Bioactive C21 Steroidal Glycosides from Euphorbia kansui Promoted HepG2 Cell Apoptosis via the Degradation of ATP1A1 and Inhibited Macrophage Polarization under Co-Cultivation
Abstract
1. Introduction
2. Results
2.1. Bioactivity of C21 Steroidal Glycosides from E.kansui
2.2. Formatting of Mathematical Components
2.2.1. Inhibition of the Proliferation and Migration of HepG2 Cells
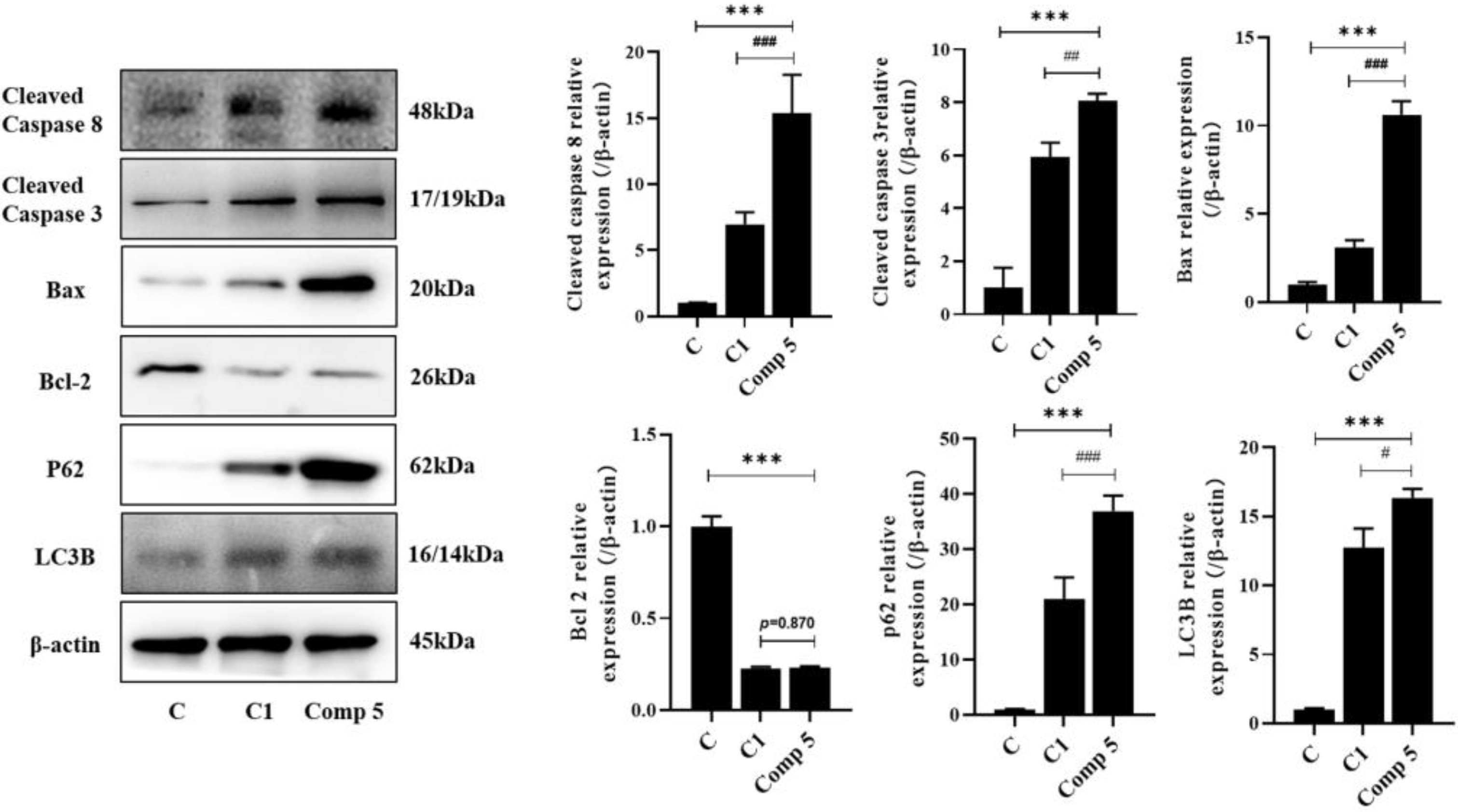
2.2.2. Promotion of HepG2 Cell Apoptosis
2.3. Against HepG2 Cells the Inhibition of the ATP1A1-Related ERK and AKT Signaling Pathways
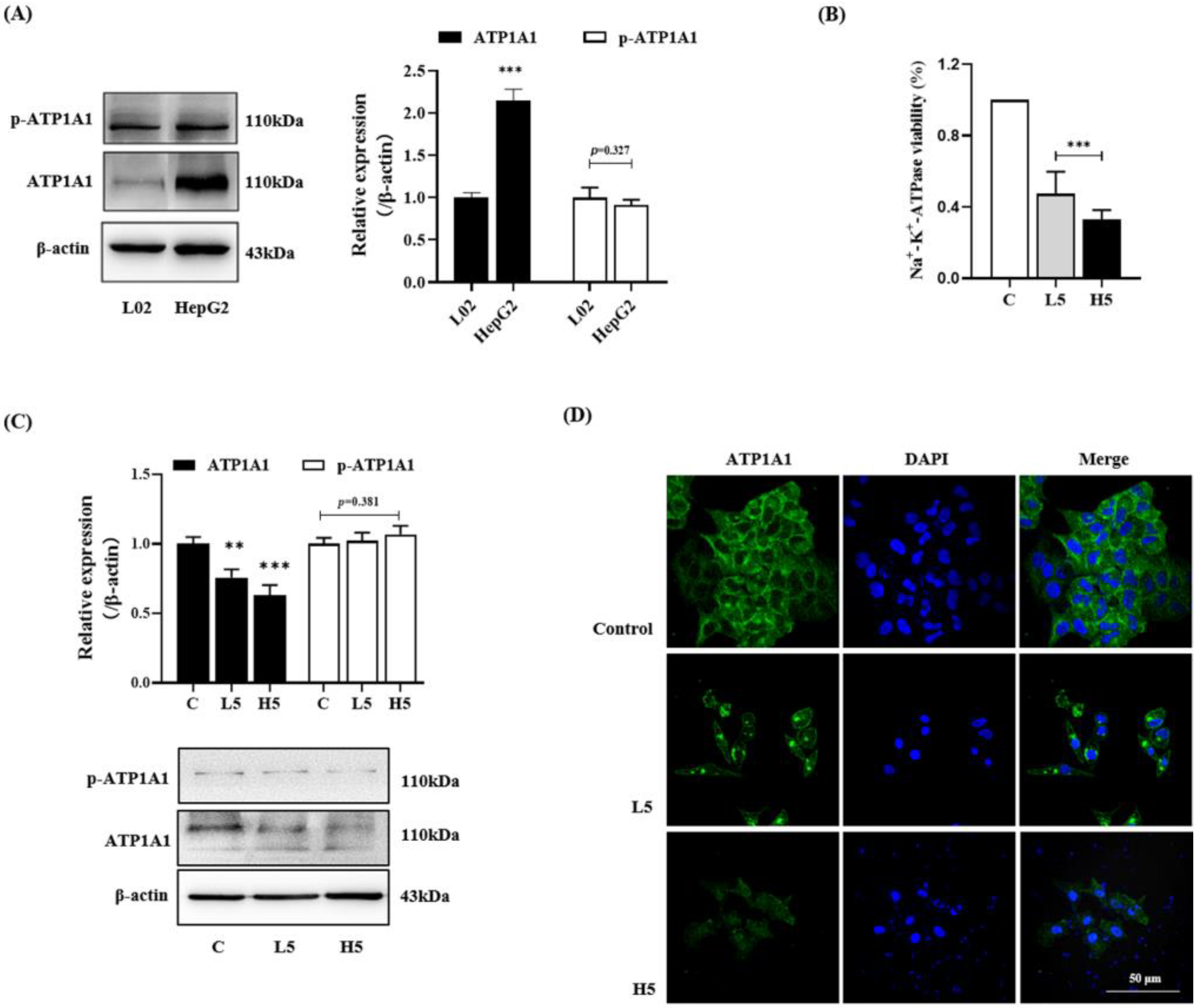
2.3.1. Decreasing ATP1A1 Expression and Inhibiting NAK Activity
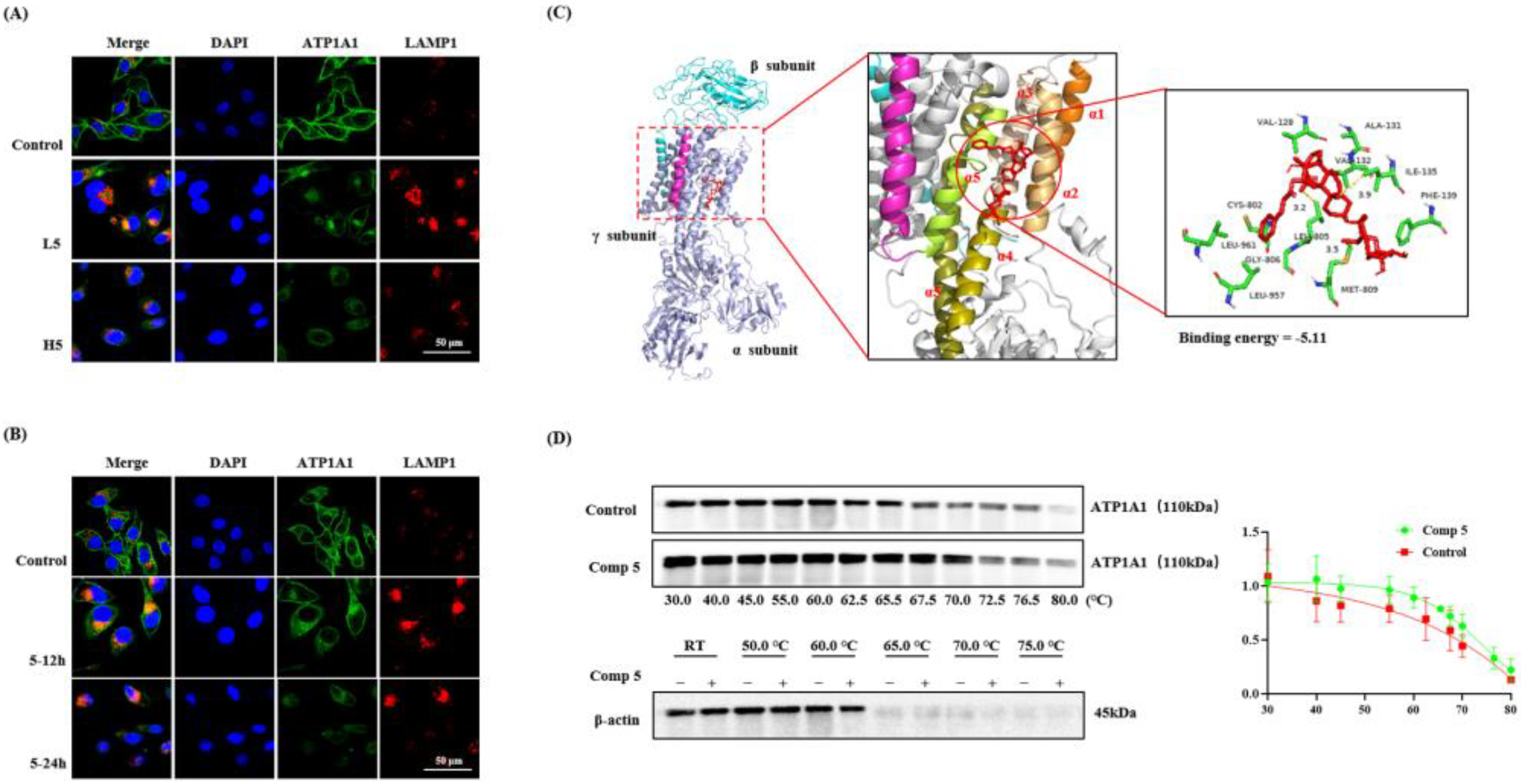
2.3.2. Promotion of the Endocytosis and Degradation of ATP1A1 Protein
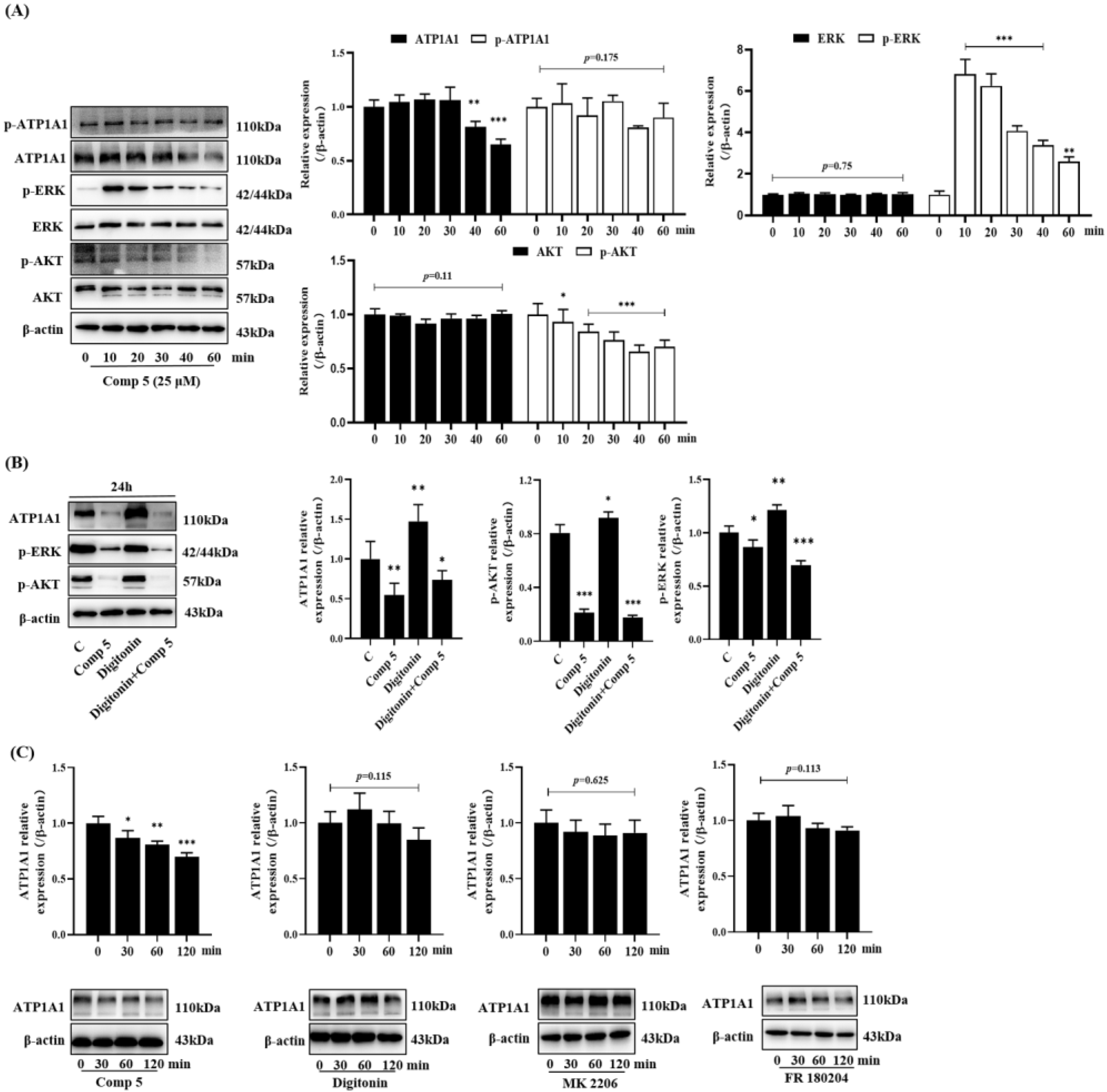
2.3.3. Inhibition of ATP1A1-Related AKT and ERK Signaling Pathways
2.4. Further Promotion of the Apoptosis of HepG2 Cells by Regulating Macrophage Function
2.4.1. Promotion of HepG2 Cell Apoptosis
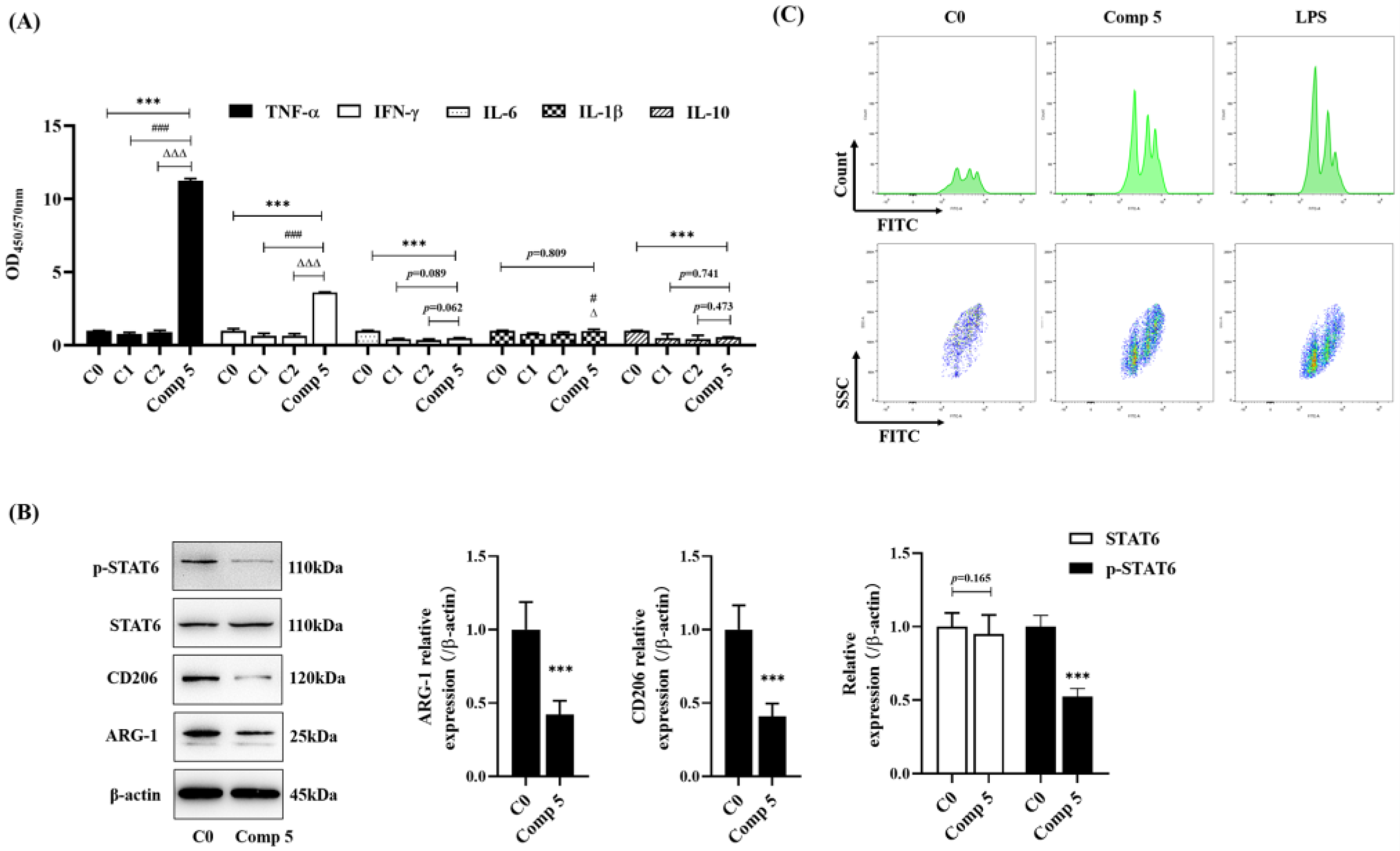
2.4.2. Regulated Macrophage Function in Cocultivation
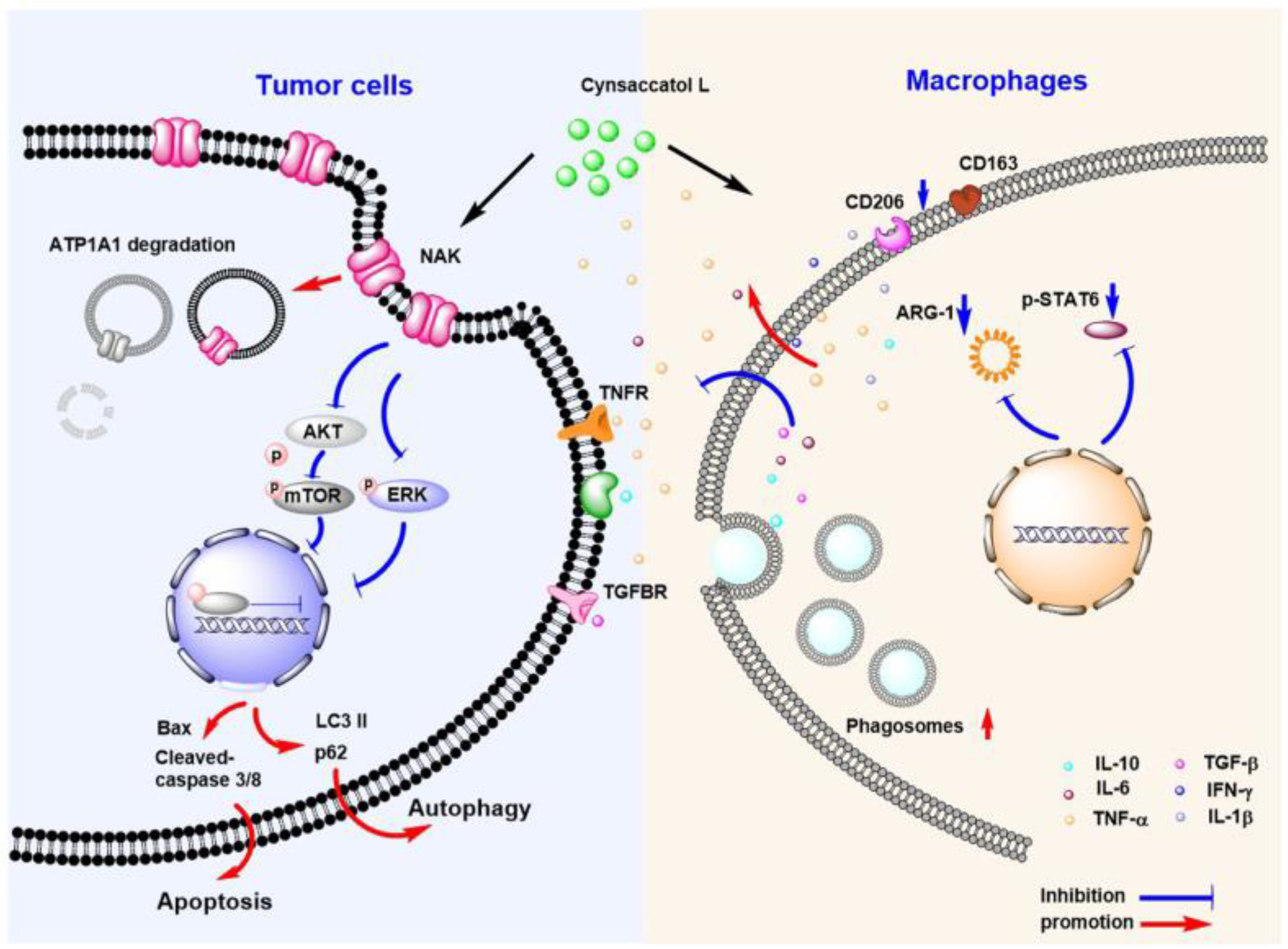
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Extraction and Isolation
4.3. Reagents
4.4. Cell Culture
4.5. MTT Assay
4.6. Cell Migration and Colony Formation Assay
4.7. Protein Kinase Inhibitor
4.8. Fluorescence Staining
4.9. Na+-K+-ATPase Analysis
4.10. Western Blot Analysis
4.11. Flow Cytometry
4.12. Measurement of Nitric Oxide and Cytokines
