants over petrochemical-based short-chain surfactants is being environmentally benign and sustainable as their feedstocks are natural renewable materials such as vegetable oil [25,26]. To provide scientific guidance for the application of CO2 foams in harsh conditions, previous research on ultra-long-chain surfactants stabilizing CO2 aqueous foams has been focused on establishing the relationships between various factors (e.g., pH, temperature, salinity and pressure) and foam properties [21,27]. However, these studies have typically been conducted in a single foam system, mainly rooted in the previous view that foam destabilization depends more on the mesoscopic properties of the foam such as bubble radius, foam film thickness and liquid fraction than on the chemical properties of the surfactant. Consequently, there are still insufficient insights into the contribution of surfactant structure to foam stability and evolution, impeding the advancement and exploitation of such foam systems.
In fact, many studies have demonstrated that surfactant structure has a noticeable impact on foam evolution and stability [16,28,29]. Fameau et al. [30] explored the role of tail length and head groups in foam properties by comparing the performance of foams made from long-chain fatty acids (myristic acid, palmitostearic acid, juniperic acid and 12-hydroxystearic acid). They found the foamability of fatty acid-based foam increased with decreasing the alkyl chain length of the fatty acid. Moreover, the presence of a hydroxyl group on the hydrophobic tail of the fatty acids increases the foamability in comparison to the non-hydroxylated fatty acids analog. A systematic study of foams made with a series of multi-tailed surfactants reported by Feitosa and co-workers demonstrated foams made with tri-cephalic double-tailed molecules have better stability than the single-tail one, regardless of the head structure [10]. Enlightened by these findings, we can safely hypothesize that aqueous foams stabilized by ultra-long-chain surfactants with different structures will exhibit differentiated foam properties and aging processes. In this context, it is desirable and beneficial to establish the correlation among the molecular structure of long-tailed surfactants, foam properties and evolution.
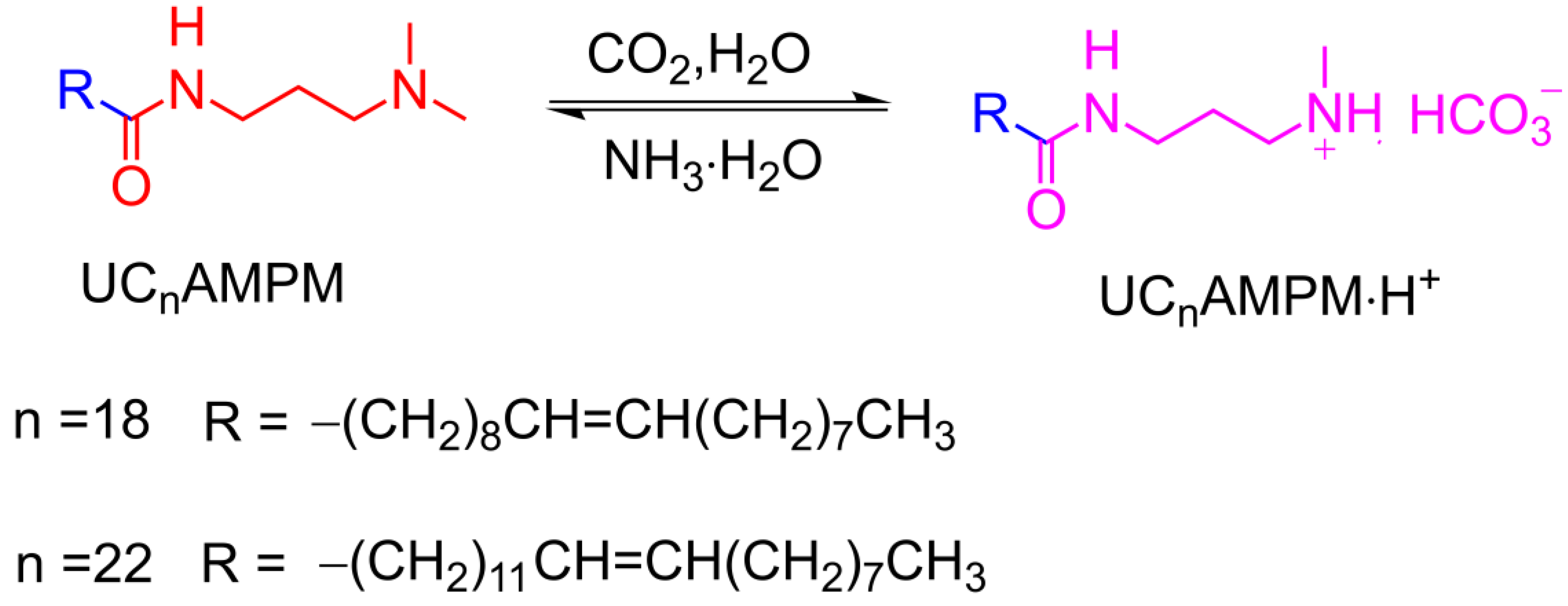
The objective of this study is to establish the surfactant structure-foam properties-foam evolution links and to deepen the understanding of the role of surfactant structure in foam properties. To attain this goal, UC22AMPM and its analog (UC18AMPM, C18 tail, Scheme 1) were used as model compounds to develop CO2 aqueous foams. Then, the foaming ability and foam stability of two CO2 aqueous foam systems were meticulously compared at various temperatures, pressures and salinity using FoamScan and a high-temperature, high-pressure (HTHP) visualization foam meter. Meanwhile, the as-prepared CO2 aqueous foams were investigated by rheometer to unravel the underlying principles driving the discrepancies in foam properties.
Scheme 1.
The chemical structures of N-erucamidopropyl-N,N-dimethylamine (UC22AMPM) and N-oleicamidopropyl-N,N-dimethylamine (UC18AMPM).
2. Results and Discussion
The section is organized as follows: First, the concentration of UC22AMPM and UC18AMPM is optimized by the static foam test in atmospheric pressure at 35 °C. Then, the switching behavior of UC18AMPM-CO2 aqueous foam is characterized in comparison with UC22AMPM-CO2 aqueous foam. Finally, the influence of temperature, salinity, and pressure on the performance of CO2 aqueous foams UC22AMPM and UC18AMPM is examined, respectively.
2.1. Determination of Optimum Concentration
