Antileishmanial and Antiplasmodial Activities of Secondary Metabolites from the Root of Antrocaryon klaineanum Pierre (Anacardiaceae)
Abstract
:1. Introduction
2. Results
2.1. Structure Elucidation of Compound 1
2.2. Physico-Chemical Characteristics of Compounds 1
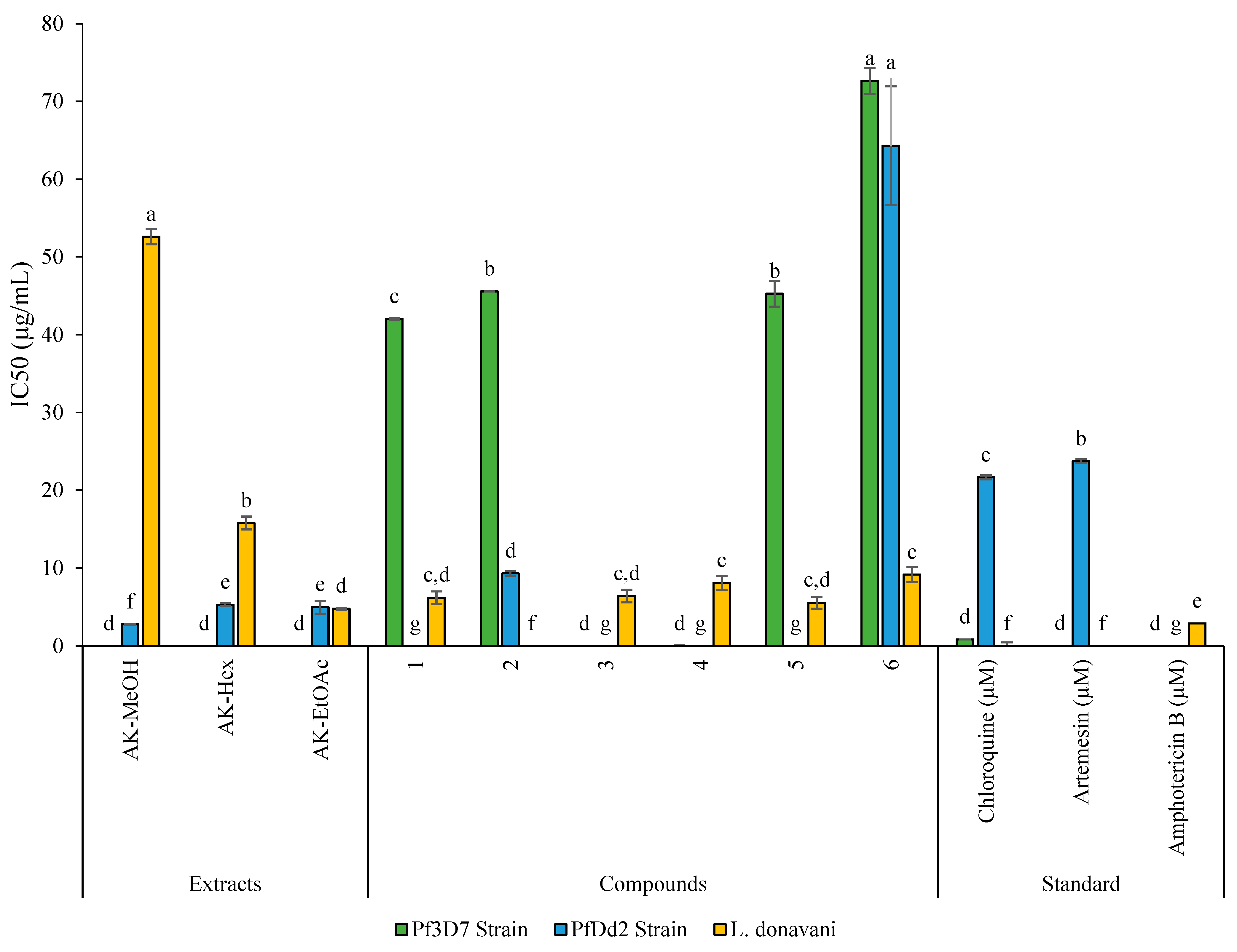
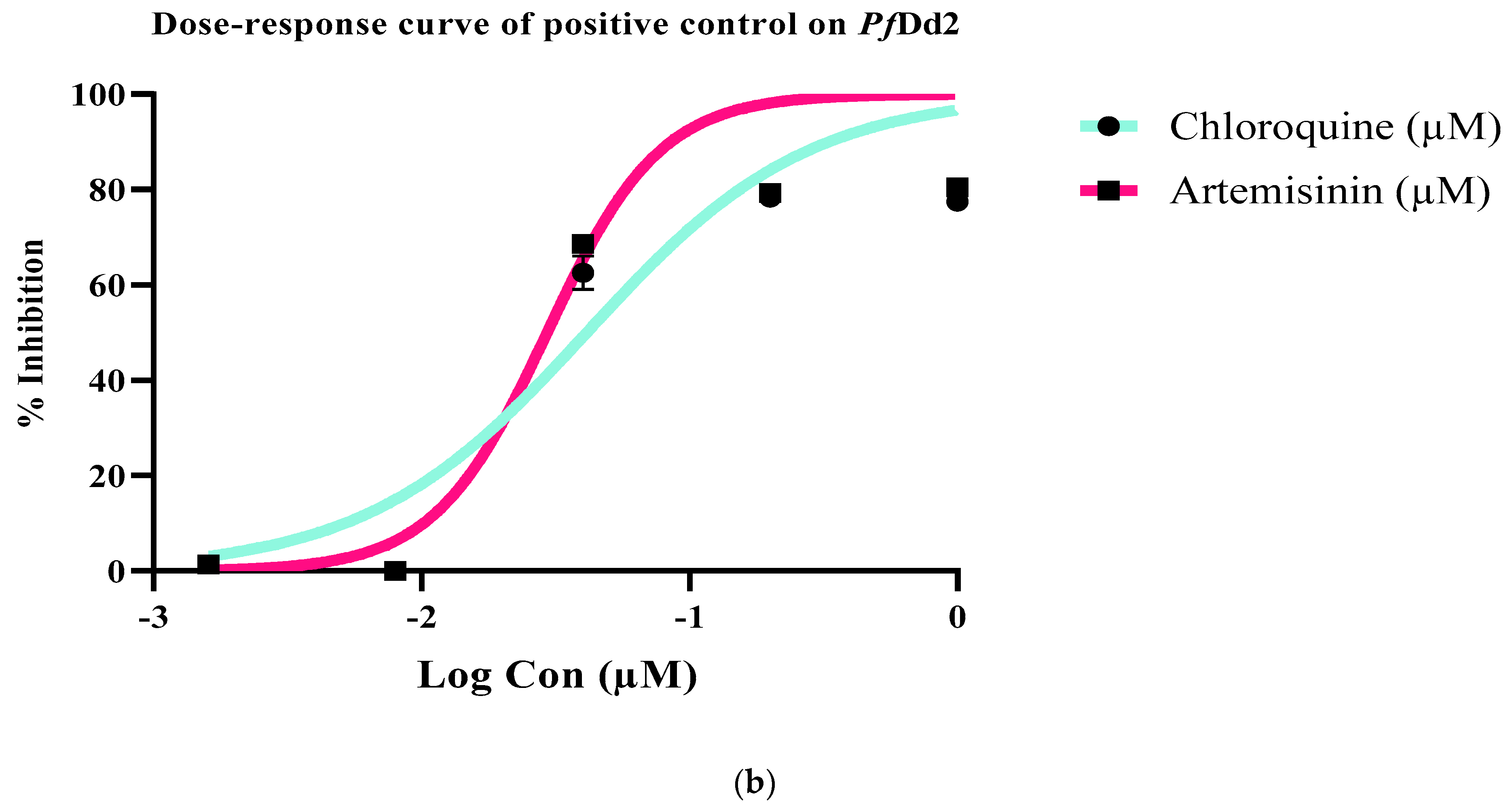
2.3. Antiplasmodial, Antileishmanial, and Cytotoxicity Activities of Extract, Fractions, and Compounds from Antrocaryon Klaineanum
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction and Isolation
4.4. Biological Activities
4.4.1. Parasite and Cell Culture
4.4.2. Sample Preparation for Biological Assays
4.4.3. Antiplasmodial Activity of Extract, Fractions, and Compounds
Synchronization of Parasite Culture
SYBR Green I-Based Fluorescence Assay
4.4.4. Antileishmanial Activity of Extract, Fractions, and Compounds
4.4.5. In Vitro Cytotoxicity Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thokouaha, Y.L.R.; Appiah-Opong, R.; Tsouh, F.P.V.; Tsabang, N.; Fekam, B.F.; Nyarko, A.K.; Wilson, M.D. Marine algae as source of novel antileishmanial drugs: A review. Mar. Drugs 2017, 15, 323. [Google Scholar] [CrossRef] [Green Version]
- Leutcha, B.P.; Sema, D.K.; Dzoyem, J.P.; Ayimele, G.A.; Nyongbela, K.D.; Delie, F.; Meli-Lannang, A. Cytotoxicity of a new tirucallane derivative isolated from Stereospermum acuminatissimum K. Schum stem bark. Nat. Prod. Res. 2021, 35, 4417–4422. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World malaria report 2022. In World Health; World Health Organization: Geneva, Switzerland, 2022; pp. 14–20. [Google Scholar]
- Lannang, A.M.; Sema, D.K.; Tatsimo, S.J.; Tankeu, V.T.; Tegha, H.F.; Wansi, J.D.; Sewald, N. A new depsidone derivative from the leaves of Garcinia polyantha. Nat. Prod. Res. 2018, 32, 1033–1038. [Google Scholar] [CrossRef]
- Nganso, Y.O.D.; Ngantchou, I.E.W.; Nkwenoua, E.; Nyasse, B.; Denier, C.; Hannert, V.; Schneider, B. Antitrypanosomal and cytotoxic activities of 22-Hydroxyclerosterol, a new sterol from Allexis cauliflora (Violaceae). Sci. Pharm. 2011, 79, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Fongang, A.L.M.; Nguemfo, E.L.; Nangue, Y.D.; Zangueu, C.B.; Fouokeng, Y.; Azebaze, A.G.B.; Vierling, W. Antinociceptive and anti-inflammatory effects of the methanolic stem bark extract of Antrocaryon klaineanum Pierre (Anacardiaceae) in mice and rat. J. Ethnopharmacol. 2017, 203, 11–19. [Google Scholar] [CrossRef]
