Theoretical and Anti-Klebsiella pneumoniae Evaluations of Substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and Imidazopyridine Hydrazide Derivatives
Abstract
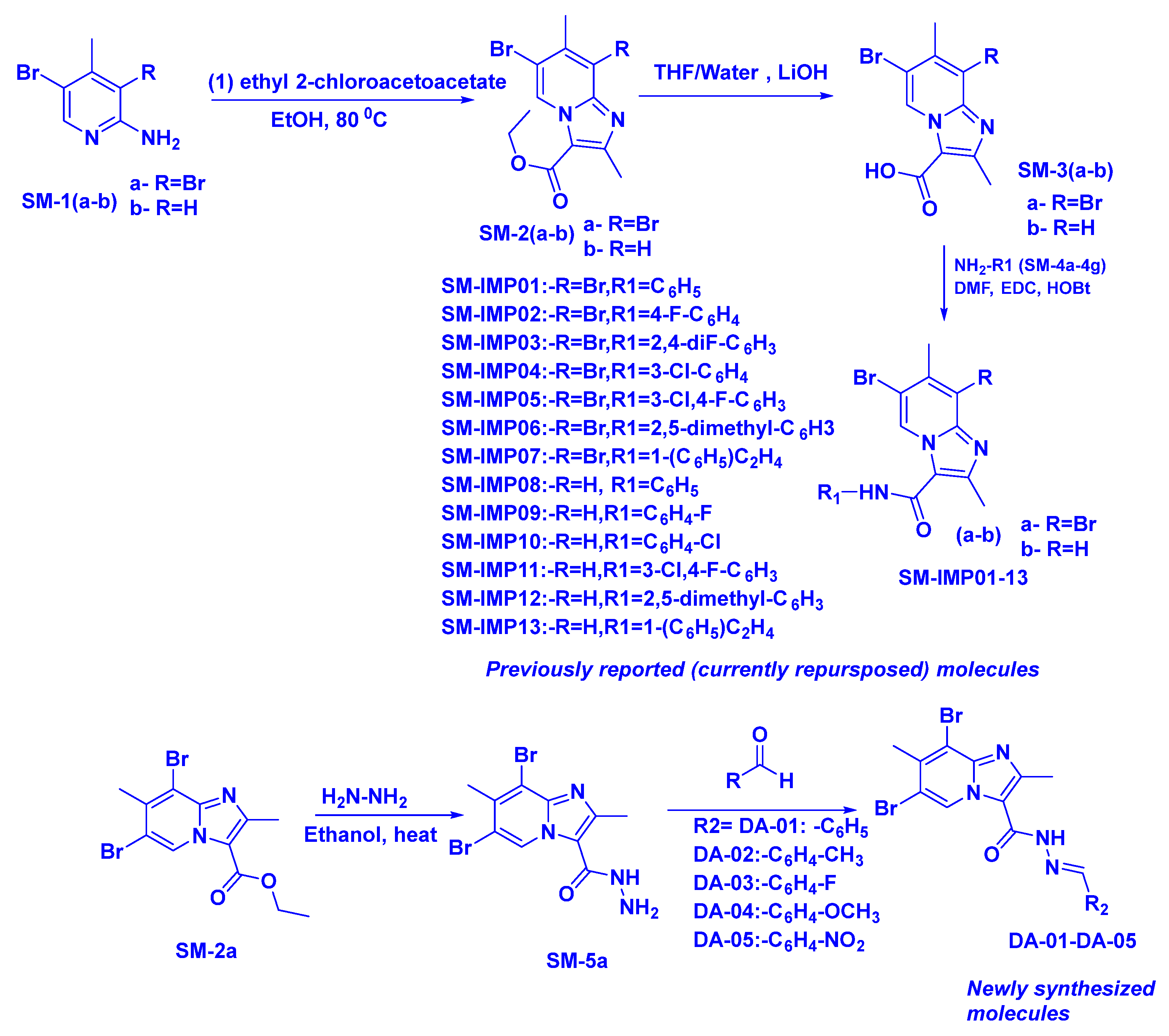
:1. Introduction
2. Results
2.1. Structures and Energetics
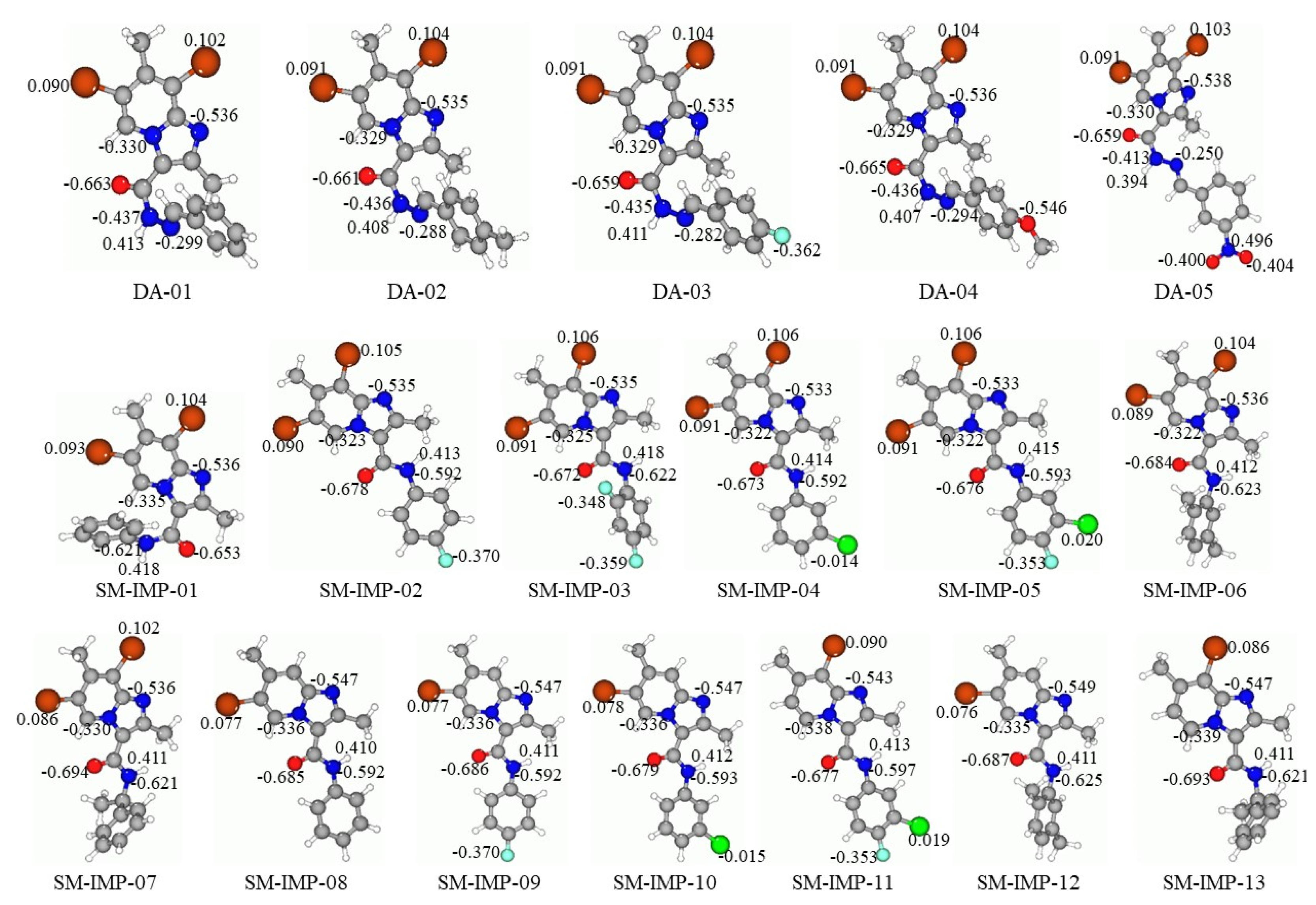
2.2. NPA Charges
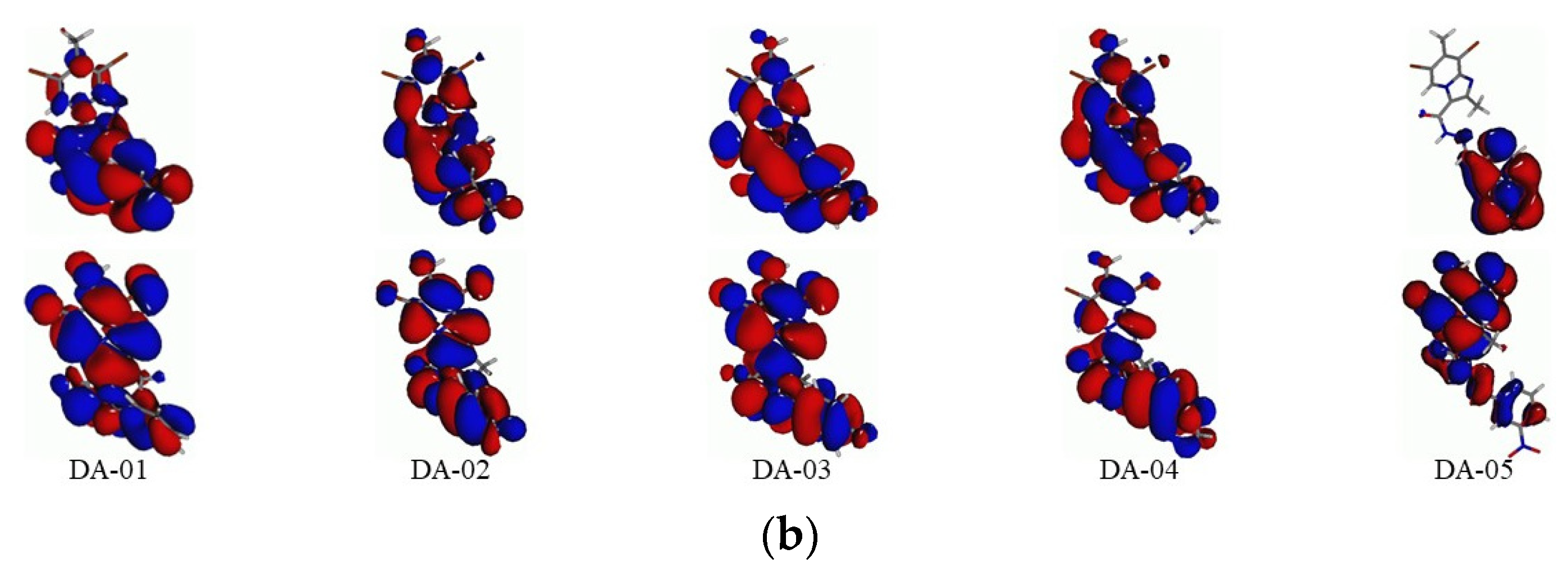
2.3. Frontier MOs
2.4. Global Reactivity Parameters (GRP)
2.5. Molecular Electrostatic Potential Plots
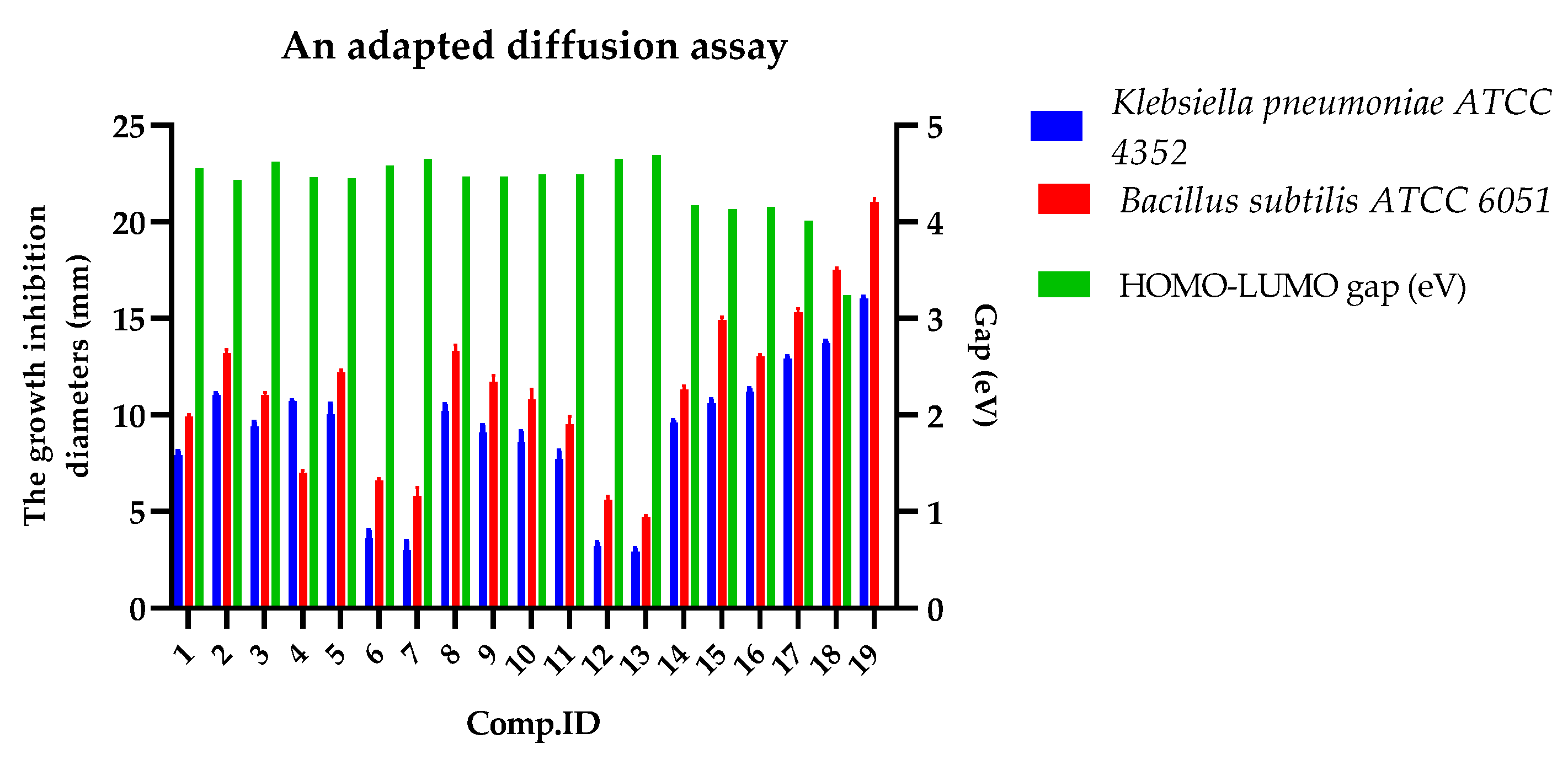
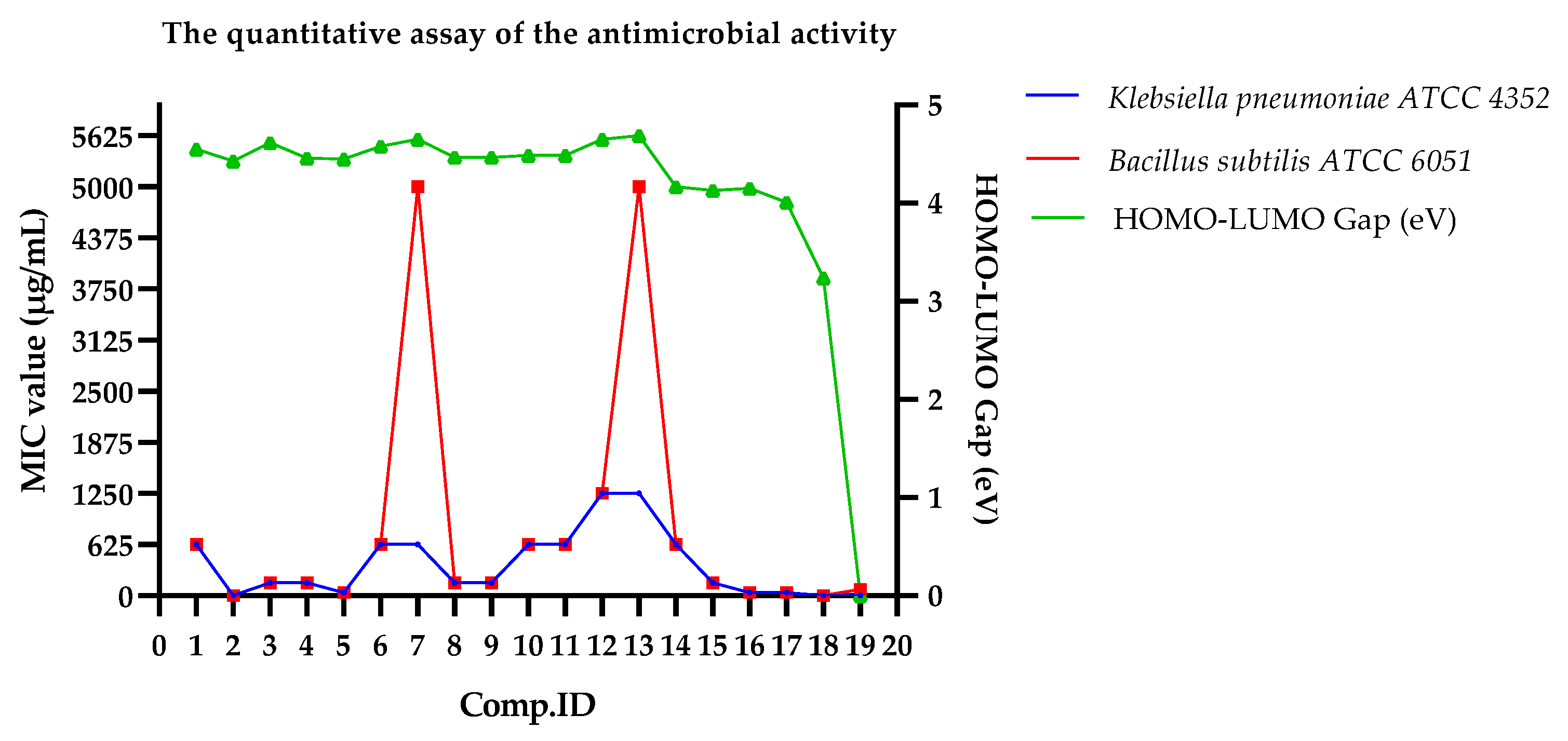
2.6. Antimicrobial Activity of the Substituted Imidazopyridines (IMPs) (SM-IMP-01 to SM-IMP-13, and DA-01-05)
Relationship of Biological Assay with DFT Properties
2.7. Spectral Data of Synthesized Compounds
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.3. Theoretical Analyses
3.4. Bioactivity Analyses
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Schrader, S.M.; Botella, H.; Vaubourgeix, J. Reframing antimicrobial resistance as a continuous spectrum of manifestations. Curr. Opin. Microbiol. 2023, 72, 102259. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Harant, A.; Fernandes, G.; Mwamelo, A.J.; Hein, W.; Dekker, D.; Sridhar, D. Measuring the global response to antimicrobial resistance, 2020–2021: A systematic governance analysis of 114 countries. Lancet Infect. Dis. 2023; in press. [Google Scholar] [CrossRef] [PubMed]
