In Vitro Antiglycation and Methylglyoxal Trapping Effect of Peppermint Leaf (Mentha × piperita L.) and Its Polyphenols
Abstract
:1. Introduction
2. Results and Discussion
2.1. Polyphenolic Profile of Peppermint Leaf Dry Extract
2.2. Anti-Glycation Activity of Peppermint Leaf Dry Extract and Its Polyphenolic Components
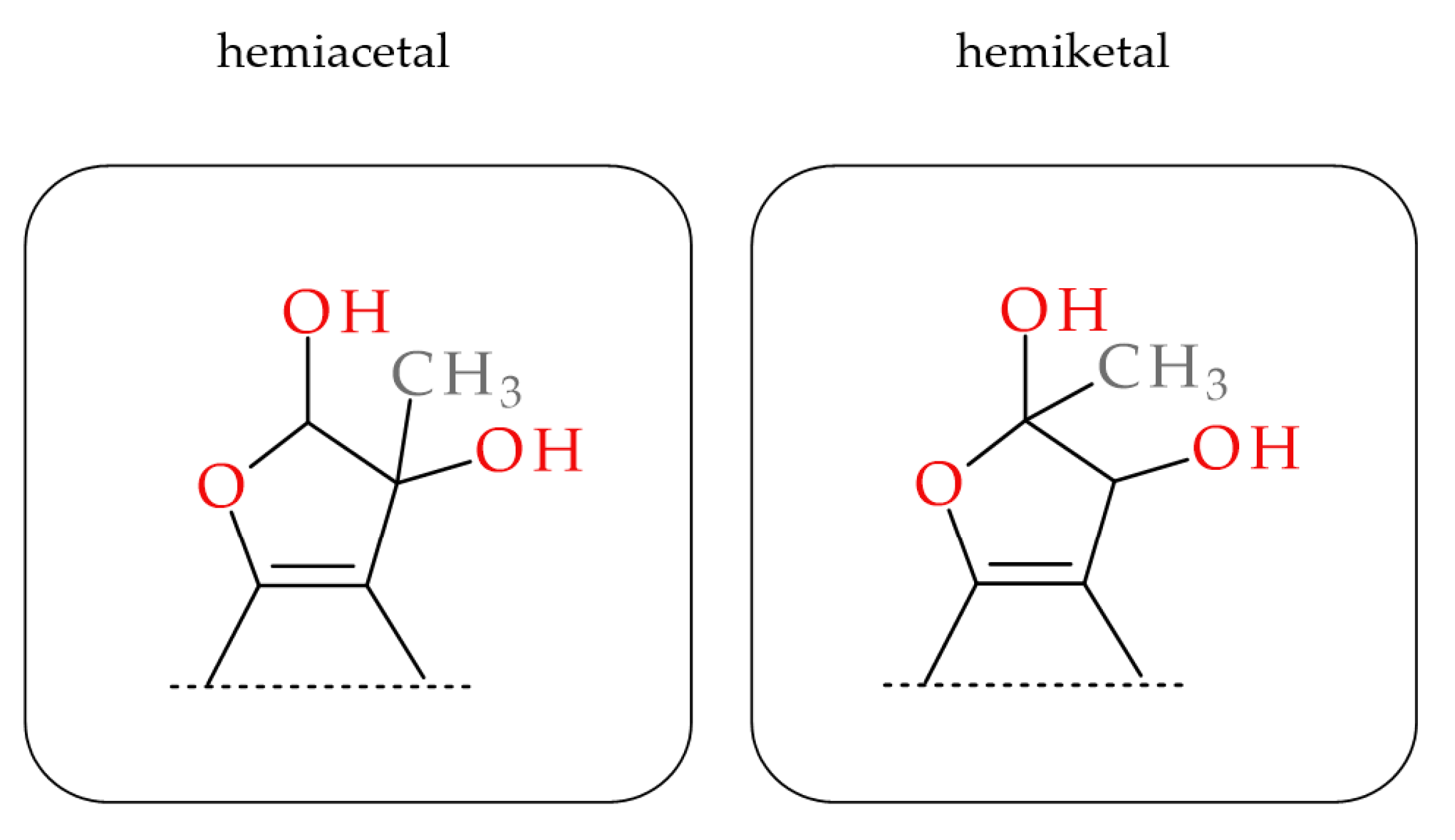
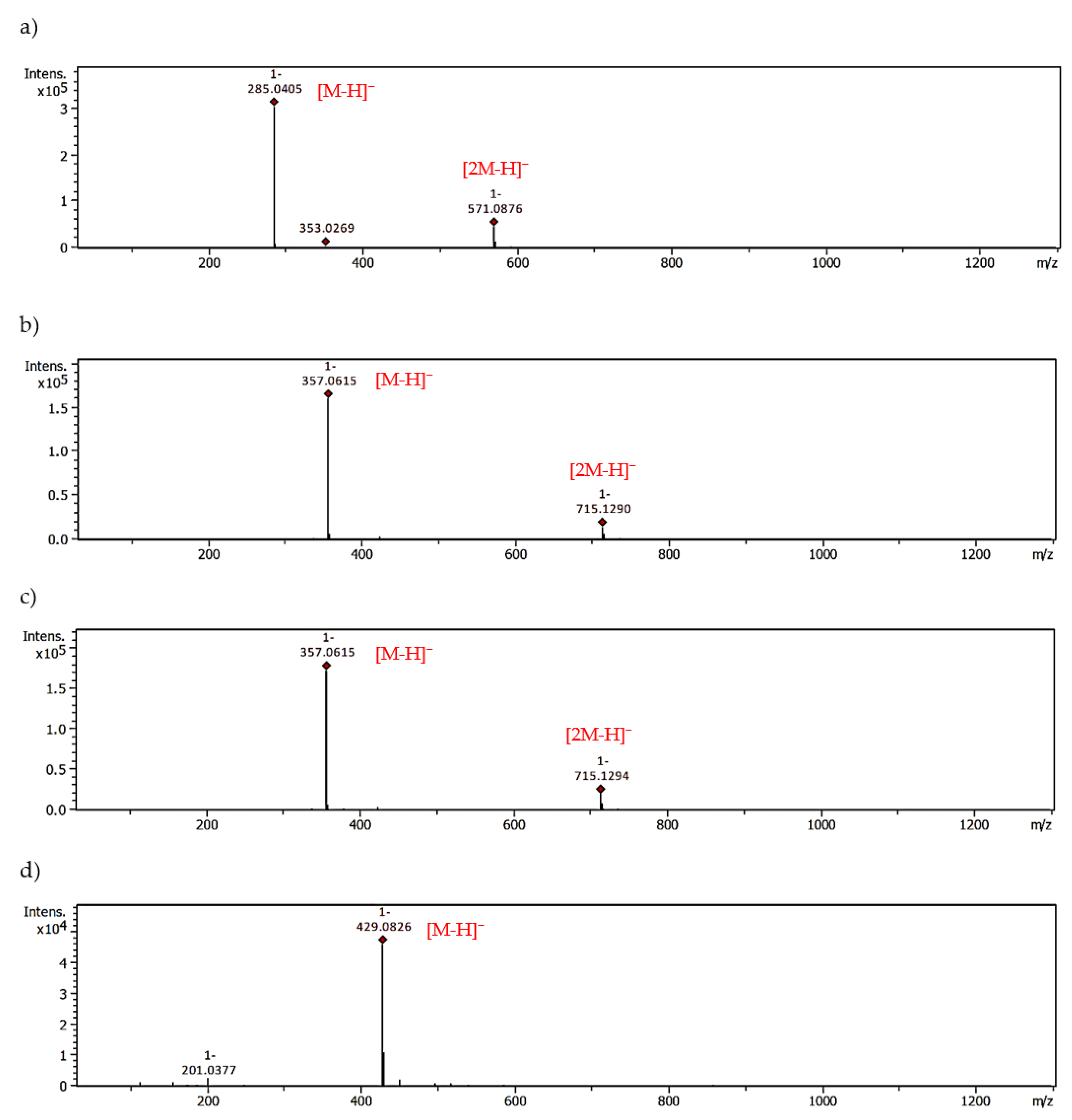
2.3. MGO-Trapping Potential of Peppermint Leaf Polyphenols
3. Materials and Methods
3.1. Chemicals
3.2. Authentic Standards
3.3. Plant Material and Extract Preparation
3.4. Chemical Composition of Peppermint Leaf Dry Extract
3.5. Antiglycation Non-Enzymatic Assay (BSA-Methylglyoxal Model)
3.6. MGO Trapping and Adduct Analysis
3.7. Analysis of the Polyphenol-MGO Adducts
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Schalkwijk, C.G.; Micali, L.R.; Wouters, K. Advanced glycation endproducts in diabetes-related macrovascular complications: Focus on methylglyoxal. Trends Endocrinol. Metab. 2023, 34, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Kold-Christensen, R.; Johannsen, M. Methylglyoxal metabolism and aging-related disease: Moving from correlation toward causation. Trends Endocrinol. Metab. 2020, 31, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Rabbani, N.; Adaikalakoteswari, A.; Larkin, J.R.; Panagiotopoulos, S.; MacIsaac, R.J.; Yue, D.K.; Fulcher, G.R.; Matthew, A.; Roberts, M.A.; Thomas, M.; et al. Analysis of serum advanced glycation end products reveals methylglyoxal-derived advanced glycation MG-H1 free adduct is a risk marker in non-diabetic and diabetic chronic kidney disease. Int. J. Mol. Sci. 2022, 24, 152. [Google Scholar] [CrossRef]
