Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Magnetic Nano-Chitosan
2.3. Characterization of Magnetic Nano-Chitosan
2.4. Adsorption Experiments
2.4.1. Adsorption Dynamic Experiments
2.4.2. Adsorption Thermodynamics Experiments
2.4.3. Influencing Factor Experiments
2.4.4. Competitive Adsorption Experiments
2.5. Data Analysis
3. Results and Discussion
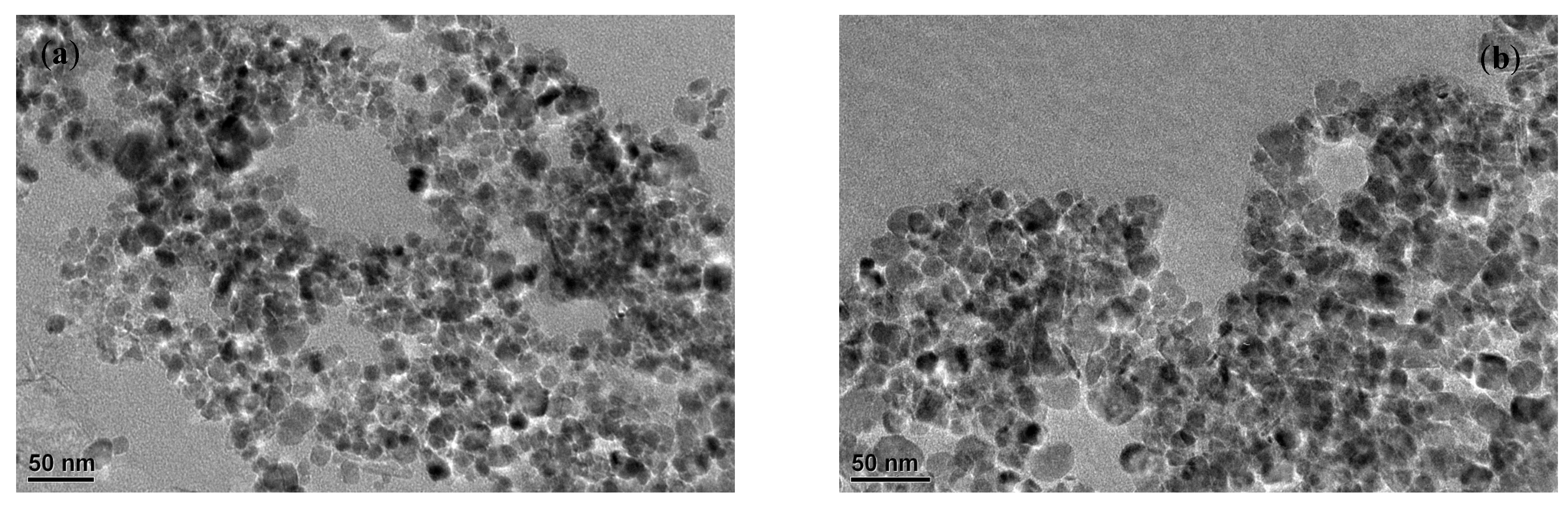
3.1. Characterization Results
3.2. Adsorption Kinetics
3.3. Adsorption Thermodynamics
3.4. Factors Influencing Adsorption
3.4.1. Adsorbent Dosage and Solution pH
3.4.2. Initial Concentration of Heavy Metal Ions and Temperature
3.5. Competitive Adsorption of Metal Ions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Martin, J.A.R.; De Arana, C.; Ramos-Miras, J.J.; Gil, C.; Boluda, R. Impact of 70 years urban growth associated with heavy metal pollution. Environ. Pollut. 2015, 196, 156–163. [Google Scholar] [CrossRef]
- Marella, T.K.; Saxena, A.; Tiwari, A. Diatom mediated heavy metal remediation: A review. Bioresour. Technol. 2020, 305, 123068. [Google Scholar] [CrossRef]
- Feng, X.F.; Long, R.X.; Wang, L.L.; Liu, C.C.; Bai, Z.X.; Liu, X.B. A review on heavy metal ions adsorption from water by layered double hydroxide and its composites. Sep. Purif. Technol. 2022, 284, 120099. [Google Scholar] [CrossRef]
- Begum, S.; Yuhana, N.Y.; Saleh, N.M.; Kamarudin, N.H.N.; Sulong, A.B. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef]
- Dey, M.; Akter, A.; Islam, S.; Dey, S.C.; Choudhury, T.R.; Fatema, K.J.; Begum, B.A. Assessment of contamination level, pollution risk and source apportionment of heavy metals in the Halda River water, Bangladesh. Heliyon 2021, 7, e08625. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.Y.; Huang, H.; Xia, F.; Liu, Y.Y.; Dahlgren, R.A.; Zhang, M.H.; Mei, K. Risk analysis of heavy metal concentration in surface waters across the rural-urban interface of the Wen-Rui Tang River, China. Environ. Pollut. 2018, 237, 639–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, T.; Wang, J.; Li, X.; Huang, S.Y.; Chen, Z.S.; Wang, S.H.; Hayat, T.; Alsaedi, A.; Wang, X.K. Production of a generic magnetic Fe3O4 nanoparticles decorated tea waste composites for highly efficient sorption of Cu (II) and Zn (II). J. Environ. Chem. Eng. 2017, 5, 3656–3666. [Google Scholar] [CrossRef]
- Shi, Z.; Deng, L. Research progresses and trends in materials for adsorption of heavy metal ions in aqueous phase. J. Archit. Civil. Eng. 2017, 34, 21–30. (In Chinese) [Google Scholar]
- Sall, M.L.; Diaw, A.K.D.; Gningue-Sall, D.; Aaron, S.E.; Aaron, J.J. Toxic heavy metals: Impact on the environment and human health, and treatment with conducting organic polymers, a review. Environ. Sci. Pollut. Res. 2020, 27, 29927–29942. [Google Scholar] [CrossRef] [PubMed]
