Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri
Abstract
1. Introduction
2. Results
2.1. Experimental Study
2.2. Theoretical Study
2.2.1. HOMO–LUMO Analysis
2.2.2. MEP Analysis
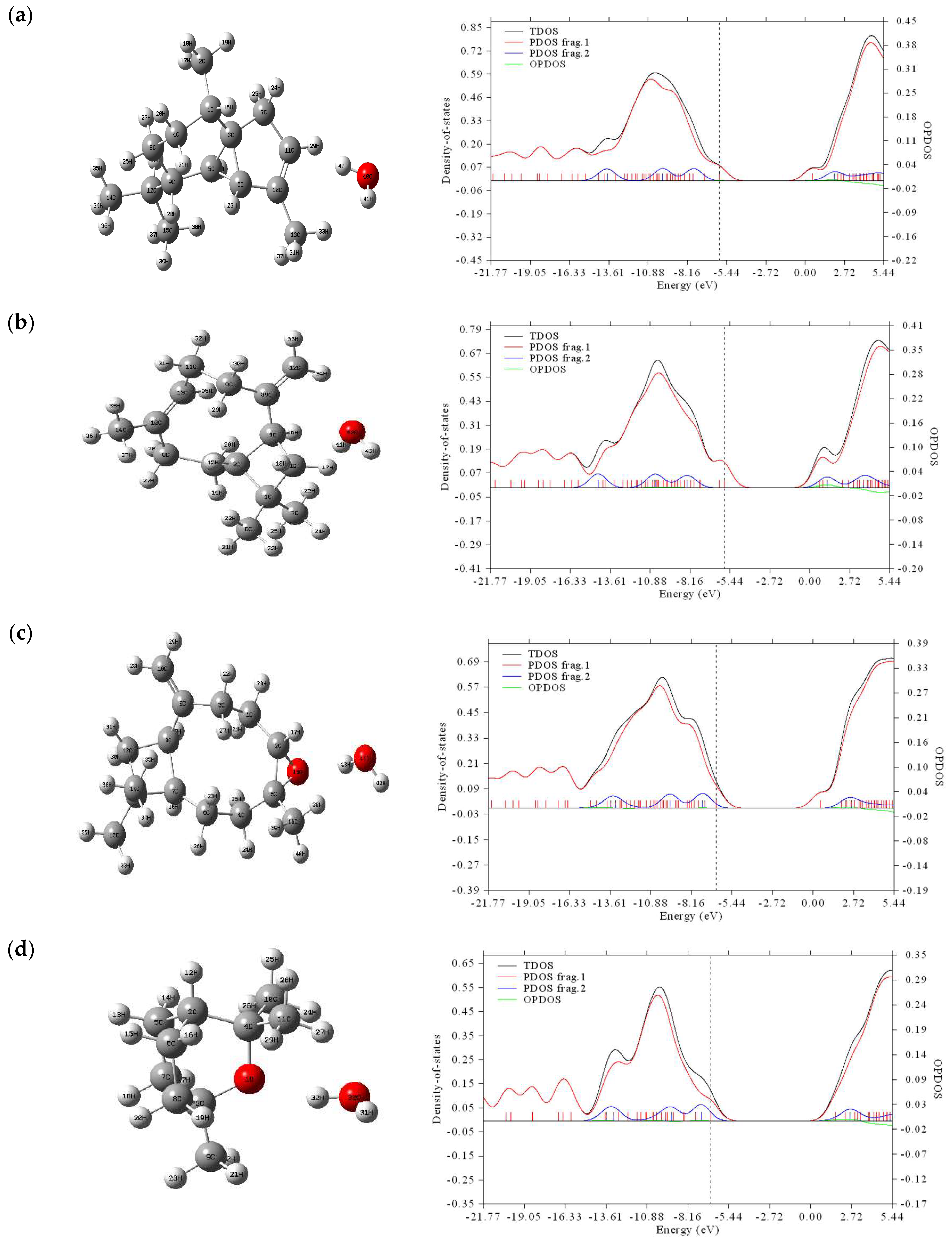
2.2.3. Energy of Population Density of States
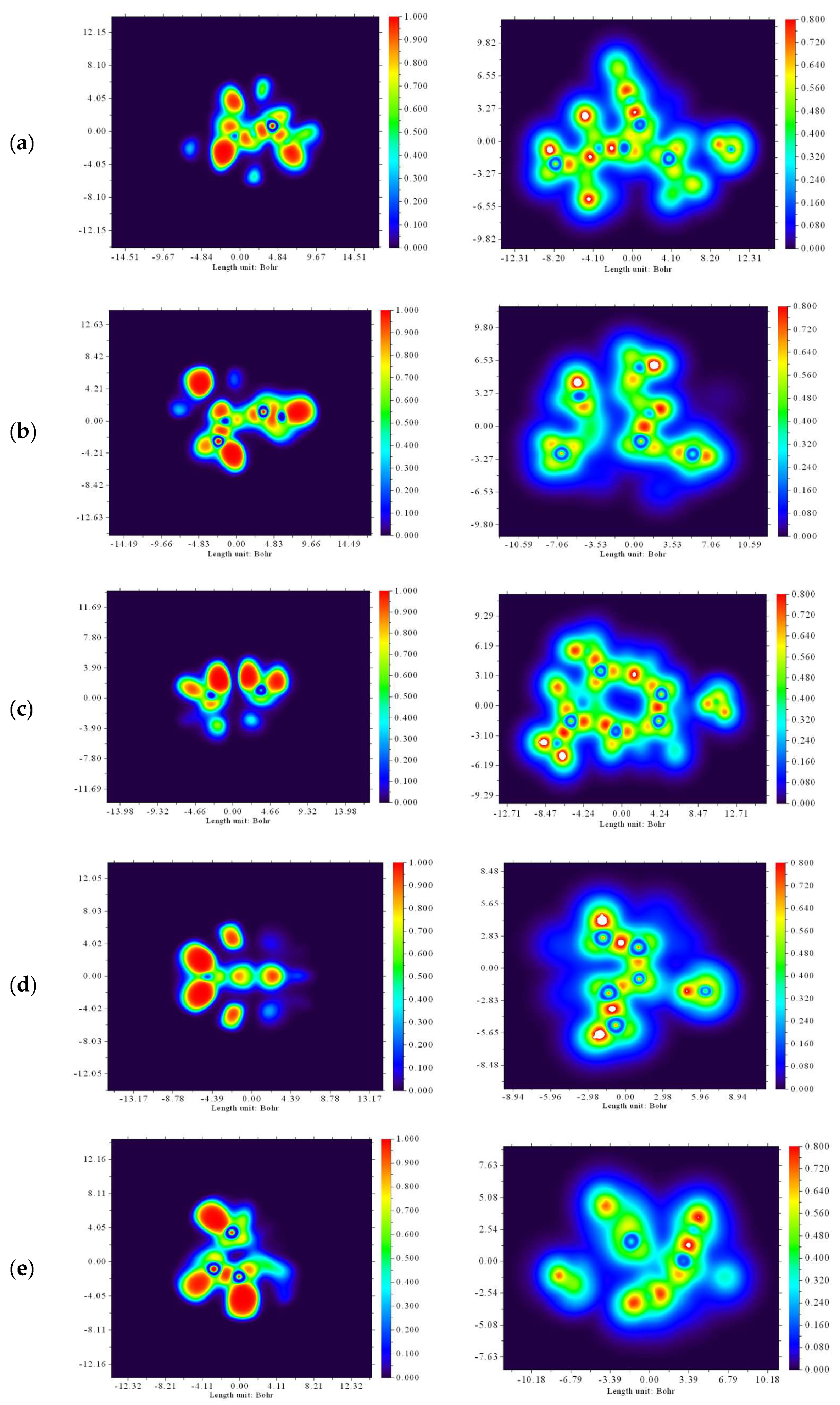
2.2.4. NCI-RDG Analysis
2.2.5. ELF and LOL Analyses
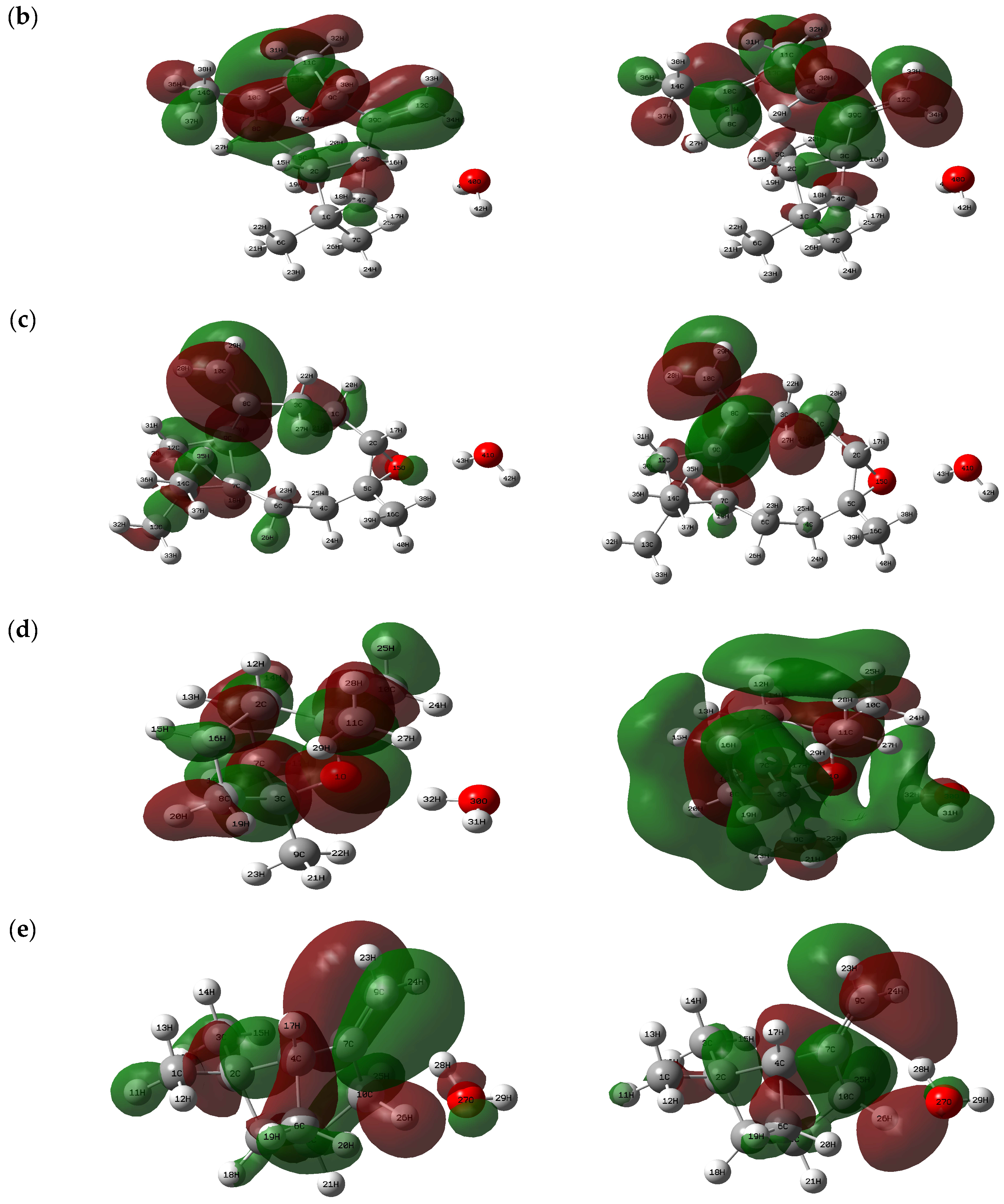
2.2.6. Fukui Analysis
3. Materials and Methods
3.1. Collection of the Plant Material
3.2. Isolation of Essential Oil and GC-MS Analysis
3.3. Theoretical Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kyriakopoulou, I.; Magiatis, P.; Skaltsounis, A.-L.; Aligiannis, N.; Harvala, C. Samioside, a New Phenylethanoid Glycoside with Free-Radical Scavenging and Antimicrobial Activities from Phlomis samia. J. Nat. Prod. 2001, 64, 1095–1097. [Google Scholar] [CrossRef]
- Demirci, B.; Baser, K.H.C.; Dadandi, M.Y. Composition of the Essential Oils of Phlomis rigida Labill. and P. samia L. J. Essent. Oil Res. 2006, 18, 328–331. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Akkaya, A. Antibacterial activities of Marrubium catariifolium and Phlomis pungens var. hirta grown wild in Eastern Anatolia, Turkey. Int. J. Agric. Biol. 2011, 13, 105–109. [Google Scholar]
- Kamel, M.S.; Mohamed, K.M.; Hassanean, H.A.; Ohtani, K.; Kasai, R.; Yamasaki, K. Iridoid and megastigmane glycosides from Phlomis aurea. Phytochemistry 2000, 55, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.-Z. Comparative analysis of essential oil components of three Phlomis species in Qinling Mountains of China. J. Pharm. Biomed. Anal. 2008, 47, 213–217. [Google Scholar] [CrossRef]
- Amor, I.L.-B.; Boubaker, J.; Sgaier, M.B.; Skandrani, I.; Bhouri, W.; Neffati, A.; Kilani, S.; Bouhlel, I.; Ghedira, K.; Chekir-Ghedira, L. Phytochemistry and biological activities of Phlomis species. J. Ethnopharmacol. 2009, 125, 183–202. [Google Scholar] [CrossRef]
- Borchardt, J.R.; Wyse, D.L.; Sheaffer, C.C.; Kauppi, K.L.; Fulcher, R.G.; Ehlke, N.J.; Biesboer, D.D.; Bey, R.F. Antimicrobial activity of native and naturalized plants of Minnesota and Wisconsin. J. Med. Plants Res. 2008, 2, 98–110. [Google Scholar]
- Parisa, S.; Hamid, R.M.E.; Gholamreza, A.; Mohammad, H.S.S.; Abbas, S. Phytochemical study of Phlomis olivieri Benth. and Phlomis persica Boiss. DARU J. Pharm. Sci. 2006, 14, 115–121. [Google Scholar]
- Akman, F. A comparative study based on molecular structure, spectroscopic, electronic, thermodynamic and NBO analysis of some nitrogen-containing monomers. Polym. Bull. 2021, 78, 663–693. [Google Scholar] [CrossRef]
- Akman, F.; Kazachenko, A.; Malyar, Y. A density functional theory study of sulfated monolignols: P-Coumaril and coniferyl alcohols. Cellul. Chem. Technol. 2021, 55, 41–54. [Google Scholar] [CrossRef]
- Akman, F. Spectroscopic investigation, HOMO–LUMO energies, natural bond orbital (NBO) analysis and thermodynamic properties of two-armed macroinitiator containing coumarin with DFT quantum chemical calculations. Can. J. Phys. 2016, 94, 583–593. [Google Scholar] [CrossRef]
- Lu, L.; Hu, H.; Hou, H.; Wang, B. An improved B3LYP method in the calculation of organic thermochemistry and reactivity. Comput. Theor. Chem. 2013, 1015, 64–71. [Google Scholar] [CrossRef]
- Demirpolat, A.; Akman, F.; Kazachenko, A.S. An Experimental and Theoretical Study on Essential Oil of Aethionema sancakense: Characterization, Molecular Properties and RDG Analysis. Molecules 2022, 27, 6129. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, A.G.Q.; Saraiva, G.D.; Albuquerque, R.L.; Nogueira, C.E.S.; Teixeira, A.M.R.; Lima, L.B.; Cruz, B.G.; de Sousa, F.F. Chemical analysis and vibrational spectroscopy study of essential oils from Lippia sidoides and of its major constituent. Vib. Spectrosc. 2020, 110, 103111. [Google Scholar] [CrossRef]
- Alves Borges Leal, A.L.; Fonseca Bezerra, C.; Ferreira e Silva, A.K.; Everson da Silva, L.; Bezerra, L.L.; Almeida-Neto, F.W.; Marinho, E.M.; Celedonio Fernandes, C.F.; Nunes da Rocha, M.; Marinho, M.M.; et al. Seasonal variation of the composition of essential oils from Piper cernuum Vell and Piper rivinoides Kunth, ADMET study, DFT calculations, molecular docking and dynamics studies of major components as potent inhibitors of the heterodimer methyltransferase complex NSP16-NSP10 SARS COV-2 protein. J. Biomol. Struct. Dyn. 2022, 1–19. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M. The essential oil composition of Phlomis bruguieri Desf. from Iran. Flavour Fragr. J. 2005, 20, 344–346. [Google Scholar] [CrossRef]