It is known that foam properties strongly depend on the concentration of the foaming agent [31,32]. Generally, the foaming properties are referred to as foamability (the maximum volume of foam system for a certain volume of foaming agent solution after a certain time of shear effect at a certain temperature, Vmax) [33,34] and foam stability (the time taken by the volume of foam system from Vmax to a half at a certain temperature, t1/2) [33,34]. To determine the optimum concentration, the properties of UC22AMPM and UC18AMPM foams were investigated separately as a function of the concentration (0.1–0.5%) using FoamScan under atmospheric pressure at 35 °C. We previously demonstrated that UC22AMPM could form stable CO2 aqueous foams but not N2 ones [21,35]. Thus, CO2 was employed as the foaming gas in this work.
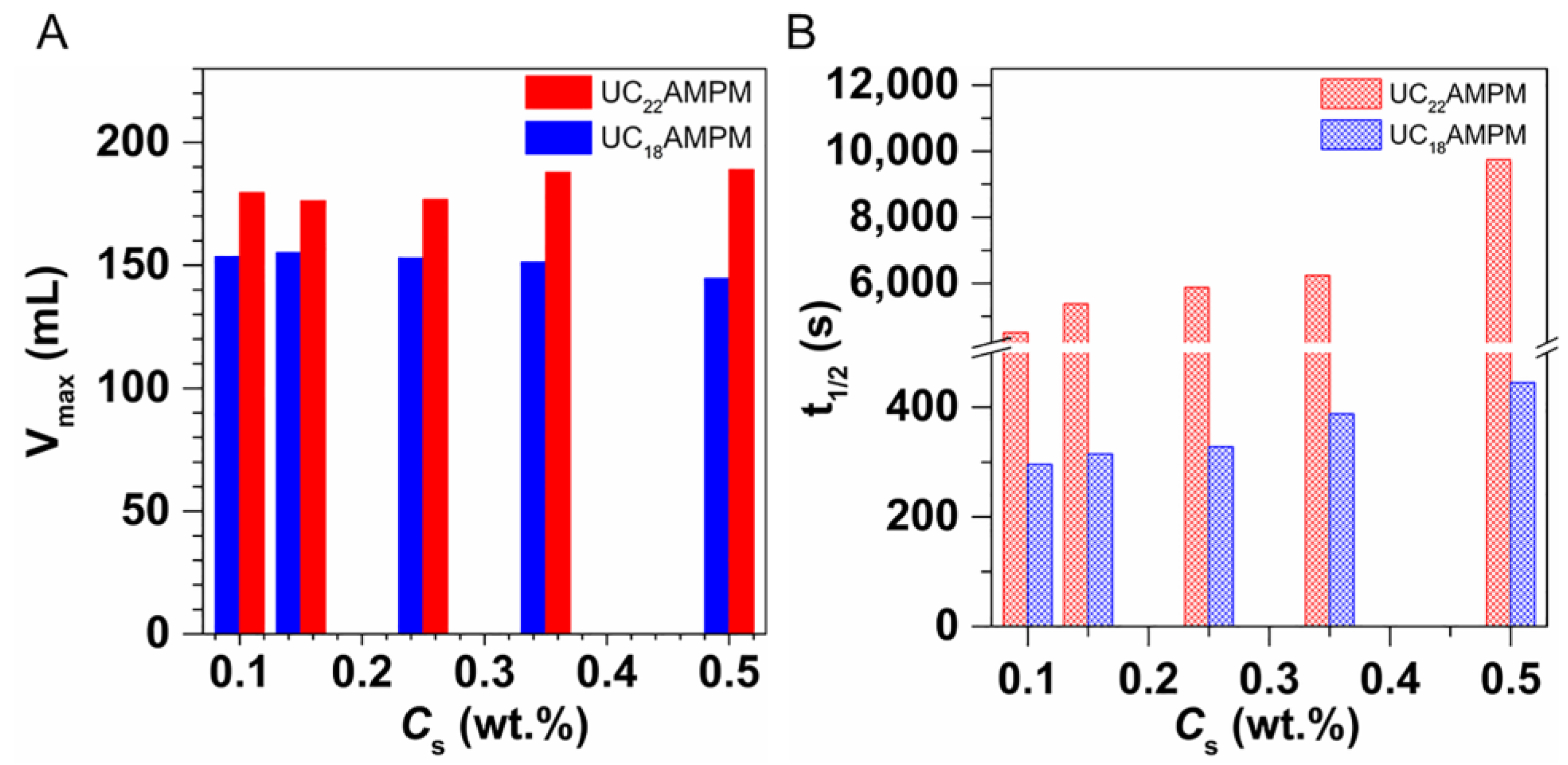
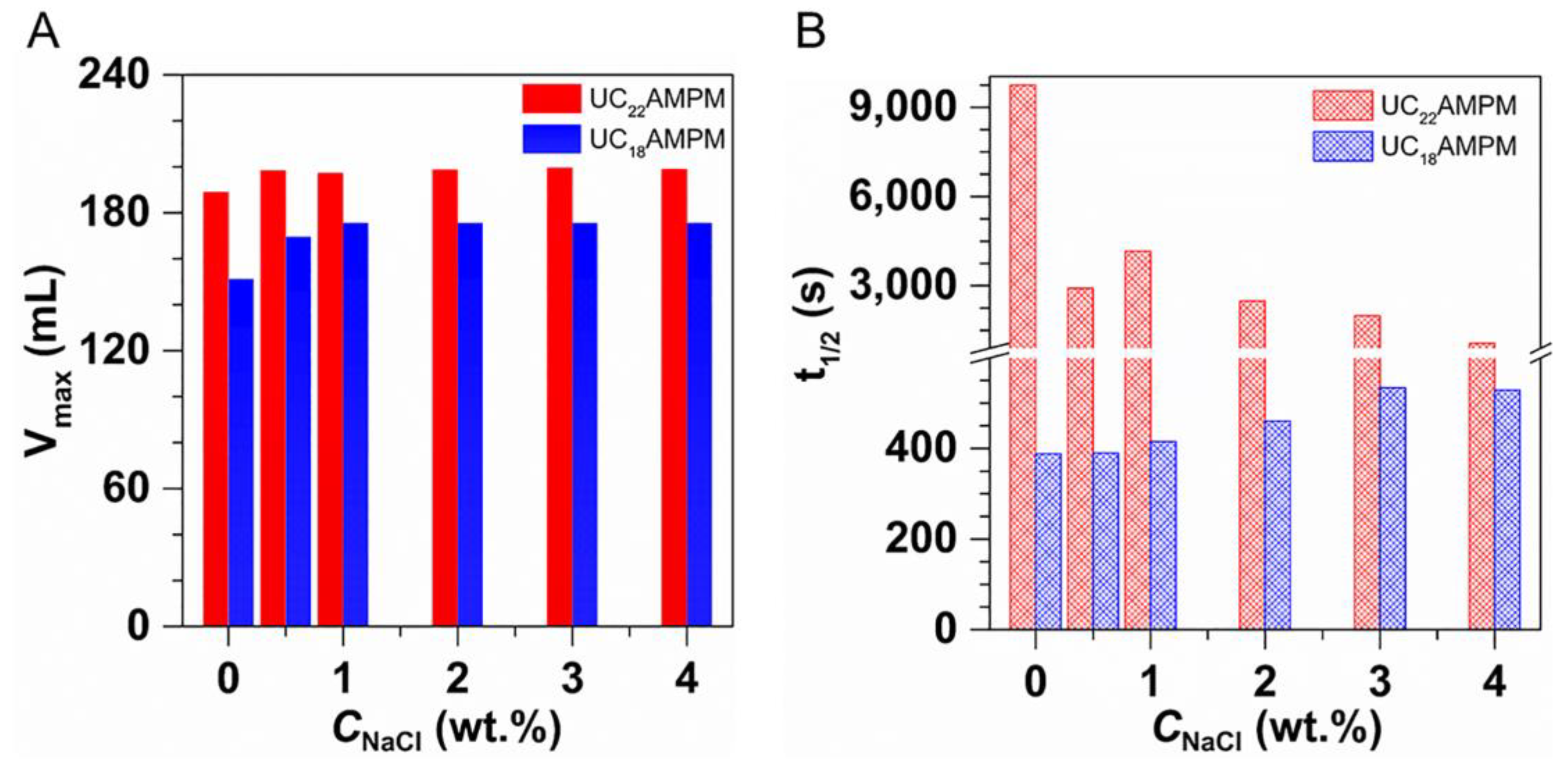
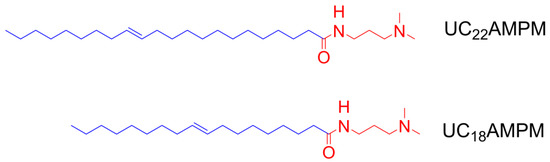
Figure 1A,B present the changes in Vmax and t1/2 of both aqueous foams with increasing concentration, respectively. It can be seen the Vmax for UC18AMPM was always constant at around 180 mL with increasing UC18AMPM concentration (CUC18AMPM), while the Vmax of UC22AMPM rose from 179 to 189 mL (Figure 1A). Meanwhile, the t1/2 of both aqueous foams rose as the concentration increased (Figure 1B). From Table 1, the t1/2 of UC22AMPM-CO2 aqueous foams improved by 2.6 fold as the concentration of UC22AMPM (CUC22AMPM) increased from 0.1% to 0.5%, higher than the increment factor of UC18AMPM-CO2 aqueous foams (~1.6). These results indicated that the CUC22AMPM exerts a more prominent influence on foamability and foam stability compared to CUC18AMPM.
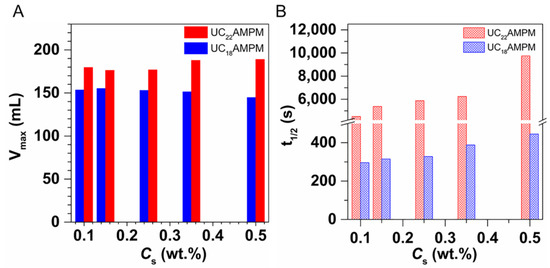
Figure 1.
The influence of surfactant concentrations on (A) Vmax and (B) t1/2 of UC22AMPM and UC18AMPM in atmospheric pressure at 35 °C, respectively.

Table 1.
CO2 foam performance with different concentrations of UC22AMPM and UC18AMPM, respectively.
The foam comprehensive index (FCI) [6], a quantitative measure to assess the foam properties, was employed to calculate the optimal concentration. The FCI can be expressed below [36]:
As listed in Table 1, the FCI of UC22AMPM and UC18AMPM reached maximum values of 1,382,062 s·mL and 49,140 s·mL at a concentration of 0.5 wt.%, respectively. Typically, the value of FCI is greater, the foam properties are better [37]. Based on the FCI criterion, 0.5 wt.% as the optimal UC22AMPM and UC18AMPM concentration was used in the following experiments. Furthermore, we also concluded that the 0.5% UC22AMPM has superior foam properties to 0.5% UC18AMPM.
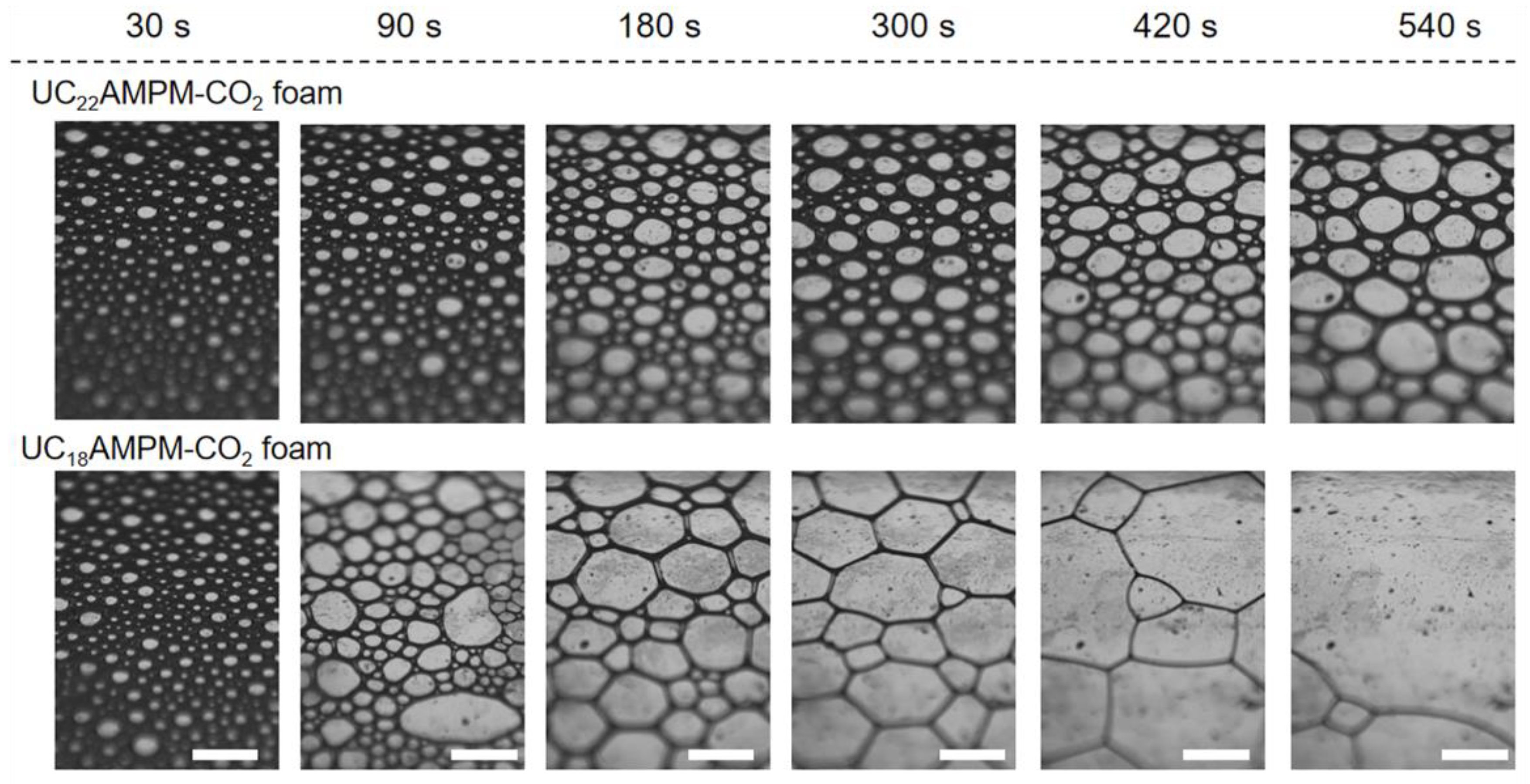
To shed light on the reasons behind the difference in properties between UC22AMPM and UC18AMPM foams at their optimum concentration, the foam evolution process, liquid content (φ) of aqueous foams (the ratio of the liquid volume to the foam volume) and continuous phase viscosity (η) of foam bulk phase were studied. As shown in Figure 2, the geometry of the bubble is spherical for both cases at the initial moment (30 s). For UC22AMPM foams, there was virtually no change in the bubble morphology as time progressed. In contrast, the bubbles in UC18AMPM aqueous foams evolved quickly into irregular polyhedral over time. At the 540th second, a substantial number of bubbles of UC18AMPM aqueous foams disappeared, indicative of foam bursting. In principle, the bubble shape is dependent on the φ of the aqueous foam [8]. In the case of high φ in the aqueous foams, the bubbles are uniformly spherical and densely packed. Decreasing the φ causes bubble deformation and the formation of defined edges. Therefore, we can conclude that the φ of UC22AMPM foams remain constant for 540 s, indicative of slow drainage. In the case of UC18AMPM foams, the faster bubble deformation could be interpreted by the rapid lowering of φ, resulting from the acceleration of the drainage process. From optical visualization, we could draw a conclusion that the foam drainage process of UC22AMPM foams is weaker than that of UC18AMPM foams.