- Forghani, M.; Azizi, A.; Livani, M.J.; Kafshgari, L.A. Adsorption of lead (II) and chromium (VI) from aqueous environment onto metal-organic framework MIL-100(Fe): Synthesis, kinetics, equilibrium and thermodynamics. J. Solid State Chem. 2020, 291, 121636. [Google Scholar] [CrossRef]
- Xiao, Z.X.; Zhang, L.J.; Wu, L.; Chen, D. Adsorptive removal of Cu (II) from aqueous solutions using a novel macroporous bead adsorbent based on poly(vinyl alcohol)/sodium alginate/KMnO4 modified biochar. J. Taiwan Inst. Chem. Eng. 2019, 102, 110–117. [Google Scholar] [CrossRef]
- Yao, Z.Y.; Qi, J.H.; Wang, L.H. Equilibrium, kinetic and thermodynamic studies on the biosorption of Cu (II) onto chestnut shell. J. Hazard. Mater. 2010, 174, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Q. Adsorption of Hexavalent Chromium on Magnetic Chitosan Composite Microspheres. Master’s Thesis, Chongqing University, Chongqing, China, 2019. (In Chinese). [Google Scholar]
- Zhang, R.S.; Tian, Y.Q. Research progress of biosorption remediation technologies for chromium contaminated sites. Environ. Eng. 2020, 38, 187–195. (In Chinese) [Google Scholar]
- Liu, J.Y.; Hu, C.W.; Huang, Q.G. Adsorption of Cu2+, Pb2+, and Cd2+ onto oil tea shell from water. Bioresour. Technol. 2019, 271, 487–491. [Google Scholar] [CrossRef]
- Liao, Q.; Tu, G.Y.; Yang, Z.H.; Wang, H.Y.; He, L.X.; Tang, J.Q.; Yang, W.C. Simultaneous adsorption of As (III), Cd (II) and Pb (II) by hybrid bio-nanocomposites of nano hydroxy ferric phosphate and hydroxy ferric sulfate particles coating on Aspergillus niger. Chemosphere 2019, 223, 551–559. [Google Scholar] [CrossRef]
- Zhu, S.D.; Khan, M.A.; Wang, F.Y.; Bano, Z.; Xia, M.Z. Rapid removal of toxic metals Cu2+ and Pb2+ by amino trimethylene phosphonic acid intercalated layered double hydroxide: A combined experimental and DFT study. Chem. Eng. J. 2020, 392, 123711. [Google Scholar] [CrossRef]
- Kumar, V.; Parihar, R.D.; Sharma, A.; Bakshi, P.; Sidhu, G.P.S.; Bali, A.S.; Karaouzas, L.; Bhardwaj, R.; Thukral, A.K.; Gyasi-Agyei, Y.; et al. Global evaluation of heavy metal content in surface water bodies: A meta-analysis using heavy metal pollution indices and multivariate statistical analyses. Chemosphere 2019, 236, 124364. [Google Scholar] [CrossRef]
- Huang, R.Y.; He, L.; Zhang, T.; Li, D.Q.; Tang, P.G.; Feng, Y.J. Novel carbon paper@magnesium silicate composite porous films: Design, fabrication, and adsorption behavior for heavy metal ions in aqueous solution. ACS Appl. Mater. Interface 2018, 10, 22776–22785. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Omer, A.M.; Dey, R.; Eltaweil, A.S.; Abd El-Monaem, E.M.; Ziora, Z.M. Insights into recent advances of chitosan-based adsorbents for sustainable removal of heavy metals and anions. Arab. J. Chem. 2022, 15, 103543. [Google Scholar] [CrossRef]