- Mishra, V.R.; Ghanavatkar, C.W.; Mali, S.N.; Qureshi, S.I.; Chaudhari, H.K.; Sekar, N. Design, synthesis, antimicrobial activity and computational studies of novel azo linked substituted benzimidazole, benzoxazole and benzothiazole derivatives. Comput. Biol. Chem. 2019, 78, 330–337. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Synthesis of New Hydrazones using a biodegradable catalyst, their Biological Evaluations and Molecular Modeling Studies (Part-II). J. Comput. Biophys. Chem. 2022, 21, 857–882. [Google Scholar] [CrossRef]
- Mali, S.N.; Tambe, S.; Pratap, A.P.; Cruz, J.N. Molecular Modeling Approaches to Investigate Essential Oils (Volatile Compounds) Interacting with Molecular Targets. In Essential Oils; Santana de Oliveira, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 1, pp. 417–442. [Google Scholar]
- Mali, S.N.; Pandey, A.; Thorat, B.R.; Lai, C.H. Multiple 3D-and 2D-quantitative structure–activity relationship models (QSAR), theoretical study and molecular modeling to identify structural requirements of imidazopyridine analogues as anti-infective agents against tuberculosis. Struct. Chem. 2022, 33, 679–694. [Google Scholar] [CrossRef]
- Mali, S.N.; Thorat, B.R.; Gupta, D.R.; Pandey, A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Eng. Proc. 2021, 11, 21. [Google Scholar]
- Mali, S.N.; Pandey, A. Multiple QSAR and molecular modelling for identification of potent human adenovirus inhibitors. J. Indian Chem. Soc. 2021, 98, 100082. [Google Scholar] [CrossRef]
