Formation of Gold Nanoclusters from Goldcarbonyl Chloride inside the Metal-Organic Framework HKUST-1
Abstract
:1. Introduction
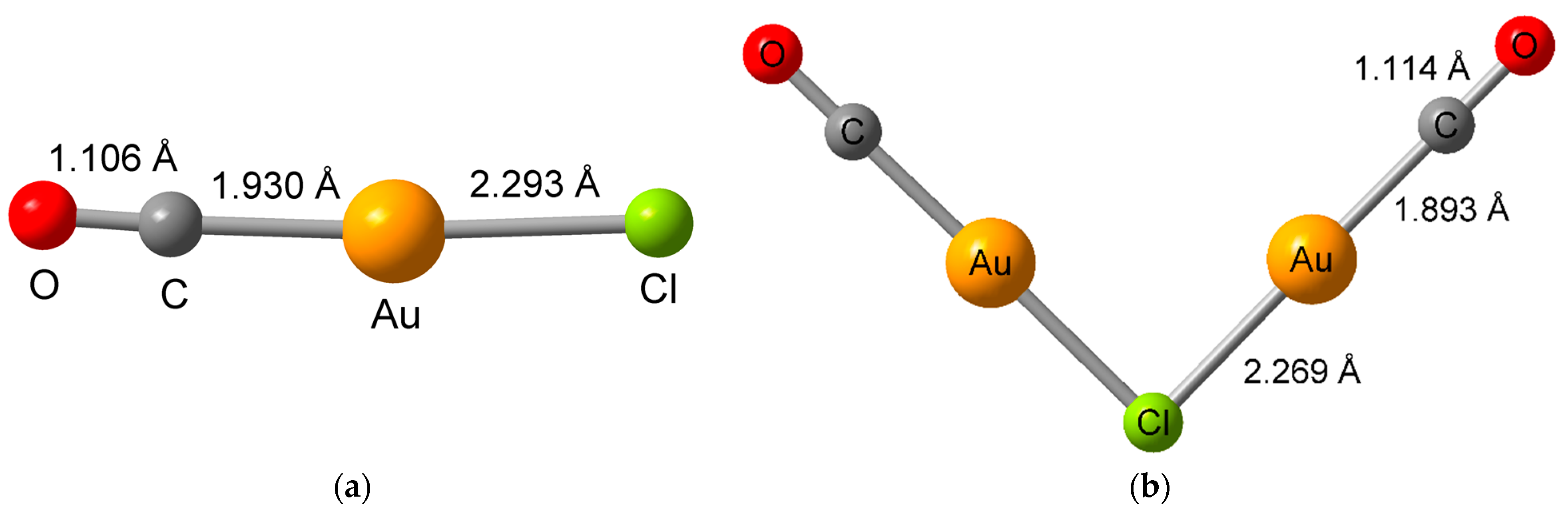
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Equipment
3.2. Fabrication of HKUST-1 SURMOFs
3.3. Fabrication of HKUST-1 Powder
3.4. Gas-Phase Loading of Au(CO)Cl into HKUST-1 SURMOFs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar] [CrossRef] [Green Version]
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Janiak, C.; Vieth, J.K. MOFs, MILs and more: Concepts, properties and applications for porous coordination networks (PCNs). New J. Chem. 2010, 34, 2366–2388. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Krivosudský, L. Metalloporphyrin Metal–Organic Frameworks: Eminent Synthetic Strategies and Recent Practical Exploitations. Molecules 2022, 27, 4917. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalyst. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Murray, L.J.; Dinca, M.; Long, J.R. Hydrogen Storage in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1294–1314. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-Organic Frameworks for Separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal-organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef]
- Wan, Y.; Li, Y.; Yue, D. Dye-Encapsulated Metal–Organic Frameworks for the Multi-Parameter Detection of Temperature. Molecules 2023, 28, 729. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Jiang, W.-J.; Cai, Z.H.; Li, D.-L.; Liu, Y.-L.; Chen, Z.-Z. Recent Progress in Metal-Organic Framework Based Fluorescent Sensors for Hazardous Materials Detection. Molecules 2022, 27, 2226. [Google Scholar] [CrossRef]
- Chen, S.; Du, W.; Qin, C.; Liu, D.; Tang, L.; Liu, Y.; Wang, S.; Zhu, M. Assembly of the Thiolated [Au1Ag22(S-Adm)12]3+ Superatom Complex into a Framework Material through Direct Linkage by SbF6− Anions. Angew. Chem. Int. Ed. 2020, 59, 7542–7547. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Kang, X.; Zuo, Z.; Song, F.; Wang, S.; Zhu, M. Hierarchical structural complexity in atomically precise nanocluster frameworks. Natl. Sci. Rev. 2021, 8, nwaa077. [Google Scholar] [CrossRef]
- Sondermann, L.; Jiang, W.; Shviro, M.; Spieß, A.; Woschko, D.; Rademacher, L.; Janiak, C. Nickel-Based Metal-Organic Frameworks as Electrocatalysts for the Oxygen Evolution Reaction (OER). Molecules 2022, 27, 1241. [Google Scholar] [CrossRef]
- Öztürk, S.; Moon, G.-H.; Spiess, A.; Budiyanto, E.; Roitsch, S.; Tüysüz, H.; Janiak, C. A Highly-Efficient Oxygen Evolution Electrocatalyst Derived from a Metal-Organic Framework and Ketjenblack Carbon Material. ChemPlusChem 2021, 86, 1106–1115. [Google Scholar] [CrossRef]
- Bloch, E.D.; Queen, W.L.; Krishna, R.; Zadrozny, J.M.; Brown, C.M.; Long, J.R. Hydrocarbon Separations in a Metal-Organic Framework with Open Iron(II) Coordination Sites. Science 2012, 335, 1606–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloch, E.D.; Murray, L.J.; Queen, W.L.; Chavan, S.; Maximoff, S.N.; Bigi, J.P.; Krishna, R.; Peterson, V.K.; Grandjean, F.; Long, G.J.; et al. Selective Binding of O2 over N2 in a Redox-Active Metal-Organic Framework with Open Iron(II) Coordination Sites. J. Am. Chem. Soc. 2011, 133, 14814–14822. [Google Scholar] [CrossRef]