- Nikan, M.; Saeidnia, S.; Manayi, A.; Saadatmand, S. Essential oils of four Phlomis species growing in Iran: Chemical composition, antimicrobial and antifungal activity. Prog. Nutr. 2017, 19, 75–79. [Google Scholar]
- Kazachenko, A.S.; Issaoui, N.; Fetisova, O.Y.; Berezhnaya, Y.D.; Al-Dossary, O.M.; Akman, F.; Kumar, N.; Bousiakou, L.G.; Kazachenko, A.S.; Ionin, V.A.; et al. Comprehensive Study of the Ammonium Sulfamate—Urea Binary System. Molecules 2023, 28, 470. [Google Scholar] [CrossRef]
- Mohammed Hussain, S.G.; Kumar, R.; Mohamed Naseer Ali, M.; Kannappan, V. Structural effect on the strength of non-covalent interactions in binary mixtures of benzyl amine and certain ethers through ultrasonic, FT-IR spectral and DFT studies at 303.15 K. J. Mol. Liq. 2019, 277, 865–875. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Abdelmoulahi, H.; Issaoui, N.; Malyar, Y.N.; Al-Dossary, O.; Wojcik, M.J. Intermolecular hydrogen bonds interactions in water clusters of ammonium sulfamate: FTIR, X-ray diffraction, AIM, DFT, RDG, ELF, NBO analysis. J. Mol. Liq. 2021, 342, 117475. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Medimagh, M.; Issaoui, N.; Al-Dossary, O.; Wojcik, M.J.; Kazachenko, A.S.; Miroshnokova, A.V.; Malyar, Y.N. Sulfamic acid/water complexes (SAA-H2O(1-8)) intermolecular hydrogen bond interactions: FTIR, X-ray, DFT and AIM analysis. J. Mol. Struct. 2022, 1265, 133394. [Google Scholar] [CrossRef]
- Lockwood, S.P.; Fuller, T.G.; Newby, J.J. Structure and Spectroscopy of Furan: H2O Complexes. J. Phys. Chem. A 2018, 122, 7160–7170. [Google Scholar] [CrossRef]
- Gougoula, E.; Cole, D.J.; Walker, N.R. Bifunctional Hydrogen Bonding of Imidazole with Water Explored by Rotational Spectroscopy and DFT Calculations. J. Phys. Chem. A 2020, 124, 2649–2659. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, R.B.; Dewberry, C.T.; Cornelius, R.D.; Smith, C.J.; Leopold, K.R. Multidimensional Large Amplitude Dynamics in the Pyridine–Water Complex. J. Phys. Chem. A 2017, 121, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Pérez, C.; Steber, A.L.; Rijs, A.M.; Temelso, B.; Shields, G.C.; Lopez, J.C.; Kisiel, Z.; Schnell, M. Corannulene and its complex with water: A tiny cup of water. Phys. Chem. Chem. Phys. 2017, 19, 14214–14223. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Green, P.G.; Bumgarner, R.E.; Dasgupta, S.; Goddard, W.A.; Blake, G.A. Benzene Forms Hydrogen Bonds with Water. Science 1992, 257, 942–945. [Google Scholar] [CrossRef]
- Gou, Q.; Feng, G.; Evangelisti, L.; Caminati, W. Lone-Pair⋅π Interaction: A Rotational Study of the Chlorotrifluoroethylene–Water Adduct. Angew. Chem. Int. Ed. 2013, 52, 11888–11891. [Google Scholar] [CrossRef]
- Potapov, A.; Asselin, P. High-resolution jet spectroscopy of weakly bound binary complexes involving water. Int. Rev. Phys. Chem. 2014, 33, 275–300. [Google Scholar] [CrossRef]
- Zwier, T.S. The spectroscopy of solvation in hydrogen-bonded aromatic clusters. Annu. Rev. Phys. Chem. 1996, 47, 205–241. [Google Scholar] [CrossRef]
- Aparicio, S.; Alcalde, R.; Dávila, M.J.; García, B.; Leal, J.M. Properties of 1,8-Cineole: A Thermophysical and Theoretical Study. J. Phys. Chem. B 2007, 111, 3167–3177. [Google Scholar] [CrossRef]
- Gyrdymova, Y.V.; Rubtsova, S.A. Caryophyllene and caryophyllene oxide: A variety of chemical transformations and biological activities. Chem. Pap. 2022, 76, 1–39. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Akman, F.; Sagaama, A.; Issaoui, N.; Malyar, N.Y.; Vasilieva, N.Y.; Borovkov, V.S. Theoretical and experimental study of guar gum sulfation. J. Mol. Model. 2021, 27, 5. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Yonezawa, T.; Shingu, H. A Molecular Orbital Theory of Reactivity in Aromatic Hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Muthu, S.; Prasath, M.; Arun Balaji, R. Experimental and theoretical investigations of spectroscopic properties of 8-chloro-1-methyl-6-phenyl-4H-[1,2,4]triazolo[4,3-a][1,4]benzodiazepine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 106, 129–145. [Google Scholar] [CrossRef]
