Bioactivity-Guided High Performance Counter-Current Chromatography and Following Semi-Preparative Liquid Chromatography Method for Rapid Isolation of Anti-Inflammatory Lignins from Dai Medicinal Plant, Zanthoxylum acanthopodium var. timbor
Abstract
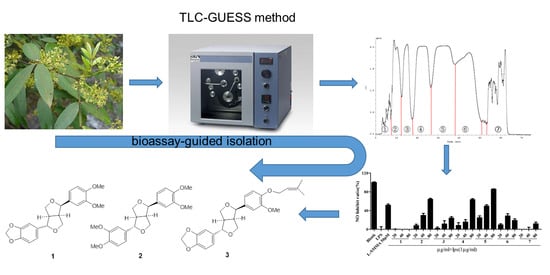
:1. Introduction
2. Results and Discussion
2.1. Nitric Oxide (NO) Production Inhibitor Screening Experiment
2.2. Optimization of HPCCC Solvent Systems
2.3. Screening of Anti-Inflammatory Activities of Different Fractions from HPCCC
2.4. Purification of the Target HPCCC Peaks
2.5. The Anti-Inflammatory Activity of Fargesin (1) and Epieudesmin (2) on TPH-1 Cells
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Sample Preparation
3.4. Selection of Two-Phase Solvent System
3.5. HPCCC Separation Process
3.6. HPLC Conditions
3.7. The Screening of NO Generation Inhibitors
3.8. Anti-Inflammatory Activity on THP-1 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- State Adeministration of Traditional Chinese Medicine of the People’ s Republic of China. Traditional Chinese Meteria Medica of Dai Nationality; Shanghai Science and Technology Press: Shanghai, China, 2005; ISBN 9787532380091. [Google Scholar]
- Ethnic Medicine Research Office of Xishuangbanna Dai Autonomous Prefecture. Annals of Xishuangbanna Dai Medicine; Board of Health of Xishuangbanna: Xishuangbanna, China, 1981. [Google Scholar]
- Pei, S.J. Overview on research and development of ethno-medicine in China. In Herbal Globalisation: A New Paradigm for Malaysian Herbal Industry: Proceedings of the Seminar on Medicinal and Aromatic Plants (MAPS 2008), Kuala Lumpur, Malaysia, 21–22 October 2009; Forest Research Institute Malaysia: Kuala Lumpur, Malaysia, 2009; pp. 1–10. [Google Scholar]
- Wang, G.H.; Yang, X.; Wang, N.H.; Tong, Y.F.; Dong, H.; Nie, Q.; Office, S. A discussion of the path to accelerate the development of dai medical education in China. J. Res. EFEM 2018, 1, 83–88. [Google Scholar]
- Rong, W.; Peng, X.; Wang, L.; Tan, B.; Liu, J.; Feng, Y.; Yang, S.J. Preparative purification of peoniflorin and albiflorin from peony rhizome using macroporous resin and medium-pressure liquid chromatography. J. Sep. Sci. 2015, 35, 1985–1992. [Google Scholar]
- Ashraf-Khorassani, M.; Taylor, L.T.; Martin, M.J.C. Supercritical fluid extraction of Kava lactones from Kava root and their separation via supercritical fluid chromatography. Chromatographia 1999, 50, 287–292. [Google Scholar] [CrossRef]
- Ito, Y.; Conway, W.D. High-speed countercurrent chromatography. Crit. Rev. Anal. Chem. 1986, 17, 65–143. [Google Scholar] [CrossRef]
- Berthod, A. Countercurrent chromatography and the journal of liquid chromatography: A love story. J. Liq. Chromatogr. Relat. Technol. 2007, 30, 1447–1463. [Google Scholar] [CrossRef]
- Ito, Y.; Knight, M.; Finn, T.M. Spiral Countercurrent Chromatography; Springer: Heidelberg, Germany, 1982. [Google Scholar]
- Sutherland, I.A.; Fisher, D.J. Role of counter-current chromatography in the modernisation of Chinese herbal medicines. J. Chromatogr. A 2009, 1216, 740–753. [Google Scholar] [CrossRef]
