Recent Advances in the Synthesis of Di- and Trisubstituted Hydroxylamines
Abstract
1. Introduction
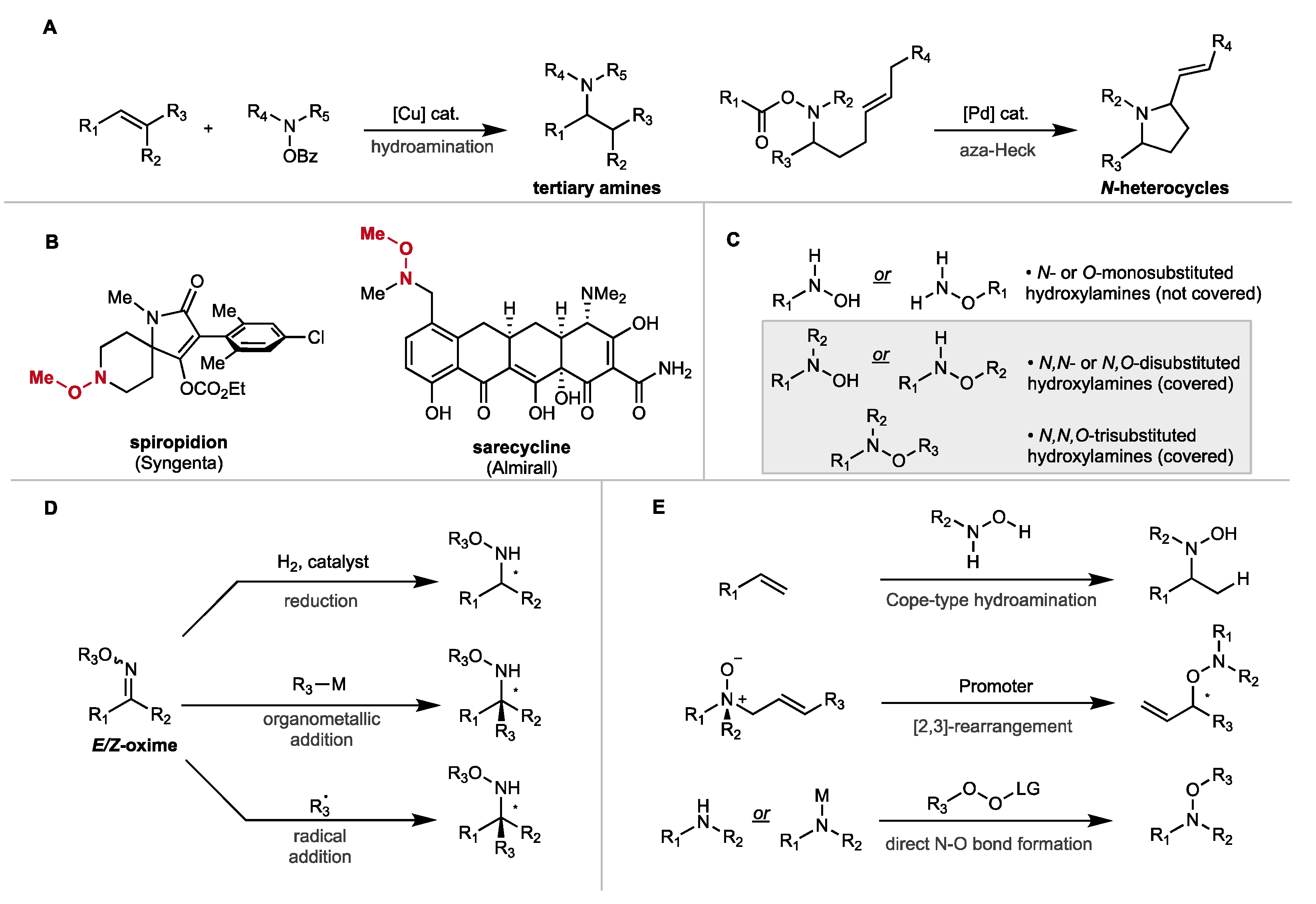
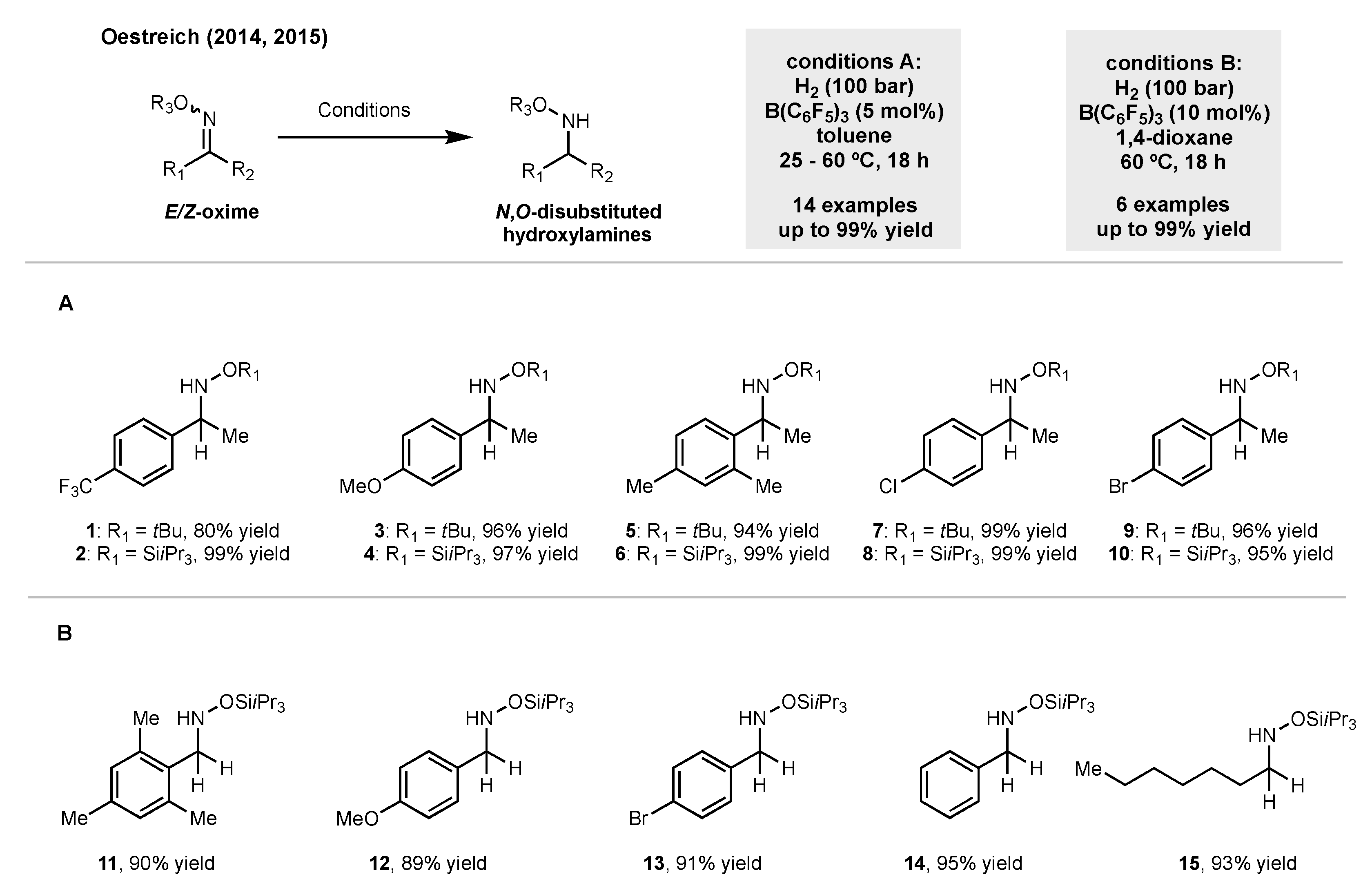
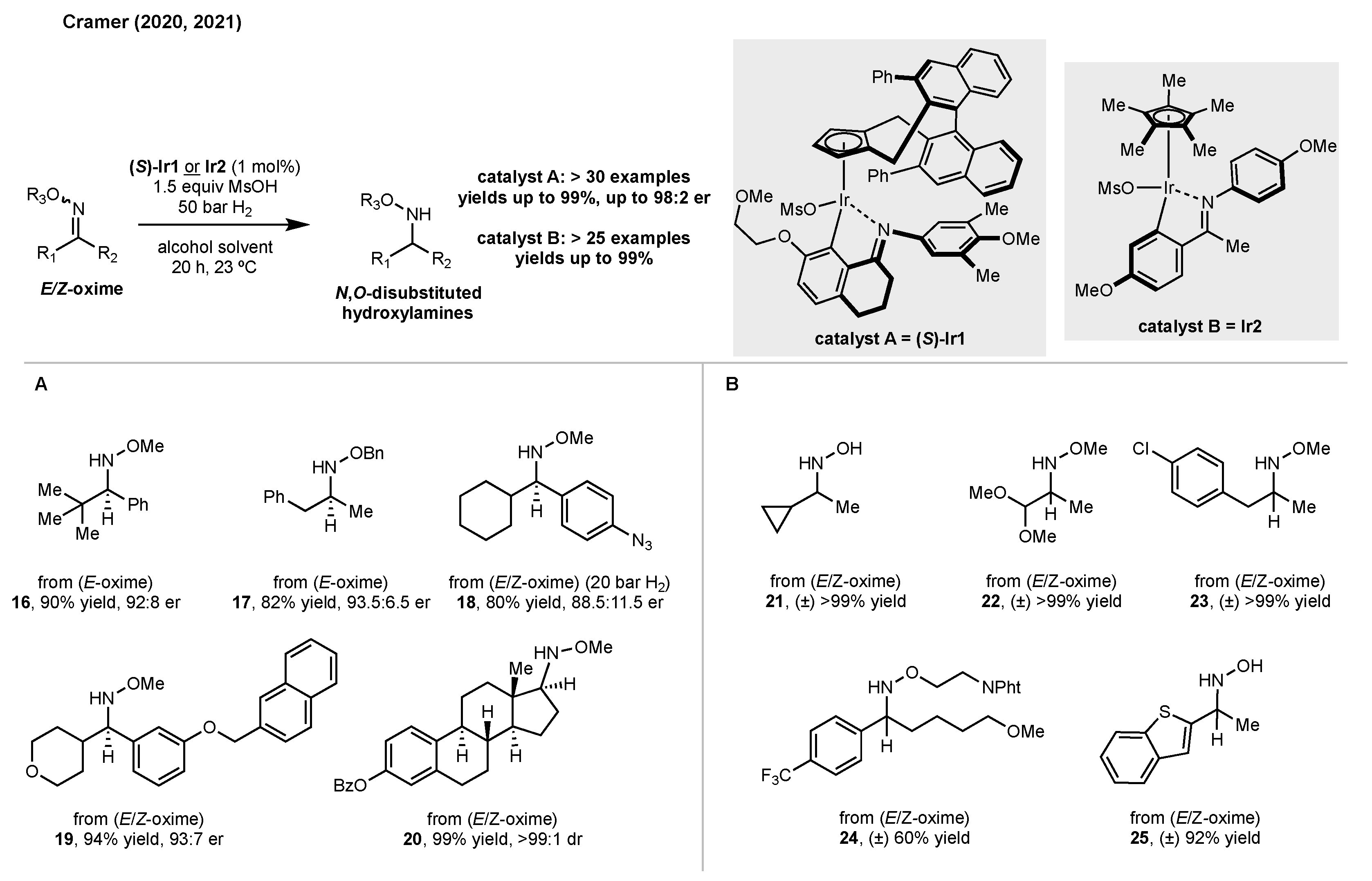
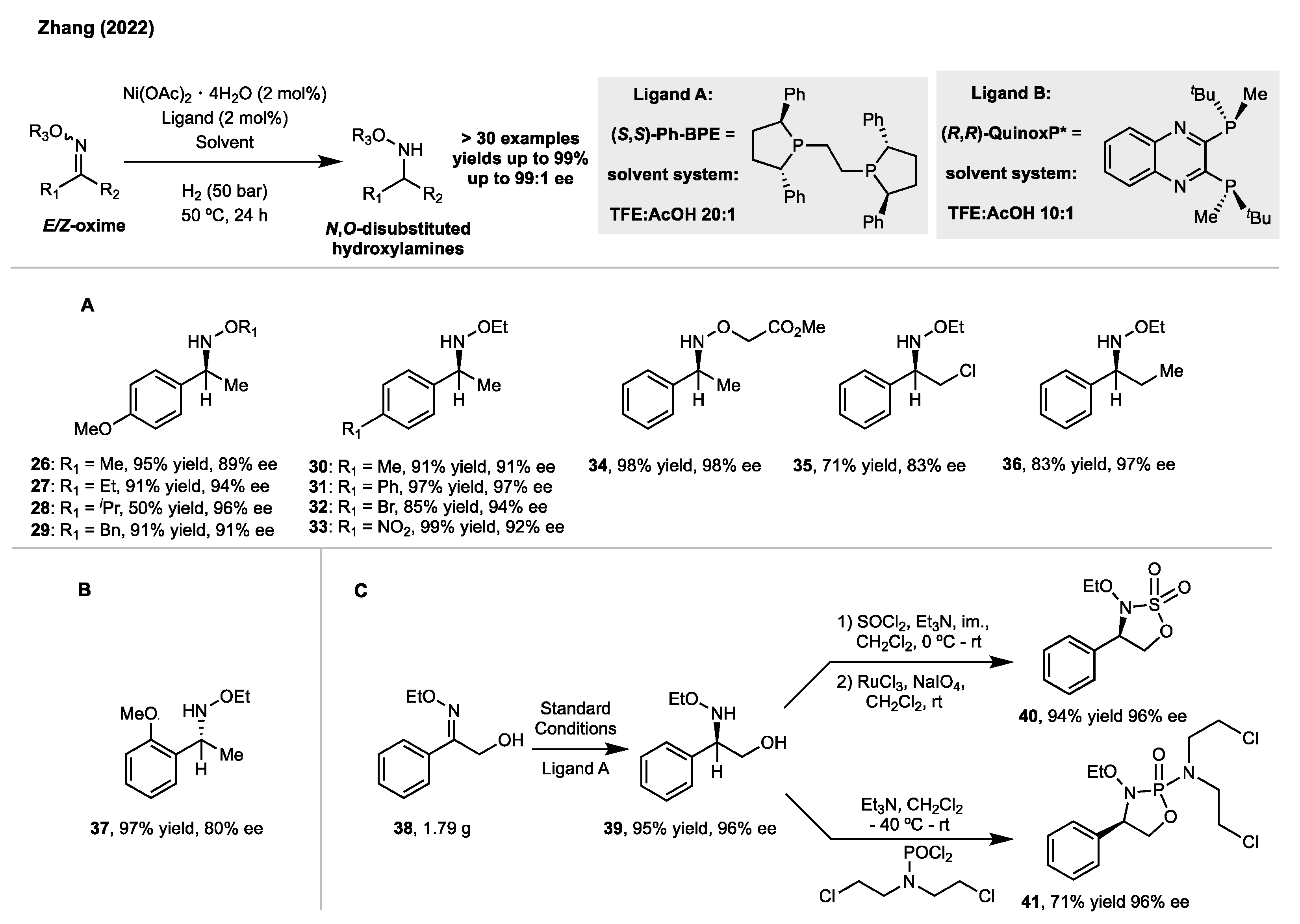
2. Synthesis of Disubstituted Hydroxylamines by Catalytic Reduction of Oxime Ethers
3. Protecting Group-Free Synthesis of Di- and Trisubstituted Hydroxylamines
4. Synthesis of Hydroxylamines by [2,3]-Sigmatropic Rearrangements of N-Oxides and Cope-Type Hydroaminations
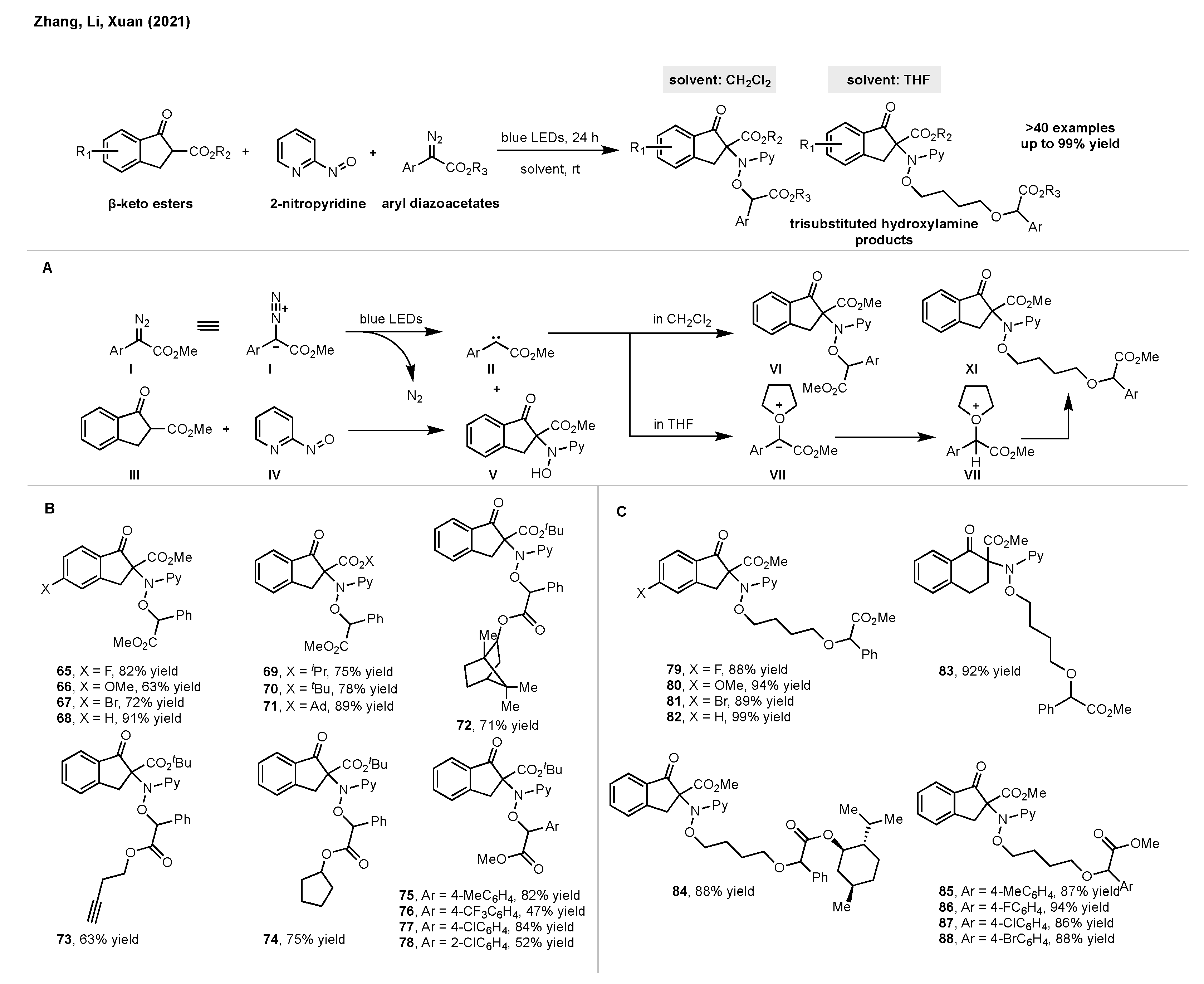
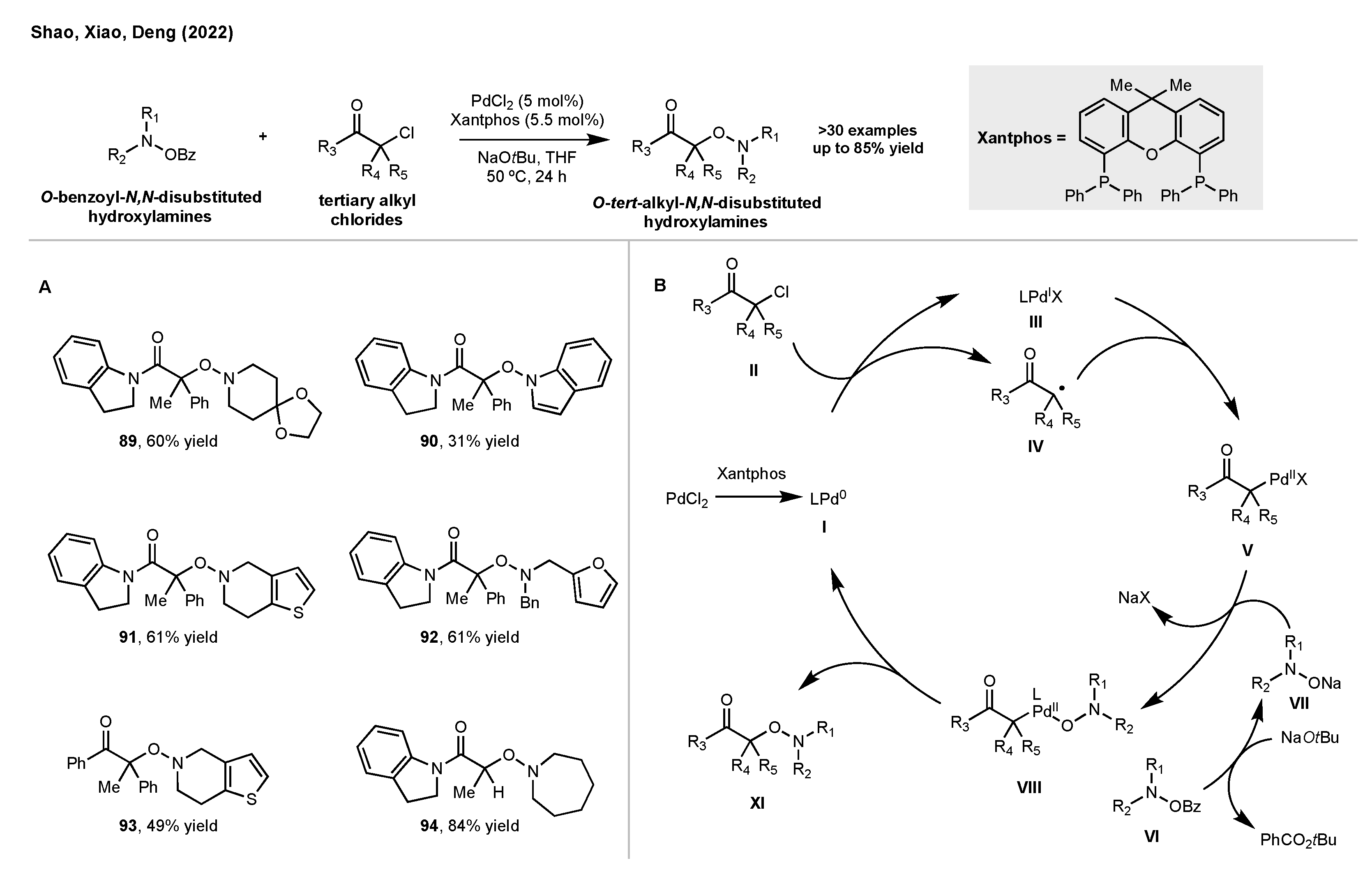
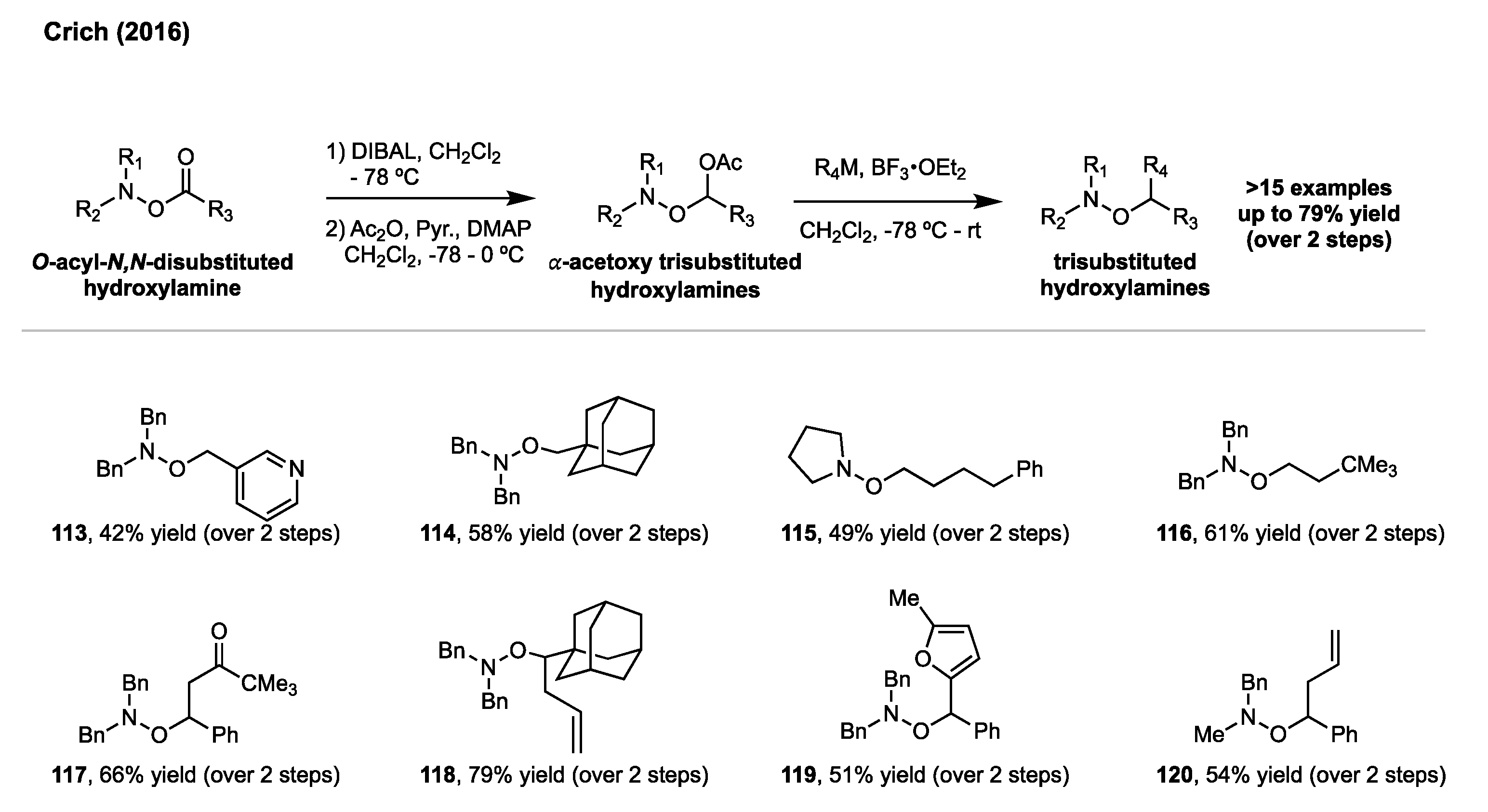
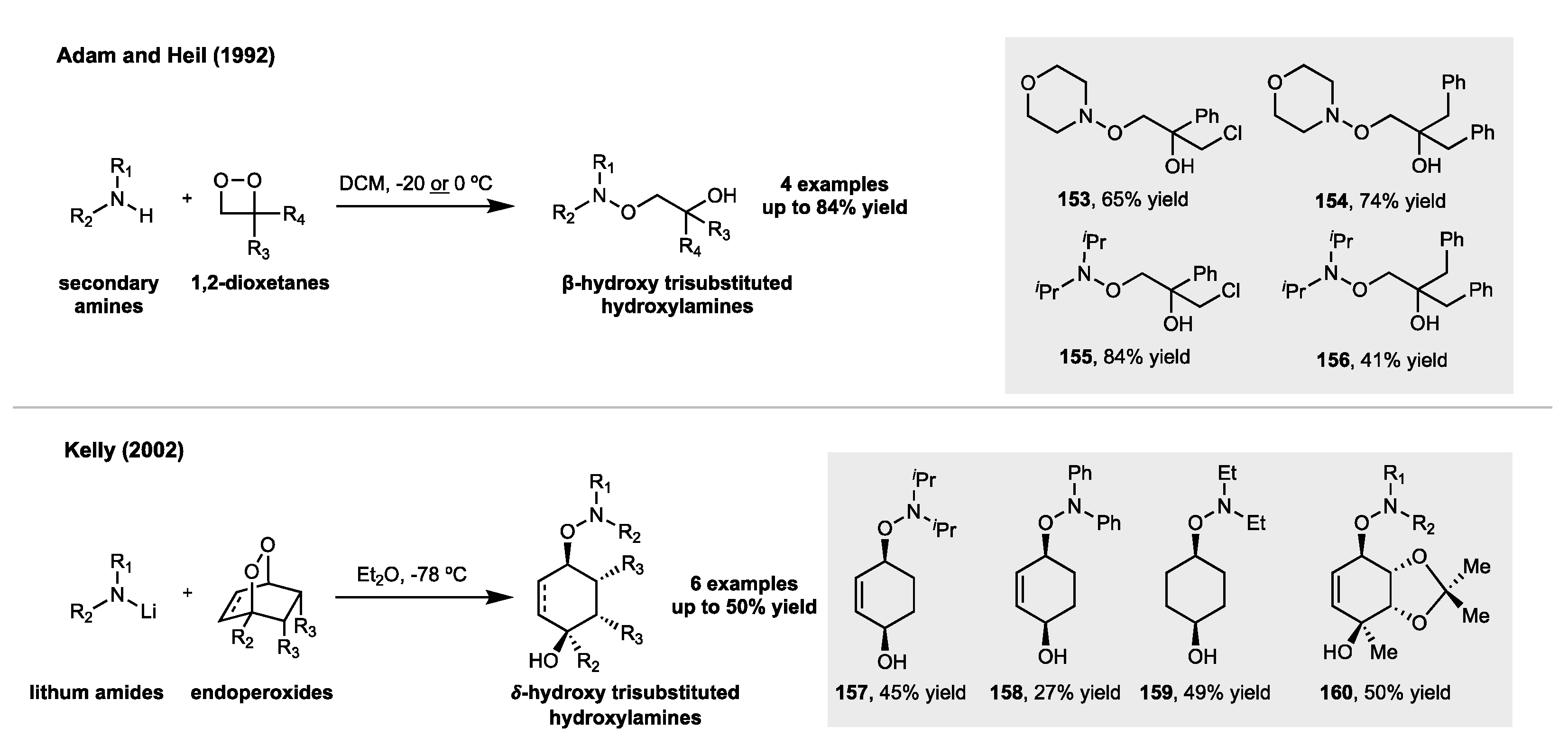
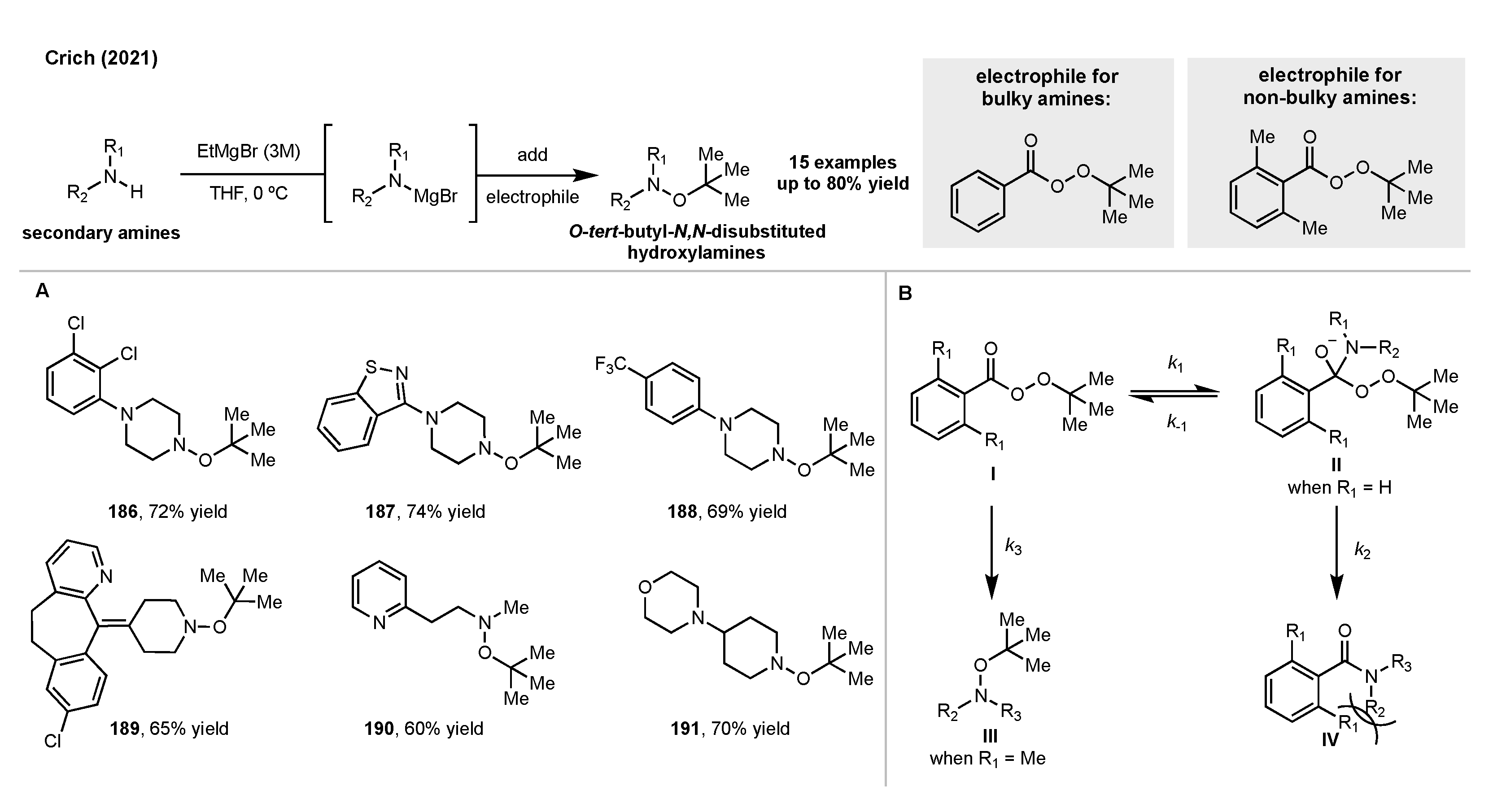
5. Synthesis of Hydroxylamines by Direct N-O Bond Formation
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bander, J.S.; Pirnot, M.T.; Buchwald, S.L. Mechanistic Studies Lead to Dramatically Improved Reaction Conditions for the Cu-Catalyzed Asymmetric Hydroamination of Olefins. J. Am. Chem. Soc. 2015, 137, 14812–14818. [Google Scholar] [CrossRef]
- Yoo, E.J.; Ma, S.; Mei, T.-S.; Chan, K.S.L.; Yu, J.-Q. Pd-Catalyzed Intermolecular C-H Amination with Alkylamines. J. Am. Chem. Soc. 2011, 133, 7652–7655. [Google Scholar] [CrossRef] [PubMed]
- Gasser, V.C.M.; Makai, S.; Morandi, B. The Advent of Electrophilic Hydroxylamine-Derived Reagents for the Direct Preparation of Unprotected Amines. Chem. Commun. 2022, 58, 9991–10003. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, L.G.; Bower, J.F. Electrophilic Aminating Agents in Total Synthesis. Angew. Chem. Int. Ed. 2021, 60, 25640–25666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Niljianskul, N.; Buchwald, S.L. Enantio- and Regioselective CuH-Catalyzed Hydroamination of Alkenes. J. Am. Chem. Soc. 2013, 135, 15746–15749. [Google Scholar] [CrossRef]
- Liu, R.Y.; Buchwald, S.L. CuH-Catalyzed Olefin Functionalization: From Hydroamination to Carbonyl Addition. Acc. Chem. Res. 2020, 53, 1229–1243. [Google Scholar] [CrossRef]
- Race, N.J.; Hazelden, I.R.; Faulkner, A.; Bower, J.F. Recent Developments in the Use of Aza-Heck Cyclizations for the Synthesis of Chiral N-Heterocycles. Chem. Sci. 2017, 8, 5248–5260. [Google Scholar] [CrossRef]
- Hazelden, I.R.; Ma, X.; Langer, T.; Bower, J.F. Diverse N-Heterocyclic Ring Systems via Aza-Heck Cyclizations of N-(Pentafluorobenzoyloxy)sulfonamides. Angew. Chem. Int. Ed. 2016, 55, 11198–11202. [Google Scholar] [CrossRef]
- Davis, C.D.; Schut, H.A.J.; Snyderwine, E.G. Enzymatic Phase II Activation of the N-Hydroxylamines of IQ and MeIQx and PhIP by Various Organs of Monkeys and Rats. Carcinogenesis 1993, 14, 2091–2096. [Google Scholar] [CrossRef]
