Study on Benzylamine(BZA) and Aminoethylpiperazine(AEP) Mixed Absorbent on Ship-Based Carbon Capture
Abstract
:1. Introduction
2. Results and Discussion
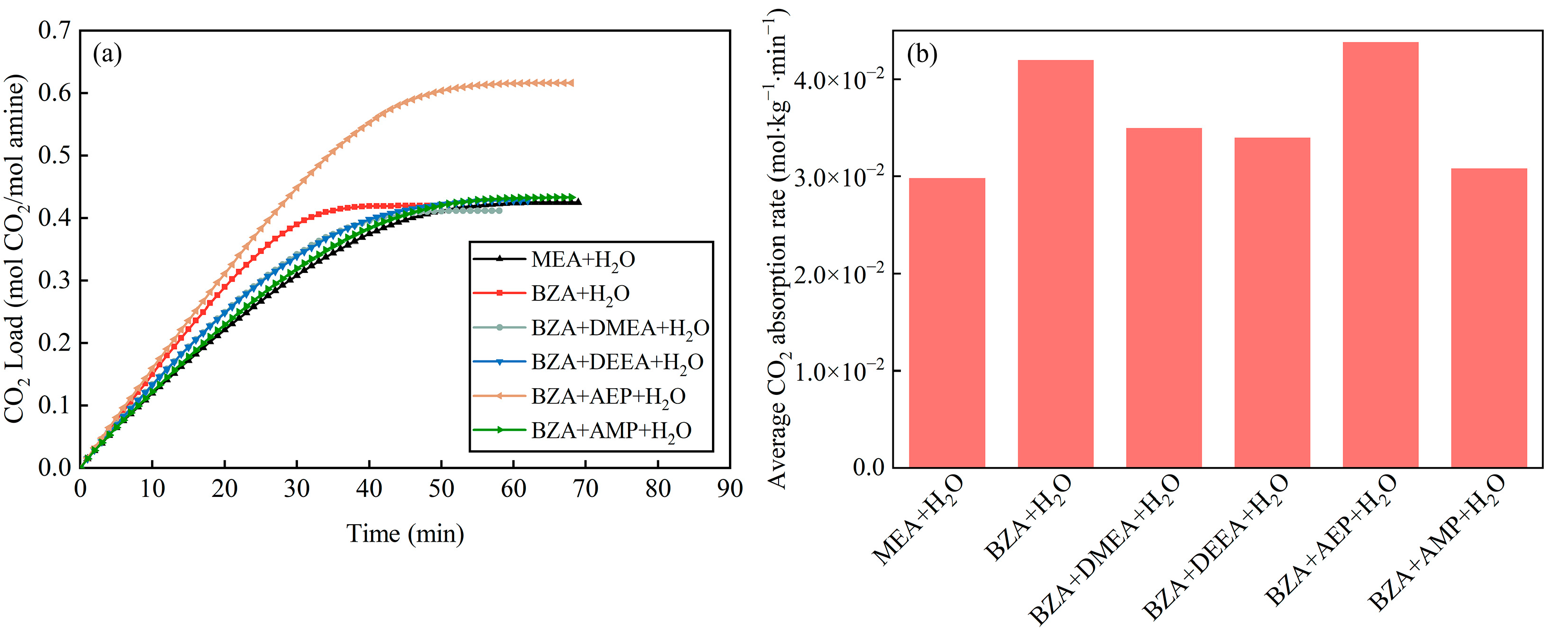
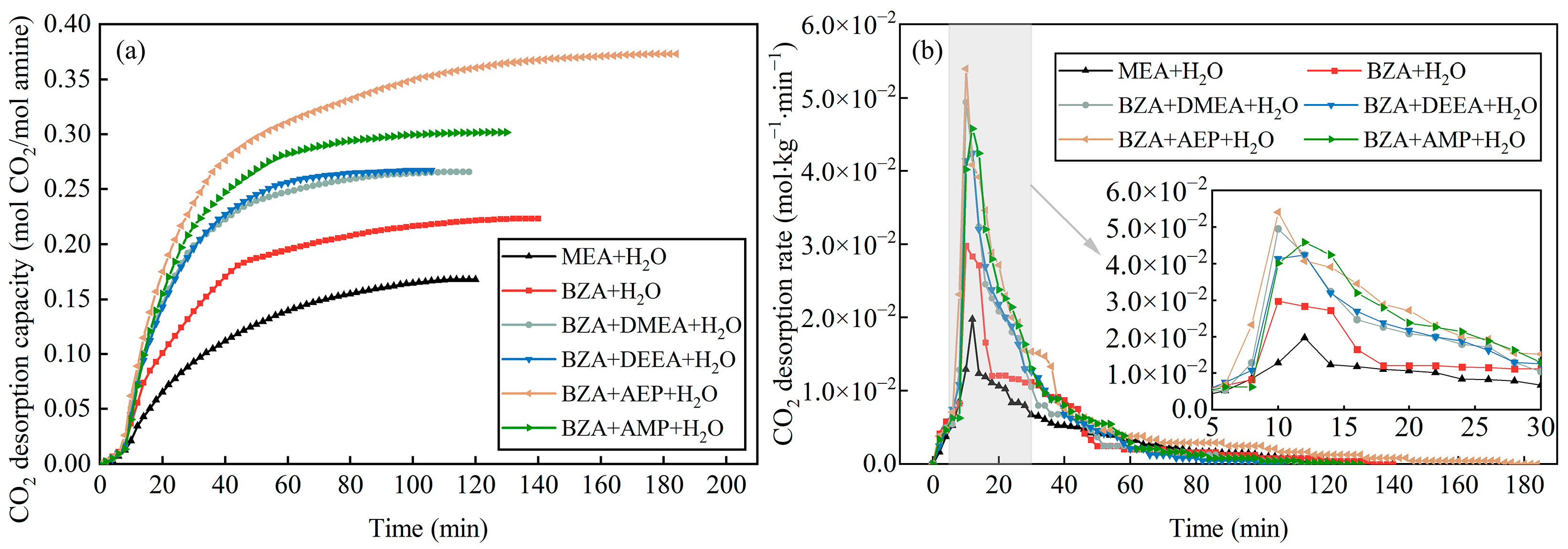
2.1. Absorption and Desorption Properties of Mixed Aqueous Amines Based on BZA
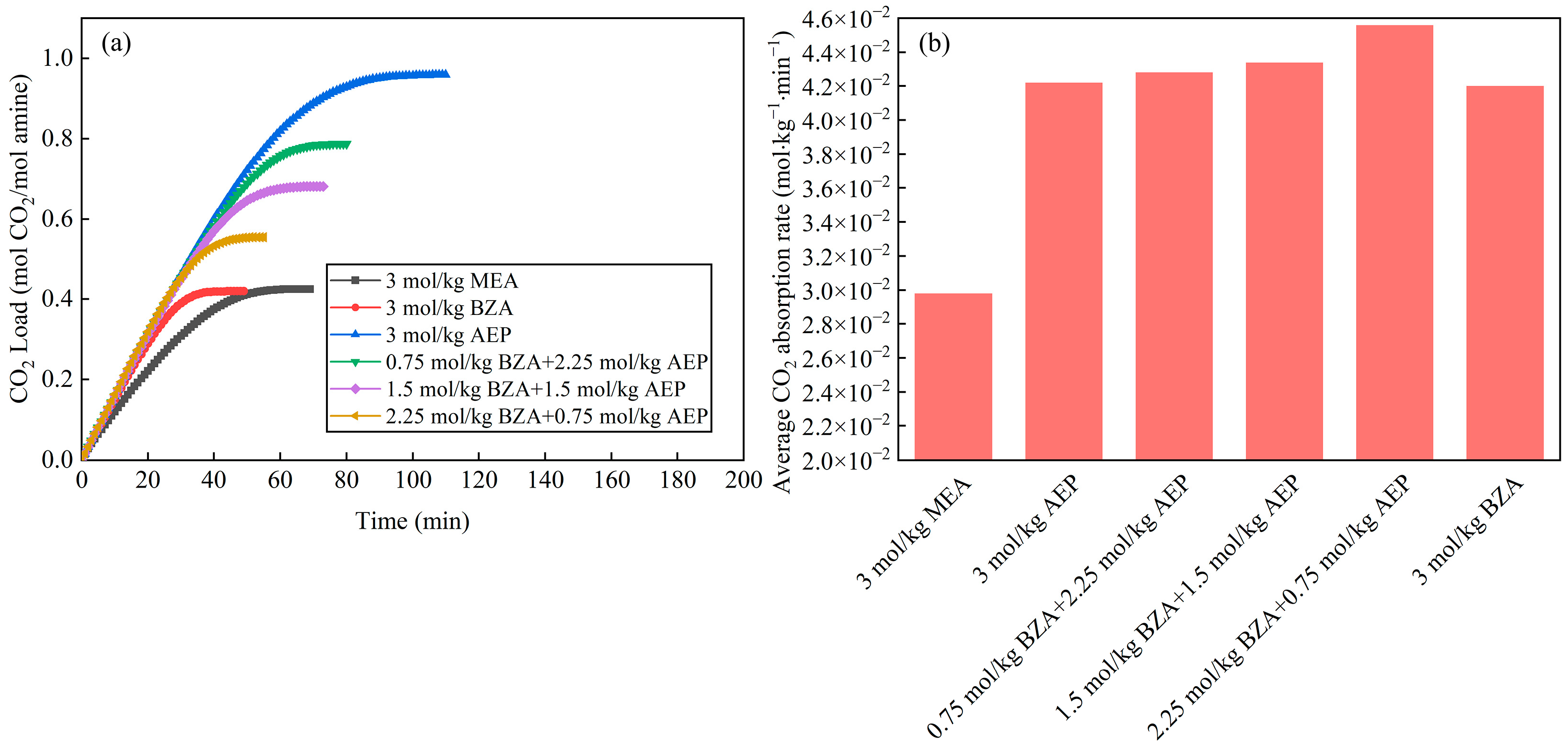
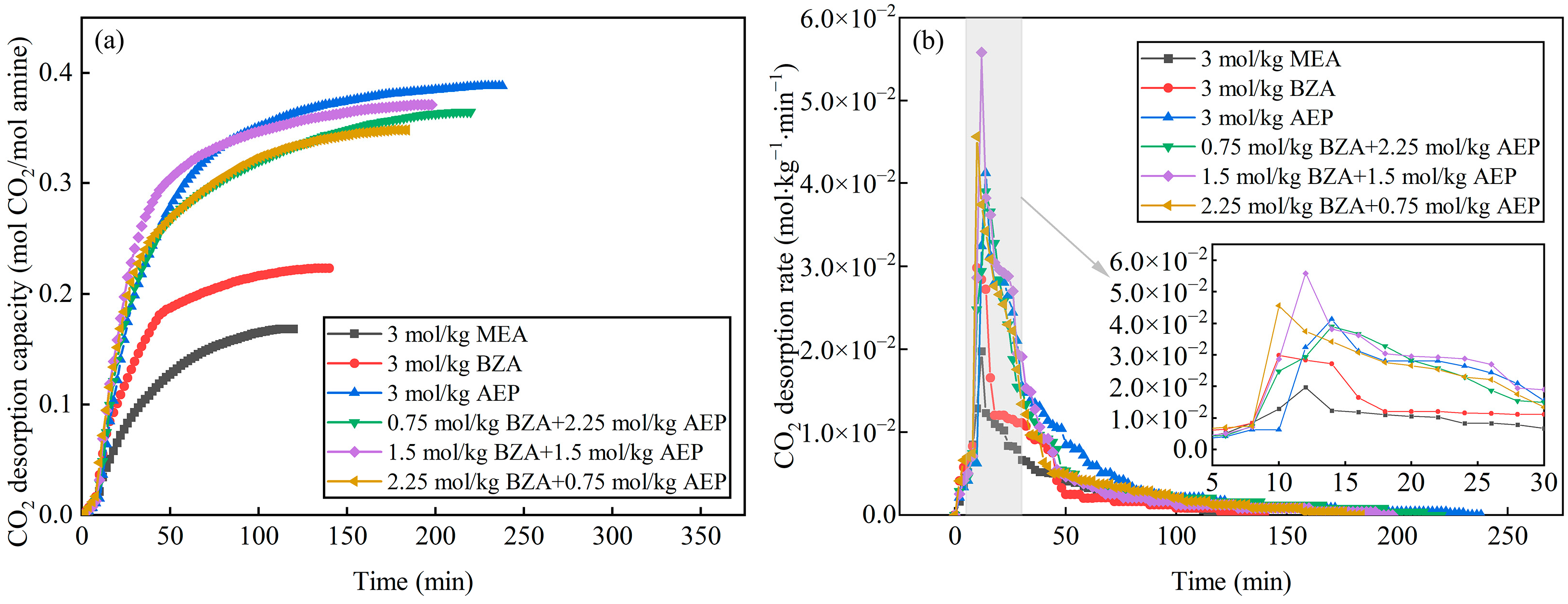
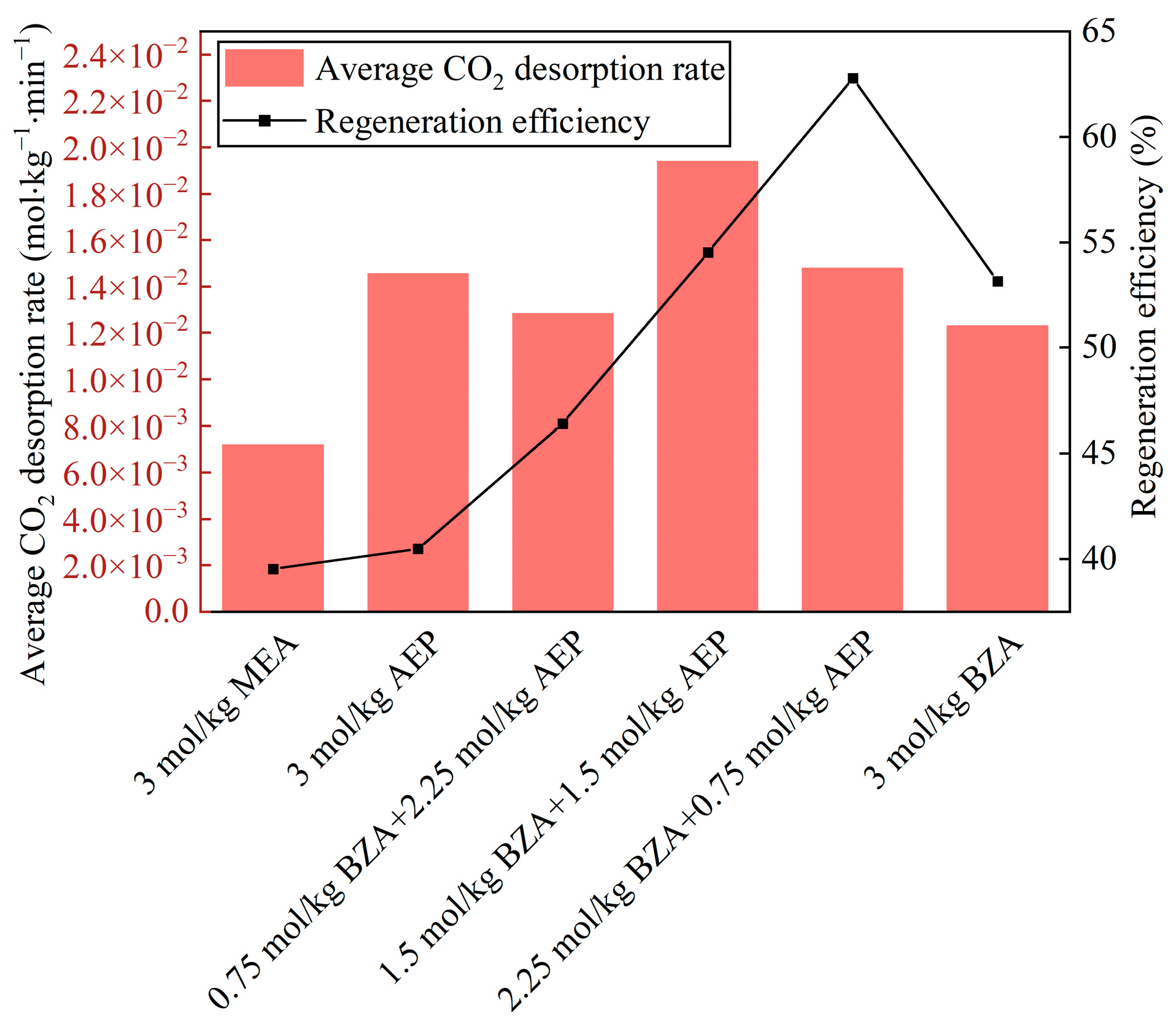
2.2. Effect of BZA and AEP Concentration Ratio
2.2.1. Absorption and Desorption Performance