- Desale, V.J.; Mali, S.N.; Thorat, B.R.; Yamgar, R.S. Synthesis, admetSAR Predictions, DPPH Radical Scavenging Activity, and Potent Anti-mycobacterial Studies of Hydrazones of Substituted 4-(anilino methyl) benzohydrazides (Part 2). Curr. Comput.-Aided Drug Des. 2021, 17, 493–503. [Google Scholar] [CrossRef]
- Mali, S.N.; Pandey, A. Molecular modeling studies on 2,4-disubstituted imidazopyridines as anti-malarials: Atom-based 3D-QSAR, molecular docking, virtual screening, in-silico ADMET and theoretical analysis. J. Comput. Biophys. Chem. 2021, 20, 267–282. [Google Scholar] [CrossRef]
- Anuse, D.G.; Mali, S.N.; Thorat, B.R.; Yamgar, R.S.; Chaudhari, H.K. Synthesis, SAR, in silico appraisal and anti-microbial study of substituted 2-aminobenzothiazoles derivatives. Curr. Comput.-Aided Drug Des. 2020, 16, 802–813. [Google Scholar] [CrossRef]
- Devi, N.; Jana, A.K.; Singh, V. Assessment of novel pyrazolopyridinone fused imidazopyridines as potential antimicrobial agents. Karbala Int. J. Mod. Sci. 2018, 4, 164–170. [Google Scholar] [CrossRef]
- Schaenzer, A.J.; Wlodarchak, N.; Drewry, D.H.; Zuercher, W.J.; Rose, W.E.; Striker, R.; Sauer, J.D. A screen for kinase inhibitors identifies antimicrobial imidazopyridine aminofurazans as specific inhibitors of the Listeria monocytogenes PASTA kinase PrkA. J. Biol. Chem. 2017, 292, 17037–17045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuthyala, S.; Shankar, M.K.; Nagaraja, G.K. Synthesis, Single-Crystal X-ray, Hirshfeld and Antimicrobial Evaluation of some New Imidazopyridine Nucleus Incorporated with Oxadiazole Scaffold. ChemistrySelect 2018, 3, 12894–12899. [Google Scholar] [CrossRef]
