Characterization of the Interactions between Minocycline Hydrochloride and Trypsin with Spectroscopic and Molecular Docking Technology
Abstract
1. Introduction
2. Results and Discussion
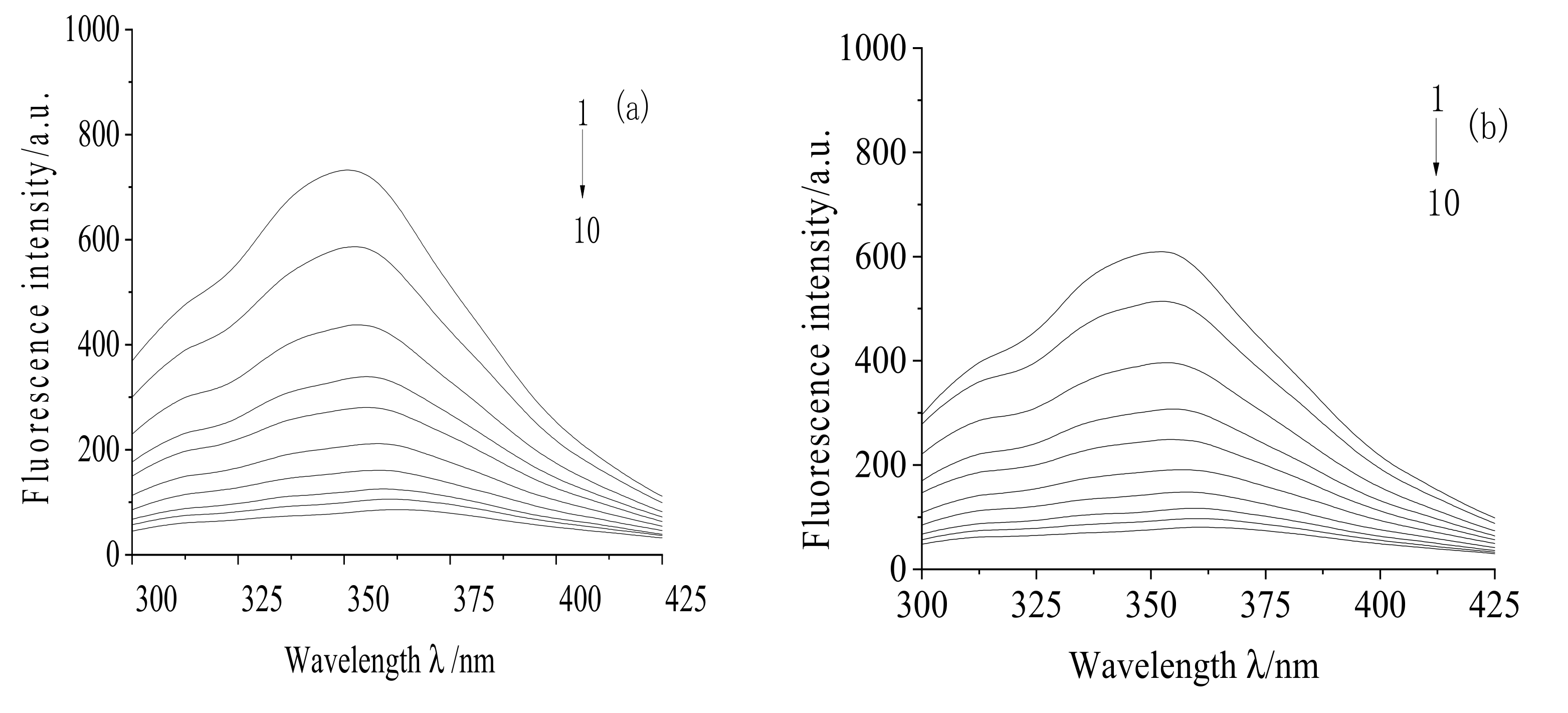
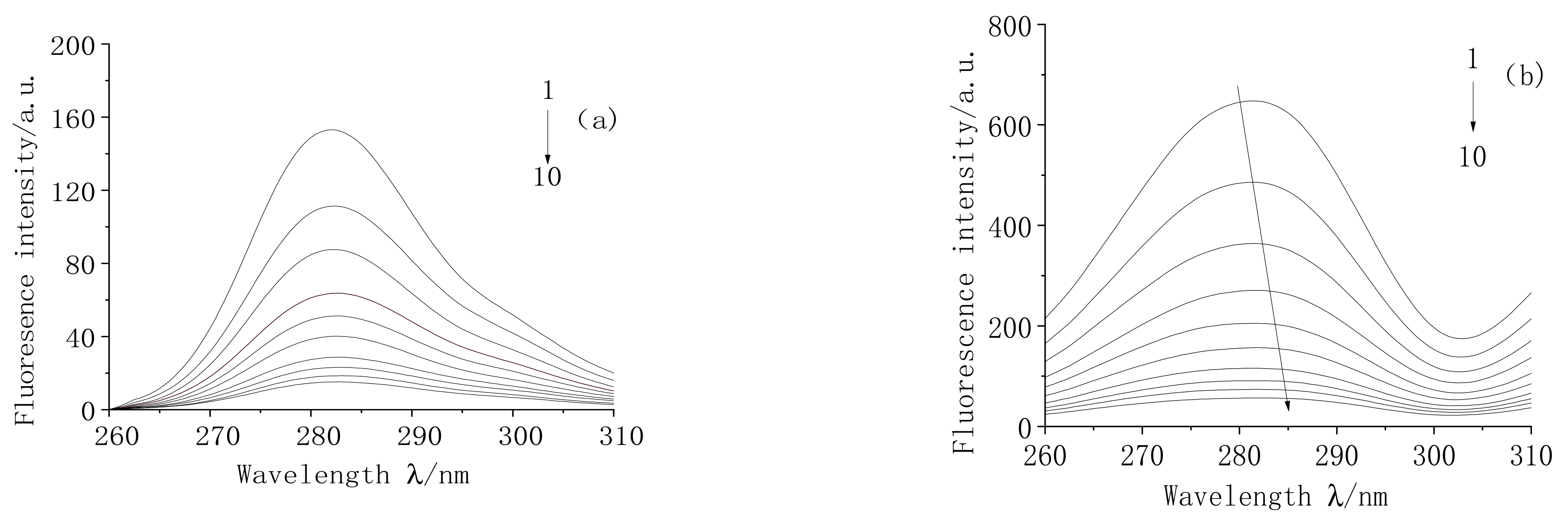
2.1. Fluorescence Quenching Spectra
2.2. Determination of Fluorescence Quenching Mechanism
2.3. The Binding Constant and Number of Binding Sites (n)
2.4. Thermodynamic Parameters and Main Forces of the Interaction between TRP and MC
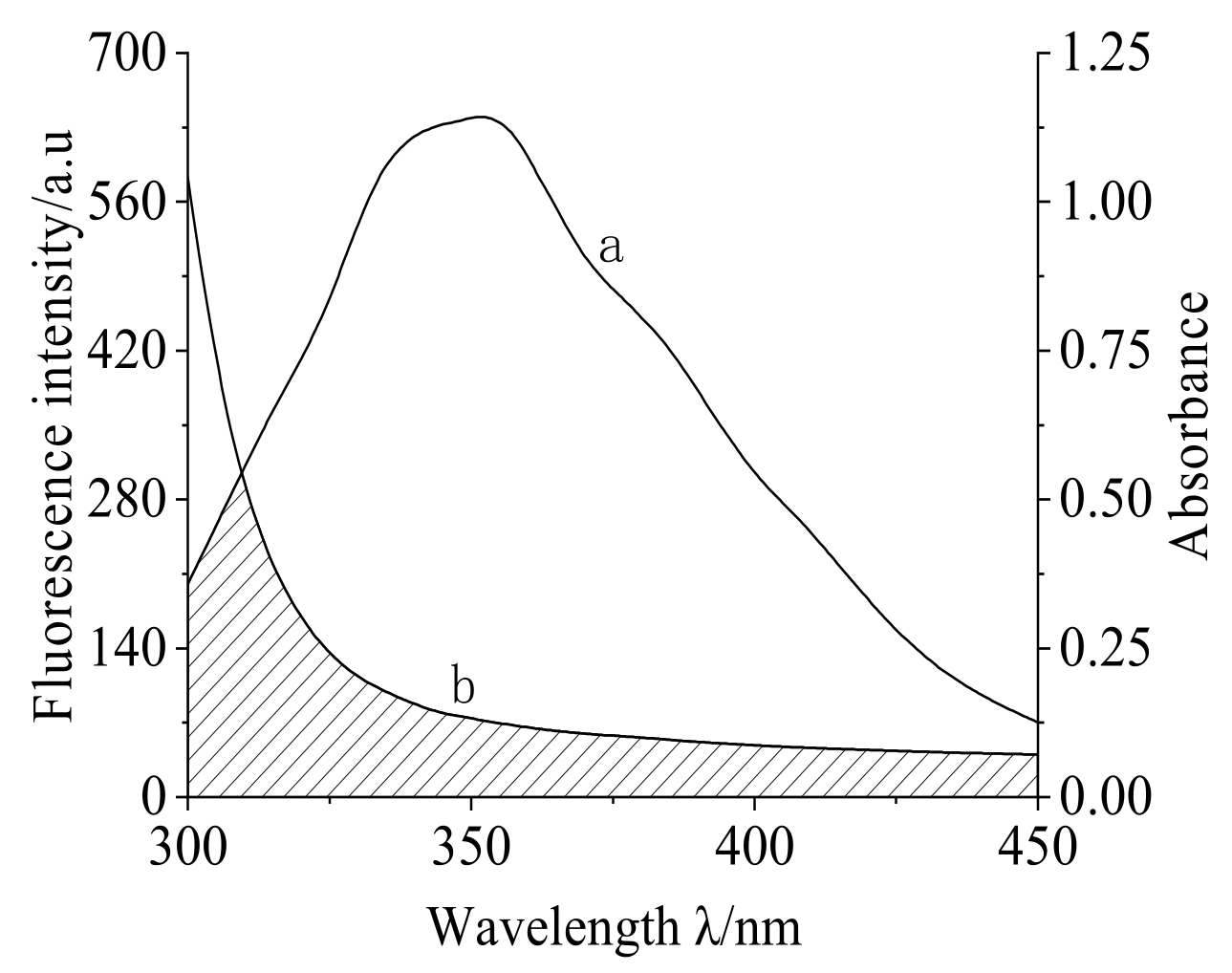
2.5. The Combination Distance between MC and TRP
2.6. Molecular Docking Study
2.7. Effect of MC on the Secondary Structure of TRP
2.7.1. Synchronous Fluorescence Spectral Analysis of the Interaction between MC and TRP
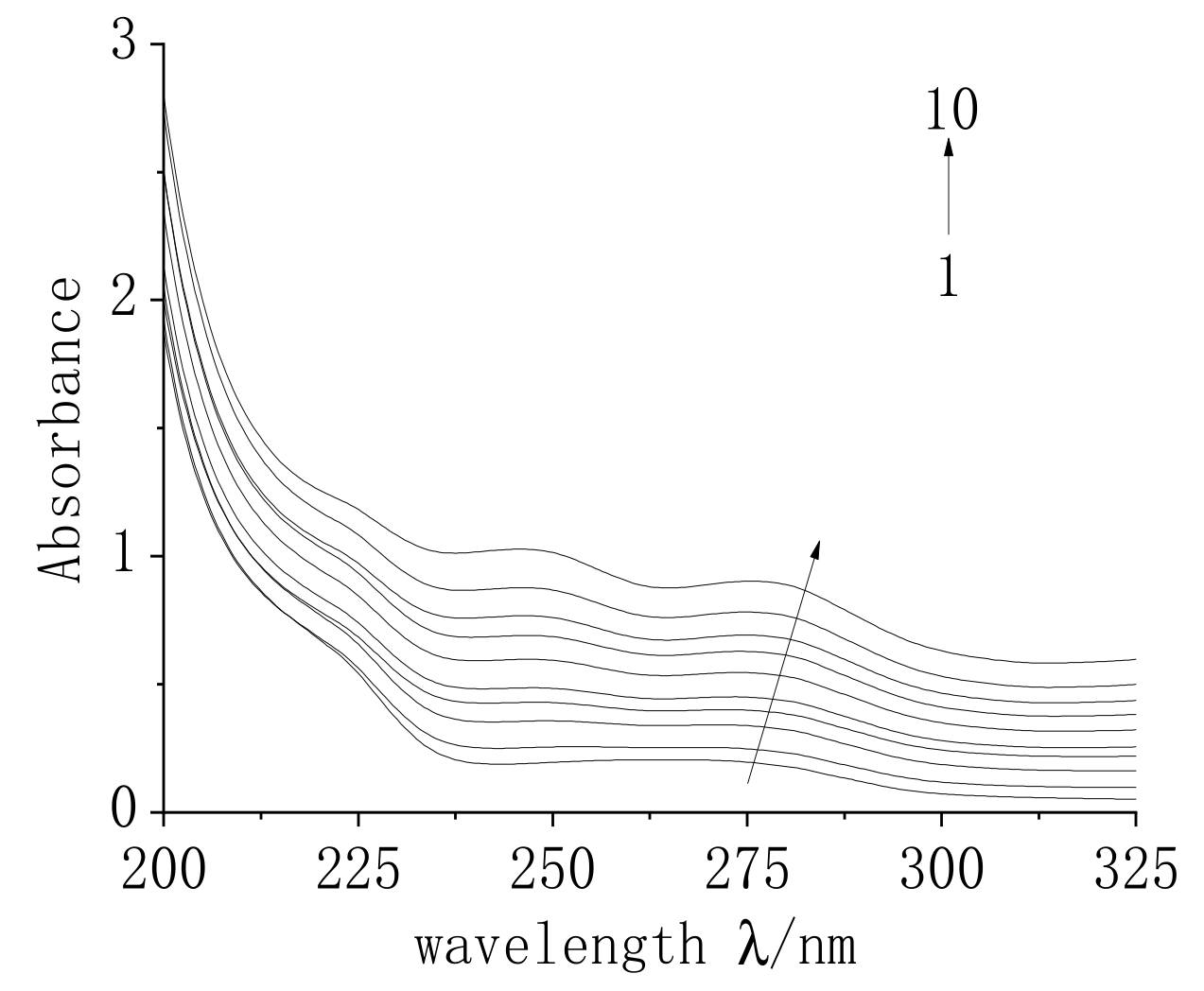
2.7.2. UV Spectroscopic Analysis of the Interaction between MC and TRP
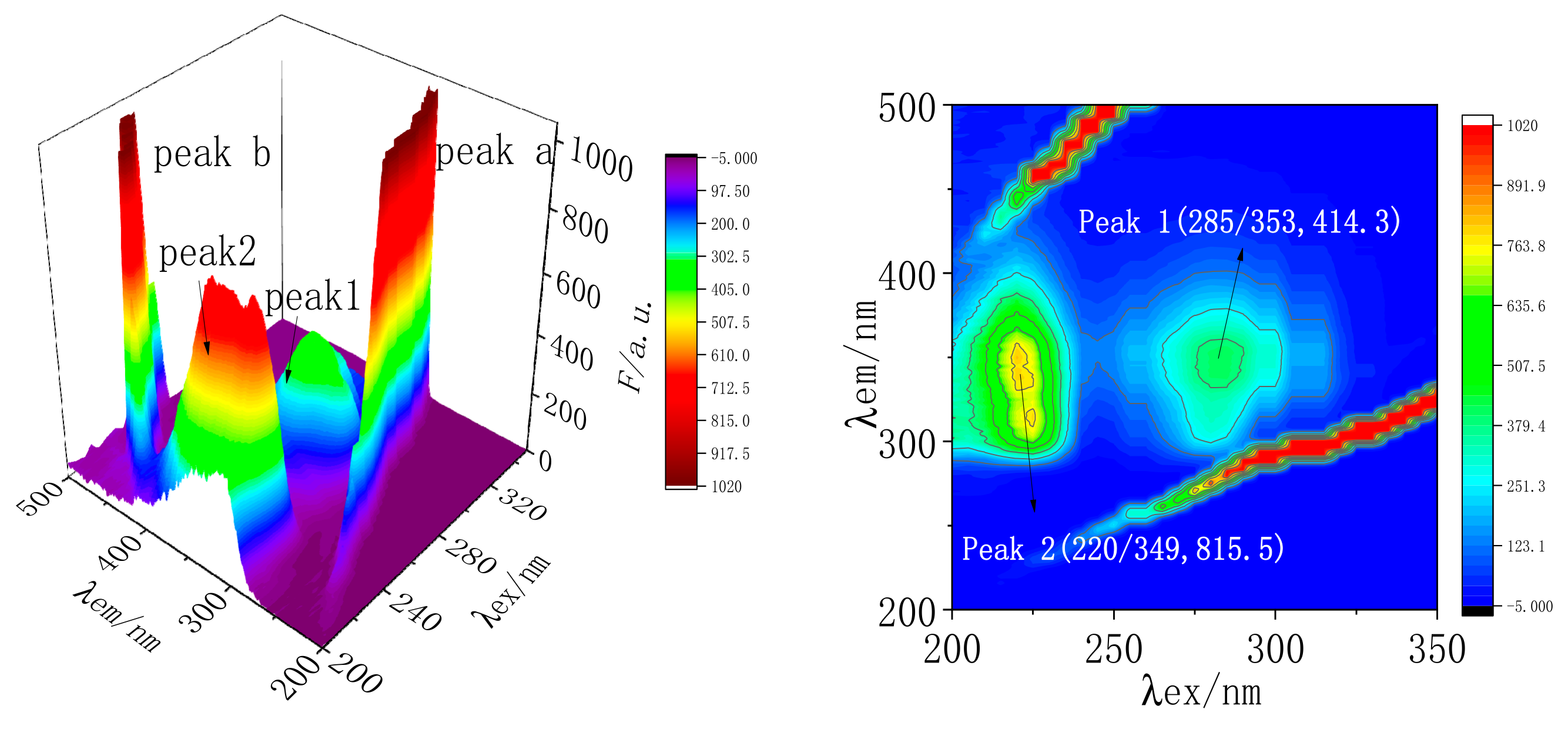
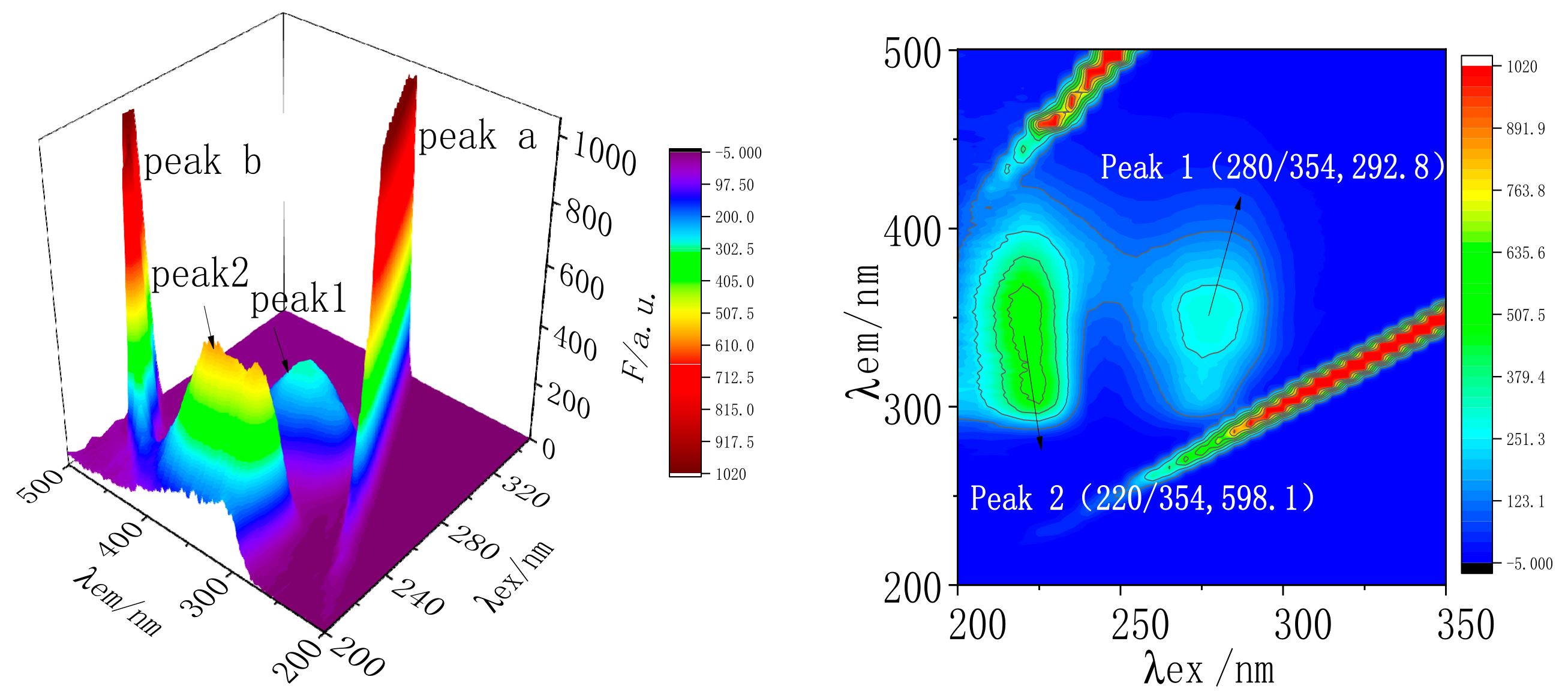
2.7.3. Three-Dimensional Fluorescence Spectral Analysis of MC and TRP
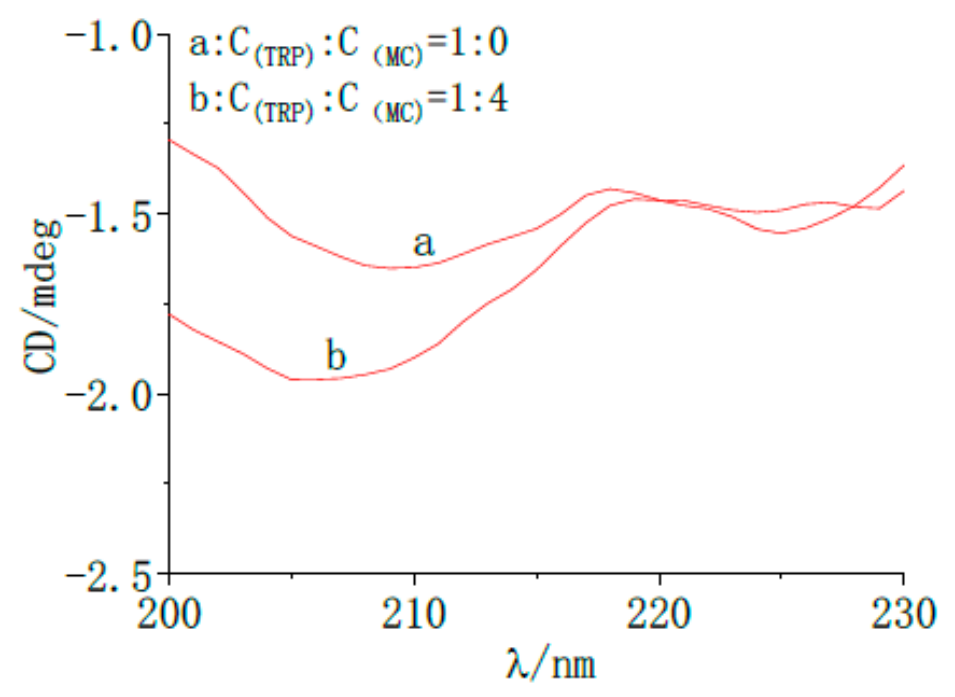
2.7.4. Circular Dichroism Analysis of MC by TRP
3. Experimental Section
3.1. Instruments
3.2. Reagents
3.3. Determination of Fluorescence Spectra, Synchronous Spectra, and Three-Dimensional Fluorescence Spectra of TRP and MC
3.4. UV-Vis Absorption Spectrum of TRP and MC
3.5. Determination of Binding Distance of TRP and MC
3.6. Molecular Docking Simulation Technology of TRP and MC