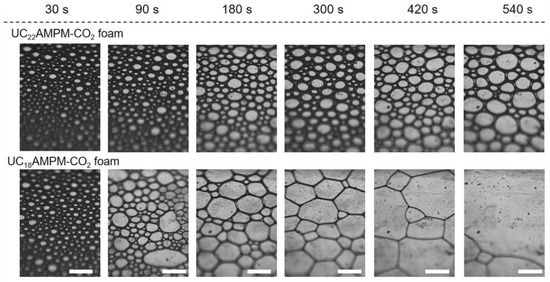
Figure 2.
Comparison of morphology evolution of CO2 aqueous foams made from 0.5% UC22AMPM and 0.5% UC18AMPM with time in atmospheric pressure at 35 °C.
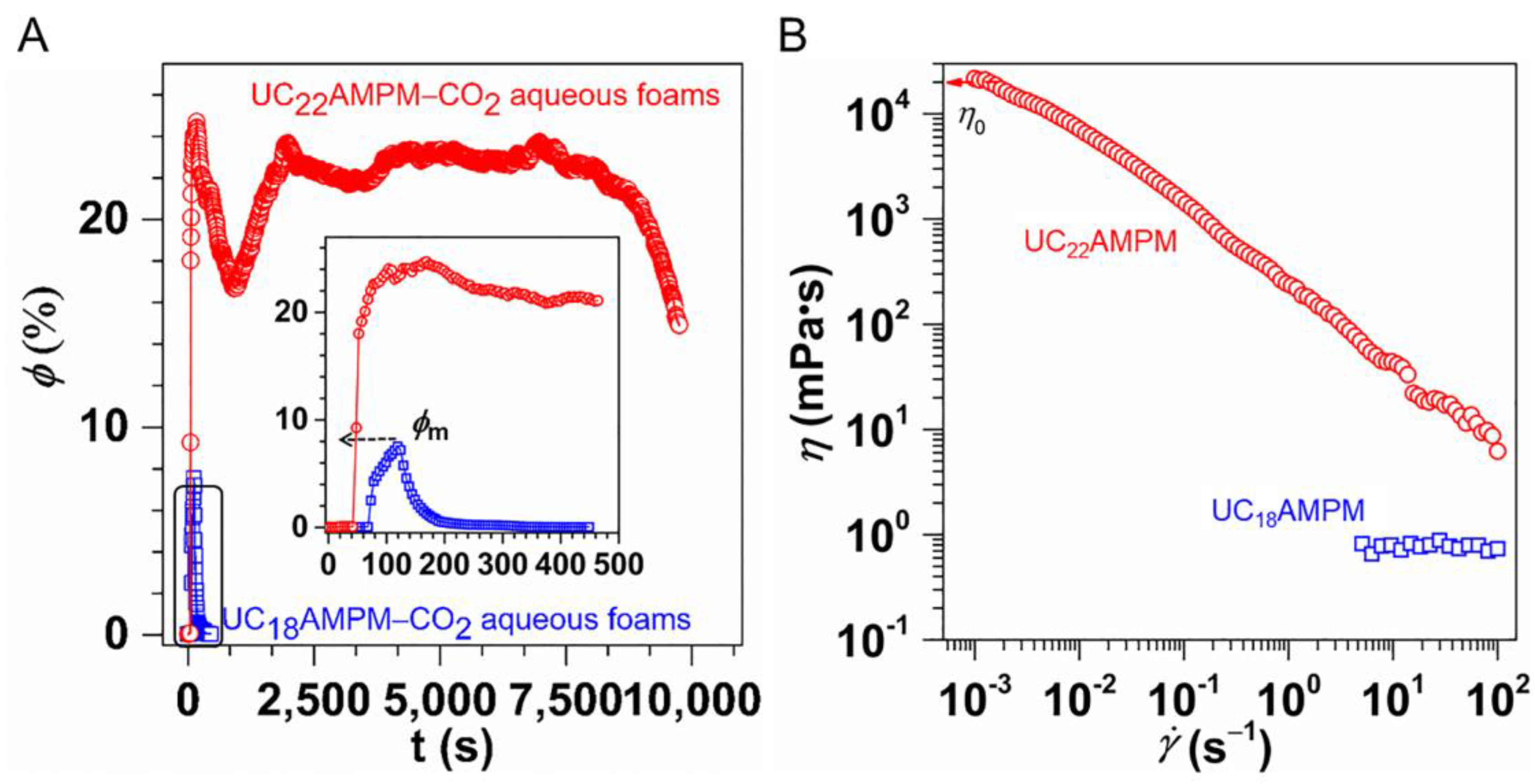
The variation in the φ as a function of time is shown in Figure 3A. Evidently, the φ in both cases increased significantly over time during the generation process of aqueous foams, reaching a maximum liquid content (φm) on completion of foaming. Comparatively speaking, the φm of the 0.5% UC22AMPM-CO2 aqueous foams was about 24.7%, greater than that of the 0.5% UC18AMPM-CO2 aqueous foams (10.6%). The lower φm is associated with its Vmax (145 mL), indicative of the inferior foaming ability of UC18AMPM. As is well-known, the foamability is positively proportional to the C-W IFT (γ) of the surfactant solution, which can be described by using the previously reported [38]:
here W and A stand for external energy applied to generate the foam and the foam area created, respectively. For a fixed W, the higher the γ is, the lower the Vmax will be. On the basis of a previous study by Feng et al. [39], with the identical head group, the γ increases with the decrease in the hydrophobic chain length. One can conclude that the γ of the UC18AMPM-CO2 solution is higher than that of the UC22AMPM counterpart due to its shorter alkyl chain. Thus, the UC18AMPM-CO2 solution presents poor foamability as compared with the UC22AMPM counterpart.
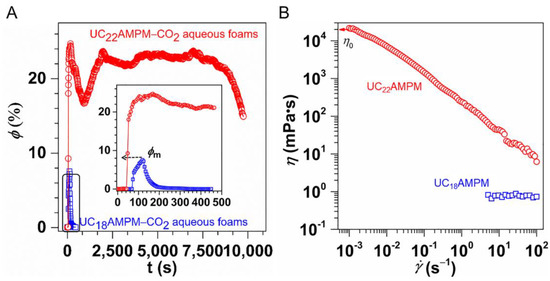
Figure 3.
(A) Variation of the liquid content (φ) for 0.5% UC22AMPM and 0.5% UC18AMPM as a function of time. (B) Shear viscosity (η) plotted as a function of shear rate for 0.5% UC22AMPM and 0.5% UC18AMPM solutions at 35 °C.
Upon CO2 sparging cease, the φ reduced gradually with time because of the drainage. It can be seen that the UC18AMPM-CO2 aqueous foams drained in the 200s to φ = 0, while the φ of UC22AMPM-CO2 aqueous foam was 20% in this period (Figure 3A), demonstrating that the drainage from UC18AMPM-CO2 aqueous foams is faster than that of UC22AMPM-CO2 aqueous solution.
The rheological results demonstrated the UC22AMPM dispersion saturated with CO2 attained very high values of zero-shear viscosity ηo (3.75 × 104 mPa·s) and showed shear-thinning behavior (Figure 3B). The high magnitude of ηo mirrors the presence of entangled wormlike micelles in solution [40,41]. In contrast, the ηo for UC18AMPM samples was only ~1.0 mPa·s (Figure 3B), reflecting the absence of wormlike micelles. Numerous studies have established that drainage velocity (V) should vary inversely with the viscosity of the continuous phase (η), as the following equation [42]:
where ΔPfilm stands for the difference in pressure between the film center and border, hf refer to the thickness of the thin film. Using Equation (3), one can conclude that the V of UC18AMPM-CO2 aqueous foams is four orders of magnitude greater than that of UC22AMPM-CO2 aqueous foams, consistent with our earlier conclusion (Figure 2). The consequence of faster drainage is that the φ decreases rapidly, concomitant with the reduction in film thickness. The thin films tend to rupture, leading to rapid foam destruction. As a result, UC18AMPM-CO2 aqueous foams show a t1/2 of 445 s, which is much shorter relative to UC22AMPM-CO2 aqueous foams (9750 s) in identical conditions.
According to the aforementioned results, we attributed the differences in performance between UC22AMPM and UC18AMPM foams to their viscosity discrepancy, rooted in the different assembled structures of UC22AMPM and UC18AMPM. More specifically, UC22AMPM with 0.5% concentration can self-assemble into wormlike micelles, but UC18AMPM cannot. For the UC22AMPM system, the entangled worm-like micelles impart high viscosity to the foam continuous phase. During the foaming process, a large amount of liquid was transported into the foam liquid channels, forming thick foam films. The thick films would increase the thermal activation energy barrier against coalescence and Ostwald ripening. More important, the drainage is retarded by high η. Overall, high continuous phase viscosity retarded the three types of foam destabilization processes simultaneously, thereby enhancing the stability of foams. In contrast, the UC18AMPM behaved as a low η Newtonian fluid due to the absence of wormlike micelles, leading to the formation relatively thin foam film. Furthermore, lamellae films drained rapidly due to the low η of the aqueous phase. The consequence of faster drainage is that the foam film becomes thinner and prone to rupture, leading to foam destruction. Therefore, the UC18AMPM-CO2 solution presents poor foam properties as compared with the UC22AMPM counterpart.