- Winglee, K.; Lun, S.; Pieroni, M.; Kozikowski, A.; Bishai, W. Mutation of Rv2887, a marR-like gene, confers Mycobacterium tuberculosis resistance to an imidazopyridine-based agent. Antimicrob. Agents Chemother. 2015, 59, 6873–6881. [Google Scholar] [CrossRef] [Green Version]
- Mali, S.N.; Pandey, A. Recent Developments in Medicinal and In Silico Applications of Imidazopyridine Derivatives: Special Emphasis on Malaria, Trypanosomiasis, and Tuberculosis. Chem. Afr. 2022, 5, 1215–1236. [Google Scholar] [CrossRef]
- Elnagdi, M.H.; Erian, A.W. New routes to polyfunctionally substituted pyridine, pyridopyridine, quinoline, and pyridazine derivatives. Arch. Pharm. 1991, 324, 853–858. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.P.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N.; et al. Synthesis of N1-arylidene-N2-quinolyl-and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorgan. Med. Chem. Lett. 2006, 16, 5384–5388. [Google Scholar] [CrossRef]
- Bijev, A. New heterocyclic hydrazones in the search for antitubercular agents: Synthesis and in vitro evaluations. Lett. Drug Des. Discov. 2006, 3, 506–512. [Google Scholar] [CrossRef]
- Ragavendran, J.V.; Sriram, D.; Patel, S.K.; Reddy, I.V.; Bharathwajan, N.; Stables, J.; Yogeeswari, P. Design and synthesis of anticonvulsants from a combined phthalimide–GABA–anilide and hydrazone pharmacophore. Eur. J. Med. Chem. 2007, 42, 146–151. [Google Scholar] [CrossRef]