- Davis, C.D.; Ghoshal, A.; Schut, H.A.J.; Snyderwine, E.G. Metabolic Activation of Heterocyclic Amine Food Mutagens in the Mammary Gland of Lactating Fischer 344 Rats. Cancer Lett. 1994, 84, 67–73. [Google Scholar] [CrossRef]
- Bach, R.D.; Schlegel, H.B. The Bond Dissociation Energy of the N-O Bond. J. Phys. Chem. A 2021, 125, 5014–5021. [Google Scholar] [CrossRef] [PubMed]
- Molli, J.; Kaspárek, F.; Lasovský, J. On the Basicity of Hydroxylamine and Its Derivatives. Chem. Zvesti 1975, 29, 39–43. [Google Scholar]
- Riddell, F.G.; Turner, E.S. The Barrier to Rotation About the N-O Bond. J. Chem. Soc. Perkin Trans. 2 1978, 2, 707–708. [Google Scholar] [CrossRef]
- Raban, M.; Kost, D. Sterolabile Configurational Units and Inversional Stereochemistry in Sulfenamides and Hydroxylamines. Tetrahedron 1984, 40, 3345–3381. [Google Scholar] [CrossRef]
- Dhanju, S.; Blazejewski, B.W.; Crich, D. Synthesis of Trialkylhydroxylamnes by Stepwise Reduction of O-Acyl N,N-Disubstituted Hydroxylamines: Substituent Effects on the Reduction of O-(1-Acyloxyalkyl)hydroxylamines and on the Conformational Dynamics of N-Alkoxypiperidines. J. Org. Chem. 2017, 82, 5345–5353. [Google Scholar] [CrossRef]
- Hill, J.; Crich, D. The N,N,O-Trisubstituted Hydroxylamine Isostere and Its Influence on Lipophilicity and Related Parameters. ACS Med. Chem. Lett. 2022, 13, 799–806. [Google Scholar] [CrossRef]
- Muehlebach, M.; Buchholz, A.; Zambach, W.; Schaetzer, J.; Daniels, M.; Hueter, O.; Kloer, D.P.; Lind, R.; Maienfisch, P.; Pierce, A.; et al. Spiro N-Methoxy Piperidine Ring Containing Aryldiones for the Control of Sucking Insects and Mites: Discovery of Spiropidion. Pest. Manag. Sci. 2020, 76, 3440–3450. [Google Scholar] [CrossRef]
- Batool, Z.; Lomakin, I.B.; Polikanov, Y.S.; Bunik, C.G. Sarecycline Interferes with tRNA Accommodation and Tethers mRNA to the 70S Ribosome. Proc. Natl. Acad. Sci. USA 2020, 117, 20530–20537. [Google Scholar] [CrossRef]
- Datta, D.; Mori, S.; Madaoui, M.; Wassarman, K.; Zlatev, I.; Manoharan, M. Aminooxy Click Chemistry as a Tool for Bis-homo and Bis-hetero Ligand Conjugation to Nucleic Acids. Org. Lett. 2022, 24, 4496–4501. [Google Scholar] [CrossRef]
- Reynisson, J.; Yu, B. Bond Stability of the “Undesirable” Heteroatom-Heteroatom Molecular Moieties for High-Throughput Screening Libraries. Eur. J. Med. Chem. 2011, 46, 5833–5837. [Google Scholar]
- Bruns, R.F.; Watson, I.A. Rules for Identifying Potentially Reactive or Promiscuous Compounds. J. Med. Chem. 2012, 55, 9763–9772. [Google Scholar] [CrossRef] [PubMed]
- Citarella, A.; Moi, D.; Pinzi, L.; Bonanni, D.; Rastelli, G. Hydroxamic Acid Derivatives: From Synthetic Strategies to Medicinal Chemistry Applications. ACS Omega 2021, 6, 21843–21849. [Google Scholar] [CrossRef] [PubMed]