- Kökçam-Demir, Ü.; Goldman, A.; Esrafili, L.; Gharib, M.; Morsali, A.; Weingart, O.; Janiak, C. Coordinatively unsaturated metal sites (open metal sites) in metal-organic frameworks: Design and applications. Chem. Soc. Rev. 2020, 49, 2751–2798. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A Chemically Functionalizable Nanoporous Material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Yun, W.S.; Kim, M.B.; Kim, J.Y.; Bae, Y.S.; Lee, J.; Jeong, N.C. A Chemical Route to Activation of Open Metal Sites in the Copper-Based Metal-Organic Framework Materials HKUST-1 and Cu-MOF-2. J. Am. Chem. Soc. 2015, 137, 10009–10015. [Google Scholar] [CrossRef] [PubMed]
- Borfecchia, E.; Maurelli, S.; Gianolio, D.; Groppo, E.; Chiesa, M.; Bonino, F.; Lamberti, C. Insights into Adsorption of NH3 on HKUST-1 Metal-Organic Framework: A Multitechnique Approach. J. Phys. Chem. C 2012, 116, 19839–19850. [Google Scholar] [CrossRef]
- Getzschmann, J.; Senkovska, I.; Wallacher, D.; Tovar, M.; Fairen-Jimenez, D.; Duren, T.; van Baten, J.M.; Krishna, R.; Kaskel, S. Methane storage mechanism in the metal-organic framework Cu3(btc)2: An in situ neutron diffraction study. Microporous Mesoporous Mater. 2010, 136, 50–58. [Google Scholar] [CrossRef]
- Bentley, J.; Foo, G.S.; Rungta, M.; Sangar, N.; Sievers, C.; Sholl, D.S.; Nair, S. Effects of Open Metal Site Availability on Adsorption Capacity and Olefin/Paraffin Selectivity in the Metal−Organic Framework Cu3(BTC)2. Ind. Eng. Chem. Res. 2016, 55, 5043–5053. [Google Scholar] [CrossRef]
- Jiang, H.L.; Liu, B.; Akita, T.; Haruta, M.; Sakurai, H.; Xu, Q. Au@ZIF-8: CO oxidation over gold nanoparticles deposited to metal-organic framework. J. Am. Chem. Soc. 2009, 131, 11302–11303. [Google Scholar] [CrossRef]
- Müller, M.; Hermes, S.; Kaehler, K.; van den Berg, M.W.E.; Muhler, M.; Fischer, R.A. Loading of MOF-5 with Cu and ZnO Nanoparticles by Gas-Phase Infiltration with Organometallic Precursors: Properties of Cu/ZnO@MOF-5 as Catalyst for Methanol Synthesis. Chem. Mater. 2008, 20, 4576–4587. [Google Scholar] [CrossRef]
- Zlotea, C.; Campesi, R.; Cuevas, F.; Leroy, E.; Dibandjo, P.; Volkringer, C.; Loiseau, T.; Férey, G.; Latroche, M. Pd Nanoparticles Embedded into a Metal-Organic Framework: Synthesis, Structural Characteristics, and Hydrogen Sorption Properties. J. Am. Chem. Soc. 2010, 132, 2991–2997. [Google Scholar] [CrossRef]
- Meilikhov, M.; Yusenko, K.; Esken, D.; Turner, S.; Van Tendeloo, G.; Fischer, R.A. Metals@MOFs—Loading MOFs with Metal Nanoparticles for Hybrid Functions. Eur. J. Inorg. Chem. 2010, 2010, 3701–3714. [Google Scholar] [CrossRef]
- Heinke, L.; Tu, M.; Wannapaiboon, S.; Fischer, R.A.; Wöll, C. Surface-mounted metal-organic frameworks for applications in sensing and separation. Microporous Mesoporous Mater. 2015, 216, 200–215. [Google Scholar] [CrossRef]