- Younes, I.; Hajji, S.; Rinaudo, M.; Chaabouni, M.; Jellouli, K.; Nasri, M. Optimization of proteins and minerals removal from shrimp shells to produce highly acetylated chitin. Int. J. Biol. Macromol. 2016, 84, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.Z.; Zhao, M.W.; Cheng, Q.; Wang, C.; Li, H.J.; Han, X.G.; Fan, Z.H.; Su, G.Y.; Pan, D.; Li, Z.Y. Research progress of adsorption and removal of heavy metals by chitosan and its derivatives: A review. Chemosphere 2021, 279, 130927. [Google Scholar] [CrossRef] [PubMed]
- Sheth, Y.; Dharaskar, S.; Khalid, M.; Sonawane, S. An environment friendly approach for heavy metal removal from industrial wastewater using chitosan based biosorbent: A review. Sustain. Energy Technol. 2021, 43, 100951. [Google Scholar] [CrossRef]
- Fan, S.L.; Chen, J.; Fan, C.; Chen, G.L.; Liu, S.G.; Zhou, H.M.; Liu, R.T.; Zhang, Y.J.; Hu, H.Y.; Huang, Z.Q.; et al. Fabrication of a CO2-responsive chitosan aerogel as an effective adsorbent for the adsorption and desorption of heavy metal ions. J. Hazard. Mater. 2021, 416, 126225. [Google Scholar] [CrossRef]
- Saheed, I.O.; Oh, W.D.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants-A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef]
- Vakili, M.; Deng, S.B.; Cagnetta, G.; Wang, W.; Meng, P.P.; Liu, D.C.; Yu, G. Regeneration of chitosan-based adsorbents used in heavy metal adsorption: A review. Sep. Purif. Technol. 2019, 224, 373–387. [Google Scholar] [CrossRef]
- Gode, F.; Pehlivan, E. Removal of chromium (III) from aqueous solutions using Lewatit S 100: The effect of pH, time, metal concentration and temperature. J. Hazard. Mater. 2006, 136, 330–337. [Google Scholar] [CrossRef]
- Ahmadi, M.; Niari, M.H.; Kakavandi, B. Development of maghemite nanoparticles supported on cross-linked chitosan (γ-Fe2O3@CS) as a recoverable mesoporous magnetic composite for effective heavy metals removal. J. Mol. Liq. 2017, 248, 184–196. [Google Scholar] [CrossRef]
- Cui, J.J. The Research on the Modification of Magnetic Chitosan and Its Adsorption Performance of Copper Ions. Master’s Thesis, Hunan University, Changsha, China, 2016. (In Chinese). [Google Scholar]
- Fu, L.M.; Gao, G.; Han, Y.; Lu, X.; Li, J.L. Adsorption properties of magnetic cross-linked chitosan microspheres to Cu2+. Chem. Res. Appl. 2020, 32, 664–670. (In Chinese) [Google Scholar]
- Chang, H.; Fan, W.; Zeng, C.; Li, Y.P. Preparation of magnetic chitosan/graphene oxide adsorbent and its adsorption for Pb (II). Metall. Anal. 2018, 38, 34–42. (In Chinese) [Google Scholar]