- Schulze-Kaysers, N.; Feuereisen, M.M.; Schieber, A. Phenolic compounds in edible species of the Anacardiaceae family—a review. RSC Adv. 2015, 5, 73301–73314. [Google Scholar] [CrossRef]
- Fouokeng, Y.; Akak, C.M.; Tala, M.F.; Azebaze, A.G.B.; Dittrich, B.; Vardamides, J.C.; Laatsch, H. The structure of antrocarine E, an ergostane isolated from Antrocaryon klaineanum Pierre (Anacardiaceae). Fitoterapia 2017, 117, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Douanla, P.D.; Tabopda, T.K.; Tchinda, A.T.; Cieckiewicz, E.; Frédérich, M.; Boyom, F.F.; Tchuendem, M.H.K. Antrocarines A–F, antiplasmodial ergostane steroids from the stem bark of Antrocaryon klaineanum. Phytochemistry 2015, 117, 521–526. [Google Scholar] [CrossRef]
- Mbougnia, J.F.; Happi, G.M.; Bitchagno, G.T.; Awouafack, M.D.; Lenta, B.N.; Kouam, S.F.; Tene, M. Chemical constituents from Ficus natalensis hochst (Moraceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021, 95, 104227. [Google Scholar] [CrossRef]
- Kaoke, M.D.; Sema, D.K.; Dzoyem, J.P.; Leutcha, P.B.; Delie, F.; Allemann, E. A new cytotoxic glycocerebroside from Rauvolfia macrophylla Stapf. Nat. Prod. Chem. Res. 2020, 8, 1–5. [Google Scholar] [CrossRef]
- Nguemo, R.T.; Mbouangouere, R.; Bitchagno, G.T.M.; Tchuenguem, R.; Temgoua, E.V.N.; Ndontsa, B.L.; Tane, P. A new ceramide from the leaves of Lannea schimperi (Hochst. ex A. Rich.) Engl. Nat. Prod. Res. 2022, 36, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Ling, T.; Xia, T.; Wan, X.; Li, D.; Wei, X. Cerebrosides from the roots of Serratula chinensis. Molecules 2006, 11, 677–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnibou, L.; Nyemb, J.; Magne, C.; Mbougnia, J.; Leutcha, B.; Henoumont, C. Chemical constituents of Spathodea campanulata (Bignoniaceae), their antimicrobial and antioxidant activities. Nat. Prod. Chem. Res. 2021, 9, 1–7. [Google Scholar] [CrossRef]