2.2. A Comparison of the Foams Switchability
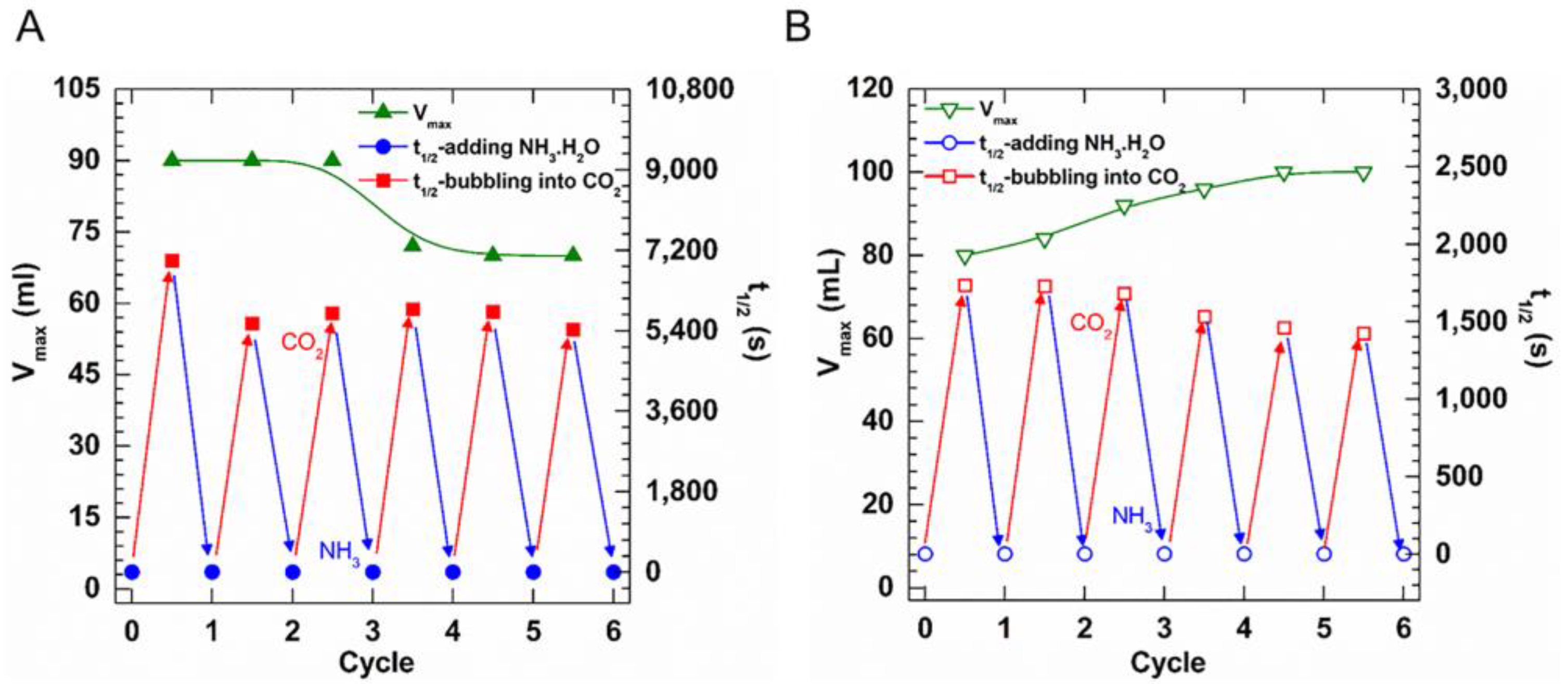
We previously demonstrated the aqueous foams stabilized by UC22AMPM could be turned “on” and “off” on demand through the bubbling of CO2 or adding NH3·H2O. It is essential to examine the switchability of the UC18AMPM-CO2 foam and to make a comparison with the UC22AMPM ones. The pressure and temperature are constant at 3 MPa and 80 °C, respectively, to ensure that the above two compounds can be protonated again after the neutralization of NH3·H2O.
Figure 4 depicts the parallel variations of Vmax and t1/2 of both CO2 foam systems after the alternating addition of NH3·H2O and CO2. It was apparent that the t1/2 rose or declined accordingly with the alternative introduction of CO2 and NH3·H2O, suggesting the foam lifetime of both foam systems can be reversibly tuned. This finding proved that CO2 aqueous foams prepared from UC18AMPM feature switchability similar to UC22AMPM, resulting from their identical hydrophilic headgroups. As shown in Scheme 2, both UC22AMPM and UC18AMPM in water can be protonated into cationic surfactants after sparging CO2, lowering C-W IFT by adsorbing at the CO2/water interface and thereby promoting foam formation. Upon NH3·H2O addition, protonated surfactant converted to a surface-inactive neutral form. Consequently, UC22AMPM and UC18AMPM would desorb from the CO2/water interface, disrupting the foam film and thereby leading to rapid foam destabilization.
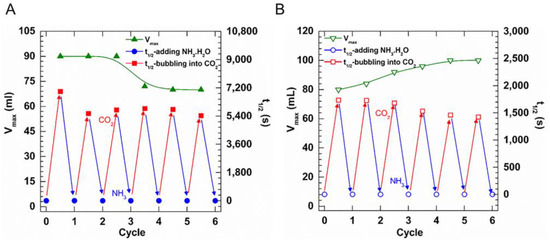
Figure 4.
Variation in Vmax and t1/2 of (A) 0.5% UC22AMPM and (B) 0.5% UC18AMPM foams treated with CO2 followed by NH3·H2O, respectively.
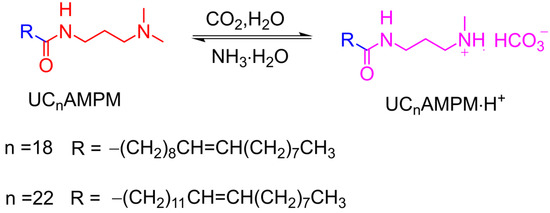
Scheme 2.
The protonation of UC22AMPM or UC18AMPM in the presence of CO2 and H2O at 80 °C, 3 MPa.
Notably, the Vmax of UC22AMPM-CO2 foams initially remained constant and then gradually declined as the cycle number increased (Figure 4A), demonstrating foamability weakening. By comparison, the Vmax of UC18AMPM-CO2 foams gradually boosted as the foaming/defoaming cycle number increased (Figure 4B), indicative of enhanced foamability. On the other hand, the t1/2 of both CO2 foam systems decreased as the number of foaming/defoaming cycles increased (Figure 4A,B), indicating that foam stability deteriorated as the cycle number increased. A similar result was observed in our earlier studies, arising from the accumulation of by-products (a mixture of ammonium carbonate and bicarbonate) [21].
2.3. Comparison of the Effect of External Factors on Foam Properties
It has been recognized that external factors such as temperature, pressure and salinity can significantly affect the foam properties [21]. In the following subsections, the influence of these external factors on the properties of the above two CO2 aqueous foams was investigated comparatively using an HTHP visualization foam meter.
2.3.1. Effect of Temperature
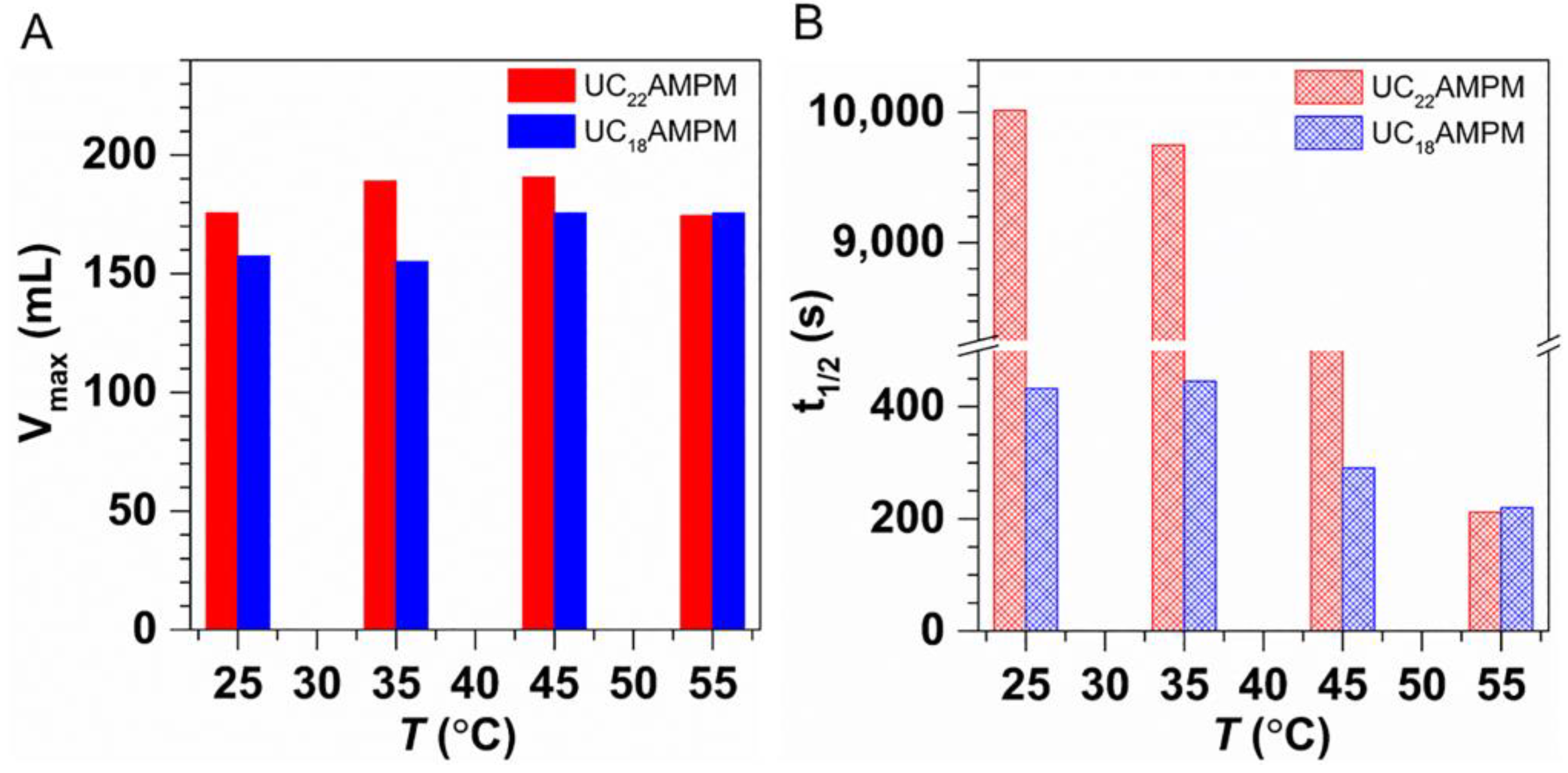
To examine the impact of temperature on the CO2 aqueous foams made with UC22AMPM or UC18AMPM, t1/2 and Vmax were determined in a temperature range of 25–120 °C at a constant pressure of 3 MPa. As shown in Figure 5A, the Vmax of both foams systems increased slightly with the temperature elevated, meaning that the increment of temperatures improves the foaming ability.
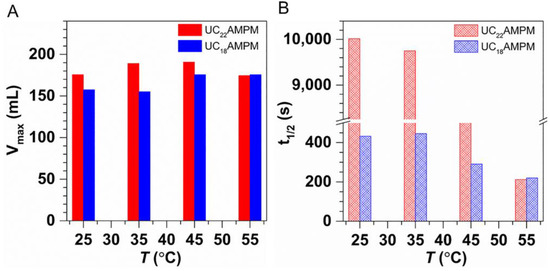
Figure 5.
The (A) Vmax and (B) t1/2 of 0.5% UC22AMPM dispersion and 0.5% UC18AMPM dispersion plotted as a function of temperature at 3 MPa in the presence of CO2, respectively.