- Han, L.W.; Yuan, Y.Q.; Zhao, L.; He, Q.X.; Li, Y.B.; Chen, X.Q.; Liu, X.H.; Liu, K.C. Tracking antiangiogenic components from Glycyrrhiza uralensis based on zebrafish assays using high-speed countercurrent chromatography. J. Sep. Sci. 2012, 35, 1167–1172. [Google Scholar] [CrossRef]
- Surup, F.; Tran, T.M.T.; Pfvtze, S.; Budde, J.; Moussa-Ayoub, T.E.; Rohn, S.; Jerz, G. Opuntisines, 14-membered cyclopeptide alkaloids from fruits of Opuntia stricta var. dillenii isolated by high-performance countercurrent chromatography. Food Chem. 2020, 334, 127552. [Google Scholar]
- Hammerschick, T.; Wagner, T.; Vetter, W. Countercurrent chromatographic fractionation followed by gas chromatography/mass spectrometry identification of alkylresorcinols in rye. Anal. Bioanal. Chem. 2020, 412, 8417–8430. [Google Scholar] [CrossRef]
- Yang, Y.F.; Sun, X.; Ni, H.; Du, X.P.; Chen, F.; Jiang, Z.D.; Li, Q.B. Identification and characterization of the tyrosinase inhibitory activity of caffeine from Camellia Pollen. J. Agric. Food Chem. 2019, 67, 12741–12751. [Google Scholar] [CrossRef]
- Li, S.N.; Tang, Y.; Liu, C.M.; Zhang, Y.C. Development of a method to screen and isolate potential alpha-glucosidase inhibitors from Panax japonicus CA Meyer by ultrafiltration, liquid chromatography, and counter-current chromatography. J. Sep. Sci. 2015, 38, 2014–2023. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.H.; Wang, Z.Q.; Hwang, S.H.; Kang, Y.H.; Lee, J.Y.; Lim, S.S. Comprehensive evaluation of the antioxidant capacity of Perilla frutescens leaves extract and isolation of free radical scavengers using step-wise HSCCC guided by DPPH-HPLC. Int. J. Food Prop. 2017, 20, 921–934. [Google Scholar] [CrossRef] [Green Version]
- Vieir, M.N.; Winterhalter, P.; Jerz, G. Flavonoids from the flowers of Impatiens glandulifera isolated by high performance countercurrent chromatography. Phytochem. Anal. 2016, 27, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Deamicis, C.; Yang, Q.; Bright, C.; Edwards, N.; Harris, G.; Kaur, S.; Wood, P.; Hewitson, P.; Ignatova, S. Development of a scalable and sustainable high performance countercurrent chromatography (HPCCC) purification for spinosyn A and spinosyn D from spinosad. Org. Process Res. 2017, 21, 1638–1643. [Google Scholar] [CrossRef] [Green Version]
- Committee of Chinese Flora. Flora of China; Science Press: Beijing, China, 1997; p. 42. [Google Scholar]
- Zhou, L. Studies on Chemical Constituents and Their Bioactivities of Zanthoxylum acanthopodium var. timbor, Fissistigma polyanthum and Mappianthus iodoides; University of Chinese Academy of Sciences: Beijing, China, 2022. [Google Scholar]
- Chen, I.S.; Tsai, I.W.; Teng, C.M.; Chen, J.J.; Chang, Y.L.; Ko, F.N.; Lu, M.C.; Pezzuto, J.M. Pyranoquinoline alkaloids from Zanthoxylum simulans. Phytochemistry 1997, 46, 525–529. [Google Scholar] [CrossRef]