- Youssef, D.T.; Ibrahim, S.R.; Shaala, L.A.; Mohamed, G.A.; Banjar, Z.M. New Cerebroside and nucleoside derivatives from a red sea strain of the marine cyanobacterium Moorea producens. Molecules 2016, 21, 324. [Google Scholar] [CrossRef] [Green Version]
- Inagaki, M.; Isobe, R.; Kawano, Y.; Miyamoto, T.; Komori, T.; Higuchi, R. Isolation and structure of three new ceramides from the starfsh Acanthaster planci. Eur. J. Org. Chem. 1998, 1998, 129–131. [Google Scholar] [CrossRef]
- Kasai, R.; Sasaki, A.; Hashimoto, T.; Kaneko, T.; Ohtani, K.; Yamasaki, K. Cycloartane glycosides from Trichosanthes tricuspidata. Phytochemistry 1999, 51, 803–808. [Google Scholar] [CrossRef]
- Sandjo, L.P.; Kuete, V. Ceramides, cerebrosides, and related long chains containing derivatives from the medicinal plants of Africa. Med. Plant Res. Afr. 2013, 607–620. [Google Scholar] [CrossRef]
- Wonkam, A.K.N.; Ngansop, C.A.N.; Wouamba, S.C.N.; Jouda, J.-B.; Happi, G.M.; Boyom, F.F.; Lenta, B.N. Rothmanniamide and other constituents from the leaves of Rothmannia hispida (K. Schum.) fagerl.(Rubiaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2020, 93, 104137. [Google Scholar] [CrossRef]
- Costantino, V.; Fattorusso, E.; Mangoni, A. Glycolipids from sponges. Part 9: Plakoside C and D, two further prenylated glycosphingolipids from the marine sponge. Tetrahedron 2000, 56, 5953–5957. [Google Scholar] [CrossRef]
- Kang, S.S.; Kim, J.S.; Son, K.H.; KIM, H.P.; CHANG, H.W. Cyclooxygenase-2 inhibitory cerebrosides from Phytolaccae Radix. Chem. Pharm. Bull. 2001, 49, 321–323. [Google Scholar] [CrossRef] [Green Version]
- Sarmiento, G.; Idelsohn, S.; Cardona, A.; Sonzogni, V. Failure internal pressure of spherical steel containments. Nucl. Eng. Des. 1985, 90, 209–222. [Google Scholar] [CrossRef]
- Natori, Y.; Rhoton, A.L.J. Transcranial approach to the orbit: Microsurgical anatomy. J. Neurosurg. 1994, 81, 78–86. [Google Scholar] [CrossRef]
- Kim, N.M.; Kim, J.; Chung, H.Y.; Choi, J.S. Isolation of luteolin 7-O-rutinoside and esculetin with potential antioxidant activity from the aerial parts of Artemisia montana. Arch. Pharm. Res. 2000, 23, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Yuan, D.A.I.; Zhi-Xin, L. Chemical constituents of Saussurea eopygmaea. Chin. J. Nat. Med. 2011, 9, 33–37. [Google Scholar] [CrossRef]