Compared in Figure 5B are the changes in t1/2 for the above two aqueous foams systems at different temperatures. Both foam systems displayed similar evolution trends, i.e., the t1/2 diminished steeply with the elevation of temperature, demonstrating that increased temperature would deteriorate foam stability. Many studies have revealed the elevating temperature resulted in increased C-W IFT [22] and decreased η [40] at constant pressure. Therefore, the foam destabilization accelerates with increasing temperature as a consequence of the higher C-W IFT and lower η, leading to poor foam stability.
Note also that the t1/2 of UC22AMPM-CO2 aqueous foams is greater than that of UC18AMPM-CO2 aqueous foams within the studied temperature scope, signifying that the CO2 aqueous foam stabilized by UC22AMPM exhibits better temperature resistance compared to UC18AMPM foams. In addition, the t1/2 of UC22AMPM-CO2 aqueous foams diminished by 5.3 fold when temperature increased from 25 to 120 °C, smaller than that of the UC18AMPM-CO2 aqueous foams (~9 fold), illustrating the impact of temperature on the stability of UC18AMPM-CO2 aqueous foams is more prevalent related to UC22AMPM. One explanation here could be that the Pc is higher than that of UC18AMPM due to its relatively lower φ.
2.3.2. Effect of Pressure
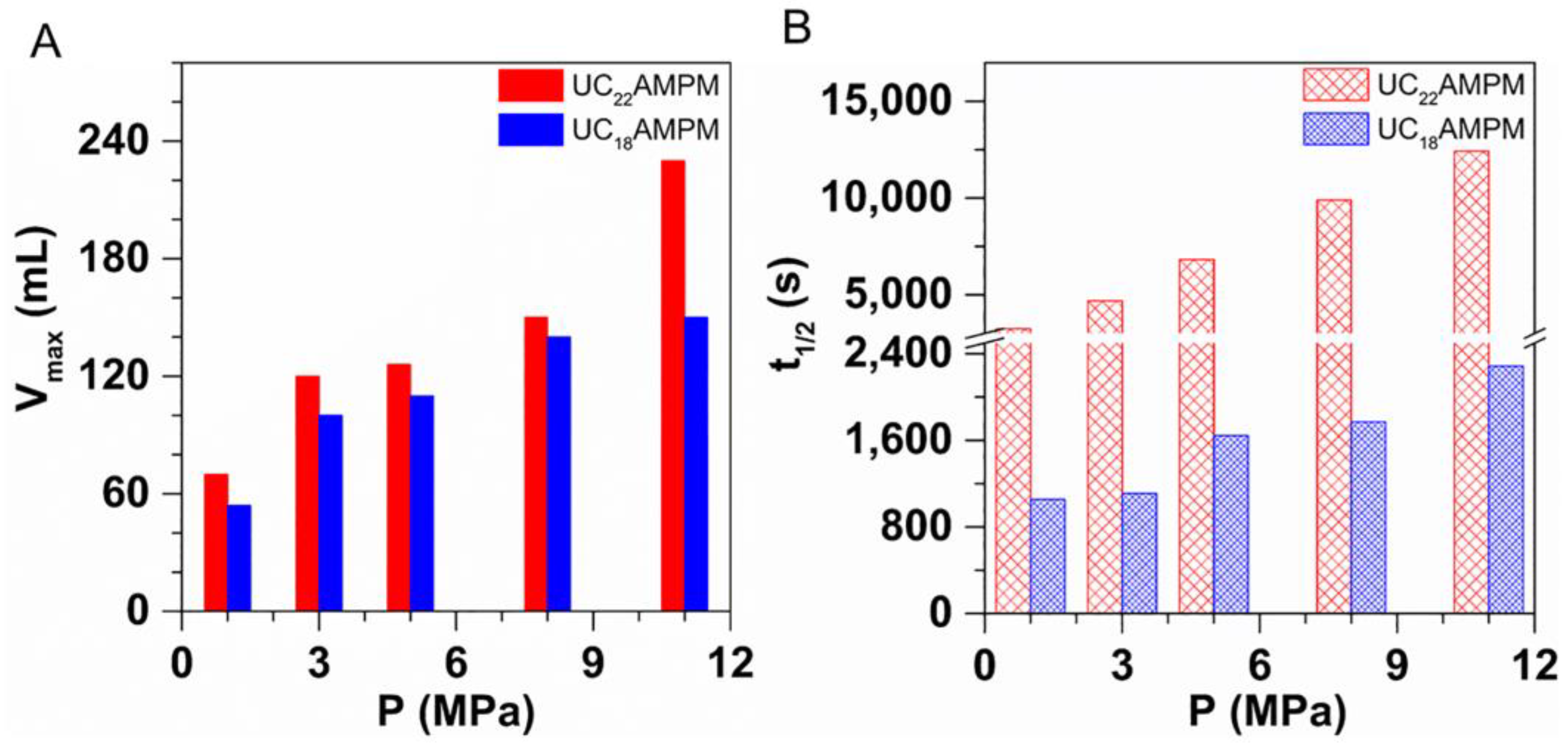
As observed in Figure 6A,B, the Vmax and t1/2 for both samples increased with the increasing pressure, demonstrating that increasing pressure is conducive to foaming ability and foam stability. The finding is consistent with previous studies [21,43,44] attributed to the decrease in the C−W IFT with the pressure increasing. Specifically, high pressure enhances the interactions between CO2 and the hydrophobic tail of surfactant molecules, reducing the contact probability between CO2 and water molecules and thus generating a lower C-W IFT [44]. Clearly, a lower C-W IFT enables the foam to easier form and to mitigate the foam aging process.
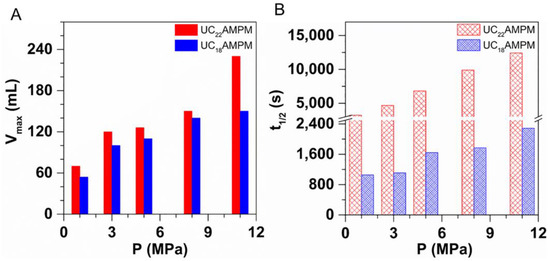
Figure 6.
The influence of pressure on (A) Vmax and (B) t1/2 of 0.5% UC22AMPM-CO2 solution and 0.5% UC18AMPM-CO2 solution, respectively. The experimental temperature is fixed at 120 °C.
Interestingly, the increased scope of Vmax of both foams showed a similar variation tendency with increasing pressure. The Vmax for UC22AMPM-CO2 aqueous foam increased from 70 to 230 mL at the tested pressures scope; the UC18AMPM-CO2 aqueous foam increased from 54 and 150 mL under identical conditions. Their Vmax increased by approximately three times, suggesting the effect of pressure on the foaming ability of both compounds is identical. Instead, the t1/2 for UC22AMPM-CO2 aqueous foam increased from 3200 and 12,400 s, showing a faint increase; while the t1/2 of UC18AMPM-CO2 aqueous foam underwent a slight increase from 1000 to 2200 s. The growth fold of t1/2 for UC22AMPM-CO2 aqueous foam is around 3.9, higher than that of UC18AMPM ones (2.2). These results highlighted that pressure is more prominent in enhancing the stability of UC22AMPM-CO2 aqueous foam compared with that of UC18AMPM-CO2 aqueous foam.
2.3.3. Effect of Salinity
Inorganic salts have been found to modulate the surface activities [45], altering the properties of the surfactant-stabilized foam [43]. Hence, a common sodium chloride (NaCl) was used as representative inorganic salt to add the above two foam systems to clarify the effect of salt on the properties of UC22AMPM and UC18AMPM CO2 aqueous foams.
As depicted in Figure 7A, the Vmax of both foams samples increased initially and then maintained constant with increasing NaCl concentration. For example, the UC18AMPM foam expanded from 151 and 175 mL when NaCl concentration increased from 0 to 1 wt.%; while the UC22AMPM foam slightly grew from 189 and 199 mL by increasing NaCl concentration from 0 to 0.5 wt.%. This means that the addition of a small amount of NaCl is beneficial for foamability. A plausible explanation could be that the addition of NaCl enhanced the adsorption of surfactant molecules at the C-W interface as a result of the charge neutralization, leading to the reducing C-W IFT, and thereby improving foaming ability [46]. Thereafter, the Vmax of both samples remained virtually constant with a further increase in NaCl concentration. We believe that electrostatic repulsions between surfactants are sufficiently shielded at high NaCl content (≥1.0 wt.%). In this scenario, the surfactants were saturated in CO2/water interfaces, and the C-W IFT achieved a minimum value. Consequently, high NaCl concentrations have a negligible effect on foamability.
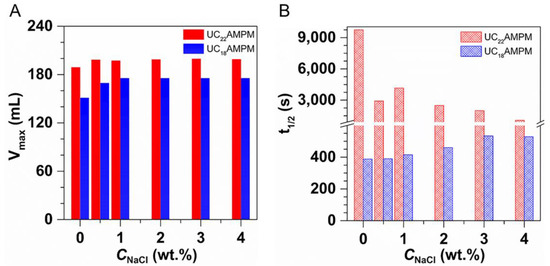
Figure 7.
Comparison of the effect of NaCl concentrations on (A) Vmax and (B) t1/2 of 0.5% UC22AMPM-CO2 solution and 0.5% UC18AMPM-CO2 solution, respectively, at 35 °C under atmospheric pressure.
Compared in Figure 7B is the t1/2 for two cases of CO2 aqueous foams as a function of NaCl concentration. Overall, the t1/2 of the UC22AMPM foam samples showed a downtrend at the tested NaCl concentrations, manifesting that the addition of NaCl undermined the foam stability of UC22AMPM. This can be interpreted with the fact that the additional NaCl causes a transformation from linear to branched micelles, leading to a decrease in η [47,48]. Upon the decrease in η, the foam aging process would speed up, leading to rapid foam destruction. As for CO2 aqueous foams made from UC18AMPM, t1/2 gradually increased and then remain unchanged with increasing salinity. We also attributed this enhanced t1/2 to the fact that the presence of NaCl enhances the adsorption density of surfactant molecules on the CO2/water interface through electrostatic screening, enhancing the strength of foam lamella and therefore resisting gas diffusion between bubbles.
It is also noteworthy that the t1/2 of UC22AMPM-CO2 aqueous foams is higher than that of UC18AMPM-CO2 aqueous foams within the studied salinity scope, signifying that the CO2 aqueous foam stabilized by UC22AMPM exhibits better salt tolerance compared to UC18AMPM foams.