2.2.2. Fitted Model
3. Materials and Methods
3.1. Materials
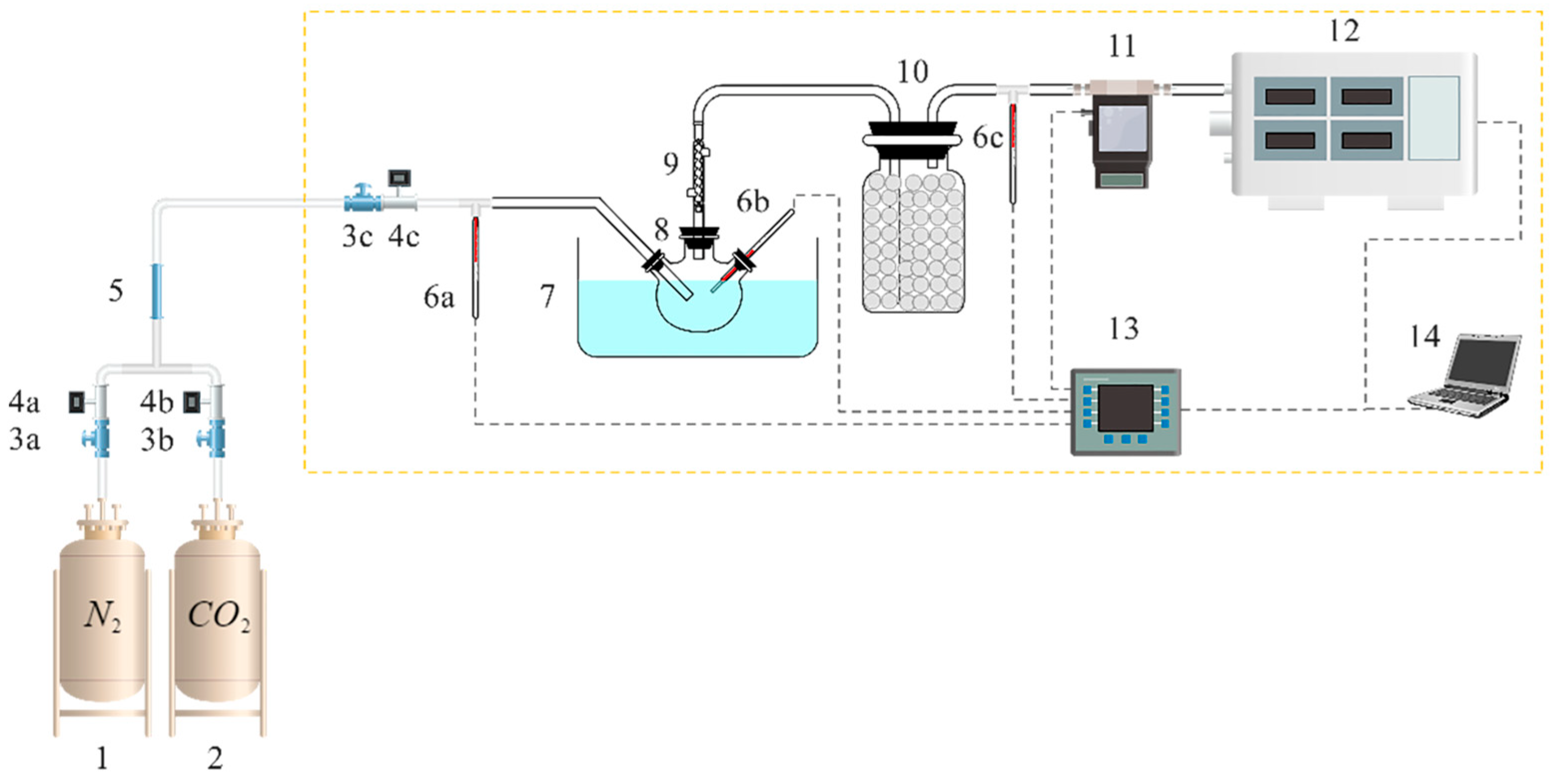
3.2. Experimental Setup
3.3. Data Processing
4. Conclusions
- The absorption and desorption performance of four mixed aqueous amines based on BZA was investigated, and it was found that BZA-AEP had the highest average CO2 absorption rate and CO2 desorption capacity. The CO2 absorption rate of BZA-DMEA, BZA-DEEA, and BZA-AMP decreased in comparison to BZA under the same conditions, while the CO2 absorption rate of BZA-AEP did not