- Khan, N.M.M.U.; Hossain, M.S. Scopoletin and β-sitosterol glucoside from roots of Ipomoea digitata. J. Pharmacogn. Phytochem. 2015, 4, 5–7. [Google Scholar]
- Bai, N.K.; He, M.; Roller, B.; Zheng, X.; Chen, Z.; Shao, T.; Peng, Q.; Zheng, Q. Active Compounds from Lagerstroemia speciosa, Insulin-like Glucose Uptake-Stimulatory/Inhibitory and Adipocyte Differentiation-Inhibitory Activities in 3T3-L1 Cells. J. Agric. Food Chem. 2008, 56, 11668–11674. [Google Scholar] [CrossRef] [PubMed]
- Lenta, B.N.; Chouna, J.R.; Nkeng-Efouet, P.A.; Sewald, N. Endiandric acid derivatives and other constituents of plants from the genera Beilschmiedia and Endiandra (Lauraceae). Biomolecules 2015, 5, 910–942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasoanaivo, P.; Petitjean, A.; Ratsimamanga-Urverg, S.; Rakoto-Ratsimamanga, A. Medicinal plants used to treat malaria in Madagascar. J. Ethnopharmacol. 1992, 37, 117–127. [Google Scholar] [CrossRef]
- Fouokeng, Y.; Feusso, H.F.; Teinkela, J.M.; Noundou, X.S.; Wintjens, R.; Isaacs, M.; Vardamides, J.C. In vitro antimalarial, antitrypanosomal and HIV-1 integrase inhibitory activities of two Cameroonian medicinal plants: Antrocaryon klaineanum (Anacardiaceae) and Diospyros conocarpa (Ebenaceae). S. Afr. J. Bot. 2019, 122, 510–517. [Google Scholar] [CrossRef] [Green Version]
- Demain, A.L.; Fang, A. The natural functions of secondary metabolites. Adv. Biochem. Eng. Biotechnol. 2000, 69, 1–39. [Google Scholar] [CrossRef]
- Guetchueng, S.T.; Nahar, L.; Ritchie, K.J.; Ismail, F.M.D.; Dempster, N.M.; Nnanga, E.N.; Sarker, S.D. Phenolic compounds from the leaves and stem bark of Pseudospondias microcarpa (A. Rich.) Engl.(Anacardiaceae). Biochem. Syst. Ecol. 2020, 91, 104078. [Google Scholar] [CrossRef]
- Mogana, R.; Adhikari, A.; Debnath, S.; Hazra, S.; Hazra, B.; Teng-Jin, K.; Wiart, C. The antiacetylcholinesterase and antileishmanial activities of Canarium patentinervium Miq. BioMed Res. Int. 2014, 2014, 903529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kowa, T.K.; Tchokouaha, L.R.Y.; Cieckiewicz, E.; Philips, T.J.; Dotse, E.; Wabo, H.K.; Tchinda, A.T.; Tane, P.; Frédérich, M. Antileishmanial