3.7. Circular Dichroism Spectrum
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edouard, S.; Silvia, C. Organizing pneumonia in a pediatric patient on minocycline: A potential culprit. Pediatr. Pulmonol. 2019, 54, 533–540. [Google Scholar]
- Guo, Z. The modification of natural products for medical use. Acta Pharm. Sin. B 2017, 7, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, R.D.S.; Amaral, I.P.G.; Ferreira, A.C.M.; Espósito, T.S.; Bezerra, R.S. Chemistry—Food Chemistry. Findings in the Area of Food Chemistry Reported from Federal University (Use of Fish Trypsin Immobilized Onto Magnetic-chitosan Composite As a New Tool To Detect Antinutrients in Aquafeeds). Food Wkly. News 2019, 31, 5348–5351. [Google Scholar]
- Tchewonpi, S.S.; Eva, L.; Henkel, I.M.; Gerd, H.; Thomas, H.; Sara, B.; Schlüter, O.K.; Harshadrai, R. Effect of Cereal α-Amylase/Trypsin Inhibitors on Developmental Characteristics and Abundance of Digestive Enzymes of Mealworm Larvae (Tenebrio molitor L.). Insects 2021, 12, 454. [Google Scholar]
- Zhirong, F.; Srinivas, A.; Michael, T.; Lars, H. Marked difference in efficiency of the digestive enzymes pepsin, trypsin, chymotrypsin, and pancreatic elastase to cleave tightly folded proteins. Biol. Chem. 2021, 402, 861–867. [Google Scholar]