- Rykaczewski, K.A.; Wearing, E.R.; Blackmun, D.E.; Schindler, C.S. Reactivity of Oximes for Diverse Methodologies and Synthetic Applications. Nat. Synth. 2022, 1, 24–36. [Google Scholar] [CrossRef]
- Berthet, M.; Cheviet, T.; Dujardin, G.; Parrot, I.; Martinez, J. Isoxazolidine: A Privileged Scaffold for Organic and Medicinal Chemistry. Chem. Rev. 2016, 116, 15235–15283. [Google Scholar] [CrossRef]
- Mas-Roselló, J.; Cramer, N. Catalytic Reduction of Oximes to Hydroxylamines: Current Methods, Challenges and Opportunities. Chem.-Eur. J. 2022, 28, e202103683. [Google Scholar]
- Borch, R.F.; Bernstein, M.D.; Durst, H.D. Cyanohydridoborate Anion as a Selective Reducing Agent. J. Am. Chem. Soc. 1971, 93, 2897–2904. [Google Scholar] [CrossRef]
- Kawase, M.; Kikugawa, Y. Chemistry of Amine-Boranes. Part 5. Reduction of Oximes, O-Acyloximes, and O-Alkyl-Oximes with Pyridine-Borane in Acid. J. Chem. Soc. Perkin Trans. 1 1979, 643–645. [Google Scholar] [CrossRef]
- Sternbach, D.D.; Jamison, W.C.L. Reduction of O-Acyl Oximes. Tetrahedron Lett. 1981, 22, 3331–3334. [Google Scholar] [CrossRef]
- Ueda, M.; Miyabe, H.; Namba, M.; Nakabayashi, T.; Naito, T. BF3-Promoted Hydrostannation of N-Heteroatom-Substituted Imines for the Reduction of C=N Bond. Tetrahedron Lett. 2002, 43, 4369–4371. [Google Scholar] [CrossRef]
- Vavon, G.; Berton, A.L. The Mechanism of the Catalytic Hydrogenation of Phenol. Bull. Soc. Chim. Fr. 1925, 37, 296–305. [Google Scholar]
- Vavon, G.; Krajcinovic, N. Catalytic Hydrogenation of Oximes and their Transformation into β-Hydroxylamines. Bull. Soc. Chim. Fr. 1928, 43, 231–237. [Google Scholar]
- Mohr, J.; Oestreich, M. B(C6F5)3-Catalyzed Hydrogenation of Oxime Ethers without Cleavage of the N−O Bond. Angew. Chem. Int. Ed. 2014, 53, 13278–13281. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W. Frustrated Lewis Pairs. J. Am. Chem. Soc. 2015, 137, 10018–10032. [Google Scholar] [CrossRef] [PubMed]
- Hounjet, L.J.; Bannwarth, C.; Garon, C.N.; Caputo, C.B.; Grimme, S.; Stephan, D.W. Combination of Ethers and B(C6F5)3 Function as Hydrogenation Catalysts. Angew. Chem. Int. Ed. 2013, 52, 7492–7495. [Google Scholar] [CrossRef]
- Mohr, J.; Porwal, D.; Chatterjee, I.; Oestreich, M. Extending the Scope of the B(C6F5)3-Catalyzed C=N Bond Reduction: Hydrogenation of Oxime Ethers and Hydrazones. Chem. Eur. J. 2015, 21, 17583–17586. [Google Scholar] [CrossRef]
- Mas-Rosello, J.; Smejkal, T.; Cramer, N. Iridium-Catalyzed Acid-Assisted Asymmetric Hydrogenation of Oximes to Hydroxylamines. Science 2020, 368, 1098–1102. [Google Scholar] [CrossRef]