- Heinke, L.; Gu, Z.G.; Wöll, C. The surface barrier phenomenon at the loading of metal-organic frameworks. Nat. Commun. 2014, 5, 4562. [Google Scholar] [CrossRef] [Green Version]
- Arslan, H.K.; Shekhah, O.; Wohlgemuth, J.; Franzreb, M.; Fischer, R.A.; Wöll, C. High-Throughput Fabrication of Uniform and Homogenous MOF Coatings. Adv. Funct. Mater. 2011, 21, 4228–4231. [Google Scholar] [CrossRef]
- Guo, W.; Chen, Z.; Yang, C.W.; Neumann, T.; Kübel, C.; Wenzel, W.; Welle, A.; Pfleging, W.; Shekhah, O.; Wöll, C.; et al. Bi2O3 nanoparticles encapsulated in surface mounted metal-organic framework thin films. Nanoscale 2016, 8, 6468–6472. [Google Scholar] [CrossRef]
- Guo, W.; Liu, J.X.; Weidler, P.G.; Liu, J.X.; Neumann, T.; Danilov, D.; Wenzel, W.; Feldmann, C.; Wöll, C. Loading of ionic compounds into metal-organic frameworks: A joint theoretical and experimental study for the case of La3+. Phys. Chem. Chem. Phys. 2014, 16, 17918–17923. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Yang, L.; Ma, Y.; Wang, X.; Zhang, J.; Bai, B.; Yu, L.; Guo, C.; Zhang, F.; Qin, S. A novel metal-organic frameworks composite-based label-free point-of-care quartz crystal microbalance aptasensing platform for tetracycline detection. Food Chem. 2022, 392, 133302. [Google Scholar] [CrossRef]
- Shang, H.; Ding, M.; Zhang, X.; Zhang, W. Dual-mode biosensing platform for sensitive and portable detection of hydrogen sulfide based on cuprous oxide/gold/copper metal organic framework heterojunction. J. Colloid Interface Sci. 2022, 629, 796–804. [Google Scholar] [CrossRef]
- Liu, T.; Hu, R.; Zhang, X.; Zhang, K.; Liu, Y.; Zhang, X.; Bai, R.; Li, D.; Yang, Y. Metal–Organic Framework Nanomaterials as Novel Signal Probes for Electron Transfer Mediated Ultrasensitive Electrochemical Immunoassay. Anal. Chem. 2016, 88, 12516–12523. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Norouzi, P.; Davami, F.; Bonakdar, A.; Marzabad, M.; Tabaei, O. Direct detection of TNF-α by copper benzene tricarboxylate MOFs/gold nanoparticles modified electrochemical label-free immunosensor using FFT admittance voltammetry. J. Electr. Chem. 2022, 925, 116897. [Google Scholar] [CrossRef]
- He, C.; Liu, L.; Korposh, S.; Correia, R.; Morgan, S.P. Volatile Organic Compound Vapour Measurements Using a Localised Surface Plasmon Resonance Optical Fibre Sensor Decorated with a Metal-Organic Framework. Sensors 2021, 21, 1420. [Google Scholar] [CrossRef]
- Cao, X.; Hong, S.; Jiang, Z.; She, Y.; Wang, S.; Zhang, C.; Li, H.; Jin, F.; Jin, M.; Wang, J. SERS-active metal-organic frameworks with embedded gold nanoparticles. Analyst 2017, 142, 2640–2647. [Google Scholar] [CrossRef]
- He, J.; Dong, J.; Hu, Y.; Li, G.; Hu, Y. Design of Raman tag-bridged core–shell Au@Cu3(BTC)2 nanoparticles for Raman imaging and synergistic chemo-photothermal therapy. Nanoscale 2019, 11, 6089–6100. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Xiao, W.; Deng, S.; Chen, C.; Zhang, N. Mechanochemistry-assisted encapsulation of metal nanoparticles in MOF matrices via a sacrificial strategy. J. Mater. Chem. A 2019, 7, 14504–14509. [Google Scholar] [CrossRef]