3. Materials and Methods
3.1. Materials
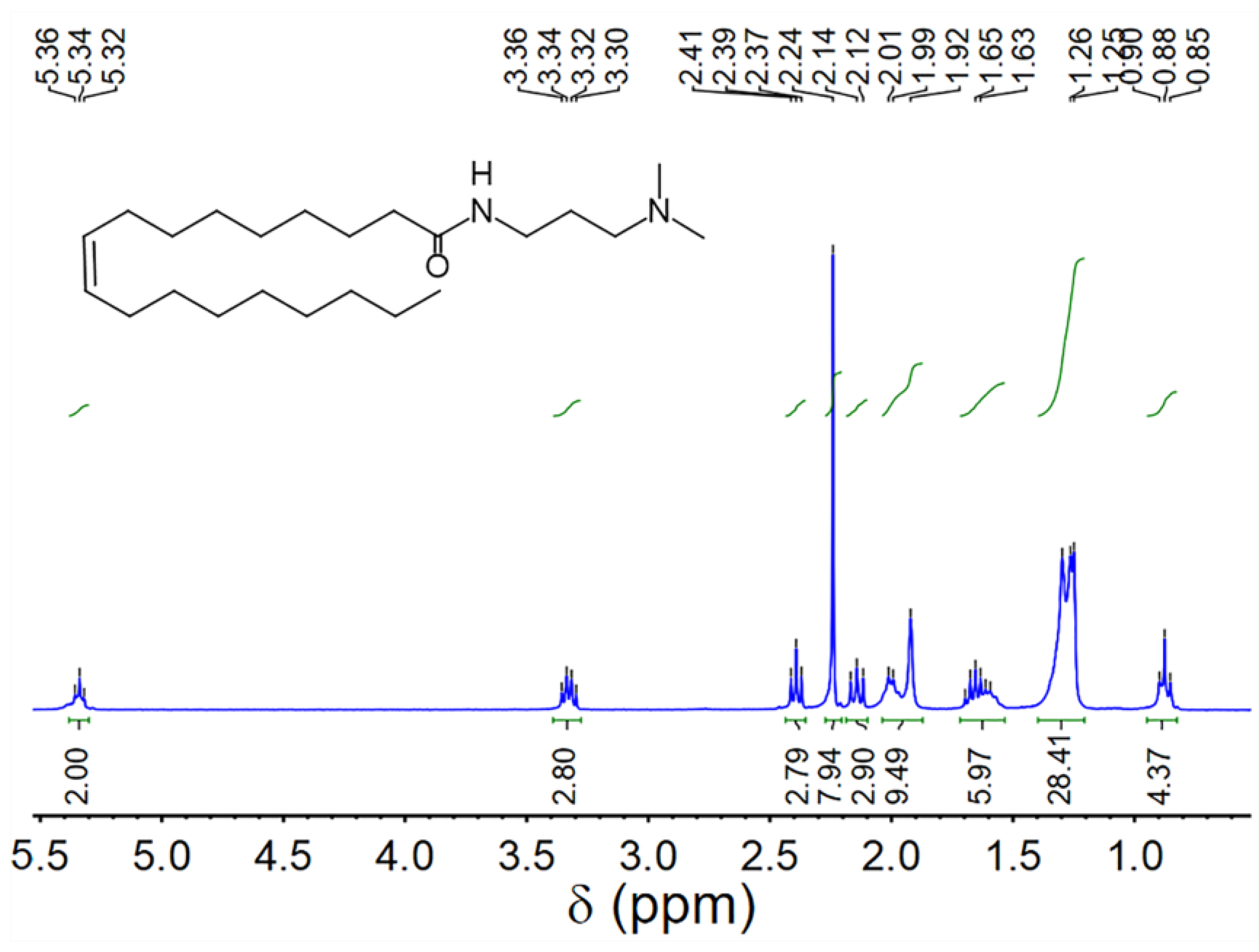
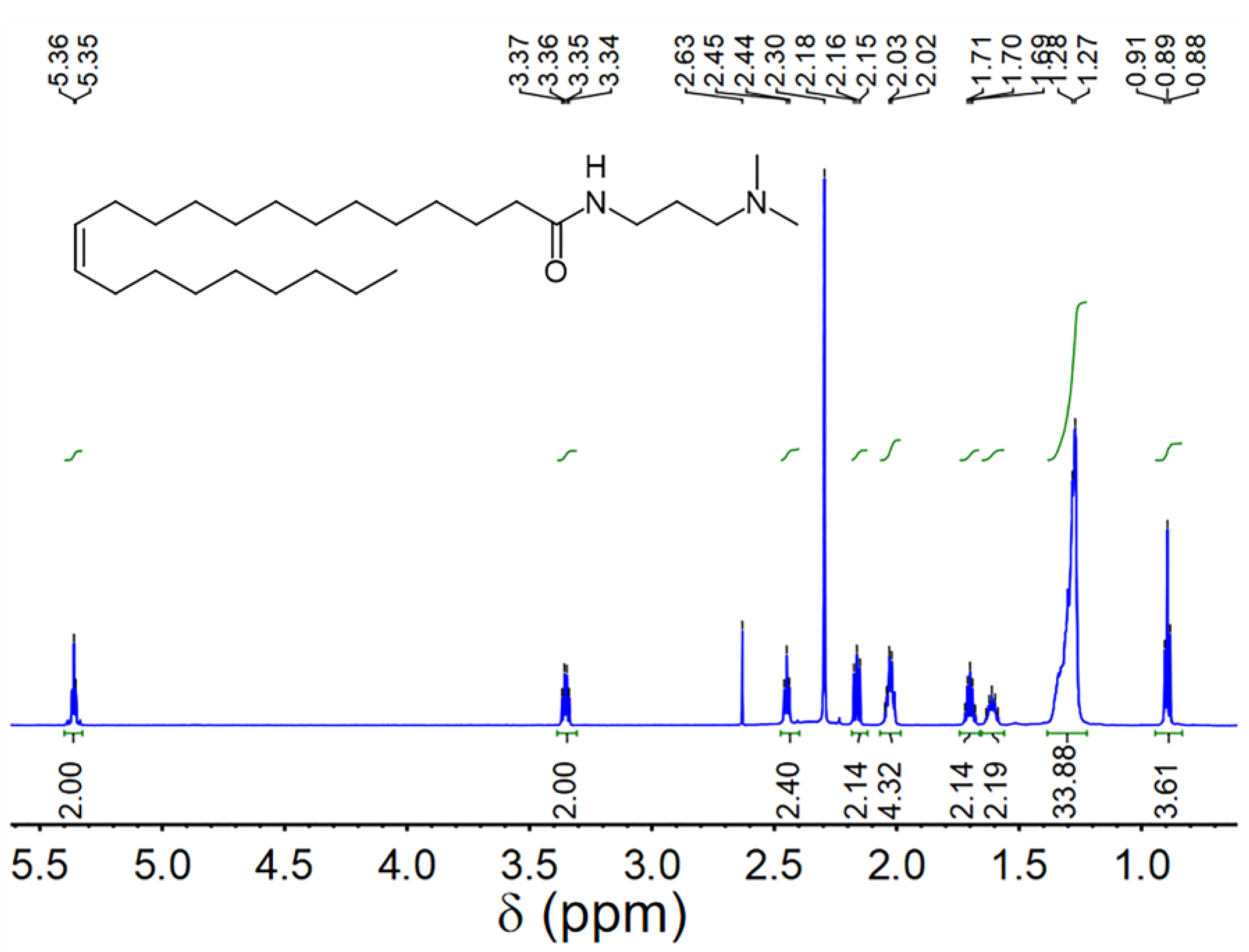
UC18AMPM and UC22AMPM were synthesized according to our previously-reported procedure [39] and confirmed by proton nuclear magnetic resonance spectroscopy (1H NMR, Figure 8 and Figure 9). CO2 (≥99.998%) was purchased from Jinnengda Gas Company (Chengdu, China) and was used as received. Sodium chloride (NaCl, 99%, GC) and NH3·H2O (25 Vol.%) were purchased from Chengdu Kelong Chemical Factory Co., Ltd. (China). CD3Cl (≥98% deuterium content) used for 1H NMR analysis was obtained from Sigma-Aldrich (Shanghai, China). The deionized water with a resistivity of 18.25 MΩ·cm used throughout this study was prepared from a quartz water purification system (UPH-I-10T, Chengdu Ultra-pure Technology Co., Ltd., Chengdu, China).
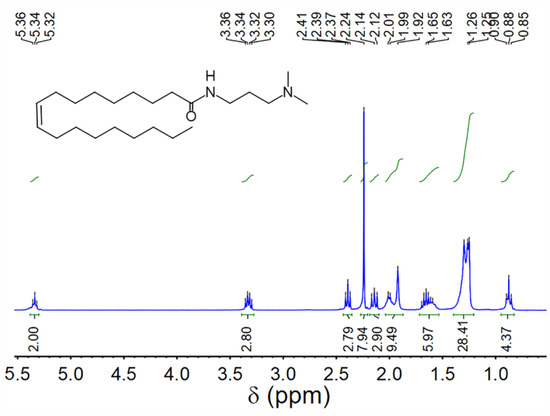
Figure 8.
1H NMR spectrum of UC18AMPM (400 MHz, CDCl3).
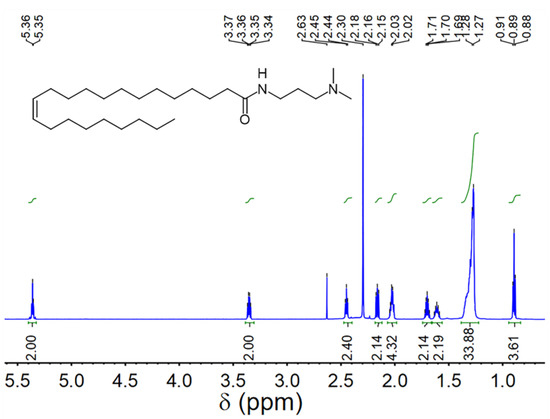
Figure 9.
1H NMR spectrum of UC22AMPM (400 MHz, CDCl3).
3.2. Preparation of Foaming Solution
A concentrated parent dispersion was prepared by adding designed amounts of surfactant samples (UC22AMPM or UC18AMPM) and deionized water to a sealed Schott-Duran bottle equipped with a magnetic bar inside. Next, the resulting mixture was stirred at 60 °C for at least 10 min, yielding low-viscosity emulsion-like dispersion. Remarkably, the dispersion concentration was calibrated by adding water to compensate for the water evaporation during the agitation process. The parent dispersions were cooled to room temperature. Then, the dispersions with desired concentration were obtained by diluting the concentrated parent dispersion with deionized water or brine.
