Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment
Abstract
:1. Introduction
2. Mushroom Production Worldwide and Consumption
3. Biological Activity of Mushrooms against Diabetes
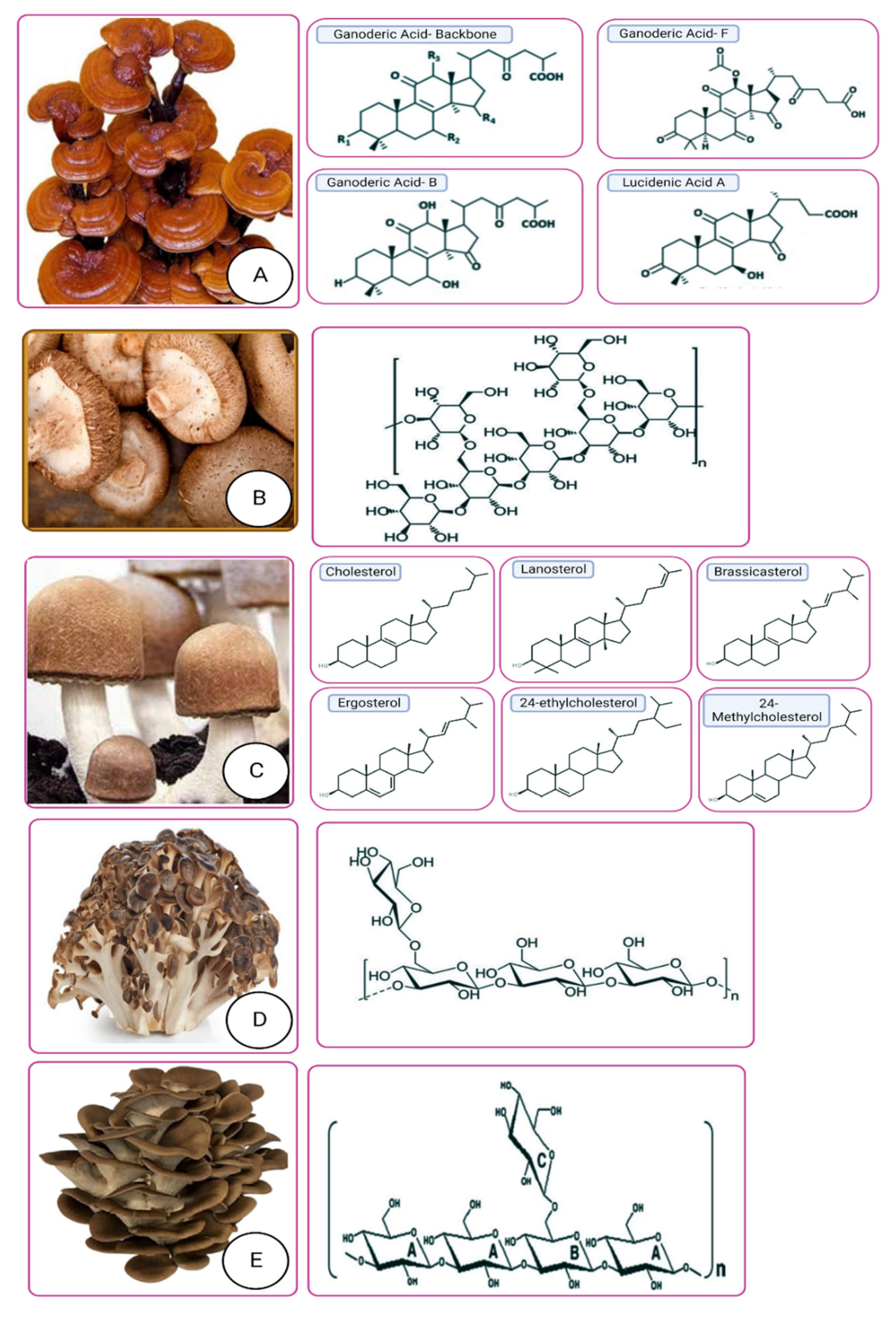
3.1. Ganoderma lucidum (Lingzhi/Reishi)
3.2. Lentinus edodes (Shiitake Mushroom)
3.3. Ophiocordyceps sinensis (Caterpillar fungus)
3.4. Agaricus blazeimurill
3.5. Grifola frondosa
3.6. Pulmonarius pleurotus (Grey Oyster Mushroom)
3.7. Panellus serotinus (Mukitake)
3.8. Auricularia auricular-judae (Jew’s Ear/Black Fungus)
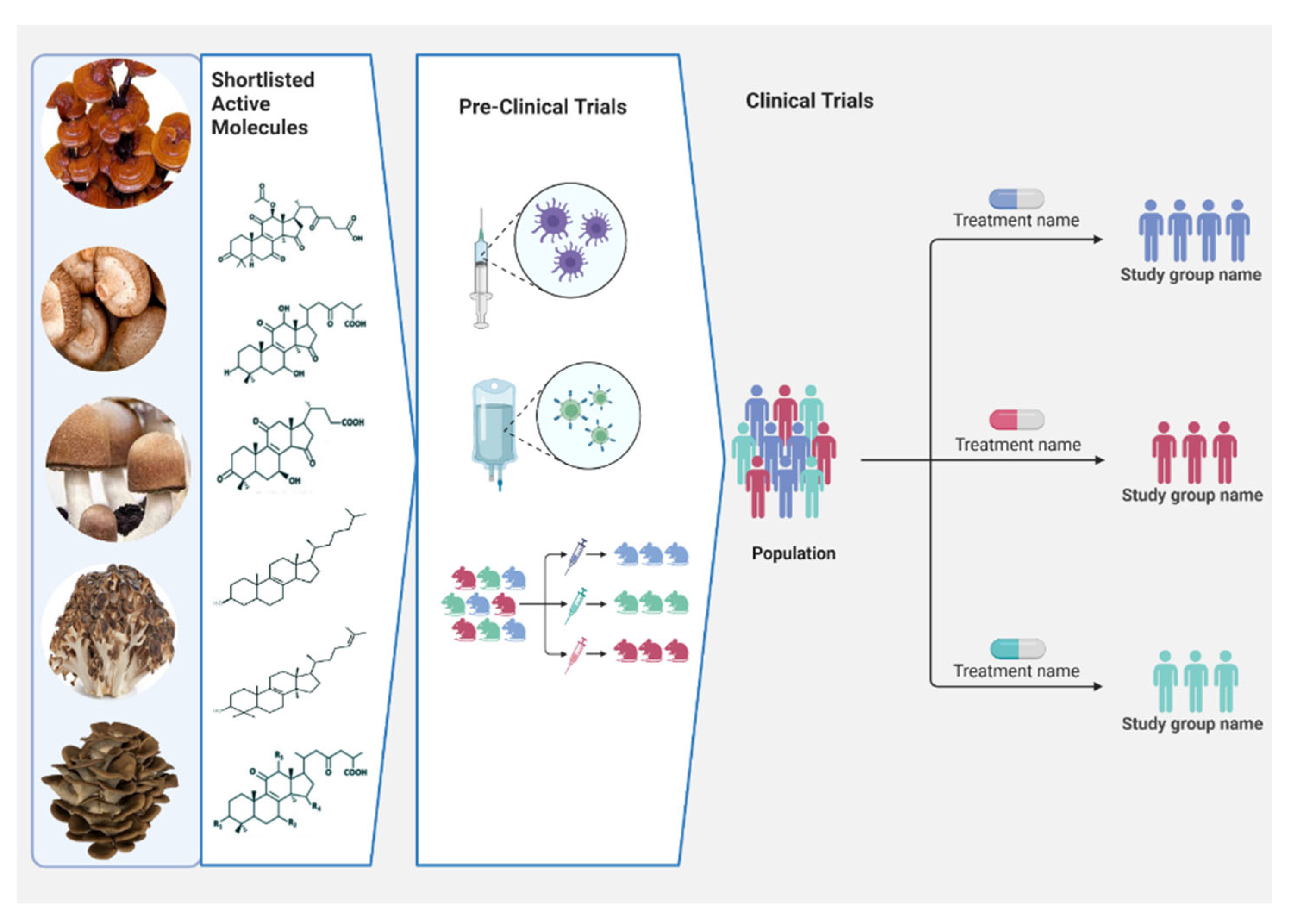
4. In Vivo Preclinical Study
5. Clinical Significance of Mushrooms
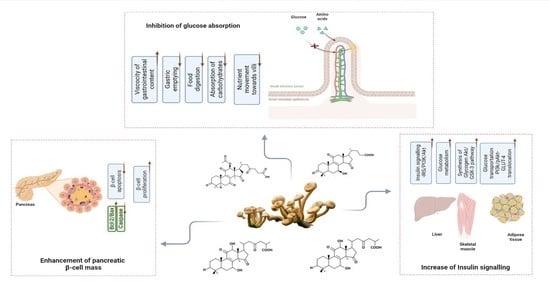
6. The Preventive Mechanistic Approach of Mushrooms against Diabetes and Insulin Resistance
6.1. The Polysaccharide-Mediated Blood Glucose-Lowering Effect
6.2. Pancreatic β Cell Activity Maintenance
6.3. Glucose Absorption Inhibition
6.4. Terpenoid-Mediated Blood Glucose-Lowering Effect
6.5. Mushroom-Based Vitamin D in Blood Glucose Regulations
7. Mushrooms as an Anti-Diabetic Functional Food
8. Challenges
9. Future Prospects and Outlook
10. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Candore, G.; Balistreri, C.R.; Listì, F.; Grimaldi, M.P.; Vasto, S.; Colonna-Romano, G.; Franceschi, C.; Lio, D.; Caselli, G.; Caruso, C. Immunogenetics, Gender, and Longevity. Ann. N. Y. Acad. Sci. 2006, 1089, 516–537. [Google Scholar] [CrossRef] [PubMed]
- Martorana, A.; Bulati, M.; Buffa, S.; Pellicanò, M.; Caruso, C.; Candore, G.; Colonna-Romano, G. Immunosenescence, Inflammation and Alzheimer’s Disease. Longev. Health 2012, 1, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oritz, A.R.R.; Garcia, J.S.; Castillo, E.P.; Ramirez, A.G.; Villalobos, M.R.; Estrada, S.S. Alpha-Glucosidase Inhibitory Activity of the Methanolic Extract from Tournefortia Hartwegiana: An Anti-Hyperglycemic Agent. J. Ethnopharmacol. 2007, 109, 48–53. [Google Scholar]
- Shobana, S.; Sreerama, Y.N.; Malleshi, N.G. Composition and Enzyme Inhibitory Properties of Finger Millet (Eleusine coracana L.) Seed Coat Phenolics: Mode of Inhibition of α-Glucosidase and Pancreatic Amylase. Food Chem. 2009, 115, 1268–1273. [Google Scholar] [CrossRef]
- Saito, N.; Sakai, H.; Sekihara, H.; Yajima, Y. Effect of an α-Glucosidase Inhibitor (Voglibose), in Combinationwith Sulphonyl Urea, on Glycemic Control in Type 2 Diabetes Patients. J. Int. Med. Res. 1998, 26, 219–232. [Google Scholar] [CrossRef]
- Melo, F.R.; Sales, M.P.; Pereira, L.S.; Bloch, C.J.R.; Franco, O.L.; Ary, M.B. α-Amylase Inhibitors from Cowpea Seeds. Protein Pept. Lett. 1999, 6, 385–390. [Google Scholar] [CrossRef]
- Koike, D. Effect of a Wheat Amylase Inhibitor on Canine Carbohydrate Digestion, Gastrointestinal Function and Pancreatic Growth. Gastroenterology 2005, 108, 1221–1229. [Google Scholar] [CrossRef]
- Fujisawa, T.; Ikegami, H.; Inoue, K.; Kawabata, Y.; Ogihara, T. Effect of Two α- Glucosidase Inhibitors, Voglibose and Acarbose, on Postprandial Hyperglycemia Correlates with Subjective Abdominal Symptoms. Metabolism 2005, 54, 387–390. [Google Scholar] [CrossRef]
- Phan, C.W.; David, P.; Naidu, M.; Wong, K.H.; Sabaratnam, V. Therapeutic Potential of Culinary-Medicinal Mushrooms for the Management of Neurodegenerative Diseases: Diversity, Metabolite, and Mechanism. Crit. Rev. Biotechnol. 2015, 35, 355–368. [Google Scholar] [CrossRef]
- Soković, M.; Ćirić, A.; Glamočlija, J.; Stojković, D. The Bioactive Properties of Mushrooms. In Wild Plants, Mushrooms and Nuts: Functional Food Properties and Applications; Ferreira, I.C.F.R., Morales, P., Barros, L., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 83–122. [Google Scholar]
- Popović, V.; Živković, J.; Davidović, S.; Stevanović, M.; Stojković, D. Mycotherapy of Cancer: An Update on Cytotoxic and Antitumor Activities of Mushrooms, Bioactive Principles and Molecular Mechanisms of Their Action. Curr. Top. Med. Chem. 2013, 13, 2791–2806. [Google Scholar] [CrossRef]
- Mustafa, F.; Chopra, H.; Baig, A.A.; Avula, S.K.; Kumari, S. Edible Mushrooms as Novel Myco-Therapeutics: Effects on Lipid Level, Obesity and BMI. J. Fungi 2022, 8, 211. [Google Scholar] [CrossRef]
- Chopra, H.; Mishra, A.K.; Baig, A.A.; Mohanta, T.K. Narrative Review: Bioactive Potential of Various Mushrooms as the Treasure of Versatile Therapeutic Natural Product. J. Fungi 2021, 7, 728. [Google Scholar] [CrossRef]
- Wasser, S.P. Medicinal Mushrooms in Human Clinical Studies. Part I. Anticancer, Oncoimmunological, and Immunomodulatory Activities: A Review. Int. J. Med. Mushrooms 2017, 19, 279–317. [Google Scholar] [CrossRef]
- Jeitler, M.; Michalsen, A.; Frings, D.; Hübner, M.; Fischer, M.; Koppold-Liebscher, D.A.; Murthy, V.; Kessler, C.S. Significance of Medicinal Mushrooms in Integrative Oncology: A Narrative Review. Front. Pharmacol. 2020, 11, 580656. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive Mushroom Polysaccharides: A Review on Monosaccharide Composition, Biosynthesis and Regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [Green Version]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef]