and cytotoxic activities of a new limonoid and a new phenyl alkene from the stem bark of Trichilia gilgiana (Meliaceae). Nat. Prod. Res. 2020, 34, 3182–3188. [Google Scholar] [CrossRef]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological Activities and Synthesis of esculetin and its derivatives: A mini-review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.M.; Ullah, F.; Ayaz, M.; Sadiq, A.; Hussain, S.; Shah, S.A.A.; Nadhman, A. β-Sitosterol from Ifloga spicata (Forssk.) Sch. Bip. as potential anti-leishmanial agent against Leishmania tropica: Docking and molecular insights. Steroids 2019, 148, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.K.; Chakraborti, S.; Bagchi, A.; Chakraborti, T. Bioassay-based Corchorus capsularis L. leaf-derived β-sitosterol exerts antileishmanial effects against Leishmania donovani by targeting trypanothione reductase. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Keumoe, R.; Koffi, J.G.; Dize, D.; Fokou, P.V.T.; Tchamgoue, J.; Ayong, L.; Ndjakou, B.L.; Sewald, N.; Ngameni, B.; Boyom, F.F. Identification of 3,3’-O-dimethylellagic acid and apigenin as the main antiplasmodial constituents of Endodesmia calophylloides Benth and Hymenostegia afzelii (Oliver.) Harms. BMC Complement. Med. Ther. 2021, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Morais, T.R.; da Costa-Silva, T.A.; Tempone, A.G.; Borborema, S.E.T.; Scotti, M.T.; de Sousa, R.M.F.; Lago, J.H.G. Antiparasitic activity of natural and semi-synthetic tirucallane triterpenoids from Schinus terebinthifolius (Anacardiaceae): Structure/activity relationships. Molecules 2014, 19, 5761–5776. [Google Scholar] [CrossRef] [Green Version]
- Keshav, P.; Goyal, D.K.; Kaur, S. In vitro and in vivo therapeutic antileishmanial potential of ellagic acid against Leishmania donovani in murine model. Med. Microbiol. Immunol. 2023, 212, 35–51. [Google Scholar] [CrossRef]
- Kumatia, E.K.; Ayertey, F.; Appiah-Opong, R.; Bolah, P.; Ehun, E.; Dabo, J. Antrocaryon micraster (A. Chev. And Guillaumin) stem bark extract demonstrated anti-malaria action and normalized hematological indices in Plasmodium berghei infested mice in the Rane’s test. J. Ethnopharmacol. 2021, 266, 113427. [Google Scholar] [CrossRef]