- Mas-Rosello, J.; Cope, C.J.; Tan, E.; Pinson, B.; Robinson, A.; Smejkal, T.; Cramer, N. Iridium-Catalyzed Acid-Assisted Hydrogenation of Oximes to Hydroxylamines. Angew. Chem. Int. Ed. 2021, 60, 15524–15532. [Google Scholar] [CrossRef]
- Li, B.; Chen, J.; Liu, D.; Gridnev, I.D.; Zhang, W. Nickel-Catalysed Asymmetric Hydrogenation of Oximes. Nat. Chem. 2022, 14, 920–927. [Google Scholar] [CrossRef]
- Melman, A. Synthesis of Hydroxylamines. In Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids; Rappoport, Z., Liebman, J.D., Eds.; Wiley: Chichester, UK, 2011; Volume 2, pp. 577–622. [Google Scholar]
- Roberts, J.S. Derivatives of Hydroxylamine. In Comprehensive Organic Chemistry; Barton, D., Ollis, W.D., Sutherland, I.O., Eds.; Pergamon: Oxford, UK, 1979; Volume 2, pp. 185–217. [Google Scholar]
- Roughley, S.D.; Jordan, A.M. The Medicinal Chemist’s Toolbox: An Analysis of Reactions Used in the Pursuit of Drug Candidates. J. Med. Chem. 2011, 54, 3451–3479. [Google Scholar] [CrossRef]
- Giovanni, P.; Eugenio, C.; Giuseppe, M.; Brenner, M.; Lu, R.; Huang, S.; Armstrong, W.P.; Gajanan, J.; Seyedi, F.; Johnston, S. Process for Making Sarecycline. Hydrochloride. Patent WO2019192614A1, 10 October 2019. [Google Scholar]
- Jespersen, T.M.; Bols, M.; Sierks, M.R.; Skrydstrup, T. Synthesis of Isofagomine, A Novel Glysocidase Inhibitor. Tetrahedron 1994, 50, 13449–13460. [Google Scholar] [CrossRef]
- Malik, G.; Guinchard, X.; Crich, D. Asymmetric Synthesis of Polyhydroxylated N-Alkoxypiperidines by Ring-Closing Reductive Amination: Facile Preparation of Isofagomne and Analogues. Org. Lett. 2012, 14, 596–599. [Google Scholar] [CrossRef] [PubMed]
- Zemplén, G.; Pacsu, E. Über die Verseifung Acetylierter Zucker und Verwandter Substanzen. Ber. Dtsch. Chem. Ges. B 1929, 62, 1613–1614. [Google Scholar] [CrossRef]
- Malik, G.; Ferry, A.; Guinchard, X.; Cresteil, T.; Crich, D. N-O Bond as a Glycosidic Bond Surrogate; Synthetic Studies Towards Polyhydroxylated N-Alkoxypiperidines. Chem.-Eur. J. 2013, 19, 2168–2179. [Google Scholar] [CrossRef] [PubMed]
- Ferry, A.; Malik, G.; Guinchard, X.; Vetvicka, V.; Crich, D. Synthesis and Evaluation of Di- and Trimeric Hydroxylamnes-Based β-(1→3)-Glucan Mimetics. J. Am. Chem. Soc. 2014, 136, 14852–14857. [Google Scholar] [CrossRef]
- Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Mechanistic Studies of a Reaction Promoted by the [YLi3{tris(bisnaphthoxide}] Complex: Are Three 1,1′-Bi-2-naphthol Units in a Rare-Earth-Alkali-Metal Heterobimetallic Complex Necessary? Angew. Chem. Int. Ed. 2004, 43, 4493–4497. [Google Scholar] [CrossRef]
- Yamagiwa, N.; Matsunaga, S.; Shibasaki, M. Heterobimetallic Catalysis in Asymmetric 1,4-Addition of O-Alkylhydroxylamine to Enones. J. Am. Chem. Soc. 2003, 125, 16178–16179. [Google Scholar] [CrossRef]