- Kazachenko, A.S.; Tanış, E.; Akman, F.; Medimagh, M.; Issaoui, N.; Al-Dossary, O.; Bousiakou, L.G.; Kazachenko, A.S.; Zimonin, D.; Skripnikov, A.M. A Comprehensive Study of N-Butyl-1H-Benzimidazole. Molecules 2022, 27, 7864. [Google Scholar] [CrossRef] [PubMed]
- Rijal, R.; Lamichhane, H.P.; Pudasainee, K. Molecular structure, homo-lumo analysis and vibrational spectroscopy of the cancer healing pro-drug temozolomide based on dft calculations. AIMS Biophys. 2022, 9, 208–220. [Google Scholar] [CrossRef]
- Kumar, P.S.; Vasudevan, K.; Prakasam, A.; Geetha, M.; Anbarasan, P.M. Quantum chemistry calculations of 3-Phenoxyphthalonitrile dye sensitizer for solar cells. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 45–50. [Google Scholar] [CrossRef]
- Parr, R.G.; Donnelly, R.A.; Levy, M.; Palke, W.E. Electronegativity: The density functional viewpoint. J. Chem. Phys. 1978, 68, 3801–3807. [Google Scholar] [CrossRef]
- Hu, S.-X.; Yu, J.-G.; Zeng, E.Y. A theoretical study on UV-spectroscopy, electronic structure and reactivity properties of sesquiterpenes. Atmos. Chem. Phys. Discuss. 2010, 10, 24325–24343. [Google Scholar]
- Joshi, B.D.; Srivastava, A.; Tandon, P.; Jain, S. Molecular structure, vibrational spectra and HOMO, LUMO analysis of yohimbine hydrochloride by density functional theory and ab initio Hartree–Fock calculations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 82, 270–278. [Google Scholar] [CrossRef]
- Nkungli, N.K.; Ghogomu, J.N. Theoretical analysis of the binding of iron(III) protoporphyrin IX to 4-methoxyacetophenone thiosemicarbazone via DFT-D3, MEP, QTAIM, NCI, ELF, and LOL studies. J. Mol. Model. 2017, 23, 200. [Google Scholar] [CrossRef]
- Krack, M.; Jug, K. Molecular electrostatic potentials for large systems. In Theoretical and Computational Chemistry; Murray, J.S., Sen, K., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 3, pp. 297–331. [Google Scholar]
- Li, P.; Wei, J.; Wei, H.; Wang, K.; Wu, J.; Li, Y.; Liu, W.; Fu, Y.; Xie, F.; Ma, J. A Systemic Insight into Exohedral Actinides and Endohedral Borospherenes: An&Bm and An@Bn (An=U, Np, Pu; m = 28, 32, 34, 36, 38, 40; n = 36, 38, 40). Molecules 2022, 27, 6047. [Google Scholar] [CrossRef]
- Chen, M.; Waghmare, U.V.; Friend, C.M.; Kaxiras, E. A density functional study of clean and hydrogen-covered α-MoO3(010): Electronic structure and surface relaxation. J. Chem. Phys. 1998, 109, 6854–6860. [Google Scholar] [CrossRef]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Arulaabaranam, K.; Mani, G.; Muthu, S. Computational assessment on wave function (ELF, LOL) analysis, molecular confirmation and molecular docking explores on 2-(5-Amino-2- Methylanilino)-4-(3-pyridyl) pyrimidine. Chem. Data Collect. 2020, 29, 100525. [Google Scholar] [CrossRef]
- Silvi, B.; Savin, A. Classification of chemical bonds based on topological analysis of electron localization functions. Nature 1994, 371, 683–686. [Google Scholar] [CrossRef]
- Arulaabaranam, K.; Muthu, S.; Mani, G.; Ben Geoffrey, A.S. Speculative assessment, molecular composition, PDOS, topology exploration (ELF, LOL, RDG), ligand-protein interactions, on 5-bromo-3-nitropyridine-2-carbonitrile. Heliyon 2021, 7, e07061. [Google Scholar] [CrossRef] [PubMed]
- Rizwana, B.F.; Muthu, S.; Prasana, J.C.; Abraham, C.S.; Raja, M. Spectroscopic (FT-IR, FT-Raman) investigation, topology (ESP, ELF, LOL) analyses, charge transfer excitation and molecular docking (dengue, HCV) studies on ribavirin. Chem. Data Collect. 2018, 17–18, 236–250. [Google Scholar] [CrossRef]
- Fuster, F.; Sevin, A.; Silvi, B. Topological Analysis of the Electron Localization Function (ELF) Applied to the Electrophilic Aromatic Substitution. J. Phys. Chem. A 2000, 104, 852–858. [Google Scholar] [CrossRef]
- Issaoui, N.; Ghalla, H.; Brandán, S.A.; Bardak, F.; Flakus, H.T.; Atac, A.; Oujia, B. Experimental FTIR and FT-Raman and theoretical studies on the molecular structures of monomer and dimer of 3-thiopheneacrylic acid. J. Mol. Struct. 2017, 1135, 209–221. [Google Scholar] [CrossRef]
- Jia, Z.; Pang, H.; Li, H.; Wang, X. A density functional theory study on complexation processes and intermolecular interactions of triptycene-derived oxacalixarenes. Theor. Chem. Acc. 2019, 138, 113. [Google Scholar] [CrossRef]