decrease. In contrast to MEA, the average CO2 absorption rate of BZA-AEP increased by 47%, and the CO2 desorption capacity increased by 122%. By adding DMEA, DEEA, AEP, and AMP to BZA, the average CO2 desorption rate was enhanced by about 150% compared with MEA and by about 45% compared with BZA.
- The absorption and desorption characteristics of BZA-AEP with different concentration ratios were investigated. The results indicated that there was an optimal concentration ratio for the average CO2 absorption rate and average CO2 desorption rate. The average CO2 absorption rate of BZA-AEP improved by 41~53%, the CO2 desorption capacity improved by 33~131%, and the average CO2 desorption rate improved by 70~170% relative to MEA. It was found that the CO2 load and the absorption equilibrium time had a linear relationship with the concentration ratio, and the desorption equilibrium time increased with the proportion of AEP in the solution.
- The model established by the experimental data indicates that it is more economical to install a complete set of carbon capture systems on a ship with a BZA/AEP concentration ratio of approximately 1.5. Compared with MEA, its average CO2 absorption rate increases by 48%, its CO2 desorption capacity increases by 120%, and its average CO2 desorption rate increases by 161%. In light of its excellent absorption and desorption characteristics, BZA-AEP mixed aqueous amine can reduce the design size of the absorption tower and desorption tower to solve the problem of limited space for SBCC. Therefore, BZA-AEP can be considered a candidate material for SBCC.