- Wai Tsuen Lai, S.; De Jesus Lopez Gonzalez, E.; Zoukari, T.; Ki, P.; Shuck, S.C. Methylglyoxal and its adducts: Induction, repair and association with disease. Chem. Res. Toxicol. 2022, 35, 1720–1746. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Aspalathin and other rooibos flavonoids trapped α-dicarbonyls and inhibited formation of advanced glycation end products in vitro. Int. J. Mol. Sci. 2022, 23, 14738. [Google Scholar] [CrossRef]
- Ho, S.C.; Wu, S.P.; Lin, S.M.; Tang, Y.L. Comparison of anti-glycation capacities of several herbal infusions with that of green tea. Food Chem. 2010, 122, 768–774. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Spada, A.P.M.; Oliveira, E.P.D.; Paiva-Filho, M.E.; Martuchi, K.A.; Leite, N.C.; Deus, R.M.; Sasaki, V.; Braganti, L.S.; Oshiiwa, M. Mentha piperita effects on wistar rats plasma lipids. Braz. Arch. Biol. Technol. 2009, 52, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Barbalho, S.M.; Damasceno, D.C.; Spada, A.P.M.; Silva, V.S.D.; Martuchi, K.A.; Oshiiwa, M.; Farinazzi Machado, F.M.V.; Mendes, C.G. Metabolic profile of offspring from diabetic Wistar rats treated with Mentha piperita (peppermint). Evid. Based Complement. Altern. Med. 2011, 2011, 430237. [Google Scholar] [CrossRef] [Green Version]
- Mesbahzadeh, B.; Akbari, M.; Zadeh, J.B. The effects of different levels of peppermint alcoholic extract on body-weight gain and blood biochemical parameters of adult male Wistar rats. Electron. Physician 2015, 7, 1376–1380. [Google Scholar] [CrossRef]