- Todeschini, A.R.; de Miranda, A.L.P.; da Silva, K.C.M.; Parrini, S.C.; Barreiro, E.J. Synthesis and evaluation of analgesic, antiinflammatory and antiplatelet properties of new 2-pyridylarylhydrazone derivatives. Eur. J. Med. Chem. 1998, 33, 189–199. [Google Scholar] [CrossRef]
- Deep, A.; Jain, S.; Sharma, P.C.; Verma, P.; Kumar, M.; Dora, C.P. Design and biological evaluation of biphenyl-4-carboxylic acid hydrazide-hydrazone for antimicrobial activity. Synthesis 2010, 182, 183OC. [Google Scholar]
- Özkay, Y.; Tunalı, Y.; Karaca, H.; Işıkdağ, İ. Antimicrobial activity and a SAR study of some novel benzimidazole derivatives bearing hydrazone moiety. Eur. J. Med. Chem. 2010, 45, 3293–3298. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Ali, M.R.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Bioallied Sci. 2014, 6, 69–80. [Google Scholar] [PubMed]
- Jadhav, B.; Kenny, R.; Nivid, Y.; Mandewale, M.; Yamgar, R. Synthesis and Evaluation of Antituberculosis Activity of Substituted 2,7-Dimethylimidazo [1,2-a]Pyridine-3-Carboxamide Derivatives. Open J. Med. Chem. 2016, 6, 59–69. [Google Scholar] [CrossRef] [Green Version]
- Gaussian 16, Revision B.01; Frisch, M.; Trucks, G.; Schlegel, H.; Scuseria, G.; Robb, M.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.; et al. (Eds.) Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Axel, D.B. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar]