- Siqueira, P.L.L.d.; Marques, R.D.d.B.; Mariana, G.e.S.; Amorim, d.S.A.C.L.; Araújo, S.Y.; Oliveira, M.A.d.; Barroso, C.L.C.B.; Guedes, P.P.M.; Viana, P.E.; Luciane, M.R.; et al. The trypsin inhibitor from Moringa oleifera flowers (MoFTI) inhibits acute inflammation in mice by reducing cytokine and nitric oxide levels. S. Afr. J. Bot. 2021, 143, 474–481. [Google Scholar]
- Liu, W.; Liu, K.; Du, H.; Zheng, T.; Zhang, N.; Xu, T.; Pang, B.; Zhang, X.; Si, C.; Zhang, K. Cellulose Nanopaper: Fabrication, Functionalization, and Applications. Nano Micro Lett. 2022, 14, 329–355. [Google Scholar] [CrossRef]
- Shukla, R.D.; Rai, B.; Kumar, A. Exploration of Catalytic Activity of Trypsin for C(sp3)-H Functionalization and Consequent C-C Bond Formation. Eur. J. Org. Chem. 2019, 2019, 2864–2868. [Google Scholar] [CrossRef]
- Momeni, L.; Shareghi, B.; Farhadian, S.; Vaziri, S.; Saboury, A.A.; Raisi, F. A molecular simulation and spectroscopic approach to the binding affinity between trypsin and 2-propanol and protein conformation. Int. J. Biol. Macromol. 2018, 119, 477–485. [Google Scholar] [CrossRef]
- Meti, M.D.; Jialiang, L.; Yuhan, W.; Zhibing, W.; Hong, X.; Xu, X.; Qingguo, H.; Ming, Y.; Zhangli, H.; Zhendan, H. Trypsin inhibition by Ligupurpuroside B as studied using spectroscopic, CD, and molecular docking techniques. J. Biomol. Struct. Dyn. 2019, 37, 3379–3387. [Google Scholar] [CrossRef]
- Selmihan, S.; Furkan, C.; Sercan, O.Y.; Ismail, O. Investigation of binding interaction behavior between antiemetic drugs and Trypsin by spectroscopy and molecular docking. Spectrochim. Acta Part A 2021, 258, 119817. [Google Scholar]