- Kolandaivel, P.; Praveena, G.; Selvarengan, P. Study of atomic and condensed atomic indices for reactive sites of molecules. J. Chem. Sci. 2005, 117, 591–598. [Google Scholar] [CrossRef]
- Zamora, P.P.; Bieger, K.; Cuchillo, A.; Tello, A.; Muena, J.P. Theoretical determination of a reaction intermediate: Fukui function analysis, dual reactivity descriptor and activation energy. J. Mol. Struct. 2021, 1227, 129369. [Google Scholar] [CrossRef]
- Vennila, M.; Rathikha, R.; Muthu, S.; Jeelani, A.; Irfan, A. Structural, spectral inspection, electronic properties in different solvents, Fukui functions, 6-acetyl-2H-1,4-benzoxazin-3(4H)-one—Multiple sclerosis and auto immune disorders therapeutics. J. Mol. Liq. 2022, 359, 119248. [Google Scholar] [CrossRef]
- Fuentealba, P.; Florez, E.; Tiznado, W. Topological Analysis of the Fukui Function. J. Chem. Theory Comput. 2010, 6, 1470–1478. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Demirpolat, A. Chemical Composition of Essential Oils of Seven Polygonum Species from Turkey: A Chemotaxonomic Approach. Molecules 2022, 27, 9053. [Google Scholar] [CrossRef]
- Kilic, A. Volatile compounds of buds, flowers and fruits of bay (Laurus nobilis L.) and their odour contribution. In Proceedings of the ICNP 2002, Trabzon, Turkiye, 16–19 October 2002; pp. 338–341. [Google Scholar]
- Van, H.T.; Thang, T.D.; Luu, T.N.; Doan, V.D. An overview of the chemical composition and biological activities of essential oils from Alpinia genus (Zingiberaceae). RSC Adv. 2021, 11, 37767–37783. [Google Scholar] [CrossRef]
- Hazzit, M.; Baaliouamer, A.; Faleiro, M.L.; Miguel, M.G. Composition of the Essential Oils of Thymus and Origanum Species from Algeria and Their Antioxidant and Antimicrobial Activities. J. Agric. Food Chem. 2006, 54, 6314–6321. [Google Scholar] [CrossRef]
- Sylvestre, M.; Pichette, A.; Longtin, A.; Nagau, F.; Legault, J. Essential oil analysis and anticancer activity of leaf essential oil of Croton flavens L. from Guadeloupe. J. Ethnopharmacol. 2006, 103, 99–102. [Google Scholar] [CrossRef]
- Mastelić, J.; Jerković, I.; Mesić, M. Volatile constituents from flowers, leaves, bark and wood of Prunus mahaleb L. Flavour Fragr. J. 2006, 21, 306–313. [Google Scholar] [CrossRef]
- Sufriadi, E.; Meilina, H.; Munawar, A.A.; Muhammad, S.; Idroes, R. Identification of β-Caryophyllene (BCP) in Aceh patchouli essential oil (PEO) using gas chromatography-mass pectrophotometry (GC-MS). IOP Conf. Ser. Earth Environ. Sci. 2021, 667, 012032. [Google Scholar] [CrossRef]
- Joshi, R.K. GC/MS Analysis of the Essential Oil of Leucas indica from India. Nat. Prod. Commun. 2014, 9, 1607–1608. [Google Scholar] [CrossRef]
- Stojanovic, G.; Palic, R.; Alagic, S.; Zeković, Z. Chemical composition and antimicrobial activity of the essential oil and CO2 extracts of semi-oriental tobacco, Otlja. Flavour Fragr. J. 2000, 15, 335–338. [Google Scholar] [CrossRef]
- Brander, C.F.; Kepner, R.E.; Webb, A.D. Identification of some Volatile Compounds of Wine of Vitis Vinifera Cultivar Pinot Noir. Am. J. Enol. Vitic. 1980, 31, 69. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Demirci, B.; Kirimer, N.e.; Satil, F.; Tümen, G. The essential oils of Thymus migricus and T. fedtschenkoi var. handelii from Turkey. Flavour Fragr. J. 2002, 17, 41–45. [Google Scholar] [CrossRef]
- Chung, T.Y.; Eiserich, J.P.; Shibamoto, T. Volatile compounds isolated from edible Korean chamchwi (Aster scaber Thunb). J. Agric. Food Chem. 1993, 41, 1693–1697. [Google Scholar] [CrossRef]
- Takeoka, G.; Perrino, C.; Buttery, R. Volatile Constituents of Used Frying Oils. J. Agric. Food Chem. 1996, 44, 654–660. [Google Scholar] [CrossRef]
- Gomez, E.; Ledbetter, C.A.; Hartsell, P.L. Volatile compounds in apricot, plum, and their interspecific hybrids. J. Agric. Food Chem. 1993, 41, 1669–1676. [Google Scholar] [CrossRef]
- Gudžić, B.; Dordević, S.; Palić, R.; Stojanović, G. Essential oils of Hypericum olympicum L. and Hypericum perforatum L. Flavour Fragr. J. 2001, 16, 201–203. [Google Scholar] [CrossRef]
- Radulović, N.; Blagojević, P.; Palić, R. Comparative Study of the Leaf Volatiles of Arctostaphylos uva-ursi (L.) Spreng. and Vaccinium vitis-idaea L. (Ericaceae). Molecules 2010, 15, 6168–6185. [Google Scholar] [CrossRef] [PubMed]