- Brenzan, M.A.; Santos, A.O.; Nakamura, C.V.; Dias Filho, B.P.; Ueda-Nakamura, T.; Young, M.C.M.; Cortez, D.A.G. Effects of (−) mammea A/BB isolated from Calophyllum brasiliense leaves and derivatives on mitochondrial membrane of Leishmania amazonensis. Phytomedicine 2012, 19, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Alves, M.M.D.M.; Brito, L.M.; Souza, A.C.; Queiroz, B.C.S.H.; de Carvalho, T.P.; Batista, J.F.; Oliveira, J.S.D.S.M.; de Mendonça, I.L.; Lira, S.R.D.S.; Chaves, M.H.; et al. Gallic and ellagic acids: Two natural immunomodulator compounds solve infection of macrophages by Leishmania major. Naunyn Schmiedeberg’s Arch. Pharm. 2017, 390, 893–903. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. Res. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Smilkstein, M.; Sriwilaijaroen, N.; Kelly, J.X.; Wilairat, P.; Riscoe, M. Simple and inexpensive fluorescence-based technique for high-throughput antimalarial drug screening. Antimicrob. Agents Chemother. 2004, 48, 1803–1806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siqueira-Neto, J.L.; Song, O.R.; Oh, H.; Sohn, J.H.; Yang, G.; Nam, J.; Jang, J.; Cechetto, J.; Lee, C.B.; Moon, S.; et al. Antileishmanial high-throughput drug screening reveals drug candidates with new scaffolds. PLoS Negl. Trop. Dis. 2010, 4, e675. [Google Scholar] [CrossRef] [Green Version]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]

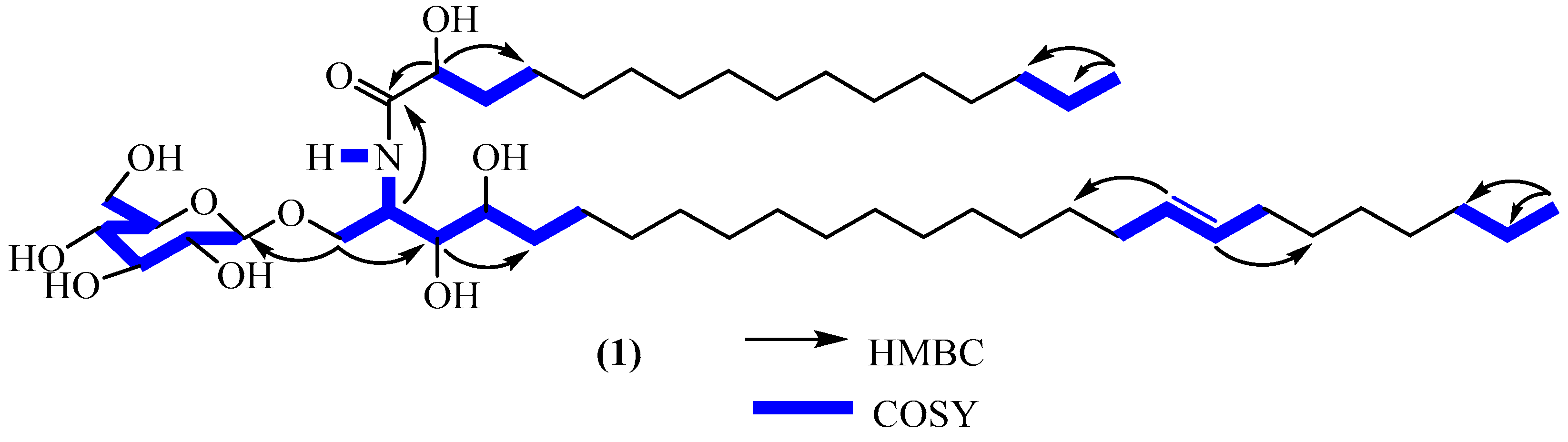
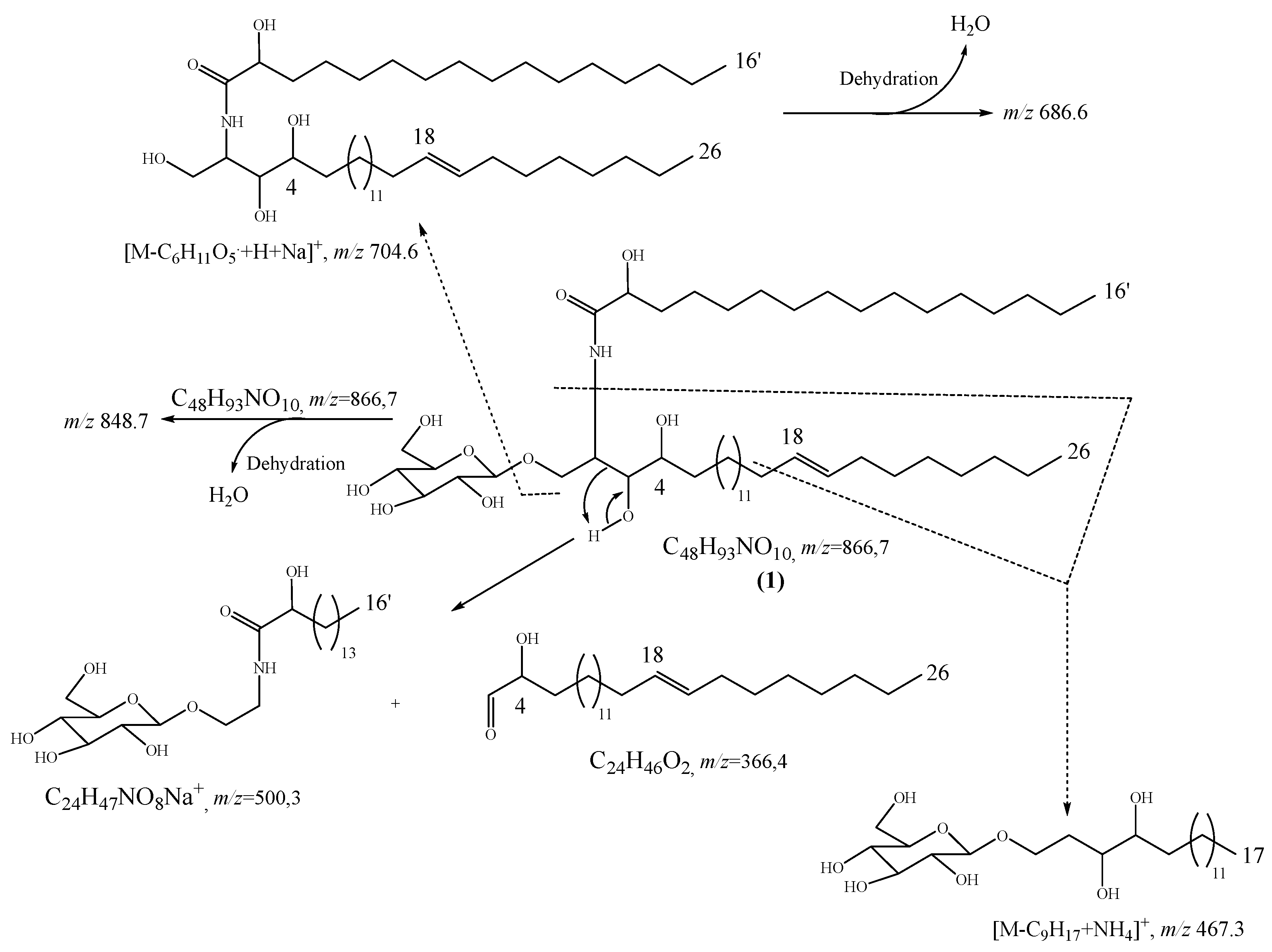

| N° | Antroklaicerebroside (1) | ||||
|---|---|---|---|---|---|
| Acetone-d6 | CD3OD | Acetone-d6 and CD3OD | |||
| δC | δH (m, J in Hz) | δC | δH (m, J in Hz) | 2,3JHC, HMBC | |
| 1 | 70.2 | 3.96 (dd, 10.9, 6.5) 3.90 (dd, 10.9, 4.5) | 69.9 | 4.05 (dd, 10.7, 6.2) 3.82 (dd, 10.7, 3.9) | 2, 3, 1″ |
| 2 | 51.7 | 4.30 (m) | 51.6 | 4.27 (m) | 3, 1′ |
| 3 | 75.9 | 3.56 (m) | 75.6 | 3.60 (t, 6.0) | 1, 2, 4, 5 |
| 4 | 72.5 | 3.58 (m) | 72.9 | 3.53 (m) | 2 |
| 5 | 33.6 | 1.42 (m) | 32.9 | 1.42 (m) | |
| 6 | 28.0 | 2.06 (m) | 28.3 | 2.06 (m) | |
| 7–16 | 30.5 | 1.29 (m) | 30.8 | 1.29 (m) | |