- Barbalho, S.M.; Machado, F.M.V.F.; Oshiiwa, M.; Abreu, M.; Guiger, E.L.; Tomazela, P.; Goulart, R.A. Investigation of the effects of peppermint (Mentha piperita) on the biochemical and anthropometric profile of university students. Food Sci. Technol. 2011, 31, 584–588. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, C.B.; Ramos, F.M.; Manthey, J.A.; Cesar, T.B. Effectiveness of Eriomin® in managing hyperglycemia and reversal of prediabetes condition: A double-blind, randomized, controlled study. Phytother. Res. 2019, 33, 1921–1933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cesar, T.B.; Ramos, F.M.M.; Ribeiro, C.B. Nutraceutical eriocitrin (Eriomin) reduces hyperglycemia by increasing glucagon-like peptide 1 and downregulates systemic inflammation: A crossover-randomized clinical trial. J. Med. Food 2022, 25, 1050–1058. [Google Scholar] [CrossRef]
- Chakraborty, K.; Chakravarti, A.R.; Bhattacharjee, S. Bioactive components of peppermint (Mentha piperita L.), their pharmacological and ameliorative potential and ethnomedicinal benefits: A review. J. Pharmacogn. Phytochem. 2022, 11, 109–114. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of water-soluble polyphenolic compounds in commercial herbal teas from Lamiaceae: Peppermint, melissa, and sage. J. Agric. Food Chem. 2007, 55, 10908–10917. [Google Scholar] [CrossRef]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of polyphenolic composition of a herbal medicinal product—peppermint tincture. Molecules 2019, 25, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Council of Europe. European Pharmacopoeia, 10th ed.; Council of Europe: Strasbourg, France, 2019; ISBN 928-718-505-0. [Google Scholar]
- European Medicines Agency. Assessment Report on Mentha × piperita L.; Folium and Aetheroleum, Final—Revision 1. Available online: https://www.ema.europa.eu/en/documents/herbal-report/assessment-report-mentha-x-piperita-l-folium-aetheroleum-revision-1_en.pdf (accessed on 15 January 2023).