- The regeneration efficiency of BZA-AEP is around 55%, and there is still much space to improve its desorption performance using a solid acid catalyst in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van den Akker, J. Carbon Capture Onboard LNG-Fueled Vessels: A Feasibility Study. Master Thesis, Delft University of Technology, Delft, The Netherlands, 2017. [Google Scholar]
- Joung, T.-H.; Kang, S.-G.; Lee, J.-K.; Ahn, J. The IMO Initial Strategy for Reducing Greenhouse Gas(GHG) Emissions, and Its Follow-up Actions towards 2050. J. Int. Marit. Saf. Environ. Aff. Shipp. 2020, 4, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Wang, M. Study of Solvent-Based Carbon Capture for Cargo Ships through Process Modelling and Simulation. Appl. Energy 2017, 195, 402–413. [Google Scholar] [CrossRef] [Green Version]
- Feenstra, M.; Monteiro, J.; van den Akker, J.T.; Abu-Zahra, M.R.M.; Gilling, E.; Goetheer, E. Ship-Based Carbon Capture Onboard of Diesel or LNG-Fuelled Ships. Int. J. Greenh. Gas Control 2019, 85, 1–10. [Google Scholar] [CrossRef]
- Mathisen, A.; Sørensen, H.; Eldrup, N.; Skagestad, R.; Melaaen, M.; Müller, G.I. Cost Optimised CO2 Capture from Aluminium Production. Energy Procedia 2014, 51, 184–190. [Google Scholar] [CrossRef] [Green Version]
- Einbu, A.; Pettersen, T.; Morud, J.; Tobiesen, A.; Jayarathna, C.K.; Skagestad, R.; Nysæther, G. Energy Assessments of Onboard CO2 Capture from Ship Engines by MEA-Based Post Combustion Capture System with Flue Gas Heat Integration. Int. J. Greenh. Gas Control 2022, 113, 103526. [Google Scholar] [CrossRef]
- Ochedi, F.O.; Yu, J.; Yu, H.; Liu, Y.; Hussain, A. Carbon Dioxide Capture Using Liquid Absorption Methods: A Review. Environ. Chem. Lett. 2021, 19, 77–109. [Google Scholar] [CrossRef]
- Davis, J.D. Thermal Degradation of Aqueous Amines Used for Carbon Dioxide Capture; The University of Texas at Austin: Austin, TX, USA, 2009; ISBN 1-109-36076-2. [Google Scholar]
- Eide-Haugmo, I. Environmental Impacts and Aspects of Absorbents Used for CO2 Capture; Norges Teknisk-Naturvitenskapelige Universitet, Fakultet for Naturvitenskap og Teknologi, Institutt for Kjemisk Prosessteknologi: Gjøvik, Norway, 2011; ISBN 978-82-471-3045-2. [Google Scholar]
- DuPart, M.S.; Bacon, T.R.; Edwards, D.J. Understanding Corrosion in Alkanolamine Gas Treating Plants: Part 1. In Hydrocarbon Processing; U.S. Department of Energy: Washington, DC, USA, 1993; Volume 72. [Google Scholar]
- DuPart, M.S.; Bacon, T.R.; Edwards, D.J. Understanding Corrosion in Alkanolamine Gas Treating Plants: Part 2. In Hydrocarbon Processing; U.S. Department of Energy: Washington, DC, USA, 1993; Volume 72. [Google Scholar]
- Mariz, C.L.; DeHart, T.R.; Hansen, D.A.; McCullough, J.G. Solving Corrosion Problems at the NEA Bellingham, Massachusetts Carbon Dioxide Recovery Plant. In CORROSION 99; OnePetro: San Antonio, TX, USA, 1999. [Google Scholar]
- PubChem Compound Summary for CID 700, Ethanolamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Ethanolamine (accessed on 1 October 2022).
- Danckwerts, P.V. The Reaction of CO2 with Ethanolamines. Chem. Eng. Sci. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Ma, C.; Pietrucci, F.; Andreoni, W. Capturing CO2 in Monoethanolamine (MEA) Aqueous Solutions: Fingerprints of Carbamate Formation Assessed with First-Principles Simulations. J. Phys. Chem. Lett. 2014, 5, 1672–1677. [Google Scholar] [CrossRef]
- Richner, G. Promoting CO2 Absorption in Aqueous Amines with Benzylamine. Energy Procedia 2013, 37, 423–430. [Google Scholar] [CrossRef] [Green Version]