- Thaís, M.M.; Caio, A.; Jairon, L.C.A.; Carlos, S.D.S.R.; Karla, M.d.V.W.; Regildo, M.G.M.; Ranilson, d.S.B.; Miranda, S.G.d.; Chenglong, L.; Luiz, N.J. Binding Mechanism between Acetylcholinesterase and Drugs Pazopanib and Lapatinib: Biochemical and Biophysical Studies. ACS Chem. Neurosci. 2021, 12, 4500–4511. [Google Scholar]
- Yajuan, Q.; Xiaoai, C.; Fei, X.; Chunhe, G.; Kexue, Z.; Yanjun, Z.; Gang, W.; Ping, W.; Lehe, T. Effects of hydroxylation at C3′ on the B ring and diglycosylation at C3 on the C ring on flavonols inhibition of α-glucosidase activity. Food Chem. 2023, 406, 135057. [Google Scholar]
- Futamura, A.; Beechem, J.M.; Peter, G.W. Gettins. Conformational equilibrium of the reactive center loop of antithrombin examined by steady state and time-resolved fluorescence measurements: Consequences for the mechanism of factor Xa inhibition by antithrombin-heparin complexes. Biochemistry 2001, 40, 6680–6687. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Uttam, R.; Bharti, A.S.; Uttam, K.N. Interaction of Zinc Oxide and Copper Oxide Nanoparticles with Chlorophyll: A Fluorescence Quenching Study. Anal. Lett. 2019, 52, 1539–1557. [Google Scholar] [CrossRef]
- Arjun, S.; Jörg, E.; Manoj, K. Photon Antibunching Reveals Static and Dynamic Quenching Interaction of Tryptophan with Atto-655. J. Phys. Chem. Lett. 2017, 8, 5821–5826. [Google Scholar]