4.13. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, Q.; Li, Z.L.; Zhang, Y.; Wang, K.; Zhang, M.; Chen, P.D.; Yao, W.F.; Tang, Y.P.; Wu, J.H.; Zhang, L. Effect of the vinegar-process on chemical compositions and biological activities of Euphorbia kansui: A review. J. Ethnopharmacol. 2020, 252, 112557. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.D. Sensitivity of the (Na+ + K+)-ATPase to state-dependent inhibitors. Biochim. Biophys. Acta—Biomembr. 1980, 598, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Shaker, S.; Sang, J.; Yan, X.L.; Fan, R.Z.; Tang, G.H.; Xu, Y.K.; Yin, S. Diterpenoids from Euphorbia royleana reverse P-glycoprotein-mediated multidrug resistance in cancer cells. Phytochemistry 2020, 176, 112395. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Kai, J.; Tang, Y.; Zhang, L.; Su, S.; Duan, J.A. The Chemical and Biological Properties of Euphorbia kansui. Am. J. Chin. Med. 2016, 44, 253–273. [Google Scholar] [CrossRef]
- Zhao, C.; Jia, Z.; Li, E.; Zhao, X.; Han, T.; Tian, J.; Li, F.; Zou, D.; Lin, R. Hepatotoxicity evaluation of Euphorbia kansui on zebrafish larvae in vivo. Phytomedicine 2019, 62, 152959. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Li, B. Neuroprotection of two C21 steroidal glycosides from Cynanchum auriculatum against H2O2-induced damage on PC12 cells. Nat. Prod. Res. 2021, 35, 1752–1755. [Google Scholar] [CrossRef]
- Si, Y.; Sha, X.-S.; Shi, L.-L.; Wei, H.-Y.; Jin, Y.-X.; Ma, G.-X.; Zhang, J. Review on Pregnane Glycosides and Their Biological Activities. Phytochem. Lett. 2022, 47, 1–17. [Google Scholar] [CrossRef]
- Li, K.; Hao, K.; Zhang, Y.; Xu, A.; Wang, Q.; Du, Y.; Wu, L.; Chen, B.; Zhang, W.; Wang, Z. C21 Fraction Refined from Marsdenia tenacissima-Induced Apoptosis is Enhanced by Suppression of Autophagy in Human Gastric Cell Lines. ACS Omega 2020, 5, 25156–25163. [Google Scholar] [CrossRef]