- Im, K.H.; Nguyen, T.K.; Choi, J.; Lee, T.S. In Vitro Antioxidant, Anti-Diabetes, Anti-Dementia, and Inflammation Inhibitory Effect of Trametes Pubescens Fruiting Body Extracts. Molecules 2016, 21, 639. [Google Scholar] [CrossRef] [Green Version]
- Lindequist, U. The Merit of Medicinal Mushrooms from a Pharmaceutical Point of View. Int. J. Med. Mushrooms 2013, 15, 517–523. [Google Scholar] [CrossRef] [Green Version]
- Paterson, R.R.; Lima, N. Biomedical Effects of Mushrooms with Emphasis on Pure Compounds. Biomed. J. 2014, 37, 357–368. [Google Scholar] [CrossRef]
- Gałgowska, M.; Pietrzak-Fiećko, R. Evaluation of the Nutritional and Health Values of Selected Polish Mushrooms Considering Fatty Acid Profiles and Lipid Indices. Molecules 2022, 27, 6193. [Google Scholar] [CrossRef]
- Huang, H.T.; Wang, S.-L.; Nguyen, V.B.; Kuo, Y.-H. Isolation and Identification of Potent Antidiabetic Compounds from Antrodia Cinnamomea—An Edible Taiwanese Mushroom. Molecules 2018, 23, 2864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, H.C.; Wasser, S.P. Medicinal Mushrooms for Glycemic Control in Diabetes Mellitus: History, Current Status, Future Perspectives, and Unsolved Problems (Review). Int. J. Med. Mushrooms 2011, 13, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms: Technology and Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 5–13. [Google Scholar] [CrossRef]
- Tsimihodimos, V.; Karanatsis, N.; Tzavela, E.; Elisaf, M. Antidiabetic Drugs and the Kidney. Curr. Pharm. Des. 2017, 23, 6310–6320. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, H.; Li, J.; Wang, Y. Research Progress on Elements of Wild Edible Mushrooms. J. Fungi 2022, 8, 964. [Google Scholar] [CrossRef]
- Das, A.; Chen, C.M.; Mu, S.C.; Yang, S.H.; Ju, Y.M.; Li, S.C. Medicinal Components in Edible Mushrooms on Diabetes Mellitus Treatment. Pharmaceutics 2022, 14, 436. [Google Scholar] [CrossRef]
- Sivanesan, I.; Muthu, M.; Gopal, J.; Oh, J.-W. Mushroom Polysaccharide-Assisted Anticarcinogenic Mycotherapy: Reviewing Its Clinical Trials. Molecules 2022, 27, 4090. [Google Scholar] [CrossRef]
- Rautela, I.; Arora, H.; Binjola, A.; Dheer, P. Potential and Nutrition Value of Mushroom and Its Cultivation; an Insight Review. Int. J. Eng. Sci. Comput. 2019, 9, 22574–22582. [Google Scholar]
- Friedman, M. Mushroom Polysaccharides: Chemistry and Antiobesity, Antidiabetes, Anticancer, and Antibiotic Properties in Cells, Rodents, and Humans. Foods 2016, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Su, C.H.; Lai, M.N.; Lin, C.C.; Ng, L.T. Comparative Characterization of Physicochemical Properties and Bioactivities of Polysaccharides from Selected Medicinal Mushrooms. Appl. Microbiol. Biotechnol. 2016, 100, 4385–4393. [Google Scholar] [CrossRef]
- Kundakovic, T.; Kolundzic, M. Therapeutic Properties of Mushrooms in Managing Adverse Effects in the Metabolic Syndrome. Curr. Top. Med. Chem. 2013, 13, 2734–2744. [Google Scholar] [CrossRef]
- Shamtsyan, M.; Antontceva, E.; Panchenko, A.; Petrishchev, N. Hyperlipidemic and Hypocholesterolic Action of Submerge Cultured Mushrooms. J. Hyg. Eng. Des. 2014, 7, 96–99. [Google Scholar]
- Wasser, S.P. Medicinal Mushroom Science: Current Perspectives, Advances, Evidences, and Challenges. Biomed. J. 2014, 37, 345–356. [Google Scholar] [CrossRef]
- Obodai, M.; Narh Mensah, D.L.; Fernandes, Â.; Kortei, N.K.; Dzomeku, M.; Teegarden, M.; Schwartz, S.J.; Barros, L.; Prempeh, J.; Takli, R.K.; et al. Chemical Characterization and Antioxidant Potential of Wild Ganoderma Species from Ghana. Molecules 2017, 22, 196. [Google Scholar] [CrossRef] [Green Version]
- Wińska, K.; MacZka, W.; Gabryelska, K.; Grabarczyk, M. Mushrooms of the Genus Ganoderma Used to Treat Diabetes and Insulin Resistance. Molecules 2019, 24, 4075. [Google Scholar] [CrossRef] [Green Version]
- Deepalakshmi, K.; Mirunalini, S. Therapeutic Properties and Current Medical Usage of Medicinal Mushroom: Ganoderma Lucidum. Inter. J. Pharm Sci Res. 2011, 2, 1922–1929. [Google Scholar]
- Klupp, N.L.; Kiat, H.; Bensoussan, A.; Steiner, G.Z.; Chang, D.H. A Double-Blind, Randomised, Placebo-Controlled Trial of Ganoderma Lucidum for the Treatment of Cardiovascular Risk Factors of Metabolic Syndrome. Sci. Rep. 2016, 6, 29540. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cai, C.; Zheng, M.; Hao, J.; Wang, Y.; Hu, M.; Fan, L.; Yu, G. Alkaline Extraction, Structural Characterization, and Bioactivities of (1→6)-β-d-Glucan from Lentinus Edodes. Molecules 2019, 24, 1610. [Google Scholar] [CrossRef] [Green Version]
- De Silva, D.D.; Rapior, S.; Hyde, K.D.; Bahkali, A.H. Medicinal Mushrooms in Prevention and Control of Diabetes Mellitus. Fungal Divers. 2012, 56, 1–29. [Google Scholar] [CrossRef]
- Firenzuoli, F.; Gori, L.; Lombardo, G. The Medicinal Mushroom Agaricus Blazei Murrill: Review of Literature and Pharmaco-Toxicological Problems. Evid. Based Complement. Altern. Med. 2008, 5, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Vitak, T.; Yurkiv, B.; Wasser, S.; Nevo, E.; Sybirna, N. Effect of Medicinal Mushrooms on Blood Cells under Conditions of Diabetes Mellitus. World J. Diabetes 2017, 8, 187. [Google Scholar] [CrossRef]