- Feng, H.X.; Li, Y.; Li, Q.Z.; Li, D.D.; Zhang, Y.F.; Zhao, D.; Chen, N.L. Adsorption of Cr (VI) ions on diatomite composite magnetic chitosan material. Metall. Anal. 2018, 35, 668–675. (In Chinese) [Google Scholar]
- Karimi, F.; Ayati, A.; Tanhaei, B.; Sanati, A.L.; Afshar, S.; Kardan, A.; Dabirifar, Z.; Karaman, C. Removal of metal ions using a new magnetic chitosan nano-bio-adsorbent; A powerful approach in water treatment. Environ. Res. 2022, 203, 111753. [Google Scholar] [CrossRef]
- Fu, J.Y.; Liu, T.; Tang, Q.L.; Dou, X.; Zhao, J. Optimization and characterization of preparation process of chitosan magnetic nanomaterial. New Chem. Mater. 2021, 49, 259–263, 266. (In Chinese) [Google Scholar]
- Han, M.; Han, Y.S.; Sun, M.Q.; He, L.T.; He, J.X.; Bi, S.D. Adsorption of heavy metal ions by magnetic chitosan materials. Liaoning Chem. Ind. 2021, 50, 36–37. (In Chinese) [Google Scholar]
- Zhou, C.Y.; Yang, J.L.; Yu, Z.D. Preparation and adsorption properties of nano-Fe3O4@chitosan. Chem. Bull. 2018, 81, 914–918, 923. (In Chinese) [Google Scholar]
- Guo, J.Y.; Gan, P.F.; Chen, C.; Zhang, G.J. Preparation of magnetic chitosan and its application in the treatment of methylene blue wastewater. China Environ. Sci. 2019, 39, 2422–2430. (In Chinese) [Google Scholar]
- Zheng, J.G.; Chen, Q.S.; Yang, T. Synthesis and characterization of nano-sized magetic ferroferric oxide particles. Inorg. Chem. Ind. 2008, 40, 15–17. (In Chinese) [Google Scholar]
- Martins, A.C.; Pezoti, O.; Cazetta, A.L.; Bedin, K.C.; Yamazaki, D.A.S.; Bandoch, G.F.G.; Asefa, T.; Visentainer, J.V.; Almeida, V.C. Removal of tetracycline by NaOH-activated carbon produced from macadamia nut shells: Kinetic and equilibrium studies. Chem. Eng. J. 2015, 260, 291–299. [Google Scholar] [CrossRef]
- Ali, I.; Alothman, Z.A.; Alwarthan, A. Uptake of propranolol on ionic liquid iron nanocomposite adsorbent: Kinetic, thermodynamics and mechanism of adsorption. J. Mol. Liq. 2017, 236, 205–213. [Google Scholar] [CrossRef]
- Yu, B.; Yu, H.C.; Song, B.X.; Qi, R.X. Adsorption of nitrobenzene on fly ash: Kinetic and thermodynamic studies. Environ. Sci. Technol. 2020, 43, 11–16. (In Chinese) [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.L.; Guo, X. Adsorption isotherm models: Classification, physical meaning, application and solving method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Shang, J.; Zhao, J. Effect of chitosan modification on Pb (II) and Cd (II) adsorption by magnetic Fe3O4. Environ. Pollut. Control 2017, 39, 746–751. (In Chinese) [Google Scholar]
- Ma, F.F.; Zhao, B.W.; Diao, J.R.; Jiang, Y.F. Adsorption characteristics of p-nitrophenol removal by magnetic biochar. China Environ. Sci. 2019, 39, 170–178. (In Chinese) [Google Scholar]