- Kenig, F.; Simons, D.-J.H.; Crich, D.; Cowen, J.P.; Ventura, G.T.; Rehbein-Khalily, T. Structure and distribution of branched aliphatic alkanes with quaternary carbon atoms in Cenomanian and Turonian black shales of Pasquia Hills (Saskatchewan, Canada). Org. Geochem. 2005, 36, 117–138. [Google Scholar] [CrossRef]
- Leffingwell, J.C.; Alford, E.D. Volatile constituents of Perique tobacco. Electron. J. Environ. Agric. Food Chem. 2005, 4, 899–915. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dennington, R.; Keith, T.; Millam, J. GaussView, 2000–2003; Guassian, Inc.: Wallingford, CT, USA; Semichem. Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Pearson, A.C.S.; Cutshall, S.M.; Hooten, W.M.; Rodgers, N.J.; Bauer, B.A.; Bhagra, A. Perspectives on the use of aromatherapy from clinicians attending an integrative medicine continuing education event. BMC Complement. Altern. Med. 2019, 19, 174. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Jaitak, V.; Horvath, G.; Bassolé, I.H.N.; Setzer, W.N.; Gori, L. Essential Oils: New Perspectives in Human Health and Wellness. Evid.-Based Complement. Altern. Med. 2014, 2014, 467363. [Google Scholar] [CrossRef]
- Reis, D.R.; Ambrosi, A.; Luccio, M.D. Encapsulated essential oils: A perspective in food preservation. Future Foods 2022, 5, 100126. [Google Scholar] [CrossRef]
- Calo, J.R.; Crandall, P.G.; O’Bryan, C.A.; Ricke, S.C. Essential oils as antimicrobials in food systems—A review. Food Control. 2015, 54, 111–119. [Google Scholar] [CrossRef]





| Parameter | Value (eV) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| a | a * | b | b * | c | c * | d | d * | e | e * | |
| EHOMO | −5.9623 | −5.070 | −5.8034 | −5.9256 | −6.4801 | −6.4080 | −6.6358 | −6.2420 | −6.5218 | −6.2352 |
| ELUMO | 0.5102 | 0.8678 | 0.6860 | 0.5170 | 0.4882 | 0.5494 | 1.6436 | 1.8561 | 0.4337 | 0.7780 |
| Egap | 6.4725 | 6.5748 | 6.4894 | 6.4426 | 6.9683 | 6.9574 | 8.2793 | 8.0981 | 6.9555 | 7.0132 |
| IP | 5.9623 | 5.7070 | 5.8034 | 5.9256 | 6.4801 | 6.4080 | 6.6358 | 6.2420 | 6.5218 | 6.2352 |
| EA | −0.5102 | −0.8678 | −0.6860 | −0.5170 | −0.4882 | −0.5494 | −1.6436 | −1.8561 | −0.4337 | −0.7780 |
| χ | 2.7260 | 2.4196 | 2.5587 | 2.7043 | 2.9960 | 2.9293 | 2.4961 | 2.1930 | 3.0440 | 2.7286 |
| μ | −2.7260 | −2.4196 | −2.5587 | −2.7043 | −2.9960 | −2.9293 | −2.4961 | −2.1930 | −3.0440 | −2.7286 |
| η | 3.2363 | 3.2874 | 3.2447 | 3.2213 | 3.4841 | 3.4787 | 4.1397 | 4.0491 | 3.4778 | 3.5066 |
| ζ | 0.3090 | 0.3042 | 0.3082 | 0.3104 | 0.2870 | 0.2875 | 0.2416 | 0.2470 | 0.2875 | 0.2852 |
| ω | 1.1481 | 0.8905 | 1.0089 | 1.1351 | 1.2881 | 1.2333 | 0.7525 | 0.5939 | 1.3322 | 1.0616 |
| ΔNmax | 0.8423 | 0.7360 | 0.7886 | 0.8395 | 0.8599 | 0.8421 | 0.6030 | 0.5416 | 0.8753 | 0.7781 |
| σo | 0.1545 | 0.1521 | 0.1541 | 0.1552 | 0.1435 | 0.1437 | 0.1208 | 0.1235 | 0.1438 | 0.1426 |
| N | 0.8710 | 1.1230 | 0.9912 | 0.8810 | 0.7763 | 0.8108 | 1.3288 | 1.6839 | 0.7507 | 0.9420 |
| Molecules | Center of TDOS (eV) | Center of PDOS for Frag. 1 (eV) | Center of PDOS for Frag. 2 (eV) | Vertical Dash Line (HOMO) (eV) |
|---|---|---|---|---|
| a | −6.642774 | −6.718485 | −5.640946 | −5.96221 |
| b | −6.827964 | −6.925306 | −5.642114 | −5.80330 |
| c | −6.866536 | −6.893760 | −6.445586 | −6.48017 |
| d | −6.806544 | −6.846677 | −6.373228 | −6.63586 |
| e | −6.629970 | −6.759459 | −5.494805 | −6.52173 |
| a | b | c | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atoms | Δf(r) | Atoms | Δf(r) | Atoms | Δf(r) | |||||||||
| 1(C) | 0.0081 | 0.0031 | 0.0056 | −0.0051 | 1(C) | 0.0074 | 0.0062 | 0.0068 | −0.0012 | 1(C) | 0.0138 | 0.0131 | 0.0134 | −0.0008 |
| 2(C) | 0.0130 | 0.0087 | 0.0108 | −0.0043 | 2(C) | 0.0045 | 0.0020 | 0.0032 | −0.0026 | 2(C) | 0.0084 | 0.0041 | 0.0062 | −0.0044 |
| 3(C) | 0.0212 | 0.0146 | 0.0179 | −0.0066 | 3(C) | 0.0065 | 0.0051 | 0.0058 | −0.0014 | 3(C) | 0.0150 | 0.0188 | 0.0169 | 0.0038 |
| 4(C) | 0.0066 | 0.0066 | 0.0066 | 0.0000 | 4(C) | 0.0233 | 0.0132 | 0.0182 | −0.0101 | 4(C) | 0.0083 | 0.0063 | 0.0073 | −0.0020 |
| 5(C) | 0.0558 | 0.0291 | 0.0424 | −0.0268 | 5(C) | 0.0104 | 0.0069 | 0.0087 | −0.0035 | 5(C) | 0.0093 | 0.0039 | 0.0066 | −0.0054 |
| 6(C) | 0.0535 | 0.0196 | 0.0365 | −0.0339 | 6(C) | 0.0073 | 0.0076 | 0.0075 | 0.0003 | 6(C) | 0.0096 | 0.0051 | 0.0074 | −0.0045 |
| 7(C) | 0.0276 | 0.0263 | 0.0270 | −0.0013 | 7(C) | 0.0064 | 0.0113 | 0.0088 | 0.0049 | 7(C) | 0.0215 | 0.0162 | 0.0189 | −0.0053 |