- Yamagiwa, N.; Qin, H.; Matsunaga, S.; Shibasaki, M. Lewis Acid-Lewis Acid Heterobimetallic Cooperative Catalysis: Mechanistic Studies and Application in Enantioselective Aza-Michael Reaction. J. Am. Chem. Soc. 2005, 127, 13419–13427. [Google Scholar] [CrossRef]
- Shibasaki, M.; Kanai, M.; Matsunaga, S.; Kumagai, N. Recent Progress in Asymmetric Bifunctional Catalysis Using Multimetallic Systems. Acc. Chem. Res. 2009, 42, 1117–1127. [Google Scholar] [CrossRef]
- Major, R.T.; Dürsch, F. 1-Alkoxy-4-phenyl-4-propionoxypiperidine and Their 3-Methyl Homolgs as New Analgesics. J. Org. Chem. 1961, 26, 1867–1874. [Google Scholar] [CrossRef]
- Shibata, K.; Takao, K.-I.; Ogura, A. Diaryliodonium Salt-Based Synthesis of N-Alkoxyindolines and Further Insights into the Ishikawa Indole Synthesis. J. Org. Chem. 2021, 86, 10067–10087. [Google Scholar] [CrossRef]
- Albini, A. Synthetic Utility of Amine N-Oxides. Synthesis 1993, 3, 263–277. [Google Scholar] [CrossRef]
- Cai, B.-G.; Li, Q.; Zhang, Q.; Li, L.; Xuan, J. Synthesis of Trisubstituted Hydroxylamines by Visible Light-Promoted Multicomponent Reaction. Org. Chem. Front. 2021, 8, 5982–5987. [Google Scholar] [CrossRef]
- Yang, Z.; Stivanin, M.L.; Jurberg, I.D.; Koenigs, R.M. Visible Light-Promoted Reactions with Diazo Compounds: A Mild and Practical Strategy Towards Free Carbene Intermediates. Chem. Soc. Rev. 2020, 49, 6833–6847. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pei, C.; Koenigs, R.M. Photochemical Fluoro-Amino Etherification Reactions of Aryldiazoacetates with NFSI Under Stoichiometric Conditions. Chem. Commun. 2020, 56, 599–602. [Google Scholar] [CrossRef]
- Chen, J.; Xu, Y.; Shao, W.; Ji, J.; Wang, B.; Yang, M.; Mao, G.; Xiao, F.; Deng, G.-J. Pd-Catalyzed C-O Bond Formation Enabling the Synthesis of Congested N,N,O-Trisubstituted Hydroxylamines. Org. Lett. 2022, 24, 8271–8276. [Google Scholar] [CrossRef]
- Kainz, Q.M.; Matier, C.D.; Bartoszewicz, A.; Zultanski, S.L.; Peters, J.C.; Fu, G.C. Asymmetric Copper-Catalyzed C-N Cross-Couplings Induced by Visible Light. Science 2016, 351, 681–684. [Google Scholar] [CrossRef]
- Peacock, D.M.; Roos, C.B.; Hartwig, J.F. Palladium-Catalyzed Cross Coupling of Secondary and Tertiary Alkyl Bromides with a Nitrogen Nucleophile. ACS Cent. Sci. 2016, 2, 647–652. [Google Scholar] [CrossRef]
- Yang, S.-Q.; Han, A.-J.; Liu, Y.; Tang, X.-Y.; Lin, G.-Q.; He, Z.-T. Catalytic Asymmetric Hydroalkoxylation and Formal Hydration and Hydroaminoxylation of Conjugated Dienes. J. Am. Chem. Soc. 2023, 145, 3915–3925. [Google Scholar] [CrossRef]