- Lei, H.; Guo, S.; Han, J.; Wang, Q.; Zhang, X.; Wu, W. Hypoglycemic and Hypolipidemic Activities of MT-α-Glucan and Its Effect on Immune Function of Diabetic Mice. Carbohydr. Polym. 2012, 89, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Tania, M. Nutritional and Medicinal Importance of Pleurotus Mushrooms: An Overview. Food Rev. Int. 2012, 28, 313–329. [Google Scholar] [CrossRef]
- Valverde, M.E.; Hernández-Pérez, T.; Paredes-López, O. Edible Mushrooms: Improving Human Health and Promoting Quality Life. Int. J. Microbiol. 2015, 2015, 376387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Royse, D.J. A Global Perspective on the High Five: Agaricus, Pleurotus. In Proceedings of the International Conference on Mushroom Biology and Mushroom Products, New Delhi, India, 19–22 November 2014; pp. 2010–2015. [Google Scholar]
- Ogbole, O.O.; Nkumah, A.O.; Linus, A.U.; Falade, M.O. Molecular Identification, in Vivo and in Vitro Activities of Calvatia Gigantea (Macro-Fungus) as an Antidiabetic Agent. Mycology 2019, 10, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Kong, D.; Cai, W.; Zhang, J.; Jia, L. Characterization and Anti-Diabetic Nephropathic Ability of Mycelium Polysaccharides from Coprinus Comatus. Carbohydr. Polym. 2021, 251, 117081. [Google Scholar] [CrossRef]
- Asrafuzzaman, M.; Rahman, M.M.; Mandal, M.; Marjuque, M.; Bhowmik, A.; Rokeya, B.; Hassan, Z.; Faruque, M.O. Oyster Mushroom Functions as an Anti-Hyperglycaemic through Phosphorylation of AMPK and Increased Expression of GLUT4 in Type 2 Diabetic Model Rats. J. Taibah Univ. Med. Sci. 2018, 13, 465–471. [Google Scholar] [CrossRef]
- Ali Sangi, S.M.; Bawadekji, A.; Al Ali, M. Comparative Effects of Metformin, Pleurotus Ostreatus, Nigella Sativa, and Zingiber Officinale on the Streptozotocin-Induced Diabetes Mellitus in Rats. Pharmacogn. Mag. 2018, 14, 268–273. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, L.; Fan, Y.; Yan, P.; Li, S.; Zhou, X. The Effect of Boletus Polysaccharides on Diabetic Hepatopathy in Rats. Chem. Biol. Interact. 2019, 308, 61–69. [Google Scholar] [CrossRef]
- Xiao, C.; Jiao, C.; Xie, Y.; Ye, L.; Li, Q.; Wu, Q. Grifola Frondosa GF5000 Improves Insulin Resistance by Modulation the Composition of Gut Microbiota in Diabetic Rats. J. Funct. Foods 2021, 77, 104313. [Google Scholar] [CrossRef]
- Patel, D.K.; Dutta, S.D.; Ganguly, K.; Cho, S.-J.; Lim, K.-T. Mushroom-Derived Bioactive Molecules as Immunotherapeutic Agents: A Review. Molecules 2021, 26, 1359. [Google Scholar] [CrossRef]
- Ekowati, N.; Yuniati, N.I.; Hernayanti; Ratnaningtyas, N.I. Antidiabetic Potentials of Button Mushroom (Agaricus Bisporus) on Alloxan-Induced Diabetic Rats. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 655–662. [Google Scholar] [CrossRef] [Green Version]
- Blumfield, M.; Abbott, K.; Duve, E.; Cassettari, T.; Marshall, S.; Fayet-Moore, F. Examining the Health Effects and Bioactive Components in Agaricus Bisporus Mushrooms: A Scoping Review. J. Nutr. Biochem. 2020, 84, 108453. [Google Scholar] [CrossRef]
- Wu, H.; Chen, J.; Li, J.; Liu, Y.; Park, H.J.; Yang, L. Recent Advances on Bioactive Ingredients of Morchella Esculenta. Appl. Biochem. Biotechnol. 2021, 193, 4197–4213. [Google Scholar] [CrossRef]
- Zhang, C.; Li, J.; Hu, C.; Wang, J.; Zhang, J.; Ren, Z.; Song, X.; Jia, L. Antihyperglycaemic and Organic Protective Effects on Pancreas, Liver and Kidney by Polysaccharides from Hericium Erinaceus SG-02 in Streptozotocin-Induced Diabetic Mice. Sci. Rep. 2017, 7, 10847. [Google Scholar] [CrossRef] [Green Version]
- Thongbai, B.; Rapior, S.; Hyde, K.D.; Wittstein, K.; Stadler, M. Hericium Erinaceus, an Amazing Medicinal Mushroom. Mycol. Prog. 2015, 14, 91. [Google Scholar] [CrossRef]
- Huang, C.-H.; Lin, W.-K.; Chang, S.-H.; Tsai, G.-J. Evaluation of the Hypoglycaemic and Antioxidant Effects of Submerged Ganoderma Lucidum Cultures in Type 2 Diabetic Rats. Mycology 2020, 12, 82–93. [Google Scholar] [CrossRef] [Green Version]
- Zeb, M.; Lee, C.H. Medicinal Properties and Bioactive Compounds from Wild Mushrooms Native to North America. Molecules 2021, 26, 251. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Advances in the Extraction, Purification, Structural-Property Relationships and Bioactive Molecular Mechanism of Flammulina Velutipes Polysaccharides: A Review. Int. J. Biol. Macromol. 2021, 167, 528–538. [Google Scholar] [CrossRef]
- Song, X.; Fua, H.; Chena, W. Effects of Flammulina Velutipes Polysaccharides on Quality Improvement of Fermented Milk and Antihyperlipidemic on Streptozotocin-Induced Mice. J. Funct. Foods 2021, 87, 104834. [Google Scholar] [CrossRef]
- Rivera, O.A.; Albarracin, W.; Lares, M. Bioactive Components of Shiitake (Lentinula Edodes Berk. Pegler) and Its Impact on Health. Arch. Venez. Farmacol. Ter. 2017, 36, 67–71. [Google Scholar]
- Laurino, L.F.; Viroel, F.J.M.; Caetano, E.; Spim, S.; Pickler, T.B.; Rosa-Castro, R.M.; Vasconcelos, E.A.; Jozala, A.F.; Hataka, A.; Grotto, D.; et al. Lentinus Edodes Exposure before and after Fetus Implantation: Materno-Fetal Development in Rats with Gestational Diabetes Mellitus. Nutrients 2019, 11, 2720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ugbogu, E.A.; Akubugwo, E.I.; Ude, V.C.; Emmanuel, O.; Okomba, N.O.; Ibe, C.; Onyero, O. Safety Evaluation of an Aqueous Extract of Termitomyces Robustus (Agaricomycetes) in Wistar Rats. Int. J. Med. Mushrooms 2019, 21, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Choudhary, N.; Varsha, N.; Kumar, S.; Seth, R. Phenolic Compounds and Their Health Benefits: A Review. J. Food Res. Technol. 2014, 2, 46–59. [Google Scholar]