| 8(C) | 0.0117 | 0.0078 | 0.0097 | −0.0039 | 8(C) | 0.0178 | 0.0156 | 0.0167 | −0.0023 | 8(C) | 0.0837 | 0.1223 | 0.1030 | 0.0386 |
| 9(C) | 0.0128 | 0.0101 | 0.0114 | −0.0027 | 9(C) | 0.0242 | 0.0168 | 0.0205 | −0.0073 | 9(C) | 0.0175 | 0.0208 | 0.0191 | 0.0033 |
| 10(C) | 0.0897 | 0.1289 | 0.1093 | 0.0393 | 10(C) | 0.0941 | 0.0684 | 0.0812 | −0.0257 | 10(C) | 0.1499 | 0.1723 | 0.1611 | 0.0224 |
| 11(C) | 0.1358 | 0.1379 | 0.1368 | 0.0021 | 11(C) | 0.0266 | 0.0182 | 0.0224 | −0.0085 | 11(C) | 0.0144 | 0.0041 | 0.0092 | −0.0103 |
| 12(C) | 0.0041 | 0.0023 | 0.0032 | −0.0018 | 12(C) | 0.1087 | 0.0828 | 0.0957 | −0.0259 | 12(C) | 0.0132 | 0.0121 | 0.0127 | −0.0012 |
| 13(C) | 0.0241 | 0.0335 | 0.0288 | 0.0094 | 13(C) | 0.0908 | 0.0628 | 0.0768 | −0.0280 | 13(C) | 0.0183 | 0.0137 | 0.0160 | −0.0046 |
| 14(C) | 0.0110 | 0.0085 | 0.0097 | −0.0025 | 14(C) | 0.0228 | 0.0193 | 0.0210 | −0.0035 | 14(C) | 0.0104 | 0.0088 | 0.0096 | −0.0016 |
| 15(C) | 0.0046 | 0.0030 | 0.0038 | −0.0015 | 15(H) | 0.0012 | 0.0030 | 0.0021 | 0.0018 | 15(O) | 0.0565 | 0.0209 | 0.0387 | −0.0356 |
| 16(H) | 0.0138 | 0.0094 | 0.0116 | −0.0043 | 16(H) | 0.0116 | 0.0075 | 0.0095 | −0.0041 | 16(C) | 0.0108 | 0.0072 | 0.0090 | −0.0036 |
| 17(H) | 0.0097 | 0.0081 | 0.0089 | −0.0016 | 17(H) | 0.0208 | 0.0172 | 0.0190 | −0.0036 | 17(H) | 0.0145 | 0.0112 | 0.0129 | −0.0033 |
| 18(H) | 0.0239 | 0.0202 | 0.0221 | −0.0037 | 18(H) | 0.0126 | 0.0122 | 0.0124 | −0.0004 | 18(H) | 0.0180 | 0.0366 | 0.0273 | 0.0186 |
| 19(H) | 0.0085 | 0.0061 | 0.0073 | −0.0024 | 19(H) | 0.0217 | 0.0168 | 0.0193 | −0.0050 | 19(H) | 0.0418 | 0.0632 | 0.0525 | 0.0215 |
| 20(H) | 0.0214 | 0.0185 | 0.0200 | −0.0030 | 20(H) | 0.0094 | 0.0064 | 0.0079 | −0.0029 | 20(H) | 0.0189 | 0.0179 | 0.0184 | −0.0010 |
| 21(H) | 0.0081 | 0.0082 | 0.0082 | 0.0001 | 21(H) | 0.0108 | 0.0108 | 0.0108 | 0.0000 | 21(H) | 0.0066 | 0.0095 | 0.0080 | 0.0030 |
| 22(H) | 0.0213 | 0.0118 | 0.0165 | −0.0095 | 22(H) | 0.0039 | 0.0066 | 0.0052 | 0.0027 | 22(H) | 0.0183 | 0.0205 | 0.0194 | 0.0022 |
| 23(H) | 0.0373 | 0.0320 | 0.0346 | −0.0053 | 23(H) | 0.0160 | 0.0145 | 0.0153 | −0.0015 | 23(H) | 0.0054 | 0.0042 | 0.0048 | −0.0012 |
| 24(H) | 0.0480 | 0.0382 | 0.0431 | −0.0098 | 24(H) | 0.0161 | 0.0176 | 0.0169 | 0.0015 | 24(H) | 0.0169 | 0.0161 | 0.0165 | −0.0008 |
| 25(H) | 0.0371 | 0.0476 | 0.0423 | 0.0104 | 25(H) | 0.0025 | 0.0088 | 0.0056 | 0.0063 | 25(H) | 0.0052 | 0.0072 | 0.0062 | 0.0020 |
| 26(H) | 0.0215 | 0.0207 | 0.0211 | −0.0008 | 26(H) | 0.0111 | 0.0151 | 0.0131 | 0.0040 | 26(H) | 0.0235 | 0.0178 | 0.0207 | −0.0057 |
| 27(H) | 0.0123 | 0.0097 | 0.0110 | −0.0026 | 27(H) | 0.0228 | 0.0200 | 0.0214 | −0.0028 | 27(H) | 0.0245 | 0.0335 | 0.0290 | 0.0089 |
| 28(H) | 0.0185 | 0.0191 | 0.0188 | 0.0006 | 28(H) | 0.0365 | 0.0277 | 0.0321 | −0.0089 | 28(H) | 0.0435 | 0.0589 | 0.0512 | 0.0154 |
| 29(H) | 0.0486 | 0.0567 | 0.0526 | 0.0081 | 29(H) | 0.0202 | 0.0151 | 0.0176 | −0.0051 | 29(H) | 0.0471 | 0.0634 | 0.0552 | 0.0164 |
| 30(H) | 0.0065 | 0.0055 | 0.0060 | −0.0010 | 30(H) | 0.0329 | 0.0352 | 0.0341 | 0.0023 | 30(H) | 0.0205 | 0.0280 | 0.0243 | 0.0075 |
| 31(H) | 0.0332 | 0.0444 | 0.0388 | 0.0112 | 31(H) | 0.0284 | 0.0260 | 0.0272 | −0.0024 | 31(H) | 0.0121 | 0.0112 | 0.0116 | −0.0009 |
| 32(H) | 0.0358 | 0.0471 | 0.0415 | 0.0113 | 32(H) | 0.0304 | 0.0196 | 0.0250 | −0.0108 | 32(H) | 0.0209 | 0.0189 | 0.0199 | −0.0020 |
| 33(H) | 0.0215 | 0.0255 | 0.0235 | 0.0040 | 33(H) | 0.0362 | 0.0353 | 0.0358 | −0.0008 | 33(H) | 0.0161 | 0.0172 | 0.0166 | 0.0011 |
| 34(H) | 0.0171 | 0.0141 | 0.0156 | −0.0030 | 34(H) | 0.0367 | 0.0302 | 0.0335 | −0.0065 | 34(H) | 0.0136 | 0.0152 | 0.0144 | 0.0016 |
| 35(H) | 0.0087 | 0.0083 | 0.0085 | −0.0004 | 35(H) | 0.0266 | 0.0214 | 0.0240 | −0.0052 | 35(H) | 0.0004 | −0.0028 | −0.0012 | −0.0032 |
| 36(H) | 0.0093 | 0.0085 | 0.0089 | −0.0008 | 36(H) | 0.0356 | 0.0280 | 0.0318 | −0.0076 | 36(H) | 0.0193 | 0.0201 | 0.0197 | 0.0008 |
| 37(H) | 0.0171 | 0.0148 | 0.0160 | −0.0023 | 37(H) | 0.0316 | 0.0271 | 0.0293 | −0.0046 | 37(H) | 0.0164 | 0.0185 | 0.0174 | 0.0021 |
| 38(H) | −0.0042 | −0.0061 | −0.0051 | −0.0019 | 38(H) | 0.0175 | 0.0155 | 0.0165 | −0.0020 | 38(H) | 0.0084 | 0.0090 | 0.0087 | 0.0006 |
| 39(H) | 0.0081 | 0.0069 | 0.0075 | −0.0012 | 39(C) | 0.0472 | 0.0486 | 0.0479 | 0.0014 | 39(H) | 0.0108 | 0.0076 | 0.0092 | −0.0032 |