- Redel, E.; Walter, M.; Thomann, R.; Vollmer, C.; Hussein, L.; Scherer, H.; Krüger, M.; Janiak, C. Synthesis, stabilization, functionalization and DFT calculations of gold nanoparticles in fluorous phases (PTFE and ILs). Chem. Eur. J. 2009, 15, 10047–10059. [Google Scholar] [CrossRef] [PubMed]
- Manchot, W.; Gall, H. Über eine Kohlenoxyd-Verbindung des Goldes. Chem. Ber. 1925, 58, 2175–2178. [Google Scholar] [CrossRef]
- Kharasch, M.S.; Isbell, H.S. The Chemistry of Organic Gold Compounds. I Aurous Chloride Carbonyl and a Method of Linking Carbon to Carbon. J. Am. Chem. Soc. 1930, 52, 2919–2927. [Google Scholar] [CrossRef]
- Calderazzo, F. Organometallic derivatives of palladium, platinum, and gold. J. Organometal. Chem. 1990, 400, 303–320. [Google Scholar] [CrossRef]
- Antes, I.; Dapprich, S.; Frenking, G.; Schwerdtfeger, P. Stability of Group 11 Carbonyl Complexes Cl-M-CO (M = Cu, Ag, Au). Inorg. Chem. 1996, 35, 2089–2096. [Google Scholar] [CrossRef]
- Schaefer, J.; Kraft, A.; Reininger, S.; Santiso-Quinones, G.; Himmel, D.; Trapp, N.; Gellrich, U.; Breit, B.; Krossing, I. A Systematic Investigation of Coinage Metal Carbonyl Complexes Stabilized by Fluorinated Alkoxy Aluminates. Chem. Eur. J. 2013, 19, 12468–12485. [Google Scholar] [CrossRef] [PubMed]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond, Version 4.6; Crystal and Molecular Structure Visualization; Crystal Impact GbR: Bonn, Germany, 1997–2022. [Google Scholar]
- Drenchev, N.; Ivanova, E.; Mihaylov, M.; Hadjiivanov, K. CO as an IR probe molecule for characterization of copper ions in a basolite C300 MOF sample. Phys. Chem. Chem. Phys. 2010, 12, 6423–6427. [Google Scholar] [CrossRef]
- Szanyi, J.; Daturi, M.; Clet, G.; Baer, D.R.; Peden, C.H. Well-studied Cu−BTC still serves surprises: Evidence for facile Cu2+/Cu+ interchange. Phys. Chem. Chem. Phys. 2012, 14, 4383–4390. [Google Scholar] [CrossRef]
- Prestipino, C.; Regli, L.; Vitillo, J.G.; Bonino, F.; Damin, A.; Lamberti, C.; Zecchina, A.; Solari, P.L.; Kongshaug, K.O.; Bordiga, S. Local Structure of Framework Cu(II) in HKUST-1 Metallorganic Framework: Spectroscopic Characterization upon Activation and Interaction with Adsorbates. Chem. Mater. 2006, 18, 1337–1346. [Google Scholar] [CrossRef]
- Todaro, M.; Alessi, A.; Sciortino, L.; Agnello, S.; Cannas, M.; Gelardi, F.M.; Buscarino, G. Investigation by Raman Spectroscopy of the Decomposition Process of HKUST-1 upon Exposure to Air. J. Spectrosc. 2016, 2016, 8074297. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Zeman, C.J., IV; Schatz, G.C.; Gu, X.W. Source of Bright Near-Infrared Luminescence in Gold Nanoclusters. ACS Nano 2021, 15, 16095–16105. [Google Scholar] [CrossRef]