- Parisa, S.; Eugene, K.; Chen, P. Thermodynamic characterization of the interaction between a peptide-drug complex and serum proteins. Langmuir 2014, 30, 11122–11130. [Google Scholar]
- Hasanzadeh, A.; Dehghan, G.; Shaghaghi, M.; Panahi, Y.; Jouyban, A.; Yekta, R. Multispectral and molecular docking studies on the interaction of human serum albumin with iohexol. J. Mol. Liq. 2017, 248, 459–467. [Google Scholar] [CrossRef]
- Tabassum, S.; Al-Asbahy, W.M.; Afzal, M.; Arjmand, F. Synthesis, characterization and interaction studies of copper based drug with Human Serum Albumin (HSA): Spectroscopic and molecular docking investigations. J. Photochem. Photobiol. B 2012, 114, 132–139. [Google Scholar] [CrossRef]
- Segovia-Zafra, A.; Zeo-Sánchez, D.E.D.; López-Gómez, C.; Prez-Valdés, Z.; García-Fuentes, E.; Angrade, R.J.; Lucena, M.I. Preclinical models of idiosyncratic drug-induced liver injury(iDILI):Moving towards prediction. Acta Pharm. Sin. B 2021, 11, 3685–3726. [Google Scholar] [CrossRef]
- Cui, C.; Liu, T.; Chen, T.; Lu, J.; Casaren, I.; Lima, D.B.; Carvalho, P.C.; Beuve, A.; Li, H. Comprehensive identification of protein disulfide bonds with pepsin/trypsin digestion, Orbitrap HCD and Spectrum Identification Machine. J. Proteom. 2019, 198, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.; Song, H.; Kim, C.; Kim, M.; Kyhm, K.; Kim, K.; Oh, J.