- Sirisidthi, K.; Kosai, P.; Jiraungkoorskul, W. Antidiabetic Activity of the Lingzhi or Reishi Medicinal Mushroom Ganoderma Lucidum: A Review. S. Afr. Pharm. J. 2016, 83, 45–47. [Google Scholar]
- Tie, L.; Yang, H.Q.; An, Y.; Liu, S.Q.; Han, J.; Xu, Y.; Hu, M.; Li, W.D.; Chen, A.F.; Lin, Z.B.; et al. Ganoderma Lucidum Polysaccharide Accelerates Refractory Wound Healing by Inhibition of Mitochondrial Oxidative Stress in Type 1 Diabetes. Cell. Physiol. Biochem. 2012, 29, 583–594. [Google Scholar] [CrossRef]
- Wang, P.C.; Zhao, S.; Yang, B.Y.; Wang, Q.H.; Kuang, H.X. Anti-Diabetic Polysaccharides from Natural Sources: A Review. Carbohydr. Polym. 2016, 148, 86–97. [Google Scholar] [CrossRef]
- Zhang, H.N.; He, J.H.; Yuan, L.; Lin, Z. Bin In Vitro and in Vivo Protective Effect of Ganoderma Lucidum Polysaccharides on Alloxan-Induced Pancreatic Islets Damage. Life Sci. 2003, 73, 2307–2319. [Google Scholar] [CrossRef]
- Zheng, J.; Yang, B.; Yu, Y.; Chen, Q.; Huang, T.; Li, D. Ganoderma Lucidum Polysaccharides Exert Anti-Hyperglycemic Effect on Streptozotocin-Induced Diabetic Rats Through Affecting & Beta Cells. Comb. Chem. High Throughput Screen. 2012, 15, 542–550. [Google Scholar] [CrossRef]
- Zheng, Y.; Bai, L.; Zhou, Y.; Tong, R.; Zeng, M.; Li, X.; Shi, J. Polysaccharides from Chinese Herbal Medicine for Anti-Diabetes Recent Advances. Int. J. Biol. Macromol. 2019, 121, 1240–1253. [Google Scholar] [CrossRef]
- Zhang, H.N.; Lin, Z. Bin Hypoglycemic Effect of Ganoderma Lucidum Polysaccharides. Acta Pharmacol. Sin. 2004, 25, 191–195. [Google Scholar]
- Xiao, C.; Wu, Q.P.; Cai, W.; Tan, J.B.; Yang, X.B.; Zhang, J.M. Hypoglycemic Effects of Ganoderma Lucidum Polysaccharides in Type 2 Diabetic Mice. Arch. Pharm. Res. 2012, 35, 1793–1801. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma Lucidum: Persuasive Biologically Active Constituents and Their Health Endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Seto, S.W.; Lam, T.Y.; Tam, H.L.; Au, A.L.S.; Chan, S.W.; Wu, J.H.; Yu, P.H.F.; Leung, G.P.H.; Ngai, S.M.; Yeung, J.H.K.; et al. Novel Hypoglycemic Effects of Ganoderma Lucidum Water-Extract in Obese/Diabetic (+db/+db) Mice. Phytomedicine 2009, 16, 426–436. [Google Scholar] [CrossRef]
- Oliver-Krasinski, J.M.; Kasner, M.T.; Yang, J.; Crutchlow, M.F.; Rustgi, A.K.; Kaestner, K.H.; Stoffers, D.A. The Diabetes Gene Pdx1 Regulates the Transcriptional Network of Pancreatic Endocrine Progenitor Cells in Mice. J. Clin. Investig. 2009, 119, 1888–1898. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.T.; Hsieh, J.F.; Chen, S.T. Anti-Diabetic Effects of Ganoderma Lucidum. Phytochemistry 2015, 114, 109–113. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.; Zhang, J.; Xie, Y.; Cai, W.; Tan, J. Antidiabetic Activity of Ganoderma Lucidum Polysaccharides F31 Down-Regulated Hepatic Glucose Regulatory Enzymes in Diabetic Mice. J. Ethnopharmacol. 2017, 196, 47–57. [Google Scholar] [CrossRef]
- Arslan, M.; Rakha, A.; Khan, M.R.; Zou, X. Complementing the Dietary Fiber and Antioxidant Potential of Gluten Free Bread with Guava Pulp Powder. J. Food Meas. Charact. 2017, 11, 1959–1968. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.; Xie, Y.; Tan, J.; Ding, Y.R.; Bai, L. Hypoglycemic Mechanisms of: Ganoderma Lucidum Polysaccharides F31 in Db/Db Mice via RNA-Seq and ITRAQ. Food Funct. 2018, 9, 6495–6507. [Google Scholar] [CrossRef]
- Hikino, H.; Ishiyama, M.; Suzuki, Y.; Konno, C. Mechanisms of Hypoglycemic Activity of Ganoderan B: A Glycan of Ganoderma Lucidum Fruit Bodies. Planta Med. 1989, 55, 423–428. [Google Scholar] [CrossRef]
- Tomoda, M.; Gonda, R.; Kasahara, Y.; Hikino, H. Glycan Structures of Ganoderans b and c, Hypoglycemic Glycans of Ganoderma Lucidum Fruit Bodies. Phytochemistry 1986, 25, 2817–2820. [Google Scholar] [CrossRef]
- Teng, B.S.; Wang, C.D.; Zhang, D.; Wu, J.S.; Pan, D.; Pan, L.F.; Yang, H.J.; Zhou, P. Hypoglycemic Effect and Mechanism of a Proteoglycan from Ganoderma Lucidum on Streptozotocin-Induced Type 2 Diabetic Rats. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 166–175. [Google Scholar] [PubMed]
- Liu, Y.; Li, Y.; Zhang, W.; Sun, M.; Zhang, Z. Hypoglycemic Effect of Inulin Combined with Ganoderma Lucidum Polysaccharides in T2DM Rats. J. Funct. Foods 2019, 55, 381–390. [Google Scholar] [CrossRef]
- Niwa, A.; Tajiri, T.; Higashino, H. Ipomoea Batatas and Agarics Blazei Ameliorate Diabetic Disorders with Therapeutic Antioxidant Potential in Streptozotocin-Induced Diabetic Rats. J. Clin. Biochem. Nutr. 2011, 48, 194–202. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, Y.; Wang, H.; Li, C.; Qi, P.; Bao, J. Agaricus Bisporus Lectins Mediates Islet β-Cell Proliferation through Regulation of Cell Cycle Proteins. Exp. Biol. Med. 2012, 237, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Yamac, M.; Kanbak, G.; Zeytinoglu, M.; Senturk, H.; Bayramoglu, G.; Dokumacioglu, A.; Van Griensven, L. Pancreas Protective Effect of Button Mushroom Agaricus Bisporus (J.E. Lange) Imbach (Agaricomycetidae) Extract on Rats with Streptozotocin-Induced Dia Betes. Int. J. Med. Mushrooms 2010, 12, 379–389. [Google Scholar] [CrossRef]