| 17 | 33.4 | 1.98 (m) | 33.1 | 1.96 (m) | 18, 19 |
| 18 | 131.2 | 5.41 (m) | 131.4 | 5.42 (brt, 5.2) | |
| 19 | 130.8 | 5.35 (m) | 130.9 | 5.37 (m) | |
| 20 | 33.5 | 2.01 (m) | 33.8 | 1.98 (m) | 18, 19 |
| 21–23 | 30.5 | 1.29 (m) | 30.8 | 1.29 (m) | |
| 24 | 32.6 | 1.29 (m) | 32.6 | 1.29 (m) | |
| 25 | 23.5 | 1.30 (m) | 23.7 | 1.32 (m) | |
| 26 | 14.5 | 0.88 (t, 7.0) | 14.5 | 0.88 (t, 7.0) | 24, 25 |
| 1′ | nd | - | 177.2 | - | |
| 2′ | 72.6 | 4.04 (m) | 73.0 | 4.02 (dd, 7.7, 3.8) | 1′, 3′, 4′ |
| 3′ | 35.6 | 1.77 (m) 1.60 (m) | 35.7 | 1.77 (m) 1.60 (m) | 1′, 2′ |
| 4′ | 25.9 | 1.44 (m) | 25.9 | 1.44 (m) | |
| 5′–13′ | 30.5 | 1.29 (m) | 30.8 | 1.29 (m) | |
| 14′ | 32.6 | 1.29 (m) | 32.6 | 1.29 (m) | |
| 15′ | 23.5 | 1.30 (m) | 23.5 | 1.32 (m) | |
| 16′ | 14.5 | 0.88 (t, 7.0) | 14.5 | 0.88 (m) | 14′, 15′ |
| 1″ | 104.9 | 4.34 (d, 7.7) | 104.7 | 4.29 (d, 7.8) | 1, 2″ |
| 2″ | 74.9 | 3.16 (brt, 8.0) | 75.0 | 3.18 (dd, 9.1, 7.8) | 1″, 3″ |
| 3″ | 78.0 | 3.38 (m) | 78.0 | 3.38 (t, 9.1) | 4″ |
| 4″ | 71.6 | 3.32 (m) | 71.6 | 3.28 (m) | 3″ |
| 5″ | 77.8 | 3.32 (m) | 77.9 | 3.28 (m) | |
| 6″ | 63.0 | 3.85 (dd, 11.5, 6.5) 3.65 (dd, 11.5, 5.9) | 62.7 | 3.87 (dd, 11.8, 1.7) 3.67 (dd, 11.8, 5.4) | 4″, 5″ |
| -NH | - | 7.51 (d, 8.9) | - | nd | |
| Samples | Cytotoxicity Raw Cells | P. falciparum | L. donovani Promastigote | |||||
|---|---|---|---|---|---|---|---|---|
| CC50 ± SD (µg/mL) | Pf3D7 IC50 ± SD (µg/mL) | SI_Pf3D7 | PfDd2 IC50 ± SD (µg/mL) | SI_PfDd2 | RI | IC50 ± SD (µg/mL) | SI | |
| Extract | ||||||||
| AK-MeOH | >500 | >100 | ND | 2.78 ± 0.06 | >179.86 | - | >100 | ND |
| Fractions | ||||||||
| AK-Hex | >500 | >100 | ND | 5.30 ± 0.19 | >94.34 | - | 15.78 ± 0.82 | >31.69 |
| AK-DCM | >500 | >100 | ND | >100 | ND | ND | >100 | ND |
| AK-EtOAc | >500 | >100 | ND | 4.98 ± 0.81 | >100.40 | - | 4.80 ± 0.13 | >104.17 |
| Compounds | ||||||||
| Antroklaicerebroside (1) | >500 | 42.04 ± 0.08 | >11.72 | >50 | ND | >1.19 | 6.19 ± 0.79 | >80.78 |
| Esculetin (2) | >500 | 45.57 ± 0.08 | >10.97 | 9.30 ± 0.29 | >53.76 | 0.2 | >50 | ND |
| Scopoletin (3) | >500 | >50 | ND | >50 | ND | ND | 6.40 ± 0.80 | >78.13 |
| 3,3′-Dimethylellagic acid (4) | >500 | >50 | ND | >50 | ND | ND | 8.07 ± 0.90 | >61.96 |