3.3. Evaluation of Aqueous Foams at Atmospheric Pressure
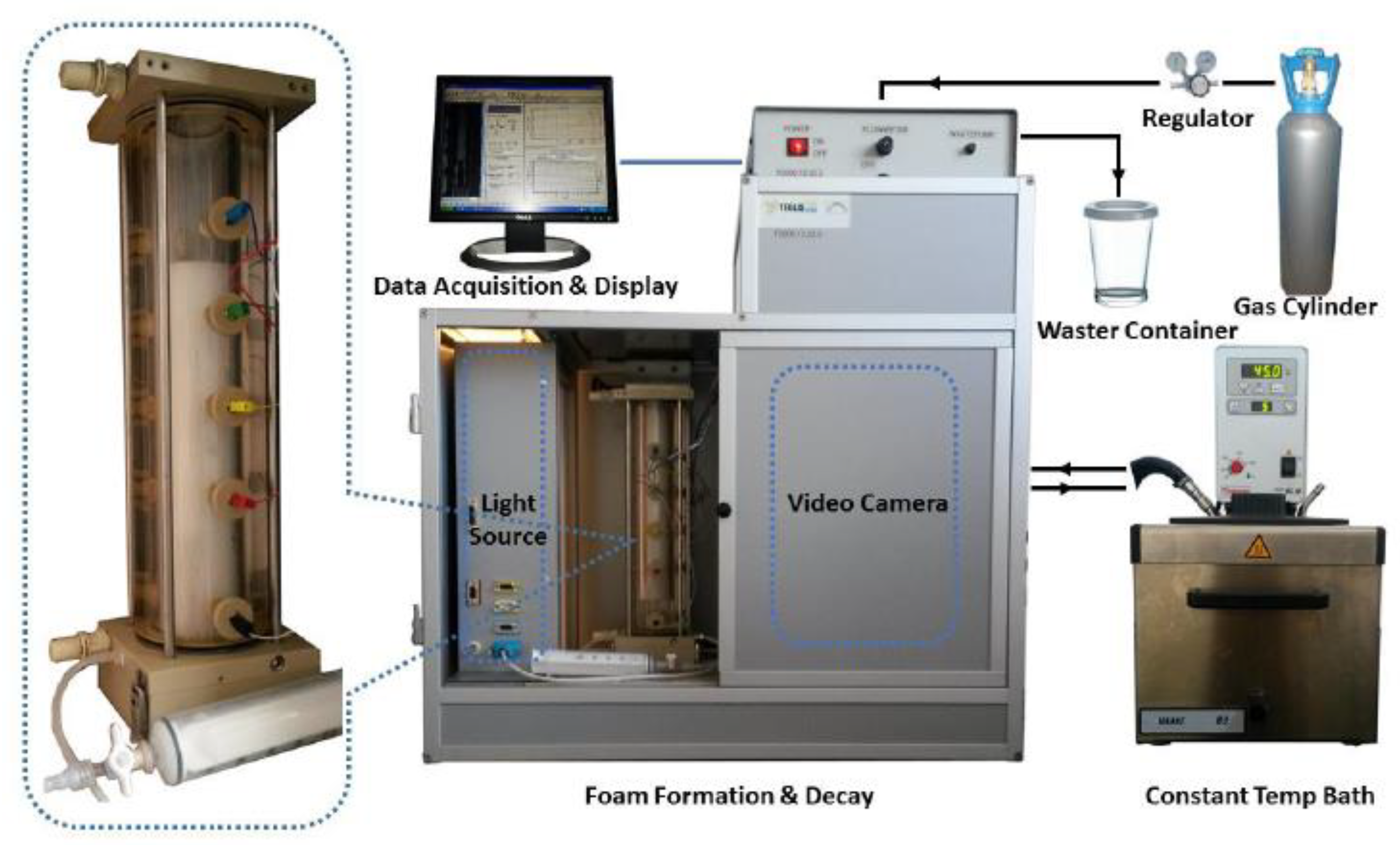
The FoamScan setup (Figure 10, TECLIS, Lyon, France), which combines image analysis and conductivity measurements to monitor foam properties, was employed to characterize the foam properties of two types of ultra-long chain tertiary amines (i.e., UC22AMPM and UC18AMPM). Briefly, 60 mL of dispersions were placed in the glass column with a porous glass filter (pore diameter 0.2 mm) and heated to the desired temperature by an embedded electric heating system. The pressure of the chamber was fixed at atmospheric pressure. Afterward, aqueous foams were formed by bubbling CO2 for two minutes. The CO2 flow rate is constant at 100 mL/min by mass flow meters. The foam volume and liquid content were measured by five pairs of electrodes located along the glass column. The bubbles evolution was captured by the CCD (charge-coupled device) camera after the gas flow stopped.
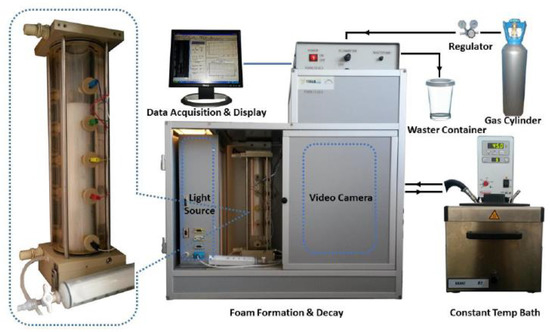
Figure 10.
A schematic diagram of FoamScan setup.
3.4. Evaluation of Aqueous Foams at High Pressure
Given that the FoamScan cannot perform at high pressure, the foam properties under high-pressure conditions were evaluated by an HTHP visualization foam meter (Jiangsu Hongbo Machinery Manufacturing Co., Ltd., Haian China). A detailed description of the HTHP visualization foam meter and operating procedures have been reported in our previous work [6,7,8]. Firstly, 100 mL dispersions were pumped into the visual chamber and heated to the desired temperature by an embedded electric heating system. The CO2 was then bubbled into the chamber to achieve the desired pressure. Afterward, the surfactant dispersions and CO2 were vigorously stirred at 1100 rpm for 3 min. Once agitation ceased, the Vmax and t1/2 were recorded by observing the foam height. All values were measured three times per experiment, and the average value was taken as the final result.
3.5. Characterization of Switchability of Aqueous Foams
To examine the switchability of aqueous foams produced from UC22AMPM and UC18AMPM, the CO2 and NH3·H2O (25 vol.%) were used as triggers to ‘‘switch’’ foam on and off. First, at a 3 MPa CO2 atmosphere, the aqueous foams were generated by the agitation of 100 mL of UC22AMPM and UC18AMPM aqueous dispersion at 1020 rpm for 3 min using an HTHP visualization foam meter, respectively. Subsequently, the appropriate amount of NH3·H2O was introduced to the CO2 aqueous foam system, during which the foaming and defoaming processes were tracked. This operation was repeated five times, and each cycle was separated by 10 min. All measurements were performed at 80 °C.
3.6. Rheological Test of Foaming Solution
The rheological measurements of the foaming solution were carried out on a Physica MCR 302 (Anton Paar, Graz, Austria) rotational rheometer equipped with a concentric cylinder geometry CC27. At atmospheric pressure, CO2 was first bubbled into the sample at a flow rate of 200 ± 1 mL/min for 2 min. Then, 16 mL of previously gas-treated sample was introduced to the measuring cell and thermostatically incubated at the desired temperature for 20 min prior to experimentation. A solvent trap was used to reduce water evaporation in the experiments. For all experiments, flow curves were registered in a stress-controlled mode, and the data were acquired by the software Rheoplus TM. The temperature was finely controlled by a Peltier temperature control device.
4. Conclusions
In this work, we investigate comparatively the properties of CO2 foams stabilized by UC22AMPM and UC18AMPAM and examined the evolution trend of foam properties concerning variation in external factors (i.e., temperature, pressure and salinity). The results showed that CO2 aqueous foams prepared from UC18AMPM exhibited similar switching properties to UC22AMPM, arising from their identical tertiary amine headgroups. However, due to the relatively long hydrophobic chain, UC22AMPM molecules self-assembled into wormlike micelles, but UC18AMPM cannot. The entanglement of these wormlike micelles into a transient network imparts high viscosity to the continuous phase of foam. During the foaming process, a large amount of liquid was transported into the foam liquid channels, forming the thicker foam film. Meanwhile, the high continuous phase viscosity of the foam system decelerates lamellae drainage. With lower drainage, the lamella remained thicker. The thicker films would enhance foam strength as well as hinder gas diffusion, arresting coalescence and Ostwald ripening, thereby enhancing the foam’s lifetime. On the contrary, the viscosity of the UC18AMPM sample decreased to ~1.0 mPa·s because of the absence of wormlike micelles. The lower viscosity accelerated the drainage process, weakening the strength of the foam film. The reduced strength and thickness of foam film, in turn, led to the bursting of bubbles. As a result, UC22AMPM foam displayed better foaming ability and foam stability compared to UC18AMPAM foam under identical concentrations. More importantly, for UC22AMPM-CO2 foam, the positive influence derived from pressure and concentration on its foam properties is much more pronounced than those of its UC18AMPM counterpart. Compared with UC18AMPM-CO2 foam, the salinity and temperature had a relatively weak negative effect on the properties of UC22AMPM-CO2 foam. In summary, this comparative study advances mechanistic insights into the role of surfactant architecture in foam properties, as well as establishes macroscopic links among foam properties, surfactant structure and environmental factors, promoting the development of such foam systems.
Author Contributions
Experiment, Writing—original draft, M.L.; Investigation, Experiment, X.Z.; Conceptualization, Writing—Review and Editing, J.W.; Conceptualization, Supervision, Y.F. All authors have read and agreed to the published version of the manuscript.
Funding
Financial support from the Natural Science Foundation of Sichuan Province (2022NSFSC0030) and the National Natural Science Foundation of China (21773161) is gratefully acknowledged.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds including UC22AMPM and UC18AMPM, are available from the authors.