- Dim, P.E.; Mustapha, L.S.; Termtanun, M.; Okafor, J.O. Adsorption of chromium (VI) and iron (III) ions onto acid-modified kaolinite: Isotherm, kinetics and thermodynamics studies. Arab. J. Chem. 2021, 14, 103064. [Google Scholar] [CrossRef]
- Azouaou, N.; Sadaoui, Z.; Djaafri, A.; Mokaddem, H. Adsorption of cadmium from aqueous solution onto untreated coffee grounds: Equilibrium, kinetics and thermodynamics. J. Hazard. Mater. 2010, 184, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.K.; Yogeshwaran, V.; Rajendran, S.; Hoang, T.K.A.; Soto-Moscoso, M.; Ghfar, A.A.; Bathula, C. Investigation of mechanism of heavy metals (Cr6+, Pb2+& Zn2+) adsorption from aqueous medium using rice husk ash: Kinetic and thermodynamic approach. Chemosphere 2022, 286, 131796. [Google Scholar]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef] [Green Version]
- Hashem, A.; Aniagor, C.O.; Taha, G.M.; Fikry, M. Utilization of low-cost sugarcane waste for the adsorption of aqueous Pb (II): Kinetics and isotherm studies. Curr. Res. Green Sustain. Chem. 2021, 4, 100056. [Google Scholar] [CrossRef]
- Kannan, N.; Sundaram, M.M. Kinetics and mechanism of removal of methylene blue by adsorption on various carbons-a comparative study. Dyes Pigment. 2001, 51, 25–40. [Google Scholar] [CrossRef]
- Hashem, A.; Azzeer, A.M.; Ayoub, A. The removal of Hg (II) ions from laboratory wastewater onto phosphorylated haloxylon ammodendron: Kinetic and equilibrium studies. Polym.-Plast. Technol. 2010, 49, 1463–1472. [Google Scholar] [CrossRef]
- Abonyi, M.N.; Aniagor, C.O.; Menkiti, M.C. Effective Dephenolation of Effluent from petroleum industry using ionic-liquid-induced hybrid adsorbent. Arab. J. Sci. Eng. 2019, 44, 10017–10029. [Google Scholar] [CrossRef]
- Ding, C.M.; Liu, Q.; Cao, Z.Y. Adsorption capability and kinetics of chitosan for lead in water. J. Environ. Health 2007, 24, 880–882. (In Chinese) [Google Scholar]
- Demiral, H.; Demiral, I.; Tumsek, F.; Karabacakoglu, B. Adsorption of chromium (VI) from aqueous solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem. Eng. J. 2008, 144, 188–196. [Google Scholar] [CrossRef]
- Sarodea, S.; Upadhyay, P.; Khosaa, M.A.; Mak, T.; Shakir, A.; Song, S.; Ullah, A. Overview of waste water treatment methods with special focus on biopoly merchitin-chitosan. Int. J. Biol. Macromol. 2019, 121, 1086–1100. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, L.; Zhu, J.; Wang, N.; Feng, J.; Yan, W. Adsorption of polythiophene/TiO2 composite for Zn (II), Pb (II) and Cu (II): Selectivity and synergistic effect investigation. Appl. Surf. Sci. 2018, 459, 318–326. [Google Scholar] [CrossRef]