- Hilgemann, D.W. Regulation of ion transport from within ion transit pathways. J. Gen. Physiol. 2020, 152, e201912455. [Google Scholar] [CrossRef]
- Lingrel, J.B.; Williams, M.T.; Vorhees, C.V.; Moseley, A.E. Na,K-ATPase and the role of alpha isoforms in behavior. J. Bioenerg. Biomembr. 2007, 39, 385–389. [Google Scholar] [CrossRef]
- Song, Y.; Lee, S.Y.; Kim, S.; Choi, I.; Kim, S.H.; Shum, D.; Heo, J.; Kim, A.R.; Kim, K.M.; Seo, H.R. Inhibitors of Na(+)/K(+) ATPase exhibit antitumor effects on multicellular tumor spheroids of hepatocellular carcinoma. Sci. Rep. 2020, 10, 5318. [Google Scholar] [CrossRef]
- Lu, S.; Cai, S.; Peng, X.; Cheng, R.; Zhang, Y. Integrative Transcriptomic, Proteomic and Functional Analysis Reveals ATP1B3 as a Diagnostic and Potential Therapeutic Target in Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 636614. [Google Scholar] [CrossRef]
- Deng, K.; Shen, J.; Wang, W.; Li, M.; Li, H.; Chen, C.; Zhao, H.; Zhang, M.; Xue, T.; Liu, Q.; et al. Sodium chloride (NaCl) potentiates digoxin-induced anti-tumor activity in small cell lung cancer. Cancer Biol. Ther. 2019, 20, 52–64. [Google Scholar] [CrossRef]
- Azalim, P.; do Monte, F.M.; Rendeiro, M.M.; Liu, X.; O’Doherty, G.A.; Fontes, C.F.; Leitao, S.G.; Quintas, L.E.M.; Noel, F. Conformational states of the pig kidney Na(+)/K(+)-ATPase differently affect bufadienolides and cardenolides: A directed structure-activity and structure-kinetics study. Biochem. Pharmacol. 2020, 171, 113679. [Google Scholar] [CrossRef]
- Peng, Y.R.; Li, Y.B.; Liu, X.D.; Zhang, J.F.; Duan, J.A. Antitumor activity of C-21 steroidal glycosides from Cynanchum auriculatum Royle ex Wight. Phytomedicine 2008, 15, 1016–1020. [Google Scholar] [CrossRef]