| 40(O) | 0.0247 | 0.0344 | 0.0295 | 0.0097 | 40(O) | −0.0017 | 0.0469 | 0.0226 | 0.0486 | 40(H) | 0.0149 | 0.0130 | 0.0139 | −0.0019 |
| 41(H) | 0.0135 | 0.0322 | 0.0228 | 0.0187 | 41(H) | 0.0054 | 0.0677 | 0.0365 | 0.0623 | 41(O) | 0.0653 | 0.0114 | 0.0384 | −0.0539 |
| 42(H) | −0.0008 | 0.0182 | 0.0087 | 0.0190 | 42(H) | 0.0053 | 0.0633 | 0.0343 | 0.0580 | 42(H) | 0.0244 | 0.0191 | 0.0217 | −0.0053 |
| 43(H) | 0.0119 | 0.0039 | 0.0079 | −0.0080 | ||||||||||
| d | e | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Atoms | Δf(r) | Atoms | Δf(r) | ||||||
| 1(O) | 0.1773 | 0.0111 | 0.0942 | −0.1661 | 1(C) | 0.0191 | 0.0154 | 0.0173 | −0.0036 |
| 2(C) | 0.0158 | 0.0165 | 0.0162 | 0.0006 | 2(C) | 0.0343 | 0.0132 | 0.0238 | −0.0210 |
| 3(C) | 0.0288 | 0.0091 | 0.0189 | −0.0197 | 3(C) | 0.0165 | 0.0091 | 0.0128 | −0.0074 |
| 4(C) | 0.0211 | 0.0058 | 0.0135 | −0.0153 | 4(C) | 0.0324 | 0.0117 | 0.0220 | −0.0207 |
| 5(C) | 0.0184 | 0.0245 | 0.0215 | 0.0061 | 5(C) | 0.0160 | 0.0083 | 0.0121 | −0.0076 |
| 6(C) | 0.0172 | 0.0253 | 0.0213 | 0.0081 | 6(C) | 0.0319 | 0.0176 | 0.0247 | −0.0143 |
| 7(C) | 0.0331 | 0.0275 | 0.0303 | −0.0056 | 7(C) | 0.0970 | 0.1397 | 0.1183 | 0.0428 |
| 8(C) | 0.0321 | 0.0285 | 0.0303 | −0.0036 | 8(C) | 0.0173 | 0.0143 | 0.0158 | −0.0030 |
| 9(C) | 0.0127 | 0.0261 | 0.0194 | 0.0134 | 9(C) | 0.1898 | 0.1739 | 0.1819 | −0.0159 |
| 10(C) | 0.0356 | 0.0249 | 0.0302 | −0.0106 | 10(C) | 0.0231 | 0.0295 | 0.0263 | 0.0064 |
| 11(C) | 0.0356 | 0.0272 | 0.0314 | −0.0085 | 11(H) | 0.0328 | 0.0242 | 0.0285 | −0.0086 |
| 12(H) | 0.0275 | 0.0517 | 0.0396 | 0.0242 | 12(H) | 0.0174 | 0.0132 | 0.0153 | −0.0041 |
| 13(H) | 0.0268 | 0.0418 | 0.0343 | 0.0151 | 13(H) | 0.0190 | 0.0153 | 0.0172 | −0.0037 |
| 14(H) | 0.0241 | 0.0325 | 0.0283 | 0.0084 | 14(H) | 0.0179 | 0.0148 | 0.0163 | −0.0031 |
| 15(H) | 0.0266 | 0.0432 | 0.0349 | 0.0166 | 15(H) | 0.0058 | 0.0020 | 0.0039 | −0.0038 |
| 16(H) | 0.0236 | 0.0334 | 0.0285 | 0.0098 | 16(H) | 0.0314 | 0.0257 | 0.0286 | −0.0058 |
| 17(H) | 0.0258 | 0.0345 | 0.0302 | 0.0087 | 17(H) | 0.0296 | 0.0218 | 0.0257 | −0.0078 |
| 18(H) | 0.0384 | 0.0533 | 0.0459 | 0.0149 | 18(H) | 0.0313 | 0.0254 | 0.0284 | −0.0059 |
| 19(H) | 0.0261 | 0.0368 | 0.0314 | 0.0107 | 19(H) | 0.0317 | 0.0296 | 0.0306 | −0.0021 |
| 20(H) | 0.0373 | 0.0543 | 0.0458 | 0.0170 | 20(H) | 0.0228 | 0.0101 | 0.0165 | −0.0127 |
| 21(H) | 0.0192 | 0.0252 | 0.0222 | 0.0060 | 21(H) | 0.0259 | 0.0300 | 0.0280 | 0.0040 |
| 22(H) | 0.0190 | 0.0240 | 0.0215 | 0.0050 | 22(H) | 0.0251 | 0.0200 | 0.0225 | −0.0051 |
| 23(H) | 0.0266 | 0.0407 | 0.0336 | 0.0142 | 23(H) | 0.0574 | 0.0671 | 0.0623 | 0.0098 |
| 24(H) | 0.0204 | 0.0207 | 0.0206 | 0.0003 | 24(H) | 0.0590 | 0.0662 | 0.0626 | 0.0072 |
| 25(H) | 0.0387 | 0.0400 | 0.0394 | 0.0014 | 25(H) | 0.0297 | 0.0346 | 0.0322 | 0.0049 |
| 26(H) | 0.0219 | 0.0261 | 0.0240 | 0.0043 | 26(H) | 0.0375 | 0.0525 | 0.0450 | 0.0151 |
| 27(H) | 0.0195 | 0.0232 | 0.0214 | 0.0038 | 27(O) | 0.0291 | 0.0430 | 0.0360 | 0.0139 |
| 28(H) | 0.0372 | 0.0428 | 0.0400 | 0.0056 | 28(H) | 0.0020 | 0.0246 | 0.0133 | 0.0226 |
| 29(H) | 0.0218 | 0.0283 | 0.0250 | 0.0065 | 29(H) | 0.0174 | 0.0469 | 0.0322 | 0.0295 |
| 30(O) | 0.0621 | 0.0425 | 0.0523 | −0.0196 | |||||
| 31(H) | 0.0241 | 0.0587 | 0.0414 | 0.0346 | |||||
| 32(H) | 0.0057 | 0.0196 | 0.0127 | 0.0138 | |||||
| Name | RI | RI Lit | RT | Area | Identification Method | Area% | |
|---|---|---|---|---|---|---|---|
| 1. | α-Pinene | 936 | 939 [17] | 5.141 | 240,990 | RI, MS | 2.76 |
| 2. | Camphene | 952 | 952 [58] | 5.330 | 19,190 | RI, MS | 0.22 |
| 3. | β-Pinene | 980 | 981 [17] | 5.684 | 22,704 | RI, MS | 9.63 |
| 4. | 1-Octen-3-ol | 987 | - | 5.813 | 56,885 | RI, MS | 0.65 |
| 5. | β-Myrcene | 990 | 990 [59] | 6.163 | 32,203 | RI, MS | 0.37 |
| 6. | α-Phellandrene | 1004 | 1004 [59] | 8.636 | 79,196 | RI, MS | 0.91 |
| 7. | Limonene | 1029 | 1029 [59] | 8.834 | 40,843 | RI, MS | 0.47 |
| 8. | 1,8-Cineol | 1095 | 1033 [59] | 9.606 | 46,927 | RI, MS | 8.64 |
| 9. | γ-Terpinene | 1060 | 1060 [59] | 9.966 | 73,320 | RI, MS | 0.84 |
| 10. | Linalool | 1145 | 1148 [60] | 11.494 | 47,165 | RI, MS | 0.54 |
| 11. | Camphor | 1185 | 1185 [60] | 11.839 | 21,546 | RI, MS | 0.25 |
| 12. | Terpinen-4-ol | 1205 | 1179 [59] | 12.421 | 53,911 | RI, MS | 0.62 |