| β-sitosterol (5) | >500 | 45.27 ± 1.66 | >11.04 | >50 | ND | >1.1 | 5.56 ± 0.74 | >89.93 |
| Stigmasterol (6) | >500 | >50 | ND | >50 | ND | 9.14 ± 0.96 | >54.70 | |
| References drugs | ||||||||
| Chloroquine (µM) | - | 0.85± 0.01 | - | 21.65 ± 0.25 | - | - | - | - |
| Artemesin (µM) | - | 0.02 ± 0.00 | - | 23.73 ± 0.24 | - | - | - | - |
| Amphotericin B (µg/mL) | - | - | - | - | - | - | 2.90 ± 0.46 | - |
| Podophyllotoxin (µM) | 0.71 ± 0.20 | - | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amang à Ngnoung, G.A.; Sidjui, L.S.; Leutcha, P.B.; Nganso Ditchou, Y.O.; Tchokouaha, L.R.Y.; Herbette, G.; Baghdikian, B.; Kowa, T.K.; Soh, D.; Kemzeu, R.; et al. Antileishmanial and Antiplasmodial Activities of Secondary Metabolites from the Root of Antrocaryon klaineanum Pierre (Anacardiaceae). Molecules 2023, 28, 2730. https://doi.org/10.3390/molecules28062730
Amang à Ngnoung GA, Sidjui LS, Leutcha PB, Nganso Ditchou YO, Tchokouaha LRY, Herbette G, Baghdikian B, Kowa TK, Soh D, Kemzeu R, et al. Antileishmanial and Antiplasmodial Activities of Secondary Metabolites from the Root of Antrocaryon klaineanum Pierre (Anacardiaceae). Molecules. 2023; 28(6):2730. https://doi.org/10.3390/molecules28062730
Chicago/Turabian StyleAmang à Ngnoung, Gabrielle Ange, Lazare S. Sidjui, Peron B. Leutcha, Yves O. Nganso Ditchou, Lauve R. Y. Tchokouaha, Gaëtan Herbette, Beatrice Baghdikian, Theodora K. Kowa, Desire Soh, Raoul Kemzeu, and et al. 2023. "Antileishmanial and Antiplasmodial Activities of Secondary Metabolites from the Root of Antrocaryon klaineanum Pierre (Anacardiaceae)" Molecules 28, no. 6: 2730. https://doi.org/10.3390/molecules28062730
APA StyleAmang à Ngnoung, G. A., Sidjui, L. S., Leutcha, P. B., Nganso Ditchou, Y. O., Tchokouaha, L. R. Y., Herbette, G., Baghdikian, B., Kowa, T. K., Soh, D., Kemzeu, R., Poka, M., Demana, P. H., Siwe Noundou, X., Tchinda, A. T., Fekam Boyom, F., Lannang, A. M., & Nyassé, B. (2023). Antileishmanial and Antiplasmodial Activities of Secondary Metabolites from the Root of Antrocaryon klaineanum Pierre (Anacardiaceae). Molecules, 28(6), 2730. https://doi.org/10.3390/molecules28062730