-W. Intermolecular distance measurement with TNT suppressor on the M13 bacteriophagebased Förster resonance energy transfer system. Sci. Rep. 2019, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.S.; Helena, G.; Jessica, S.; George, B.B.; Thorsten, S. Quantification of Förster resonance energy transfer by monitoring sensitized emission in living plant cells. Front. Plant Sci. 2013, 4, 413. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhang, Y.; Wang, P.; Zhang, J.; Chen, H.; Zhang, L.; Du, X.; Zhao, C.; Wu, D.; Liu, F.; et al. A comprehensive review of integrative pharmacology-based investigation:A paradigm shift in traditional Chinese medicine. Acta Pharm. Sin. B 2021, 11, 1379–1399. [Google Scholar] [CrossRef]
- Pan, X.; Pei, J.; Wang, A.; Shuai, W.; Feng, L.; Bu, F.; Zhu, Y.; Zhang, L.; Wang, G.; Ouyang, L. Development of small molecule extracellular signal-regulated kinases(ERKs) inhibitors for cancer therapy. Acta Pharm. Sin. B 2022, 12, 2171–2192. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, S.; Bourke, J.F. Image Gallery: Minocycline-induced photo-onycholysis with pigmentation. Br. J. Dermatol. 2019, 180, e102. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Liu, M.; Hu, Y.; Wang, L.; Wang, C.; Jiang, Y.; Zhou, Q.; Qi, X.; Dong, N.; Wu, Q. Ectopic expression of human airway trypsin-like protease 4 in acute myeloid leukemia promotes cancer cell invasion and tumor growth. Cancer Med. 2019, 8, 2348–2359. [Google Scholar] [CrossRef]