- Qian, X.; Li, B.; Li, P.; Wang, D.; Dai, W.; Zhang, M. C(21) steroidal glycosides from Cynanchum auriculatum and their neuroprotective effects against H(2)O(2)-induced damage in PC12 cells. Phytochemistry 2017, 140, 1–15. [Google Scholar] [CrossRef]
- Li, J.C.; Li, S.Y.; Tang, J.X.; Liu, D.; Feng, X.Y.; Rao, K.R.; Zhao, X.D.; Li, H.M.; Li, R.T. Triterpenoids, steroids and other constituents from Euphorbia kansui and their anti-inflammatory and anti-tumor properties. Phytochemistry 2022, 204, 113449. [Google Scholar] [CrossRef]
- Wu, K.; Lin, K.; Li, X.; Yuan, X.; Xu, P.; Ni, P.; Xu, D. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 2020, 11, 1731. [Google Scholar] [CrossRef]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Das, S.; Shukla, N.; Singh, S.S.; Kushwaha, S.; Shrivastava, R. Mechanism of interaction between autophagy and apoptosis in cancer. Apoptosis 2021, 26, 512–533. [Google Scholar] [CrossRef]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Overvatn, A.; Bjorkoy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, H.; Chen, Z.; Zhuang, L.; Xu, L.; Ning, Z.; Zhu, Z.; Wang, P.; Meng, Z. Carbonic anhydrase 2 inhibits epithelial-mesenchymal transition and metastasis in hepatocellular carcinoma. Carcinogenesis 2018, 39, 562–570. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.; Xu, L.; Wang, P.; Jiang, Y.; Yong, P.; Zhang, C.; Zhang, H.; Meng, Z.; Yang, P. Na+/K+-ATPase alpha1 subunit, a novel therapeutic target for hepatocellular carcinoma. Oncotarget 2015, 6, 28183–28193. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ribas, H.T.; Heath, K.; Wu, S.; Ren, J.; Shriwas, P.; Chen, X.; Johnson, M.E.; Cheng, X.; Burdette, J.E.; et al. Na(+)/K(+)-ATPase-Targeted Cytotoxicity of (+)-Digoxin and Several Semisynthetic Derivatives. J. Nat. Prod. 2020, 83, 638–648. [Google Scholar] [CrossRef]