- Conway, W.; Beyad, Y.; Richner, G.; Puxty, G.; Feron, P. Rapid CO2 Absorption into Aqueous Benzylamine (BZA) Solutions and Its Formulations with Monoethanolamine (MEA), and 2-Amino-2-Methyl-1-Propanol (AMP) as Components for Post Combustion Capture Processes. Chem. Eng. J. 2015, 264, 954–961. [Google Scholar] [CrossRef]
- Richner, G.; Puxty, G.; Carnal, A.; Conway, W.; Maeder, M.; Pearson, P. Thermokinetic Properties and Performance Evaluation of Benzylamine-Based Solvents for CO2 Capture. Chem. Eng. J. 2015, 264, 230–240. [Google Scholar] [CrossRef]
- Oexmann, J.; Kather, A. Minimising the Regeneration Heat Duty of Post-Combustion CO2 Capture by Wet Chemical Absorption: The Misguided Focus on Low Heat of Absorption Solvents. Int. J. Greenh. Gas Control 2010, 4, 36–43. [Google Scholar] [CrossRef]
- Gao, J.; Yin, J.; Zhu, F.; Chen, X.; Tong, M.K.; Wan, Z. Study of the CO2 Absorption into Aqueous Benzylamine (BZA) and Its Formulations with Monoethanolamine (MEA) as a Component for Post-Combustion Capture Process. China Pet. Process. Petrochem. Technol. 2016, 18, 7. [Google Scholar]
- Mukherjee, S.; Bandyopadhyay, S.S.; Samanta, A.N. Experimental Measurements and Modelling of CO2 Solubility in Aqueous Mixtures of Benzylamine and N-(2-Aminoethyl) Ethanolamine. Asia-Pac. J. Chem. Eng. 2018, 13, e2264. [Google Scholar] [CrossRef]
- Zheng, W.; Yan, Z.; Zhang, R.; Jiang, W.; Luo, X.; Liang, Z.; Yang, Q.; Yu, H. A Study of Kinetics, Equilibrium Solubility, Speciation and Thermodynamics of CO2 Absorption into Benzylamine (BZA) Solution. Chem. Eng. Sci. 2022, 251, 117452. [Google Scholar] [CrossRef]
- Puxty, G.; Conway, W.; Botma, H.; Feron, P.; Maher, D.; Wardhaugh, L. A New CO2 Absorbent Developed from Addressing Benzylamine Vapour Pressure Using Co-Solvents. Energy Procedia 2017, 114, 1956–1965. [Google Scholar] [CrossRef]
- Chen, G.; Chen, G.; Peruzzini, M.; Barzagli, F.; Zhang, R. Investigating the Performance of Ethanolamine and Benzylamine Blends as Promising Sorbents for Postcombustion CO2 Capture through 13C NMR Speciation and Heat of CO2 Absorption Analysis. Energy Fuels 2022, 36, 9203–9212. [Google Scholar] [CrossRef]
- Domalski, E.S.; Hearing, E.D. Heat Capacities and Entropies of Organic Compounds in the Condensed Phase. Volume III. J. Phys. Chem. Ref. Data 1996, 25, 1–525. [Google Scholar] [CrossRef]
- Chiu, L.-F.; Li, M.-H. Heat Capacity of Alkanolamine Aqueous Solutions. J. Chem. Eng. Data 1999, 44, 1396–1401. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bandyopadhyay, S.S.; Samanta, A.N. Vapor-Liquid Equilibrium (VLE) of CO2 in Aqueous Solutions of Benzylamine: New Data and Modeling Using ENRTL-Equation. Int. J. Greenh. Gas Control 2017, 56, 12–21. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bandyopadhyay, S.S.; Samanta, A.N. Kinetic Study of CO2 Absorption in Aqueous Benzylamine Solvent Using a Stirred Cell Reaction Calorimeter. Energy Fuels 2018, 32, 3668–3680. [Google Scholar] [CrossRef]
- Mukherjee, S.; Samanta, A.N. Heat of Absorption of CO2 and Heat Capacity Measurements in Aqueous Solutions of Benzylamine, N-(2-Aminoethyl)-Ethanolamine, and Their Blends Using a Reaction Calorimeter. J. Chem. Eng. Data 2019, 64, 3392–3406. [Google Scholar] [CrossRef]
- Martin, S.; Lepaumier, H.; Picq, D.; Kittel, J.; de Bruin, T.; Faraj, A.; Carrette, P.-L. New Amines for CO2 Capture. IV. Degradation, Corrosion, and Quantitative Structure Property Relationship Model. Ind. Eng. Chem. Res. 2012, 51, 6283–6289. [Google Scholar] [CrossRef]
- Puxty, G.; Conway, W.; Yang, Q.; Bennett, R.; Fernandes, D.; Pearson, P.; Maher, D.; Feron, P. The Evolution of a New Class of CO2 Absorbents: Aromatic Amines. Int. J. Greenh. Gas Control 2019, 83, 11–19. [Google Scholar] [CrossRef]
- PubChem Compound Summary for CID 7504, Benzylamine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Benzylamine (accessed on 1 October 2022).