- Yang, J.; Sohn, I. Compositional dependence of thermophysical properties in binary alkaline earth borate melts: Insights from structure in short-range and intermediate-range order. J. Mater. Sci. Technol. 2022, 131, 195–203. [Google Scholar] [CrossRef]
- Wallace, B. The role of circular dichroism spectroscopy in the era of integrative structural biology. Curr. Opin. Struct. Biol. 2019, 58, 191–196. [Google Scholar] [CrossRef]

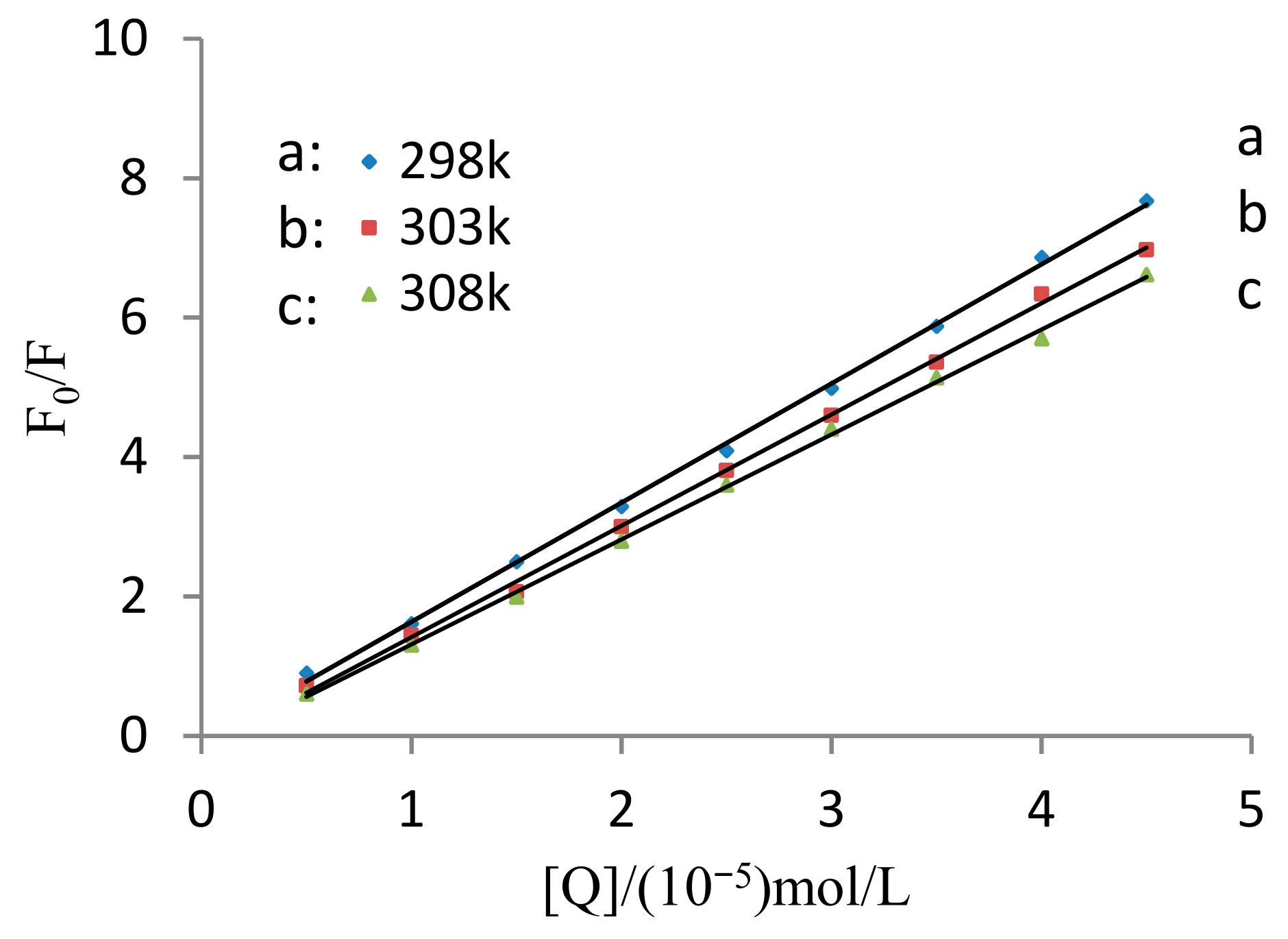
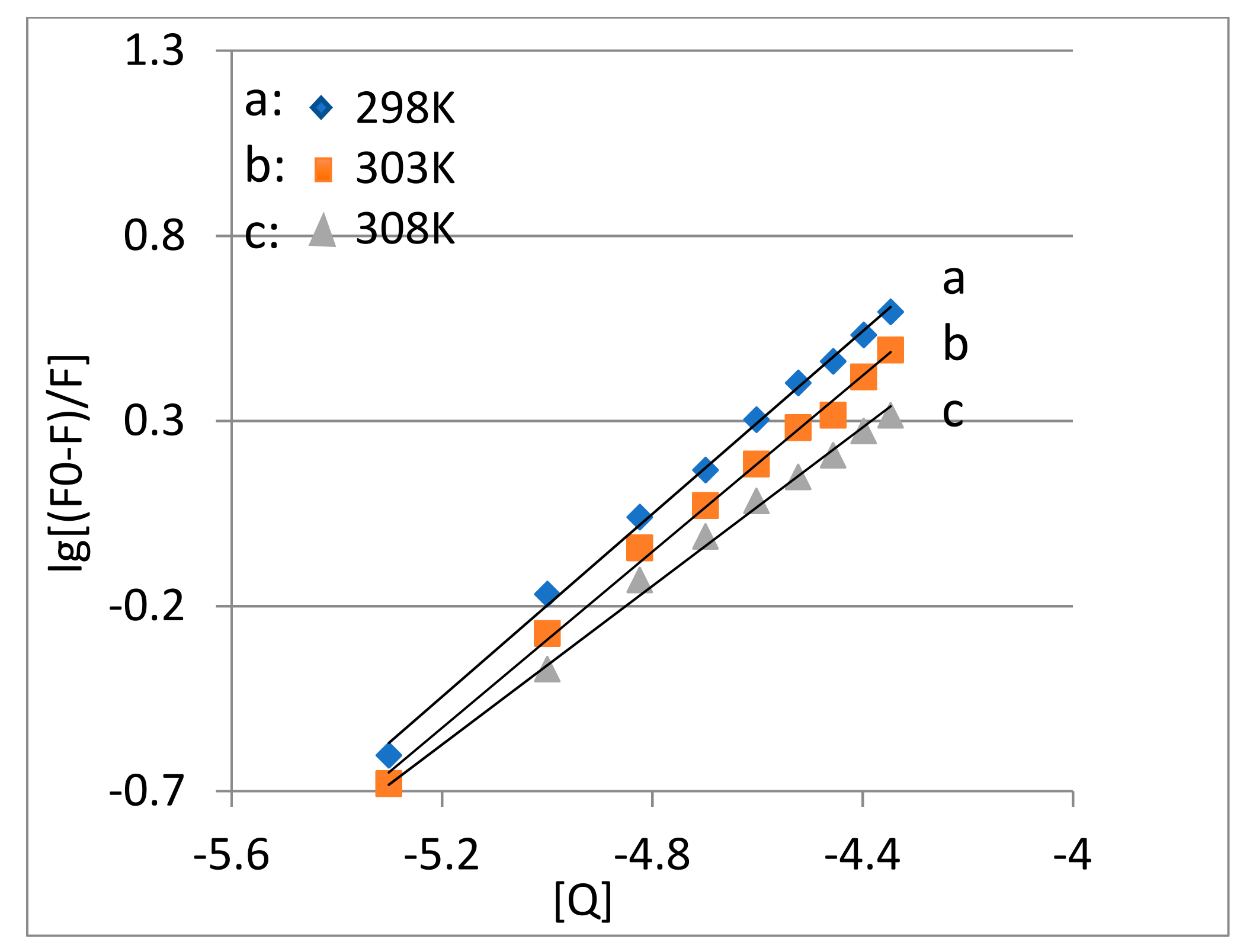

| T/K | Linear Equation | Correlation Coefficient R2 | Ksv/(L/mol) | Kq/(L/mol·s) |
|---|---|---|---|---|
| 298 | y = 1.643x + 0.147 | 0.9920 | 1.643 × 105 | 1.643 × 1013 |
| 303 | y = 1.532x + 0.034 | 0.9917 | 1.532 × 105 | 1.532 × 1013 |
| 308 | y = 1.442x + 0.018 | 0.9933 | 1.442 × 105 | 1.442 × 1013 |
| T/K | Linear Equation | Correlation Coefficient R2 | KA/(L/mol) | Binding-Site Number n |
|---|---|---|---|---|
| 298K | y = 1.234x + 5.972 | 0.9973 | 9.381 × 105 | 1.234 |
| 303K | y = 1.189x + 5.655 | 0.9959 | 4.536 × 105 | 1.189 |
| 308K | y = 1.071x + 4.995 | 0.9950 | 9.910 × 104 | 1.071 |
| T/K | KA/(L/mol) | ΔH/(KJ/mol) | ΔS/(J/(mol.k)) | ΔG/(KJ/mol) |
|---|---|---|---|---|
| 298 | 9.381 × 105 | −34.07 | ||
| 303 | 4.536 × 105 | −109.31 | −252.48 | −32.81 |
| 308 | 9.910 × 104 | −29.46 |
| System | Component | Peak 1 (λex/λem, F) | Peak 2 (λex/λem, F) |
|---|---|---|---|
| A | TRP | 285/353, 414.3 | 220/349, 815.5 |