- Chang, H.; Karan, N.S.; Shin, K.; Bootharaju, M.S.; Nah, S.; Chae, S.I.; Baek, W.; Lee, S.; Kim, J.; Son, Y.J.; et al. Highly Fluorescent Gold Cluster Assembly. J. Am. Chem. Soc. 2021, 143, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Gentile, F.S.; Pannico, M.; Causà, M.; Mensitieri, G.; Di Palma, G.; Scherillo, G.; Musto, P. Metal defects in HKUST-1 MOF revealed by vibrational spectroscopy: A combined quantum mechanical and experimental study. J. Mater. Chem. A 2020, 8, 10796–10812. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Crystallogr. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Kobayashi, A.; Halder, G.J.; Peterson, V.K.; Chapman, K.W.; Lock, N.; Southon, P.D.; Kepert, C.J. Negative Thermal Expansion in the Metal-Organic Framework Material Cu3(1,3,5-benzenetricarboxylate)2. Angew. Chem. Int. Ed. 2008, 47, 8929–8932. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, W.; Eiichiro, M.; Kozo, S. X-ray Diffraction Crystallography; Springer: Berlin/Heidelberg, Germany, 2011; Chapter 3; pp. 67–106. [Google Scholar]
- Jeremias, F.; Fröhlich, D.; Janiak, C.; Henninger, S.K. Water and methanol adsorption on MOFs for cycling heat transformation processes. New J. Chem. 2014, 38, 1846–1852. [Google Scholar] [CrossRef] [Green Version]
- Henninger, S.K.; Jeremias, F.; Kummer, H.; Janiak, C. MOFs for Use in Adsorption Heat Pump Processes. Eur. J. Inorg. Chem. 2012, 2012, 2625–2634. [Google Scholar] [CrossRef]
- Menzel, S.; Heinen, T.; Boldog, I.; Beglau, T.H.Y.; Xing, S.; Spieß, A.; Woschko, D.; Janiak, C. Metal-organic framework structures of fused hexagonal motifs with cuprophilic interactions of a triangular Cu(I)3(pyrazolate-benzoate) metallo-linker. CrystEngComm 2022, 24, 3675–3691. [Google Scholar] [CrossRef]
- Available online: https://www.thermofisher.com/de/de/home/materials-science/learning-center/periodic-table/transition-metal/copper.html (accessed on 6 February 2023).
- Casaletto, M.P.; Longo, A.; Martorana, A.; Prestianni, A.; Venezia, A.M. XPS study of supported gold catalysts: The role of Au0 and Au+ species as active sites. Surf. Interface Anal. 2006, 38, 215–218. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, L.; Ji, L.; Chen, Z. Preparation of nanostructured Cu(OH)2 and CuO electrocatalysts for water oxidation by electrophoresis deposition. J. Mater. Res. 2018, 33, 581–589. [Google Scholar] [CrossRef] [Green Version]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, Z.M.; Guo, W.; Welle, A.; Oestreich, R.; Janiak, C.; Redel, E. Formation of Gold Nanoclusters from Goldcarbonyl Chloride inside the Metal-Organic Framework HKUST-1. Molecules 2023, 28, 2716. https://doi.org/10.3390/molecules28062716
Hassan ZM, Guo W, Welle A, Oestreich R, Janiak C, Redel E. Formation of Gold Nanoclusters from Goldcarbonyl Chloride inside the Metal-Organic Framework HKUST-1. Molecules. 2023; 28(6):2716. https://doi.org/10.3390/molecules28062716
Chicago/Turabian StyleHassan, Zeinab Mohamed, Wei Guo, Alexander Welle, Robert Oestreich, Christoph Janiak, and Engelbert Redel. 2023. "Formation of Gold Nanoclusters from Goldcarbonyl Chloride inside the Metal-Organic Framework HKUST-1" Molecules 28, no. 6: 2716. https://doi.org/10.3390/molecules28062716
APA StyleHassan, Z. M., Guo, W., Welle, A., Oestreich, R., Janiak, C., & Redel, E. (2023). Formation of Gold Nanoclusters from Goldcarbonyl Chloride inside the Metal-Organic Framework HKUST-1. Molecules, 28(6), 2716. https://doi.org/10.3390/molecules28062716