- McLean, A.D.; Chandler, G. Contracted Gaussian-basis sets for molecular calculations. 1. 2nd row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.; Raymond, J.; People, J. Self-Consistent Molecular Orbital Methods. 20. Basis set for correlated wave-functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum mechanical continuum solvation models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Geerlings, P.; De Proft, F.; Langenaeker, W. Conceptual density functional theory. Chem. Rev. 2003, 103, 1793–1874. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Pan, S.; Chattaraj, P. Biological activity and toxicity: A conceptual DFT approach. Appl. Density Funct. Theory Biol. Bioinorg. Chem. 2013, 150, 143–180. [Google Scholar]
- Jorio, S.; Salah, M.; El Makarim, H.A.; Tabyaoui, M. Reactivity indices related to DFT theory, the electron localization function (ELF) and non-covalent interactions (NCI) calculations in the formation of the non-halogenated pyruvic esters in solution. Mediterr. J. Chem. 2019, 8, 476–485. [Google Scholar] [CrossRef] [Green Version]
- Schaftenaar, G.; Noordik, J. Molden: A pre- and post-processing program for molecular and electronic structures. J. Comput.-Aided Mol. Des. 2000, 14, 123–134. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.; Lonie, D.; Vandermeersch, T.; Zurek, E.; Hutchison, G. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminforma. 2012, 41, 17. [Google Scholar] [CrossRef] [Green Version]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool. Version 1. Available online: http://avogadro.cc/ (accessed on 3 December 2022).