- Wu, Q.L.; Dong, S.Z.; Wang, L.J.; Li, X.Y. Single and Competitive Adsorption Behaviors of Cu2+, Pb2+ and Zn2+ on the Biochar and Magnetic Biochar of Pomelo Peel in Aqueous Solution. Water 2021, 13, 868. [Google Scholar] [CrossRef]
- Caporale, A.G.; Pigna, M.; Sommella, A.; Conte, P. Effect of pruning-derived biochar on heavy metals removal and water dynamics. Biol. Fertil. Soils 2014, 50, 1211–1222. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.T.; Branford-White, C.; Nie, H.L.; Zhu, L.M. Adsorption mechanism of Cu2+ from aqueous solution by chitosan-coated magnetic nanoparticles modified with-ketoglutaric acid. Colloid Surf. B 2009, 74, 244–252. [Google Scholar] [CrossRef]







| Ions | qexp (µmol/g) | Pseudo-First-Order | Pseudo-Second-Order | Elovich | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| K1 (g·µmol−1·min−1) | qe (µmol/g) | R2 | K2 (g·µmol−1·min−1) | qe (µmol/g) | R2 | α (g·µmol−1·min−1) | K (g·µmol−1·min−1) | R2 | ||
| Cr6+ | 61.208 | 0.4185 | 57.283 | 0.9397 | 0.0119 | 60.626 | 0.9757 | 39.465 | 4.7523 | 0.9946 |
| Cu2+ | 81.141 | 0.1492 | 81.260 | 0.8702 | 0.0023 | 81.575 | 0.9390 | 33.499 | 11.878 | 0.9622 |
| Pb2+ | 45.276 | 0.1299 | 43.287 | 0.9451 | 0.0037 | 45.748 | 0.9722 | 15.360 | 6.559 | 0.9643 |
| Zn2+ | 43.092 | 0.2043 | 37.951 | 0.8119 | 0.0071 | 42.126 | 0.9021 | 14.951 | 5.692 | 0.9695 |
| Ions | Intra-Particle Diffusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| K1d (µmol·g−1·min−0.5) | C1 | R2 | K2d (µmol·g−1·min−0.5) | C2 | R2 | K3d (µmol·g−1·min−0.5) | C3 | R2 | |
| Cr6+ | 1.878 | 46.217 | 0.8470 | 3.371 | 37.629 | 0.9938 | 0.6537 | 57.386 | 0.8404 |
| Cu2+ | 3.953 | 43.843 | 0.9986 | 13.474 | 36.014 | 0.8778 | 0.898 | 84.348 | 0.3308 |
| Pb2+ | 11.653 | 21.726 | 0.6685 | 1.891 | 31.474 | 0.9310 | 1.118 | 37.141 | 0.9129 |
| Zn2+ | 1.450 | 24.154 | 0.9300 | 0.758 | 27.656 | 0.9511 | 2.001 | 31.342 | 0.8667 |
| Ions | T (K) | qexp (µmol/g) | Langmuir | Freundlich | Temkin | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| KL (L/µmol) | qm (µmol/g) | R2 | 1/n | KF (µmol/g)/(µmol/L)1/n | R2 | A (J/mol) | KT (L/µmol) | R2 | |||
| Cr6+ | 298 | 151.635 | 0.004 | 203.360 | 0.9915 | 0.74 | 1.619 | 0.8917 | 35.61 | 0.039 | 0.9462 |
| 308 | 155.365 | 0.002 | 248.083 | 0.9982 | 0.64 | 2.751 | 0.9829 | 35.87 | 0.062 | 0.9340 | |
| 318 | 162.096 | 0.001 | 301.057 | 0.9977 | 0.52 | 5.840 | 0.9695 | 36.47 | 0.112 | 0.9318 | |
| Cu2+ | 298 | 97.580 | 0.027 | 98.617 | 0.9849 | 0.33 | 11.071 | 0.9337 | 16.17 | 0.507 | 0.9751 |
| 308 | 147.270 | 0.020 | 127.263 | 0.9458 | 0.35 | 17.512 | 0.9133 | 23.17 | 0.676 | 0.9404 | |
| 318 | 192.430 | 0.030 | 198.861 | 0.9801 | 0.39 | 29.272 | 0.9570 | 41.20 | 0.405 | 0.9934 | |