- Bonkowski, M.S.; Sinclair, D.A. Slowing Ageing by Design: The Rise of NAD+ and Sirtuin-Activating Compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Sosnowska, B.; Mazidi, M.; Penson, P.; Gluba-Brzózka, A.; Rysz, J.; Banach, M. The Sirtuin Family Members SIRT1, SIRT3 and SIRT6: Their Role in Vascular Biology and Atherogenesis. Atherosclerosis 2017, 265, 275–282. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Chen, H.Z.; Wan, Y.Z.; Zhang, Q.J.; Wei, Y.S.; Huang, S.; Liu, J.J.; Lu, Y.B.; Zhang, Z.Q.; Yang, R.F.; et al. Repression of P66Shc Expression by SIRT1 Contributes to the Prevention of Hyperglycemia-Induced Endothelial Dysfunction. Circ. Res. 2011, 109, 639–648. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Giovane, A.; Casale, R.; Vitiello, M.; Marfella, R.; Paolisso, G.; Balestrieri, M.L. Ergothioneine Oxidation in the Protection against High-Glucose Induced Endothelial Senescence: Involvement of SIRT1 and SIRT6. Free Radic. Biol. Med. 2016, 96, 211–222. [Google Scholar] [CrossRef]
- Lappas, M. Anti-Inflammatory Properties of Sirtuin 6 in Human Umbilical Vein Endothelial Cells. Mediat. Inflamm. 2012, 2012, 597514. [Google Scholar] [CrossRef] [Green Version]
- Song, T.Y.; Yang, N.C.; Chen, C.L.; Thi, T.L.V. Protective Effects and Possible Mechanisms of Ergothioneine and Hispidin against Methylglyoxal-Induced Injuries in Rat Pheochromocytoma Cells. Oxid. Med. Cell. Longev. 2017, 2017, 4824371. [Google Scholar] [CrossRef] [Green Version]
- Guijarro, M.V.; Indart, A.; Aruoma, O.I.; Viana, M.; Bonet, B. Effects of Ergothioneine on Diabetic Embryopathy in Pregnant Rats. Food Chem. Toxicol. 2002, 40, 1751–1755. [Google Scholar] [CrossRef]
- Cho, E.J.; Hwang, H.J.; Kim, S.W.; Oh, J.Y.; Baek, Y.M.; Choi, J.W.; Bae, S.H.; Yun, J.W. Hypoglycemic Effects of Exopolysaccharides Produced by Mycelial Cultures of Two Different Mushrooms Tremella Fuciformis and Phellinus Baumii in Ob/Ob Mice. Appl. Microbiol. Biotechnol. 2007, 75, 1257–1265. [Google Scholar] [CrossRef]
- Rushita, S.; Vijayakumar, M.; Noorlidah, A.; Ameen Abdulla, M.; Vikineswary, S. Effect of Pleurotus Citrinopileatus on Blood Glucose, Insulin and Catalase of Streptozotocin-Induced Type 2 Diabetes Mellitus Rats. J. Anim. Plant Sci. 2013, 23, 1566–1571. [Google Scholar]
- Ahmad, N.; Bansal, R.; Rastogi, A.K.; Kidwai, J.R. Effect of PHA-B Fraction of Agaricus Bisporus Lectin on Insulin Release and 45Ca2+ Uptake by Islets of Langerhans in Vitro. Acta Diabetol. Lat. 1984, 21, 63–70. [Google Scholar] [CrossRef]
- Ravi, B.; Renitta, R.E.; Prabha, M.L.; Issac, R.; Naidu, S. Evaluation of Antidiabetic Potential of Oyster Mushroom (Pleurotus Ostreatus) in Alloxan-Induced Diabetic Mice. Immunopharmacol. Immunotoxicol. 2013, 35, 101–109. [Google Scholar] [CrossRef]
- Lam-Sidun, D.; Peters, K.M.; Borradaile, N.M. Mushroom-Derived Medicine? Preclinical Studies Suggest Potential Benefits of Ergothioneine for Cardiometabolic Health. Int. J. Mol. Sci. 2021, 22, 3246. [Google Scholar] [CrossRef]
- Roupas, P.; Keogh, J.; Noakes, M.; Margetts, C.; Taylor, P. The Role of Edible Mushrooms in Health: Evaluation of the Evidence. J. Funct. Foods 2012, 4, 687–709. [Google Scholar] [CrossRef]
- Ramos, M.; Burgos, N.; Barnard, A.; Evans, G.; Preece, J.; Graz, M.; Ruthes, A.C.; Jiménez-Quero, A.; Martínez-Abad, A.; Vilaplana, F.; et al. Agaricus Bisporus and Its By-Products as a Source of Valuable Extracts and Bioactive Compounds. Food Chem. 2019, 292, 176–187. [Google Scholar] [CrossRef] [Green Version]
- Calvo, M.S.; Mehrotra, A.; Beelman, R.B.; Nadkarni, G.; Wang, L.; Cai, W.; Goh, B.C.; Kalaras, M.D.; Uribarri, J. A Retrospective Study in Adults with Metabolic Syndrome: Diabetic Risk Factor Response to Daily Consumption of Agaricus Bisporus (White Button Mushrooms). Plant Foods Hum. Nutr. 2016, 71, 245–251. [Google Scholar] [CrossRef]
- Gao, Y.; Chen, G.; Dai, X.; Ye, J.; Zhou, S. A Phase I/II Study of Ling Zhi Mushroom Ganoderma Lucidum (W.Curt.:Fr.) Lloyd (Aphyllophoromycetideae) Extract in Patients with Coronary Heart Disease. Int. J. Med. Mushrooms 2004, 6, 327–334. [Google Scholar] [CrossRef]
- Ganesan, K.; Xu, B. Anti-Diabetic Effects and Mechanisms of Dietary Polysaccharides. Molecules 2019, 24, 2556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banukie, N.; Jayasuriya, W.J.A.; Wanigatunge, C.A.; Fernando, G.H.; Abeytunga, D.T.U.; Suresh, T.S. Hypoglycaemic Activity of Culinary Pleurotus Ostreatus and P. Cystidiosus Mushrooms in Healthy Volunteers and Type 2 Diabetic Patients on Diet Control and the Possible Mechanisms of Action. Phyther. Res. 2015, 29, 303–309. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Liao, Y.-L.; Lin, S.-C.; Hwang, K.-C.; Chou, P. The Mushroom Agaricus Blazei Murill in Combination with Metformin and Gliclazide Improves Insulin Resistance in Type 2 Diabetes: A Randomized, Double-Blinded, and Placebo-Controlled Clinical Trial. J. Altern. Complement. Med. 2007, 13, 97–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osonoi, Y.; Mita, T.; Osonoi, T.; Saito, M.; Tamasawa, A.; Nakayama, S.; Someya, Y.; Ishida, H.; Kanazawa, A.; Gosho, M.; et al. Relationship between Dietary Patterns and Risk Factors for Cardiovascular Disease in Patients with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Nutr. J. 2016, 15, 15. [Google Scholar] [CrossRef] [Green Version]