- Cherniavsky-Lev, M.; Golani, O.; Karlish, S.J.; Garty, H. Ouabain-induced internalization and lysosomal degradation of the Na+/K+-ATPase. J. Biol. Chem. 2014, 289, 1049–1059. [Google Scholar] [CrossRef]
- Holthouser, K.A.; Mandal, A.; Merchant, M.L.; Schelling, J.R.; Delamere, N.A.; Valdes, R.R., Jr.; Tyagi, S.C.; Lederer, E.D.; Khundmiri, S.J. Ouabain stimulates Na-K-ATPase through a sodium/hydrogen exchanger-1 (NHE-1)-dependent mechanism in human kidney proximal tubule cells. Am. J. Physiol. Renal. Physiol. 2010, 299, F77–F90. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, C.; Huo, G.; Deng, J.; Zhao, H.; Xu, R.; Jiang, L.; Chen, S.; Wang, S. ATP1A1 Integrates AKT and ERK Signaling via Potential Interaction With Src to Promote Growth and Survival in Glioma Stem Cells. Front. Oncol. 2019, 9, 320. [Google Scholar] [CrossRef]
- L’Hote, V.; Courbeyrette, R.; Pinna, G.; Cintrat, J.C.; Le Pavec, G.; Delaunay-Moisan, A.; Mann, C.; Thuret, J.Y. Ouabain and chloroquine trigger senolysis of BRAF-V600E-induced senescent cells by targeting autophagy. Aging Cell 2021, 20, e13447. [Google Scholar] [CrossRef]
- Wang, W.; Bian, H.; Li, F.; Li, X.; Zhang, D.; Sun, S.; Song, S.; Zhu, Q.; Ren, W.; Qin, C.; et al. HBeAg induces the expression of macrophage miR-155 to accelerate liver injury via promoting production of inflammatory cytokines. Cell. Mol. Life Sci. 2018, 75, 2627–2641. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, J.; Hu, F.; Liu, S.; Zhou, Z. Metformin affects the features of a human hepatocellular cell line (HepG2) by regulating macrophage polarization in a co-culture microenviroment. Diabetes Metab. Res. Rev. 2015, 31, 781–789. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Q.R.; Lou, J.W.; Chen, P.D.; Yao, W.F.; Tao, W.W.; Tang, Y.P.; Dai, G.C.; Wang, K.; Zhang, L. Chemical Constituents from Euphorbia kansui. Molecules 2017, 22, 2176. [Google Scholar] [CrossRef]
- Hou, J.-J.; Shen, Y.; Yang, Z.; Fang, L.; Cai, L.-Y.; Yao, S.; Long, H.-L.; Wu, W.-Y.; Guo, D.-A. Anti-proliferation activity of terpenoids isolated from Euphorbia kansui in human cancer cells and their structure-activity relationship. Chin. J. Nat. Med. 2017, 15, 766–774. [Google Scholar] [CrossRef]
- Jiang, X.; Liu, Q.; Xue, S. LC-MS/MS method for determination of kansuinine a in rat plasma and its application to a rat pharmacokinetic study. Biomed. Chromatogr. 2021, 36, e5282. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Ying, Y.; Chen, B.; Hao, K.; Chen, B.; Zheng, Y.; Lyu, J.; Tong, X.; Chen, X.; et al. C21 steroid-enriched fraction refined from Marsdenia tenacissima inhibits hepatocellular carcinoma through the coordination of Hippo-Yap and PTEN-PI3K/AKT signaling pathways. Oncotarget 2017, 8, 110576–110591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gao, M.; Rao, Z.; Lei, Z.; Zeng, J.; Huang, Z.; Shen, C.; Zeng, N. The antitumour activity of C(21) steroidal glycosides and their derivatives of Baishouwu: A review. J. Ethnopharmacol. 2022, 293, 115300. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Pham, S.; Ming, D.S.; Chin, M.Y.; Adomat, H.; Hurtado-Coll, A.; Gleave, M.E.; Guns, E.S.T. Characterization of Precursor-Dependent Steroidogenesis in Human Prostate Cancer Models. Cancers 2018, 10, 343. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Aparicio-Siegmund, S.; Rose-John, S. The IL-6/gp130/STAT3 signaling axis: Recent advances towards specific inhibition. Curr. Opin. Immunol. 2015, 34, 75–82. [Google Scholar] [CrossRef]
- Holotiuk, V.V.; Kryzhanivska, A.Y.; Churpiy, I.K.; Tataryn, B.B.; Ivasiutyn, D.Y. Role of nitric oxide in pathogenesis of tumor growth and its possible application in cancer treatment. Exp. Oncol. 2019, 41, 210–215. [Google Scholar] [CrossRef]
- Lopez-Sanchez, L.M.; Aranda, E.; Rodriguez-Ariza, A. Nitric oxide and tumor metabolic reprogramming. Biochem. Pharmacol. 2020, 176, 113769. [Google Scholar] [CrossRef]


| Drug | HepG2 IC50 (μg/mL) |
|---|---|
| Control | - |
| Adr 1 | 5.98 ± 2.06 |
| A | >100 |
| B | >100 |
| C | 39.68 ± 4.53 |
| D | 63.72 ± 6.01 |
| E | 13.92 ± 3.28 |
| Compounds | Name | HepG2 IC50 (μM) |
|---|---|---|
| Control | - | - |
| Adr 1 | - | 6.93 ± 1.23 |
| 1 | Eupokanu G | 46.38 ± 3.09 |
| 2 | Cynotophylloside B | 41.11 ± 6.05 |
| 3 | Deacylmetaplexigenin | >50 |
| 4 | Kidjolanin | >50 |
| 5 | Cynsaccatol L | 12.61 ± 2.13 |
| 6 | Kidjoranin-3-O-β-D-cymaropyranoside | 27.30 ± 3.48 |
| 7 | Wilfoside G | 12.55 ± 2.98 |
| 8 | Cynotophylloside J | >50 |
| 9 | Maslinic acid | >50 |
| 10 | Kidjoranin 3-O-α-diginopyranosyl-(1→4)-β-cymaropyranoside | 22.73 ± 4.02 |
| 11 | Kidjoranin 3-O-β-digitoxopyranoside | >50 |
| 12 | Caudatin 3-O-β-D-digitoxopyranoside | >50 |
| Compounds | RAW264.7 | Compounds | RAW264.7 | ||
|---|---|---|---|---|---|
| CC50 1 (μM) | IC50 2 (μM) | CC50 1 (μM) | IC50 2 (μM) | ||