- Kshatriya, R.; Shelke, P.; Mali, S.; Yashwantrao, G.; Pratap, A.; Saha, S. Synthesis and evaluation of anticancer activity of pyrazolone appended triarylmethanes (TRAMs). ChemistrySelect 2021, 6, 6230–6239. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Aldawsari, F.D.; Al-Showiman, S.S.; Barakat, A.; Soliman, S.M.; Choudhary, M.I.; Yousuf, S.; Mubarak, M. Novel enaminone derived from thieno[2, 3-b]thiene: Synthesis, X-ray crystal structure, HOMO, LUMO, NBO analyses and biological activity. Chem. Cent. J. 2015, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Saini, V.; Maurya, I.K.; Sindhu, J.; Kumari, M.; Kataria, R.; Kumar, V. Design, synthesis, DFT, docking studies and ADME prediction of some new coumarinyl linked pyrazolylthiazoles: Potential standalone or adjuvant antimicrobial agents. PLoS ONE 2018, 13, e0196016. [Google Scholar] [CrossRef] [Green Version]



| Compound | E0, A.U. | E0 + ZPE, A.U. | E(HOMO/ LUMO), A.U. | DE(HOMO/ LUMO), eV | TDDFT Gap, eV |
|---|---|---|---|---|---|
| SM-IMP-01 | −6005.551842 | −6005.293120 | −0.23600/−0.06880 | 4.55 | 3.95 |
| SM-IMP-02 | −6104.828771 | −6104.577934 | −0.22966/−0.06689 | 4.43 | 3.92 |
| SM-IMP-03 | −6204.092300 | −6203.850115 | −0.23619/−0.06648 | 4.62 | 4.09 |
| SM-IMP-04 | −6465.183041 | −6464.933668 | −0.23269/−0.06869 | 4.46 | 3.92 |
| SM-IMP-05 | −6564.446170 | −6564.205187 | −0.23207/−0.06865 | 4.45 | 3.91 |
| SM-IMP-06 | −6084.213075 | −6083.900065 | −0.23289/−0.06456 | 4.58 | 4.07 |
| SM-IMP-07 | −6084.204338 | −6083.889031 | −0.23353/−0.06250 | 4.65 | 4.10, 4.22 |
| SM-IMP-08 | −3432.025895 | −3431.756301 | −0.22610/−0.06173 | 4.47 | 3.94 |
| SM-IMP-09 | −3531.294066 | −3531.032988 | −0.22603/−0.06189 | 4.47 | 3.94 |
| SM-IMP-10 | −3891.648466 | −3891.388547 | −0.22936/−0.06435 | 4.49 | 3.93 |
| SM-IMP-11 | −3990.912017 | −3990.660204 | −0.22852/−0.06370 | 4.49 | 3.94 |
| SM-IMP-12 | −3510.678376 | −3510.354618 | −0.22928/−0.05854 | 4.65 | 4.11 |
| SM-IMP-13 | −3510.670015 | −3510.344084 | −0.22852/−0.05634 | 4.69 | 4.15 |
| DA-01 | −6098.995509 | −6098.715663 | −0.23274/−0.07953 | 4.17 | 3.49 |
| DA-02 | −6138.324011 | −6138.016908 | −0.23020/−0.07854 | 4.13 | 3.46 |
| DA-03 | −6198.264953 | −6197.993317 | −0.23282/−0.08043 | 4.15 | 3.46 |
| DA-04 | −6213.555413 | −6213.243263 | −0.22354/−0.07633 | 4.01 | 3.41 |
| DA-05 | −6303.573759 | −6303.291645 | −0.23588/−0.11688 | 3.24 | 3.16, 3.65 |
| Compound | IP | EA | Gap | X | η | μ | S | ω |
|---|---|---|---|---|---|---|---|---|
| SM-IMP-01 | 6.42 | 1.87 | 4.55 | 4.145 | 2.275 | −4.145 | 0.220 | 3.861 |
| SM-IMP-02 | 6.25 | 1.82 | 4.43 | 4.035 | 2.215 | −4.035 | 0.226 | 3.675 |