- Li, W.; Sun, Y.N.; Yan, X.T.; Yang, S.Y.; Kim, E.-J.; Kang, H.K.; Kim, Y.H. Coumarins and lignans from Zanthoxylum schinifolium and their anticancer activities. J. Agric. Food Chem. 2013, 61, 10730–10740. [Google Scholar] [CrossRef]
- Sun, L.; Yu, D.; Wu, Z.; Wang, C.; Yu, L.; Wei, A.; Wang, D.J. Comparative transcriptome analysis and expression of genes reveal the biosynthesis and accumulation patterns of key flavonoids in different varieties of Zanthoxylum bungeanum leaves. J. Agric. Food Chem. 2019, 67, 13258–13268. [Google Scholar] [CrossRef]
- Pang, W.W.; Liu, S.; He, F.T.; Li, X.Y.; Saira, B.; Zheng, T.L.; Chen, J.Y.; Dong, K.; Pei, X.F. Anticancer activities of Zanthoxylum bungeanum seed oil on malignant melanoma. J. Ethnopharmacol. 2019, 229, 180–189. [Google Scholar] [CrossRef]
- Wang, Z.J.; Zhou, Y.; Shi, X.L.; Xia, X.; Luo, X.D. Comparison of chemical constituents in diverse Zanthoxylum herbs, and evaluation of their relative antibacterial and nematicidal activity. Food Biosci. 2021, 42, 101206. [Google Scholar] [CrossRef]
- Barus, M.N.G.; Syafruddinilyas, M.S.; Bachtiar, A. Andaliman fruit extract (Zanthoxylum acantophodium) and it’s effect on preeclampsia as anti-inflammatory. Int. J. Curr. Pharm. Res. 2020, 12, 75–77. [Google Scholar] [CrossRef] [Green Version]
- Nooreen, Z.; Tandon, S.; Yadav, N.P.; Kumar, P.; Ahmad, A. Zanthoxylum: A systematic review of its traditional uses, naturally occurring constituents and pharmacological properties. Curr.Org. Chem. 2019, 23, 1307–1341. [Google Scholar] [CrossRef]
- Ito, Y.; Conway, W.D. Development of countercurrent chromatography. Nature 1984, 326, 65–143. [Google Scholar] [CrossRef]
- Sun, J.; Sun, J.; Heping, L.I.; Yan, X.; Dianpeng, L.I.; Fenglai, L.U. Preparation of cucurbitacin compounds in Siraitia grosvenorii roots by high speed countercurrent chromatography. Chin. J. Chromatogr. 2022, 40, 364–371. [Google Scholar] [CrossRef]
- Fan, Q.F.; Zhang, H.L.; Hu, H.B.; Xu, Y.K.; Song, Q.S. Quick method for separating target compounds from the bark of Maqian (Zanthoxylum myriacanthum var. pubescens) by high-performance countercurrent chromatography. J. Sep. Sci. 2016, 39, 4049–4052. [Google Scholar] [CrossRef]
- Yang, L.; Friesen, J.B.; Grzelak, E.M.; Fan, Q.F.; Pauli, G.F. Sweet spot matching: A thin-layer chromatography-based countercurrent solvent system selection strategy. J. Chromatogr. A 2017, 1504, 46–54. [Google Scholar]
- Li, D.X.; Liu, M.; Zhou, X.J. A new dimeric lignan from Zanthoxylum simulans. China J. Chin. Mater. Med. 2015, 40, 2843–2848. [Google Scholar]
- Bastos, J.K.; Gottlieb, O.R.; Sarti, S.J.; Filho, D.S. Isolation of lignans and sesquiterpenoids from leaves of Zanthoxylum naranjillo. Nat. Prod. Let. 1996, 9, 65–70. [Google Scholar] [CrossRef]
- Cai, X.; Yu, H.Z.; Yu, Y.; Li, Q.; Chen, B.; Huang, Y.; Zou, X.W.; Huang, B.S.; Tang, J.T. Separation of five naphtho-γ-pyrones from Pleurotus ostreatus by high-speed countercurrent chromatography. J. Sep. Sci. 2018, 41, 4551–4558. [Google Scholar] [CrossRef]