- Howard, P.H. Handbook of Environmental Fate and Exposure Data: For Organic Chemicals, Volume III Pesticides; CRC Press: Boca Raton, FL, USA, 1991; ISBN 978-0-87371-328-3. [Google Scholar]
- Xiao, M.; Liu, H.; Idem, R.; Tontiwachwuthikul, P.; Liang, Z. A Study of Structure–Activity Relationships of Commercial Tertiary Amines for Post-Combustion CO2 Capture. Appl. Energy 2016, 184, 219–229. [Google Scholar] [CrossRef]
- Muchan, P.; Narku-Tetteh, J.; Saiwan, C.; Idem, R.; Supap, T. Effect of Number of Amine Groups in Aqueous Polyamine Solution on Carbon Dioxide (CO2) Capture Activities. Sep. Purif. Technol. 2017, 184, 128–134. [Google Scholar] [CrossRef]
- Sartori, G.; Savage, D.W. Sterically Hindered Amines for Carbon Dioxide Removal from Gases. Ind. Eng. Chem. Fundam. 1983, 22, 239–249. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Mumford, K.; Stevens, G.W.; Fei, W.; Wang, Y. Recent Advances in Carbon Dioxide Capture and Utilization with Amines and Ionic Liquids. Green Chem. Eng. 2020, 1, 16–32. [Google Scholar] [CrossRef]
- Narku-Tetteh, J.; Muchan, P.; Saiwan, C.; Supap, T.; Idem, R. Selection of Components for Formulation of Amine Blends for Post Combustion CO2 Capture Based on the Side Chain Structure of Primary, Secondary and Tertiary Amines. Chem. Eng. Sci. 2017, 170, 542–560. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, X.; Yang, Q.; Yu, H.; Liang, Z.; Luo, X. Analysis of the Reduction of Energy Cost by Using MEA-MDEA-PZ Solvent for Post-Combustion Carbon Dioxide Capture (PCC). Appl. Energy 2017, 205, 1002–1011. [Google Scholar] [CrossRef]
- Cooper, D.A. Exhaust Emissions from High Speed Passenger Ferries. Atmos. Environ. 2001, 35, 4189–4200. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Gallego, L.M.; Camino, S.; Camino, J.A.; Navarrete, B. Evaluation of MEA 5 M Performance at Different CO2 Concentrations of Flue Gas Tested at a CO2 Capture Lab-Scale Plant. Energy Procedia 2017, 114, 6222–6228. [Google Scholar] [CrossRef]
- Van Wagener, D.H.; Rochelle, G.T. Stripper Configurations for CO2 Capture by Aqueous Monoethanolamine and Piperazine. Energy Procedia 2011, 4, 1323–1330. [Google Scholar] [CrossRef] [Green Version]
- Plaza, J.M.; Rochelle, G.T. Modeling Pilot Plant Results for CO2 Capture by Aqueous Piperazine. Energy Procedia 2011, 4, 1593–1600. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Zhao, X.; Chen, J.; Fang, M. Pilot-Scale Experimental Study of a New High-Loading Absorbent for Capturing CO2 from Flue Gas. Processes 2022, 10, 599. [Google Scholar] [CrossRef]


| Std | Run | BZA (mol/kg) | AEP (mol/kg) |
|---|---|---|---|
| 6 | 1 | 3 | 0 |
| 2 | 2 | 0 | 3 |
| 1 | 3 | 3 | 0 |
| 3 | 4 | 1.5 | 1.5 |
| 5 | 5 | 0.75 | 2.25 |
| 7 | 6 | 0 | 3 |
| 8 | 7 | 1.5 | 1.5 |
| 4 | 8 | 2.25 | 0.75 |
| Reagent Name | Abbreviation | Boiling Point/°C (101.325 kPa) | Dynamic Viscosity/mPa·s (20 °C) |
|---|---|---|---|
| ethanolamine | MEA | 170 | 7.5 |
| benzylamine | BZA | 185 | 1.78 |
| 2-amino-2-methyl-1-propanol | AMP | 165 | 147 |
| N, N-dimethylethanolamine | DMEA | 134 | 3.58 |
| N, N-diethylethanolamine | DEEA | 163 | 5 |
| aminoethylpiperazine | AEP | 220 | 15.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Chen, H.; Wang, Y.; Zhu, X.; Yang, G. Study on Benzylamine(BZA) and Aminoethylpiperazine(AEP) Mixed Absorbent on Ship-Based Carbon Capture. Molecules 2023, 28, 2661. https://doi.org/10.3390/molecules28062661
Mao X, Chen H, Wang Y, Zhu X, Yang G. Study on Benzylamine(BZA) and Aminoethylpiperazine(AEP) Mixed Absorbent on Ship-Based Carbon Capture. Molecules. 2023; 28(6):2661. https://doi.org/10.3390/molecules28062661
Chicago/Turabian StyleMao, Xudong, Hao Chen, Yubing Wang, Xinbo Zhu, and Guohua Yang. 2023. "Study on Benzylamine(BZA) and Aminoethylpiperazine(AEP) Mixed Absorbent on Ship-Based Carbon Capture" Molecules 28, no. 6: 2661. https://doi.org/10.3390/molecules28062661
APA StyleMao, X., Chen, H., Wang, Y., Zhu, X., & Yang, G. (2023). Study on Benzylamine(BZA) and Aminoethylpiperazine(AEP) Mixed Absorbent on Ship-Based Carbon Capture. Molecules, 28(6), 2661. https://doi.org/10.3390/molecules28062661