- Sayeed, M.A.; Banu, A.; Khatun, K.; Khanam, P.A.; Begum, T.; Mahtab, H.; Haq, J.A. Effect of Edible Mushroom (Pleurotus Ostreatus) on Type-2 Diabetics. Ibrahim Med. Coll. J. 2015, 8, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Aramabasic, J.J.; Mihailović, M.; Uskoković, A.; Grdović, N.; Dinić, S.; Vidaković, M. The Effects of Major Mushroom Bioactive Compounds on Mechanisms That Control Blood Glucose Level. J. Fungi 2021, 7, 58. [Google Scholar] [CrossRef]
- Grondin, J.M.; Tamura, K.; Déjean, G.; Abbott, D.W.; Brumer, H. Polysaccharide Utilization Loci: Fueling Microbial Communities. J. Bacteriol. 2017, 199, e00860-16. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.K.; Chaturvedi, V.K.; Mishra, D.; Bajpeyee, A.; Tiwari, A.; Singh, M.P. Role of Edible Mushroom as a Potent Therapeutics for the Diabetes and Obesity. 3 Biotech 2019, 9, 450. [Google Scholar] [CrossRef]
- Ratnaningtyas, N.I.; Hernayanti; Andarwanti, S.; Ekowati, N.; Purwanti, E.S.; Sukmawati, D. Effects of Ganoderma Lucidum Extract on Diabetic Rats. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 642–647. [Google Scholar] [CrossRef] [Green Version]
- Lin, X.; Brennan-Speranza, T.C.; Levinger, I.; Yeap, B.B. Undercarboxylated Osteocalcin: Experimental and Human Evidence for a Role in Glucose Homeostasis and Muscle Regulation of Insulin Sensitivity. Nutrients 2018, 10, 847. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Hu, Y.; Duan, X.; Tang, T. Characterization and Antioxidant Activities of Polysaccharides from Thirteen Boletus Mushrooms. Int. J. Biol. Macromol. 2018, 113, 1–7. [Google Scholar] [CrossRef]
- Khursheed, R.; Singh, S.K.; Wadhwa, S.; Gulati, M.; Awasthi, A. Therapeutic Potential of Mushrooms in Diabetes Mellitus: Role of Polysaccharides. Int. J. Biol. Macromol. 2020, 164, 1194–1205. [Google Scholar] [CrossRef]
- Panigrahy, S.K.; Bhatt, R.; Kumar, A. Targeting Type II Diabetes with Plant Terpenes: The New and Promising Antidiabetic Therapeutics. Biologia 2020, 76, 241–254. [Google Scholar] [CrossRef]
- Dasgupta, A.; Acharya, K. Mushrooms: An Emerging Resource for Therapeutic Terpenoids. 3 Biotech 2019, 9, 369. [Google Scholar] [CrossRef]
- Cardwell, G.; Bornman, J.F.; James, A.P.; Black, L.J. A Review of Mushrooms as a Potential Source of Dietary Vitamin D. Nutrients 2018, 10, 1498. [Google Scholar] [CrossRef] [Green Version]
- Sung, C.-C.; Liao, M.-T.; Lu, K.-C.; Wu, C.-C. Role of Vitamin D in Insulin Resistance. J. Biomed. Biotechnol. 2012, 2012, 634195. [Google Scholar] [CrossRef] [Green Version]
- Al-Shoumer, K.A.; Al-Essa, T.M. Is There a Relationship between Vitamin D with Insulin Resistance and Diabetes Mellitus? World J. Diabetes 2015, 6, 1057–1064. [Google Scholar] [CrossRef]
- Urbain, P.; Singler, F.; Ihorst, G. Bioavailability of Vitamin D2 from UV-B-Irradiated Button Mushrooms in Healthy Adults Deficient in Serum 25-Hydroxyvitamin D: A Randomized Controlled Trial. Eur. J. Clin. Nutr. 2011, 65, 965–971. [Google Scholar] [CrossRef] [Green Version]
- Han, N.S.; Wan Ahmad, W.A.N.; Wan Ishak, W.R. Quality Characteristics of Pleurotus Sajor-Caju Powder: Study on Nutritional Compositions, Functional Properties and Storage Stability. Sains Malays. 2016, 45, 1617–1623. [Google Scholar]
- Ho, L.-H.; Asyikeen Zulkifli, N.; Tan, T.-C. Edible Mushroom: Nutritional Properties, Potential Nutraceutical Values, and Its Utilisation in Food Product Development. In An Introduction to Mushroom; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef]
- Rahi, D.K.; Malik, D. Diversity of Mushrooms and Their Metabolites of Nutraceutical and Therapeutic Significance. J. Mycol. 2016, 2016, 7654123. [Google Scholar] [CrossRef]
- Zhong, J.-J.J.; Tang, Y.-J.J. Submerged Cultivation of Medicinal Mushrooms for Production of Valuable Bioactive Metabolites BT-Biomanufacturing. In Advances in Biochemical Engineering/Biotechnology; Zhong, J.-J., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 87, pp. 25–59. ISBN 978-3-540-39998-8. [Google Scholar]
- Zhong, J.J.; Xiao, J.H. Secondary Metabolites from Higher Fungi: Discovery, Bioactivity, and Bioproduction; Springer International Publishing: Berlin/Heidelberg, Germany, 2009; Volume 113. [Google Scholar]
- Subhadip, M.; Banerjee, D. Fungal Exopolysaccharide: Production, Composition and Applications. Microbiol. Insights 2013, 6, 1–16. [Google Scholar]
- Zhang, W.X.; Zhong, J.J. Effect of Oxygen Concentration in Gas Phase on Sporulation and Individual Ganoderic Acids Accumulation in Liquid Static Culture of Ganoderma Lucidum. J. Biosci. Bioeng. 2010, 109, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Cui, B.; Han, L.; Qu, J.; Lv, Y. Hypoglycemic Activity of Grifola Frondosa Rich in Vanadium. Biol. Trace Elem. Res. 2009, 131, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Dhingra, G.S.; Shri, R. Antidiabetic Potential of Mushrooms. Asian J. Pharm. Res. 2015, 5, 111–125. [Google Scholar]