| Control | - | - | 7 | 26.15 ± 2.22 | 1.77 ± 0.34 |
| LPS 3 | - | - | 8 | >50 | 17.39 ± 3.01 |
| 1 | >50 | >50 | 9 | >50 | 17.38 ± 2.21 |
| 2 | >50 | 9.10 ± 2.27 | 10 | 24.12 ± 3.24 | 2.78 ± 0.44 |
| 3 | >50 | >50 | 11 | >50 | >50 |
| 4 | >50 | 30.72 ± 4.08 | 12 | >50 | >50 |
| 5 | 30.12 ± 3.14 | 0.02 ± 0.02 | l-NMMA 4 | >50 | 21.22 ± 3.26 |
| 6 | 35.24 ± 5.12 | 3.32 ± 0.43 | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, X.; Li, J.; Li, H.; Chen, X.; Liu, D.; Li, R. Bioactive C21 Steroidal Glycosides from Euphorbia kansui Promoted HepG2 Cell Apoptosis via the Degradation of ATP1A1 and Inhibited Macrophage Polarization under Co-Cultivation. Molecules 2023, 28, 2830. https://doi.org/10.3390/molecules28062830
Feng X, Li J, Li H, Chen X, Liu D, Li R. Bioactive C21 Steroidal Glycosides from Euphorbia kansui Promoted HepG2 Cell Apoptosis via the Degradation of ATP1A1 and Inhibited Macrophage Polarization under Co-Cultivation. Molecules. 2023; 28(6):2830. https://doi.org/10.3390/molecules28062830
Chicago/Turabian StyleFeng, Xiaoyi, Jianchun Li, Hongmei Li, Xuanqin Chen, Dan Liu, and Rongtao Li. 2023. "Bioactive C21 Steroidal Glycosides from Euphorbia kansui Promoted HepG2 Cell Apoptosis via the Degradation of ATP1A1 and Inhibited Macrophage Polarization under Co-Cultivation" Molecules 28, no. 6: 2830. https://doi.org/10.3390/molecules28062830
APA StyleFeng, X., Li, J., Li, H., Chen, X., Liu, D., & Li, R. (2023). Bioactive C21 Steroidal Glycosides from Euphorbia kansui Promoted HepG2 Cell Apoptosis via the Degradation of ATP1A1 and Inhibited Macrophage Polarization under Co-Cultivation. Molecules, 28(6), 2830. https://doi.org/10.3390/molecules28062830