| Pb2+ | 298 | 58.797 | 0.051 | 103.438 | 0.9768 | 0.76 | 8.8254 | 0.9696 | 29.08 | 0.169 | 0.9488 |
| 308 | 63.659 | 0.050 | 115.161 | 0.9897 | 0.60 | 15.226 | 0.9849 | 29.73 | 0.444 | 0.8860 | |
| 318 | 68.923 | 0.045 | 121.942 | 0.9948 | 0.59 | 16.971 | 0.9922 | 31.19 | 0.463 | 0.9178 | |
| Zn2+ | 298 | 47.569 | 0.018 | 45.776 | 0.9425 | 0.35 | 6.874 | 0.9207 | 8.646 | 0.244 | 0.9482 |
| 308 | 50.769 | 0.016 | 51.868 | 0.9712 | 0.35 | 6.584 | 0.9593 | 9.885 | 0.205 | 0.9837 | |
| 318 | 54.520 | 0.013 | 62.727 | 0.9509 | 0.37 | 7.950 | 0.9339 | 11.44 | 0.182 | 0.9612 | |
| Ions | ΔG (kJ/mol) | ΔH (kJ/mol) | ΔS (J/(mol·K)) | ||
|---|---|---|---|---|---|
| 298K | 308K | 318K | |||
| Cr6+ | −5.959 | −6.166 | −6.598 | 3.53 | 31.701 |
| Cu2+ | −1.243 | −1.734 | −2.883 | 23.099 | 81.302 |
| Pb2+ | −3.422 | −3.941 | −4.233 | 8.709 | 40.805 |
| Zn2+ | −1.949 | −2.175 | −2.572 | 7.317 | 30.986 |
| Ions | Competition Experiment | qexp (µmol/g) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|---|
| KL (L/µmol) | qm (µmol/g) | R2 | 1/n | KF (µmol/g)/(µmol/L)1/n | R2 | |||
| Cu2+ | Single | 97.584 | 0.0156 | 102.644 | 0.9096 | 0.304 | 17.125 | 0.9881 |
| Cu2+-Pb2+ | 81.959 | 0.0057 | 89.400 | 0.9353 | 0.480 | 4.433 | 0.9479 | |
| Cu2+-Zn2+ | 92.272 | 0.0037 | 119.390 | 0.9991 | 0.520 | 4.724 | 0.9838 | |
| Ternary | 79.684 | 0.0229 | 78.4616 | 0.8818 | 0.287 | 11.3541 | 0.9971 | |
| Pb2+ | Single | 62.637 | 0.0034 | 250.406 | 0.9989 | 0.874 | 1.458 | 0.9996 |
| Pb2+-Cu2+ | 42.415 | 0.0023 | 145.305 | 0.9993 | 0.841 | 0.618 | 0.9990 | |
| Pb2+-Zn2+ | 43.864 | 0.0043 | 106.041 | 0.9969 | 0.742 | 1.264 | 0.9902 | |
| Ternary | 35.474 | 0.0140 | 60.205 | 0.9369 | 0.647 | 3.109 | 0.8975 | |
| Zn2+ | Single | 47.566 | 0.0173 | 48.938 | 0.9045 | 0.257 | 9.848 | 0.9316 |
| Zn2+-Cu2+ | 44.182 | 0.0014 | 53.965 | 0.9507 | 0.603 | 0.969 | 0.9755 | |
| Zn2+-Pb2+ | 26.157 | 0.0025 | 39.558 | 0.9648 | 0.478 | 1.053 | 0.9891 | |
| Ternary | 41.372 | 0.0011 | 30.659 | 0.8201 | 0.6268 | 1.022 | 0.8705 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, Y.; Zhang, P.; Wang, L. Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan. Molecules 2023, 28, 2607. https://doi.org/10.3390/molecules28062607
He Y, Zhang P, Wang L. Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan. Molecules. 2023; 28(6):2607. https://doi.org/10.3390/molecules28062607
Chicago/Turabian StyleHe, Yuran, Panqing Zhang, and Lijun Wang. 2023. "Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan" Molecules 28, no. 6: 2607. https://doi.org/10.3390/molecules28062607
APA StyleHe, Y., Zhang, P., & Wang, L. (2023). Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan. Molecules, 28(6), 2607. https://doi.org/10.3390/molecules28062607