- Chaturvedi, V.K.; Dubey, S.K.; Singh, M.P. Antidiabetic Potential of Medicinal Mushrooms. Phytochem. Med. Plants 2020, 5, 137–158. [Google Scholar] [CrossRef]
- Bharti, S.K.; Krishnan, S.; Kumar, A.; Kumar, A. Antidiabetic Phytoconstituents and Their Mode of Action on Metabolic Pathways. Ther. Adv. Endocrinol. Metab. 2018, 9, 81–100. [Google Scholar] [CrossRef]
- Arunachalam, K.; Sreeja, P.S.; Yang, X. The Antioxidant Properties of Mushroom Polysaccharides Can Potentially Mitigate Oxidative Stress, Beta-Cell Dysfunction and Insulin Resistance. Front. Pharmacol. 2022, 13, 874474. [Google Scholar] [CrossRef]
- Chowdhury, P.; Paul, S. The Potential Role of Mushrooms in The Prevention and Treatment of Diabetes: A Review. J. Biol. Act. Prod. Nat. 2020, 10, 429–454. [Google Scholar] [CrossRef]
- Yang, B.-K.; Kim, D.-H.; Jeong, S.-C.; Das, S.; Choi, Y.-S.; Shin, J.-S.; Lee, S.-C.; Song, C.-H. Hypoglycemic effect of a Lentinus edodes exo-polymer produced from a submerged mycelial culture. Biosci. Biotechnol. Biochem. 2002, 66, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.R.; Lee, Y.P.; Kim, D.W.; Song, H.Y.; Yoo, K.-Y.; Won, M.H.; Kang, T.-C.; Lee, K.J.; Kim, K.H.; Joo, J.H.; et al. Amelioration of streptozotocin-induced diabetes by Agrocybe chaxingu polysaccharide. Mol. Cells 2010, 29, 349–354. [Google Scholar] [CrossRef]
- Yuan, Z.; He, P.; Takeuchi, H. Ameliorating effects of water-soluble polysaccharides from woody ear (Auricularia auricula-judae Quel.) in genetically diabetic KK-Ay mice. J. Nutr. Sci. Vitaminol. 1998, 44, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Sun, J.; Rao, S.; Su, Y.; Yang, Y. Antihyperglycemic, antihyperlipidemic and antioxidant activities of polysaccharides from Catathelasma ventricosum in streptozotocin-induced diabetic mice. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2013, 57, 39–45. [Google Scholar] [CrossRef]
- Zhang, G.; Huang, Y.; Bian, Y.; Wong, J.H.; Ng, T.B.; Wang, H. Hypoglycemic activity of the fungi Cordyceps militaris, Cordyceps sinensis, Tricholoma mongolicum, and Omphalia lapidescens in streptozotocin-induced diabetic rats. Appl. Microbiol. Biotechnol. 2006, 72, 1152–1156. [Google Scholar] [CrossRef]
- Kou, L.; Du, M.; Liu, P.; Zhang, B.; Zhang, Y.; Yang, P.; Shang, M.; Wang, X. Anti-diabetic and anti-nephritic activities of Grifola frondosa mycelium polysaccharides in diet-streptozotocin-induced diabetic rats via modulation on oxidative stress. Appl. Biochem. Biotechnol. 2019, 187, 310–322. [Google Scholar] [CrossRef]
- Xiao, C.; Wu, Q.; Xie, Y.; Zhang, J.; Tan, J. Hypoglycemic effects of Grifola frondosa (Maitake) polysaccharides F2 and F3 through improvement of insulin resistance in diabetic rats. Food Funct. 2015, 6, 3567–3575. [Google Scholar] [CrossRef]
- Jang, J.S.; Lee, J.S.; Lee, J.H.; Kwon, D.S.; Lee, K.E.; Lee, S.Y.; Hong, E.K. Hispidin produced from Phellinus linteus protects pancreatic beta-cells from damage by hydrogen peroxide. Arch. Pharm. Res. 2010, 33, 853–861. [Google Scholar] [CrossRef]
- Zhao, C.; Liao, Z.; Wu, X.; Liu, Y.; Liu, X.; Lin, Z.; Huang, Y.; Liu, B. Isolation, purification, and structural features of a polysaccharide from Phellinus linteus and its hypoglycemic effect in alloxan-induced diabetic mice. J. Food Sci. 2014, 79, H1002-10. [Google Scholar] [CrossRef]
- Zhao, H.; Lai, Q.; Zhang, J.; Huang, C.; Jia, L. Antioxidant and hypoglycemic effects of acidic-extractable polysaccharides from Cordyceps militaris on type 2 diabetes mice. Oxid. Med. Cell. Longev. 2018, 2018, 9150807. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.R.; Ng, T.B.; Li, L.; Fang, J.C.; Jiang, Y.; Wen, T.Y.; Qiao, W.T.; Li, N.; Liu, F. Isolation of a polysaccharide with antiproliferative, hypoglycemic, antioxidant and HIV-1 reverse transcriptase inhibitory activities from the fruiting bodies of the abalone mushroom Pleurotus abalonus. J. Pharm. Pharmacol. 2011, 63, 825–832. [Google Scholar] [CrossRef]
- Hu, S.-H.; Wang, J.-C.; Lien, J.-L.; Liaw, E.-T.; Lee, M.-Y. Antihyperglycemic effect of polysaccharide from fermented broth of Pleurotus citrinopileatus. Appl. Microbiol. Biotechnol. 2006, 70, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meng, G.; Zhang, C.; Lin, L.; Xu, N.; Liu, M.; Cui, F.; Jia, L. The antioxidative effects of acidic-, alkalic-, and enzymatic-extractable mycelium zinc polysaccharides by Pleurotus djamor on liver and kidney of streptozocin-induced diabetic mice. BMC Complement. Altern. Med. 2015, 15, 440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Zhang, L.; Liu, H.; Zhang, J.; Hu, C.; Jia, L. Antioxidation, anti-hyperglycaemia and renoprotective effects of extracellular polysaccharides from Pleurotus eryngii SI-04. Int. J. Biol. Macromol. 2018, 111, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ganeshpurkar, A.; Kohli, S.; Rai, G. Antidiabetic potential of polysaccharides from the white oyster culinary-medicinal mushroom Pleurotus florida (higher Basidiomycetes). Int. J. Med. Mushrooms 2014, 16, 207–217. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, T.; Zhou, H.; Zhang, Y.; Jin, G.; Yang, Y. Antidiabetic effect of polysaccharides from Pleurotus ostreatus in streptozotocin-induced diabetic rats. Int. J. Biol. Macromol. 2016, 83, 126–132. [Google Scholar] [CrossRef]