- Reif, D.W.; McCreedy, S.A. N-nitro-L-arginine and N-monomethyl-L-arginine exhibit a different pattern of inactivation toward the three nitricoxide synthases. Arch. Biochem. Biophys. 1995, 320, 170–176. [Google Scholar] [CrossRef]
- Jin, K.K.; Hyun, C.G. 4-Hydroxy-7-Methoxycoumarin inhibits inflammation in LPS-activated RAW264.7 macrophages by suppressing NF-κB and MAPK activation. Molecules 2020, 25, 4424–4433. [Google Scholar]
- Li, R.; Yang, J.J.; Shi, Y.X.; Zhao, M.; Ji, K.L.; Zhang, P.; Xu, Y.K.; Hu, H.B. Chemical composition, antimicrobial and anti-inflammatory activities of the essential oil from Maqian (Zanthoxylum myriacanthum var. pubescens) in Xishuangbanna, SW China. J. Ethnopharmacol. 2014, 158, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.D.; Huang, S.X.; Han, Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef] [PubMed]




| Sample Name | Concentration | Inhibition Rate of NO Production (%) |
|---|---|---|
| L-NMMA | 50 μM | 51.88 ± 1.17 |
| Residual water fraction | 40 μg/mL | - |
| N-butanol extract | 40 μg/mL | 8.83 ± 1.67 |
| Ethyl acetate extract | 40 μg/mL | 74.59 ± 1.40 |
| Petroleum ether extract | 40 μg/mL | 93.88 ± 0.67 |
| Total extract | 40 μg/mL | 39.35 ± 0.42 |
| NO | Solvent Systems (v/v/v/v) Petroleum Ether/Ethyl Acetate/Methanol/Water | Solvent Systems (v/v) Petroleum Ether / Ethyl Acetateof TLC | Average Rf Value |
|---|---|---|---|
| 1 | 4:1:4:1 | 4:1 | 0.3 |
| 2 | 5:2:5:2 | 5:2 | 0.4 |
| 3 | 1:1:1:1 | 1:1 | 0.6 |
| 4 | 3:2:3:2 | 3:2 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Q.-F.; Zhou, L.; Gongpan, P.-C.; Lu, C.-L.; Chang, H.; Xiang, X. Bioactivity-Guided High Performance Counter-Current Chromatography and Following Semi-Preparative Liquid Chromatography Method for Rapid Isolation of Anti-Inflammatory Lignins from Dai Medicinal Plant, Zanthoxylum acanthopodium var. timbor. Molecules 2023, 28, 2592. https://doi.org/10.3390/molecules28062592
Fan Q-F, Zhou L, Gongpan P-C, Lu C-L, Chang H, Xiang X. Bioactivity-Guided High Performance Counter-Current Chromatography and Following Semi-Preparative Liquid Chromatography Method for Rapid Isolation of Anti-Inflammatory Lignins from Dai Medicinal Plant, Zanthoxylum acanthopodium var. timbor. Molecules. 2023; 28(6):2592. https://doi.org/10.3390/molecules28062592
Chicago/Turabian StyleFan, Qing-Fei, Lan Zhou, Pian-Chou Gongpan, Chuan-Li Lu, Hua Chang, and Xun Xiang. 2023. "Bioactivity-Guided High Performance Counter-Current Chromatography and Following Semi-Preparative Liquid Chromatography Method for Rapid Isolation of Anti-Inflammatory Lignins from Dai Medicinal Plant, Zanthoxylum acanthopodium var. timbor" Molecules 28, no. 6: 2592. https://doi.org/10.3390/molecules28062592
APA StyleFan, Q.-F., Zhou, L., Gongpan, P.-C., Lu, C.-L., Chang, H., & Xiang, X. (2023). Bioactivity-Guided High Performance Counter-Current Chromatography and Following Semi-Preparative Liquid Chromatography Method for Rapid Isolation of Anti-Inflammatory Lignins from Dai Medicinal Plant, Zanthoxylum acanthopodium var. timbor. Molecules, 28(6), 2592. https://doi.org/10.3390/molecules28062592