- European Medicines Agency. European Union Herbal Monograph on Mentha × piperita L.; Folium, Final—Revision 1. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/draft-european-union-herbal-monograph-mentha-x-piperita-l-folium-revision-1_en.pdf (accessed on 15 January 2023).
- Sroka, Z.; Fecka, I.; Cisowski, W. Antiradical and anti-H2O2 properties of polyphenolic compounds from an aqueous peppermint extract. Z. Naturforsch. C J. Biosci. 2005, 60, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Dorman, H.D.; Koşar, M.; Başer, K.H.C.; Hiltunen, R. Phenolic profile and antioxidant evaluation of Mentha × piperita L.(peppermint) extracts. Nat. Prod. Commun. 2009, 4, 1934578X0900400419. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Chen, H.; Zhu, Y.; Sedighi, R.; Ho, C.T.; Sang, S. Essential structural requirements and additive effects for flavonoids to scavenge methylglyoxal. J. Agric. Food Chem. 2014, 62, 3202–3210. [Google Scholar] [CrossRef]
- Sarmah, S.; Das, S.; Roy, A.S. Protective actions of bioactive flavonoids chrysin and luteolin on the glyoxal induced formation of advanced glycation end products and aggregation of human serum albumin: In vitro and molecular docking analysis. Int. J. Biol. Macromol. 2020, 165, 2275–2285. [Google Scholar] [CrossRef]
- Shamsi, A.; Ahmed, A.; Khan, M.S.; Husain, F.M.; Bano, B. Rosmarinic acid restrains protein glycation and aggregation in human serum albumin: Multi spectroscopic and microscopic insight-possible therapeutics targeting diseases. Int. J. Biol. Macromol. 2020, 161, 187–193. [Google Scholar] [CrossRef]
- Piwowar, A.; Rorbach-Dolata, A.; Fecka, I. The antiglycoxidative ability of selected phenolic compounds—An in vitro study. Molecules 2019, 24, 2689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mortera, R.R.; Bains, Y.; Gugliucci, A. Fructose at the crossroads of the metabolic syndrome and obesity epidemics. Front. Biosci. (Landmark Ed.) 2019, 24, 186–211. [Google Scholar] [CrossRef] [PubMed]
- Vekic, J.; Vujcic, S.; Bufan, B.; Bojanin, D.; Al-Hashmi, K.; Al-Rasadi, K.; Stoian, A.P.; Zeljkovic, A.; Rizzo, M. The role of advanced glycation end products on dyslipidemia. Metabolites 2023, 13, 77. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, P.V.; Tavares, J.F.; Costa, M.A.; Mattar, J.B.; Alfenas, R.C. Effect of reducing dietary advanced glycation end products on obesity-associated complications: A systematic review. Nutr. Rev. 2019, 77, 725–734. [Google Scholar] [CrossRef]
- Bucala, R.; Cerami, A. Advanced glycosylation: Chemistry, biology, and implications for diabetes and aging. Adv. Pharmacol. 1992, 23, 1–34. [Google Scholar] [CrossRef]
- Lo, T.W.; Westwood, M.E.; McLellan, A.C.; Selwood, T.; Thornalley, P.J. Binding and modification of proteins by methylglyoxal under physiological conditions. A kinetic and mechanistic study with N alpha-acetylarginine, N alpha-acetylcysteine, and N alpha-acetyllysine, and bovine serum albumin. J. Biol. Chem. 1994, 269, 32299–32305. [Google Scholar] [CrossRef]
- Kilhovd, B.K.; Giardino, I.; Torjesen, P.A.; Birkeland, K.I.; Berg, T.J.; Thornalley, P.J.; Brownlee, M.; Hanssen, K.F. Increased serum levels of the specific AGE-compound methylglyoxal-derived hydroimidazolone in patients with type 2 diabetes. Metabolism 2003, 52, 163–167. [Google Scholar] [CrossRef]