- Kanagasabapathy, G.; Kuppusamy, U.R.; Abd Malek, S.N.; Abdulla, M.A.; Chua, K.-H.; Sabaratnam, V. Glucan-rich polysaccharides from Pleurotus sajor-caju (Fr.) Singer prevents glucose intolerance, insulin resistance and inflammation in C57BL/6J mice fed a high-fat diet. BMC Complement. Altern. Med. 2012, 12, 261. [Google Scholar] [CrossRef] [Green Version]
- Kanagasabapathy, G.; Chua, K.H.; Malek, S.N.A.; Vikineswary, S.; Kuppusamy, U.R. AMP-activated protein kinase mediates insulin-like and lipo-mobilising effects of β-glucan-rich polysaccharides isolated from Pleurotus sajor-caju (Fr.), Singer mushroom, in 3T3-L1 cells. Food Chem. 2014, 145, 198–204. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Korivi, M.; Yang, H.-T.; Huang, C.-C.; Chaing, Y.-Y.; Tsai, Y.-C. Effect of Pleurotus tuber-regium polysaccharides supplementation on the progression of diabetes complications in obese-diabetic rats. Chin. J. Physiol. 2014, 57, 198–208. [Google Scholar] [CrossRef] [Green Version]
- Lo, H.-C.; Tsai, F.-A.; Wasser, S.P.; Yang, J.-G.; Huang, B.-M. Effects of ingested fruiting bodies, submerged culture biomass, and acidic polysaccharide glucuronoxylomannan of Tremella mesenterica Retz.:Fr. on glycemic responses in normal and diabetic rats. Life Sci. 2006, 78, 1957–1966. [Google Scholar] [CrossRef]
- Hong, L.; Xun, M.; Wutong, W. Anti-diabetic effect of an alpha-glucan from fruit body of maitake (Grifola frondosa) on KK-Ay mice. J. Pharm. Pharmacol. 2007, 59, 575–582. [Google Scholar] [CrossRef]



| S. No. | Name of the Species | Secondary Metabolites | Bioactivity | References |
|---|---|---|---|---|
| 1 | Calvatia gigantea | 2-Pyrrolidinone, 1-Dodecene, ergosterol, hexadecane, benzeneacetic acid | Anti-diabetic, antioxidant, anti-inflammatory | [47] |
| 2 | Coprinus comatus | Mycelium, polysaccharides | Immunomodulatory, anti-diabetic, antioxidant, anti-cancer | [48] |
| 3 | Pleurotusostreatus, P. pulmonarius, and P. fossulatus | Terpenoids, heterocyclic amines, phenols, glucan, proteoglycan | Anti-cholesterol, anti-cancer effects, anti-inflammatory, anti-diabetic | [49,50] |
| 4 | Boletus edulis | Tocopherol, quinic acid, hydroxy benzoic acid | Antioxidant, anti-inflammatory, hypoglycemic | [51] |
| 5 | Grifola frondosa | Grifolan polysaccharide, D-fraction, MD-fraction, polysaccharide, galactomannan, heteroglycan | Hypoglycemic, anti-inflammatory, anti-modulatory, anti-tumor | [52,53] |
| 6 | Agaricus bisporus | Pyrogallol, hydroxybenzoic acid derivatives glavonoid | Anti-inflammatory, anti-diabetic | [54,55] |
| 7 | Morchella esculenta | Polysaccharides (mannose, galactose, and glucose), phenolic compounds | Antioxidant, anti-inflammation, immunoregulation, hypoglycemic | [56] |
| 8 | Hericium erinaceus | 4-chloro-3, 5-dimethoxybenzoic acid-O-arabitol ester, 2-hydroxymethyl-5-α-hydroxyethyl-γ-pyranone, 6-methyl-2,5-dihydroxymethyl-γ-pyranone, 4-chloro-3,5-dihydroxybenzaldehyde, 4-chloro-3,5-dihydroxybenzyl alcohol | Immunomodulatory, hypoglycemic, antimicrobial | [57,58] |
| 9 | Ganoderma lucidium | Ganoderic acid, danoderiol, danderenic acid, lucidenic acid, Ganoderma leucidum Polysaccharide | Anti-diabetic, anti-inflammatory | [53,59] |
| 10 | Lenzites betulina | α-glucan, β-glucan, β-glucan protein, galacturonic acid | Antioxidant, anti-hyperglycaemic, anti-inflammatory, anti-proliferative, antibacterial | [60] |
| 11 | Flammulina velutipes | Flammulinolide, enokipodin, proflamin and other polysaccharide | Anti-tumor, anti-hypertension, antihypercholesterolemia, hypoglycemic | [61,62] |
| 12 | Lentinula edodes | Lentinan, eritadenina | Anti-carcinogenic, antioxidant, hypocholesterolemic action | [63,64] |
| 13 | Termitomyces robustus | glutamyl-βphenylethylamine, tryptophan 1,4-hydroxyphenylacetic acid, hydroxyphenyl propionic acid and phenyllactic acid | Hypoglycemic effect | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamim, M.Z.; Mishra, A.K.; Kausar, T.; Mahanta, S.; Sarma, B.; Kumar, V.; Mishra, P.K.; Panda, J.; Baek, K.-H.; Mohanta, Y.K. Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment. Molecules 2023, 28, 2837. https://doi.org/10.3390/molecules28062837
Shamim MZ, Mishra AK, Kausar T, Mahanta S, Sarma B, Kumar V, Mishra PK, Panda J, Baek K-H, Mohanta YK. Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment. Molecules. 2023; 28(6):2837. https://doi.org/10.3390/molecules28062837
Chicago/Turabian StyleShamim, Mohammad Zaki, Awdhesh Kumar Mishra, Tahreem Kausar, Saurov Mahanta, Bhaskar Sarma, Vijay Kumar, Piyush Kumar Mishra, Jibanjyoti Panda, Kwang-Hyun Baek, and Yugal Kishore Mohanta. 2023. "Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment" Molecules 28, no. 6: 2837. https://doi.org/10.3390/molecules28062837
APA StyleShamim, M. Z., Mishra, A. K., Kausar, T., Mahanta, S., Sarma, B., Kumar, V., Mishra, P. K., Panda, J., Baek, K.-H., & Mohanta, Y. K. (2023). Exploring Edible Mushrooms for Diabetes: Unveiling Their Role in Prevention and Treatment. Molecules, 28(6), 2837. https://doi.org/10.3390/molecules28062837