- Shirokane, K.; Kurosaki, Y.; Sato, T.; Chida, N. A Direct Entry to Substituted N-Methyoxyamines from N-Methoxyamides via N-Oxyiminium Ions. Angew. Chem. Int. Ed. 2010, 49, 6369–6372. [Google Scholar] [CrossRef]
- Dhanju, S.; Crich, D. Synthesis of N,N,O-Trisubstituted Hydroxylamines by Stepwise Reduction and Substitution of O-Acyl N,N-Disubstituted Hydroxylamines. Org. Lett. 2016, 18, 1820–1823. [Google Scholar] [CrossRef]
- Kopecky, D.J.; Rychnovsky, S.D. Improved Procedure for the Reductive Acetylation of Acylic Esters and a New Synthesis of Ethers. J. Org. Chem. 2000, 65, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Meisenheimer, J. Über eine eigenartige Umlagerung des Methyl-allyl-anilin-N-oxyds. Ber. Dtsch. Chem. Ges. 1919, 52, 1667–1677. [Google Scholar] [CrossRef]
- Buston, J.E.H.; Coldham, I.; Mulholland, K.R. Studies into the Asymmetric Meisenheimer Rearrangeent. Tetrahedron Asymmetry 1998, 9, 1995–2009. [Google Scholar] [CrossRef]
- Bao, H.; Qi, X.; Tambar, U.K. Catalytic Enantioselective [2,3]-Rearrangements of Amine N-Oxides. J. Am. Chem. Soc. 2011, 133, 1206–1208. [Google Scholar] [CrossRef] [PubMed]
- Watson, M.P.; Overman, L.E.; Bergman, R.G. Kinetic and Computational Analysis of the Palladium(II)-Catalyzed Asymmetric Allylic Trichloroacetimidate Rearrangement: Development of a Model for Enantioselectivity. J. Am. Chem. Soc. 2007, 129, 5031–5044. [Google Scholar] [CrossRef]
- Grob, C.A.; Schiess, P.W. Heterolytic Fragmentation. A Class of Organic Reactions. Angew. Chem. Int. Ed. 1967, 6, 1–15. [Google Scholar] [CrossRef]
- Yu, X.; Wannenmacher, N.; Peters, R. Stereospecific Asymmetric Synthesis of Tertiary Allyl Alcohol Derivatives by Catalytic [2,3]-Meisenheimer Rearrangements. Angew. Chem. Int. Ed. 2020, 59, 10944–10948. [Google Scholar] [CrossRef]
- Cope, A.C.; Foster, T.T.; Towle, P.H. Thermal Decomposition of Amine Oxides to Olefins and Dialkylhydroxylamines. J. Am. Chem. Soc. 1949, 71, 3929–3934. [Google Scholar] [CrossRef]
- Cooper, N.J.; Knight, D.W. The Reverse Cope Cyclisation: A Classical Reaction Goes Backwards. Tetrahedron 2004, 60, 243–269. [Google Scholar] [CrossRef]
- Beauchemin, A.M. Recent Developments in Cope-Type Hydroamination Reactions of Hydroxylamine and Hydrazine Derivatives. Org. Biomol. Chem. 2013, 11, 7039–7050. [Google Scholar] [CrossRef]
- Bourgeois, J.; Dion, I.; Cebrowski, P.H.; Loiseau, F.; Bedard, A.-C.; Beauchemin, A.M. The Tandem Cope-Type Hydroxamination/[2,3]-Rearrangements Sequence: A Strategy to Favour the Formation of Intermolecular Hydroamination Products and Enable Difficult Cyclizations. J. Am. Chem. Soc. 2009, 131, 874–875. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, M.J.; Schipper, D.J.; Ng, P.J.; Moran, J.; Beauchemin, A.M. A Catalytic Tethering Strategy: Simple Aldehydes Catalyze Intermolecular Alkene Hydroaminations. J. Am. Chem. Soc. 2011, 133, 20100–20103. [Google Scholar] [CrossRef] [PubMed]