| 13. | Terpinolene | 1210 | 1193 [59] | 12.813 | 254,979 | RI, MS | 2.92 |
| 14. | Myrtenol | 1216 | 1216 [60] | 12.850 | 224,979 | RI, MS | 0.54 |
| 15. | Thymol | 1297 | 1297 [61] | 12.989 | 47,814 | RI, MS | 0.55 |
| 16. | Carvacrol | 1300 | 1317 [61] | 12.923 | 47,823 | RI, MS | 0.64 |
| 17. | α-Cubebene | 1323 | 1337 [60] | 13.140 | 47,270 | RI, MS | 8.89 |
| 18. | Eugenol | 1345 | 1359 [59] | 13.317 | 50,434 | RI, MS | 0.58 |
| 19. | α-Copaene | 1352 | 1376 [61] | 13.690 | 101,376 | RI, MS | 1.16 |
| 20. | δ-Cadinene | 1358 | 1529 [59] | 14.362 | 195,023 | RI, MS | 2.24 |
| 21. | β-Bourbonene | 1388 | 1382 [62] | 14.765 | 210,657 | RI, MS | 2.42 |
| 22. | Tiglate -3(Z)-hexenyl- | 1390 | 1316 [63] | 16.276 | 30,313 | RI, MS | 0.35 |
| 23. | β-Caryophyllene | 1393 | 1392 [64,65] | 33.265 | 111,541 | RI, MS | 8.28 |
| 24. | Capraldehyde | 1400 | 1204 [66] | 25.144 | 54,421 | RI, MS | 0.62 |
| 25. | Bicyclogermacrene | 1443 | 1445 [60] | 33.681 | 57,567 | RI, MS | 0.66 |
| 26. | α-Humulene | 1418 | 1418 [60] | 33.903 | 28,076 | RI, MS | 0.32 |
| 27. | Isobornil asetat | 1467 | - | 34.636 | 33,822 | RI, MS | 0.39 |
| 28. | 2-Methyl-4-pentenal | 1466 | - | 40.941 | 46,066 | RI, MS | 3.08 |
| 29. | 2-Hexen-1-ol | 1470 | 1420 [67] | 41.224 | 21,884 | RI, MS | 0.25 |
| 30. | Dodecanal | 1477 | 1722 [68] | 41.538 | 102,832 | RI, MS | 1.18 |
| 31. | Germacrene D | 1490 | 1490 [60] | 35.239 | 290,085 | RI, MS | 0.33 |
| 32. | Pentadecane | 1502 | 1504 [69] | 42.138 | 81,843 | RI, MS | 0.94 |
| 33. | Decanal | 1504 | 1506 [68] | 42.396 | 74,986 | RI, MS | 0.86 |
| 34. | Pentadecane | 1510 | 1500 [69] | 42.697 | 356,908 | RI, MS | 1.09 |
| 35. | γ-Cadinene | 1514 | 1511 [61] | 36.587 | 37,582 | RI, MS | 0.43 |
| 36. | Isolongifolene | 1518 | 1517 [60] | 36.775 | 84,774 | RI, MS | 0.97 |
| 37. | β-Selinene | 1521 | 1441 [60] | 36.986 | 81,178 | RI, MS | 0.93 |
| 38. | Lauric acid | 1547 | 1547 [70] | 43.814 | 23,501 | RI, MS | 0.27 |
| 39. | Germacrene B | 1562 | 1524 [60] | 37.247 | 50,873 | RI, MS | 0.58 |
| 40. | α-Curcumene | 1569 | 1483 [61] | 37.927 | 191,605 | RI, MS | 1.20 |
| 41. | Spathulenol | 1572 | 1571 [61] | 38.601 | 203,413 | RI, MS | 0.33 |
| 42. | Phthalate diethyl | 1587 | 1587 [71] | 52.791 | 101,397 | RI, MS | 1.16 |
| 43. | Caryophyllene oxide | 1595 | 1578 [61] | 39.221 | 93,680 | RI, MS | 10.56 |
| 44. | Undecane | 1598 | 1100 [69] | 42.214 | 720,462 | RI, MS | 1.91 |
| 45. | Nonadecane | 1656 | 1900 [69] | 53.812 | 84,321 | RI, MS | 0.97 |
| 46. | Phytone | 1820 | 1827 [66] | 51.922 | 308,615 | RI, MS | 3.54 |
| 47. | p-Cymen-8-ol | 1850 | 1847 [66] | 44.352 | 61,374 | RI, MS | 4.70 |
| 48. | Cetyl alcohol | 1885 | 1882 [72] | 52.480 | 56,068 | RI, MS | 0.64 |
| 49. | Heptadecyl alcohol | 1980 | 1982 [73] | 50.046 | 60,498 | RI, MS | 0.69 |
| 50. | 2,2-Dimethyloctadecane | 1910 | 1917 [74] | 50.290 | 129,919 | RI, MS | 1.49 |
| 51. | Butylated hydroxytoluene | 1920 | 1920 [69] | 54.609 | 641,971 | RI, MS | 3.36 |
| 52. | Hexadecanoic acid, methyl ester | 1930 | 1928 [75] | 54.730 | 72,402 | RI, MS | 0.83 |
| 53. | Eicosane | 2000 | 2000 [69] | 57.202 | 66,550 | RI, MS | 0.76 |
| 54. | Heneicosane | 2110 | 2100 [69] | 48.955 | 56,028 | RI, MS | 0.49 |
| Total | 100.00 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akman, F.; Demirpolat, A.; Kazachenko, A.S.; Kazachenko, A.S.; Issaoui, N.; Al-Dossary, O. Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri. Molecules 2023, 28, 2684. https://doi.org/10.3390/molecules28062684
Akman F, Demirpolat A, Kazachenko AS, Kazachenko AS, Issaoui N, Al-Dossary O. Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri. Molecules. 2023; 28(6):2684. https://doi.org/10.3390/molecules28062684
Chicago/Turabian StyleAkman, Feride, Azize Demirpolat, Aleksandr S. Kazachenko, Anna S. Kazachenko, Noureddine Issaoui, and Omar Al-Dossary. 2023. "Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri" Molecules 28, no. 6: 2684. https://doi.org/10.3390/molecules28062684
APA StyleAkman, F., Demirpolat, A., Kazachenko, A. S., Kazachenko, A. S., Issaoui, N., & Al-Dossary, O. (2023). Molecular Structure, Electronic Properties, Reactivity (ELF, LOL, and Fukui), and NCI-RDG Studies of the Binary Mixture of Water and Essential Oil of Phlomis bruguieri. Molecules, 28(6), 2684. https://doi.org/10.3390/molecules28062684