| B | MC-TRP | 280/354, 292.8 | 220/354, 598.1 |
| System | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Bandom Coil (%) |
|---|---|---|---|---|
| TRP | 10.8 | 50.5 | 19.2 | 27 |
| TRP-MC | 12.6 | 53.2 | 18.9 | 25.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Sun, J.; Ma, L.; Nie, Z.; Sai, H.; Cheng, J.; Duan, J. Characterization of the Interactions between Minocycline Hydrochloride and Trypsin with Spectroscopic and Molecular Docking Technology. Molecules 2023, 28, 2656. https://doi.org/10.3390/molecules28062656
Wang X, Sun J, Ma L, Nie Z, Sai H, Cheng J, Duan J. Characterization of the Interactions between Minocycline Hydrochloride and Trypsin with Spectroscopic and Molecular Docking Technology. Molecules. 2023; 28(6):2656. https://doi.org/10.3390/molecules28062656
Chicago/Turabian StyleWang, Xiaoxia, Jisheng Sun, Litong Ma, Zhihua Nie, Huazheng Sai, Jianguo Cheng, and Jianguo Duan. 2023. "Characterization of the Interactions between Minocycline Hydrochloride and Trypsin with Spectroscopic and Molecular Docking Technology" Molecules 28, no. 6: 2656. https://doi.org/10.3390/molecules28062656
APA StyleWang, X., Sun, J., Ma, L., Nie, Z., Sai, H., Cheng, J., & Duan, J. (2023). Characterization of the Interactions between Minocycline Hydrochloride and Trypsin with Spectroscopic and Molecular Docking Technology. Molecules, 28(6), 2656. https://doi.org/10.3390/molecules28062656