- Guimond, N.; MacDonald, M.J.; Lemieux, V.; Beauchemin, A.M. Catalysis Through Temporary Intramolecularity: Mechanistic Investigations on Aldehyde-Catalyzed Cope-Type Hydroamination Lead to the Discovery of a More Efficient Tetherng Catalyst. J. Am. Chem. Soc. 2012, 134, 16571–16577. [Google Scholar] [CrossRef] [PubMed]
- MadDonald, M.J.; Hesp, C.R.; Schipper, D.J.; Pesant, M.; Beauchemin, A.M. Highly Enantioselective Intermolecular Hydroamination of Allylic Amines with Chiral Aldehydes as Tetherng Catalysts. Chem. Eur. J. 2013, 19, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Bilodeau, E.; Lemieux, V.; Beauchemin, A.M. Hydrogen Bonding-Directed Intermolecular Cope-Type Hydroamination of Alkenes. Org. Lett. 2012, 14, 5082–5085. [Google Scholar] [CrossRef]
- Moran, J.; Gorelsky, S.I.; Dimitrijevic, E.; Lebrun, M.-E.; Bedard, A.-C.; Seguin, C.; Beauchemin, A.M. Intermolecular Cope-Type Hydroamination of Alkenes and Alkynes Using Hydroxylamines. J. Am. Chem. Soc. 2008, 130, 17893–17906. [Google Scholar] [CrossRef]
- Beauchemin, A.M.; Moran, J.; Lebrun, M.-E.; Seguin, C.; Dimitrijevic, E.; Zhang, L.; Gorelsky, S.I. Intermolecular Cope-Type Hydroaminations of Alkenes and Alkynes. Angew. Chem. Int. Ed. 2008, 47, 1410–1413. [Google Scholar] [CrossRef]
- Zefirov, N.S.; Makhon’kov, D.I. X-Philic Reactions. Chem. Rev. 1982, 82, 615–624. [Google Scholar] [CrossRef]
- Waldman, A.J.; Ng, T.L.; Wang, P.; Balskus, E.P. Heteroatom-Heteroatom Bond Formation in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5784–5863. [Google Scholar] [CrossRef]
- Adam, W.; Heil, M. Reaction of 1,2-Dioxetanes with Heteroatom Nucleophiles: Adduct Formation by Nucleophilic Attack at the Peroxide Bond. J. Am. Chem. Soc. 1992, 114, 5591–5598. [Google Scholar] [CrossRef]
- Kelly, D.R.; Bansal, H.; Morgan, J.J.G. The Mechanism of the Tertiary Amine Catalysed Isomerization of Endoperoxides to Hydroxyketones: Synthesis and Chemistry of the Intermediate Postulated in the Peroxide Attack Mechanism. Tetrahedron Lett. 2002, 43, 9331–9333. [Google Scholar] [CrossRef]
- Biloski, A.J.; Ganem, B. Improved Oxidation of Amines with Dibenzoyl Peroxide. Synthesis 1983, 7, 537–538. [Google Scholar] [CrossRef]
- Banerjee, A.; Yamamoto, H. Direct N-O Bond Formation via Oxidation of Amines with Benzoyl Peroxide. Chem. Sci. 2019, 10, 2124–2129. [Google Scholar] [CrossRef]
- Hill, J.; Hettikankanamalage, A.A.; Crich, D. Diversity-Oriented Synthesis of N,N,O-Trisubstituted Hydroxylamines from Alcohols and Amines by N-O Bond Formation. J. Am. Chem. Soc. 2020, 142, 14820–14825. [Google Scholar] [CrossRef]