| SM-IMP-03 | 6.43 | 1.81 | 4.62 | 4.12 | 2.310 | −4.12 | 0.216 | 3.674 |
| SM-IMP-04 | 6.33 | 1.87 | 4.46 | 4.10 | 2.230 | −4.10 | 0.224 | 3.769 |
| SM-IMP-05 | 6.31 | 1.86 | 4.45 | 4.085 | 2.225 | −4.085 | 0.225 | 3.750 |
| SM-IMP-06 | 6.34 | 1.76 | 4.58 | 4.05 | 2.29 | −4.05 | 0.218 | 3.581 |
| SM-IMP-07 | 6.35 | 1.70 | 4.65 | 4.025 | 2.325 | −4.025 | 0.215 | 3.484 |
| SM-IMP-08 | 6.15 | 1.68 | 4.47 | 3.915 | 2.235 | −3.915 | 0.224 | 3.429 |
| SM-IMP-09 | 6.15 | 1.68 | 4.47 | 3.915 | 2.235 | −3.915 | 0.224 | 3.429 |
| SM-IMP-10 | 6.24 | 1.75 | 4.49 | 3.995 | 2.245 | −3.995 | 0.223 | 3.555 |
| SM-IMP-11 | 6.22 | 1.73 | 4.49 | 3.975 | 2.245 | −3.975 | 0.223 | 3.519 |
| SM-IMP-12 | 6.24 | 1.59 | 4.65 | 3.915 | 2.325 | −3.915 | 0.215 | 3.296 |
| SM-IMP-13 | 6.22 | 1.53 | 4.69 | 3.875 | 2.345 | −3.875 | 0.213 | 3.202 |
| DA-01 | 6.33 | 2.16 | 4.17 | 4.245 | 2.085 | −4.245 | 0.240 | 4.321 |
| DA-02 | 6.26 | 2.13 | 4.13 | 4.195 | 2.065 | −4.195 | 0.242 | 4.261 |
| DA-03 | 6.34 | 2.19 | 4.15 | 4.265 | 2.075 | −4.265 | 0.241 | 4.383 |
| DA-04 | 6.08 | 2.07 | 4.01 | 4.075 | 2.005 | −4.075 | 0.249 | 4.141 |
| DA-05 | 6.42 | 3.18 | 3.24 | 4.8 | 1.62 | −4.8 | 0.309 | 7.111 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mali, S.N.; Anand, A.; Zaki, M.E.A.; Al-Hussain, S.A.; Jawarkar, R.D.; Pandey, A.; Kuznetsov, A. Theoretical and Anti-Klebsiella pneumoniae Evaluations of Substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and Imidazopyridine Hydrazide Derivatives. Molecules 2023, 28, 2801. https://doi.org/10.3390/molecules28062801
Mali SN, Anand A, Zaki MEA, Al-Hussain SA, Jawarkar RD, Pandey A, Kuznetsov A. Theoretical and Anti-Klebsiella pneumoniae Evaluations of Substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and Imidazopyridine Hydrazide Derivatives. Molecules. 2023; 28(6):2801. https://doi.org/10.3390/molecules28062801
Chicago/Turabian StyleMali, Suraj N., Amit Anand, Magdi E. A. Zaki, Sami A. Al-Hussain, Rahul D. Jawarkar, Anima Pandey, and Aleksey Kuznetsov. 2023. "Theoretical and Anti-Klebsiella pneumoniae Evaluations of Substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and Imidazopyridine Hydrazide Derivatives" Molecules 28, no. 6: 2801. https://doi.org/10.3390/molecules28062801
APA StyleMali, S. N., Anand, A., Zaki, M. E. A., Al-Hussain, S. A., Jawarkar, R. D., Pandey, A., & Kuznetsov, A. (2023). Theoretical and Anti-Klebsiella pneumoniae Evaluations of Substituted 2,7-dimethylimidazo[1,2-a]pyridine-3-carboxamide and Imidazopyridine Hydrazide Derivatives. Molecules, 28(6), 2801. https://doi.org/10.3390/molecules28062801