References
- Langevin, D. Aqueous foams: A field of investigation at the frontier between chemistry and physics. Chemphyschem 2008, 9, 510–522. [Google Scholar] [CrossRef]
- Wei, P.; Guo, K.; Pu, W.; Xie, Y.; Huang, X.; Zhang, J. Aqueous Foam Stabilized by an in Situ Hydrophobic Polymer via Interaction with Alkyl Polyglycoside for Enhancing Oil Recovery. Energy Fuels 2020, 34, 1639–1652. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Hu, Y.; Yu, X.; He, G.; Fu, W. Froth flotation separation of lepidolite ore using a new Gemini surfactant as the flotation collector. Sep. Purif. Technol. 2022, 282, 119122–119136. [Google Scholar] [CrossRef]
- Sheng, Y.; Jiang, N.; Lu, S.; Wang, Q.; Zhao, Y.; Liu, X. Study of environmental-friendly firefighting foam based on the mixture of hydrocarbon and silicone surfactants. Fire Technol. 2020, 56, 1059–1075. [Google Scholar] [CrossRef]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. Foamability of sodium dodecyl sulfate solutions: Anomalous effect of dodecanol unexplained by conventional theories. Colloids Surf. A 2016, 495, 110–117. [Google Scholar] [CrossRef]
- Farajzadeh, R.; Krastev, R.; Zitha, P.L.J. Foam films stabilized with alpha olefin sulfonate (AOS). Colloids Surf. A 2008, 324, 35–40. [Google Scholar] [CrossRef]
- Saint-Jalmes, A. Physical chemistry in foam drainage and coarsening. Soft Matter 2006, 2, 836–849. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nguyen, A.V.; Farrokhpay, S. A critical review of the growth, drainage and collapse of foams. Adv. Colloid Interface Sci. 2016, 228, 55–70. [Google Scholar] [CrossRef]
- Langevin, D. Aqueous foams and foam films stabilised by surfactants. Gravity-free studies. C. R. Mécanique 2017, 345, 47–55. [Google Scholar] [CrossRef]
- Heerschap, S.; Marafino, J.N.; McKenna, K.; Caran, K.L.; Feitosa, K. Foams stabilized by tricationic amphiphilic surfactants. Colloids Surf. A 2015, 487, 190–197. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Wang, P. Experimental Study of the Stabilization of CO2 foam by sodium dodecyl sulfate and hydrophobic nanoparticles. Ind. Eng. Chem. Res. 2016, 55, 1243–1253. [Google Scholar] [CrossRef]
- Fukuoka, K.; Tomikawa, A.; Nakamura, Y.; Fujii, S. Aqueous foams stabilized with several tens of micrometer-sized polymer particles: Effects of surface hydrophilic–hydrophobic balance on foamability and foam stability. Chem. Lett. 2016, 45, 667–669. [Google Scholar] [CrossRef]
- Pu, W.-F.; Wei, P.; Sun, L.; Jin, F.-Y.; Wang, S. Experimental investigation of viscoelastic polymers for stabilizing foam. Ind. Eng. Chem. Ind. Ed. 2017, 47, 360–367. [Google Scholar] [CrossRef]
- Rouimi, S.; Schorsch, C.; Valentini, C.; Vaslin, S. Foam stability and interfacial properties of milk protein-surfactant systems. Food Hydrocoll. 2005, 19, 467–478. [Google Scholar] [CrossRef]
- Murray, B.S.; Ettelaie, R. Foam stability: Proteins and nanoparticles. Curr. Opin. Colloid Interface Sci. 2004, 9, 314–320. [Google Scholar] [CrossRef]
- Davis, J.P.; Foegeding, E.A. Comparisons of the foaming and interfacial properties of whey protein isolate and egg white proteins. Colloids Surf. B 2007, 54, 200–210. [Google Scholar] [CrossRef]
- Stocco, A.; Rio, E.; Binks, B.P.; Langevin, D. Aqueous foams stabilized solely by particles. Soft Matter 2011, 7, 1260–1267. [Google Scholar] [CrossRef]
- Binks, B.P.; Horozov, T.S. Aqueous foams stabilized solely by silica nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 3722–3725. [Google Scholar] [CrossRef] [PubMed]
- Schad, T.; Preisig, N.; Blunk, D.; Piening, H.; Drenckhan, W.; Stubenrauch, C. Less is more: Unstable foams clean better than stable foams. J. Colloid Interface Sci. 2021, 590, 311–320. [Google Scholar] [CrossRef]
- Alzobaidi, S.; Da, C.; Tran, V.; Prodanovic, M.; Johnston, K.P. High temperature ultralow water content carbon dioxide-in-water foam stabilized with viscoelastic zwitterionic surfactants. J. Colloid Interface Sci. 2017, 488, 79–91. [Google Scholar] [CrossRef]
- Wang, J.; Luo, X.; Rogers, S.; Li, P.; Feng, Y. Stabilization of CO2 aqueous foams at high temperature and high pressure: Small-angle neutron scattering and rheological studies. Colloids Surf. A 2022, 647, 129015–129026. [Google Scholar] [CrossRef]
- Chen, Y.; Elhag, A.S.; Worthen, A.J.; Reddy, P.P.; Ou, A.M.; Hirasaki, G.J.; Nguyen, Q.P.; Biswal, S.L.; Johnston, K.P. High Temperature CO2-in-water foams stabilized with cationic quaternary ammonium Surfactants. J. Chem. Eng. Data 2016, 61, 2761–2770. [Google Scholar] [CrossRef]
- Elhag, A.S.; Da, C.; Chen, Y.; Mukherjee, N.; Noguera, J.A.; Alzobaidi, S.; Reddy, P.P.; AlSumaiti, A.M.; Hirasaki, G.J.; Biswal, S.L.; et al. Viscoelastic diamine surfactant for stable carbon dioxide/water foams over a wide range in salinity and temperature. J. Colloid Interface Sci. 2018, 522, 151–162. [Google Scholar] [CrossRef]
- Xue, Z.; Worthen, A.; Qajar, A.; Robert, I.; Bryant, S.L.; Huh, C.; Prodanovic, M.; Johnston, K.P. Viscosity and stability of ultra-high internal phase CO2-in-water foams stabilized with surfactants and nanoparticles with or without polyelectrolytes. J. Colloid Interface Sci. 2016, 461, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Chu, Z.; Feng, Y. Vegetable-derived long-chain surfactants synthesized via a “Green” route. ACS Sustain. Chem. Eng. 2012, 1, 75–79. [Google Scholar] [CrossRef]
- Feng, Y.; Chu, Z. A Facile route towards the preparation of ultra-long-chain amidosulfobetaine surfactants. Synlett 2009, 2009, 2655–2658. [Google Scholar] [CrossRef]
- Zhang, P.; Bai, G.; Cui, G.; Zhang, L.; Peng, X.; Pei, S.; Ren, S. Enhanced CO2 foam based on amide and amine surfactants and synergistically coupled with sodium dodecyl sulfate at high temperature and high pressure. J. Pet. Sci. Eng. 2019, 179, 266–275. [Google Scholar] [CrossRef]
- Salonen, A.; In, M.; Emile, J.; Saint-Jalmes, A. Solutions of surfactant oligomers: A model system for tuning foam stability by the surfactant structure. Soft Matter 2010, 6, 2271–2281. [Google Scholar] [CrossRef]
- Hu, X.; Li, Y.; He, X.; Li, C.; Li, Z.; Cao, X.; Xin, X.; Somasundaran, P. Structure-behavior-property relationship study of surfactants as foam stabilizers explored by experimental and molecular simulation approaches. J. Phys. Chem. B 2012, 116, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Fameau, A.-L.; Ventureira, J.; Novales, B.; Douliez, J.-P. Foaming and emulsifying properties of fatty acids neutralized by tetrabutylammonium hydroxide. Colloids Surf. A 2012, 403, 87–95. [Google Scholar] [CrossRef]
- Oh, S.G.; Shah, D.O. Relationship between micellar lifetime and foamability of sodium dodecyl sulfate and sodium dodecyl sulfate/1-hexanol mixtures. Langmuir 1991, 7, 1316–1318. [Google Scholar] [CrossRef]
- Petkova, B.; Tcholakova, S.; Chenkova, M.; Golemanov, K.; Denkov, N.; Thorley, D.; Stoyanov, S. Foamability of aqueous solutions: Role of surfactant type and concentration. Adv. Colloid Interface Sci. 2020, 276, 102084–102103. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Qi, X.; Sun, H.; Zhao, H.; Li, Y. Understanding about How Different Foaming Gases Effect the Interfacial Array Behaviors of Surfactants and the Foam Properties. Langmuir 2016, 32, 7503–7511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Qu, C.; Lu, G.; Li, Z.; Wang, X.; Yin, H.; Feng, Y. Deliquification of low-productivity natural gas wells with in situ generated foams and heat. Energy Fuels 2021, 35, 9873–9882. [Google Scholar] [CrossRef]
- Wang, J.; Liang, M.; Tian, Q.; Feng, Y.; Yin, H.; Lu, G. CO2-switchable foams stabilized by a long-chain viscoelastic surfactant. J. Colloid Interface Sci. 2018, 523, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Dehdari, B.; Parsaei, R.; Riazi, M.; Rezaei, N.; Zendehboudi, S. New insight into foam stability enhancement mechanism, using polyvinyl alcohol (PVA) and nanoparticles. J. Mol. Liq. 2020, 307, 112755–112768. [Google Scholar] [CrossRef]
- Pang, Z.; Wu, Y.; Zhao, M. Novel evaluation method of foam agents for thermal recovery in heavy oil Reservoirs. Energy Fuels 2016, 30, 2948–2957. [Google Scholar] [CrossRef]
- Hill, C.; Eastoe, J. Foams: From nature to industry. Adv. Colloid Interface Sci. 2017, 247, 496–513. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Zhang, Y.; Chen, Q.; Wang, J.; Li, B.; Feng, Y. Synthesis and surface activities of amidobetaine surfactants with ultra-long unsaturated hydrophobic chains. J. Surfactants Deterg. 2012, 15, 657–661. [Google Scholar] [CrossRef]
- Wang, J.; Feng, Y.; Agrawal, N.R.; Raghavan, S.R. Wormlike micelles versus water-soluble polymers as rheology-modifiers: Similarities and differences. Phys. Chem. Chem. Phys. 2017, 19, 24458–24466. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, Z.; Dreiss, C.A.; Wang, Y.; Fei, C.; Feng, Y. Smart wormlike micelles switched by CO2 and air. Soft Matter 2013, 9, 6217–6221. [Google Scholar] [CrossRef]
- Langevin, D. On the rupture of thin films made from aqueous surfactant solutions. Adv. Colloid Interface Sci. 2020, 275, 102075–102084. [Google Scholar] [CrossRef]
- Qu, C.; Wang, J.; Yin, H.; Lu, G.; Li, Z.; Feng, Y. Condensate oil-tolerant foams stabilized by an anionic-sulfobetaine surfactant mixture. ACS Omega 2019, 4, 1738–1747. [Google Scholar] [CrossRef]
- Fan, C.; Jia, J.; Peng, B.; Liang, Y.; Li, J.; Liu, S. Molecular dynamics study on CO2 foam films with sodium dodecyl sulfate: Effects of surfactant concentration, temperature, and pressure on the interfacial tension. Energy Fuels 2020, 34, 8562–8574. [Google Scholar] [CrossRef]
- Maiti, K.; Mitra, D.; Guha, S.; Moulik, S.P. Salt effect on self-aggregation of sodium dodecylsulfate (SDS) and tetradecyltrimethylammonium bromide (TTAB): Physicochemical correlation and assessment in the light of Hofmeister (lyotropic) effect. J. Mol. Liq. 2009, 146, 44–51. [Google Scholar] [CrossRef]
- Kumar, B.; Tikariha, D.; Ghosh, K.K. Effects of Electrolytes on Micellar and Surface Properties of Some Monomeric Surfactants. J. Dispers. Sci. Technol. 2012, 33, 265–271. [Google Scholar] [CrossRef]
- McCoy, T.M.; Valiakhmetova, A.; Pottage, M.J.; Garvey, C.J.; Campo, L.; Rehm, C.; Kuryashov, D.A.; Tabor, R.F. Structural Evolution of Wormlike Micellar Fluids Formed by Erucyl Amidopropyl Betaine with Oil, Salts, and Surfactants. Langmuir 2016, 32, 12423–12433. [Google Scholar] [CrossRef]
- Parker, A.; Fieber, W. Viscoelasticity of anionic wormlike micelles: Effects of ionic strength and small hydrophobic molecules. Soft Matter 2013, 9, 1203–1213. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).