- Kayasa, S.; Meier, R.N.; Pardini, R.A.; Truttmann, K.; Kuwata, K.T.; Dussault, P.H. Synthesis of Ethers via Reaction of Carbanions and Monoperoxyacetals. J. Org. Chem. 2015, 80, 12100–12114. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.M.; Lees, N.R.; Herzon, S.B. Programmable Synthesis of 2-Deoxyglycosides. J. Am. Chem. Soc. 2019, 141, 8098–8103. [Google Scholar] [CrossRef] [PubMed]
- Hoang, K.M.; Lees, N.R.; Herzon, S.B. General Method for the Synthesis of α- or β-Deoxyaminoglycosides Bearing Basic Nitrogen. J. Am. Chem. Soc. 2021, 143, 2777–2783. [Google Scholar] [CrossRef]
- Upadhyaya, K.; Crich, D. Synthesis of 10-Aza-9-oxakalkitoxin by N-O Bond Formation. Org. Lett. 2022, 24, 1833–1836. [Google Scholar] [CrossRef]
- Dhanju, S.; Upadhyaya, K.; Rice, C.A.; Pegan, S.D.; Media, J.; Valeriote, F.A.; Crich, D. Synthesis, Cytotoxicity, and Genotoxicity of 10-Aza-9-oxakalkitoxin, an N,N,O-Trisubstituted Hydroxylamine Analog, or Hydroxalog, of a Marina Natural Product. J. Am. Chem. Soc. 2020, 142, 9147–9151. [Google Scholar] [CrossRef]
- Hill, J.; Crich, D. Synthesis of O-tert-Butyl-N,N-disubstituted Hydroxylamines by N-O Bond Formation. Org. Lett. 2021, 23, 6396–6400. [Google Scholar] [CrossRef]
- Locklear, M.; Dussault, P.H. The Chemistry of Peresters. Eur. J. Org. Chem. 2020, 2020, 4814–4840. [Google Scholar] [CrossRef]
- Fuson, R.C.; Fisher, H.C.; Ullyot, G.E.; Fugate, W.O. Reactions of Bromomagnesium Enolates of Mesityl Ketones. I. J. Org. Chem. 1939, 4, 111–118. [Google Scholar] [CrossRef]
- Kohler, E.P.; Baltzly, R. Steric Hindrance in Certain Mesitylenic Ketones. J. Am. Chem. Soc. 1932, 54, 4015–4026. [Google Scholar] [CrossRef]
- Nishioka, T.; Fujita, T.; Kitamura, K.; Nakajima, M. The Ortho Effect in Hydrolysis of Phenyl Esters. J. Org. Chem. 1975, 40, 2520–2525. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hill, J.; Beckler, T.D.; Crich, D. Recent Advances in the Synthesis of Di- and Trisubstituted Hydroxylamines. Molecules 2023, 28, 2816. https://doi.org/10.3390/molecules28062816
Hill J, Beckler TD, Crich D. Recent Advances in the Synthesis of Di- and Trisubstituted Hydroxylamines. Molecules. 2023; 28(6):2816. https://doi.org/10.3390/molecules28062816
Chicago/Turabian StyleHill, Jarvis, Thomas D. Beckler, and David Crich. 2023. "Recent Advances in the Synthesis of Di- and Trisubstituted Hydroxylamines" Molecules 28, no. 6: 2816. https://doi.org/10.3390/molecules28062816
APA StyleHill, J., Beckler, T. D., & Crich, D. (2023). Recent Advances in the Synthesis of Di- and Trisubstituted Hydroxylamines. Molecules, 28(6), 2816. https://doi.org/10.3390/molecules28062816