- Lee, J.H.; Samsuzzaman, M.; Park, M.G.; Park, S.J.; Kim, S.Y. Methylglyoxal-derived hemoglobin advanced glycation end products induce apoptosis and oxidative stress in human umbilical vein endothelial cells. Int. J. Biol. Macromol. 2021, 187, 409–421. [Google Scholar] [CrossRef]
- Ito, K.; Sakata, N.; Nagai, R.; Shirakawa, J.I.; Watanabe, M.; Mimata, A.; Abe, Y.; Yasuno, T.; Sasatomi, Y.; Miyake, K.; et al. High serum level of methylglyoxal-derived AGE, N δ-(5-hydro-5-methyl-4-imidazolone-2-yl)-ornithine, independently relates to renal dysfunction. Clin. Exp. Nephrol. 2017, 21, 398–406. [Google Scholar] [CrossRef]
- Jia, X.; Olson, D.J.; Ross, A.R.; Wu, L. Structural and functional changes in human insulin induced by methylglyoxal. FASEB J. 2006, 20, 1555–1557. [Google Scholar] [CrossRef]
- Westwood, M.E.; Thornalley, P.J. Molecular characteristics of methylglyoxal-modified bovine and human serum albumins. Comparison with glucose-derived advanced glycation endproduct-modified serum albumins. J. Protein Chem. 1995, 14, 359–372. [Google Scholar] [CrossRef]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a highly reactive dicarbonyl compound, in diabetes, its vascular complications, and other age-related diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Boersma, M.G.; Van der Woude, H.; Bogaards, J.; Boeren, S.; Vervoort, J.; Cnubben, N.H.; van Iersel, M.L.P.S.; van Bladeren, P.J.; Rietjens, I.M.C.M. Regioselectivity of phase II metabolism of luteolin and quercetin by UDP-glucuronosyl transferases. Chem. Res. Toxicol. 2002, 15, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, N.; Shimizu, N.; Komoda, T.; Mohri, S.; Tsushida, T.; Eitsuka, T.; Miyazawa, T.; Nakagawa, K. Absorption and metabolism of luteolin in rats and humans in relation to in vitro anti-inflammatory effects. J. Agric. Food Chem. 2018, 66, 11320–11329. [Google Scholar] [CrossRef]
- Hitl, M.; Kladar, N.; Gavarić, N.; Božin, B. Rosmarinic acid–human pharmacokinetics and health benefits. Planta Med. 2021, 87, 273–282. [Google Scholar] [CrossRef]
- Bednarska, K.; Fecka, I. Potential of vasoprotectives to inhibit non-enzymatic protein glycation, and reactive carbonyl and oxygen species uptake. Int. J. Mol. Sci. 2021, 22, 10026. [Google Scholar] [CrossRef]
- Zhou, Q.; Cheng, K.W.; Xiao, J.; Wang, M. The multifunctional roles of flavonoids against the formation of advanced glycation end products (AGEs) and AGEs-induced harmful effects. Trends Food Sci. Technol. 2020, 103, 333–347. [Google Scholar] [CrossRef]
- Kinsky, O.R.; Hargraves, T.L.; Anumol, T.; Jacobsen, N.E.; Dai, J.; Snyder, S.A.; Monks, T.J.; Lau, S.S. Metformin scavenges methylglyoxal to form a novel imidazolinone metabolite in humans. Chem. Res. Toxicol. 2016, 29, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhuiyan, M.N.I.; Mitsuhashi, S.; Sigetomi, K.; Ubukata, M. Quercetin inhibits advanced glycation end product formation via chelating metal ions, trapping methylglyoxal, and trapping reactive oxygen species. Biosci. Biotechnol. Biochem. 2017, 81, 882–890. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Q.; Cheng, K.W.; Gong, J.; Li, E.T.; Wang, M. Apigenin and its methylglyoxal-adduct inhibit advanced glycation end products-induced oxidative stress and inflammation in endothelial cells. Biochem. Pharmacol. 2019, 166, 231–241. [Google Scholar] [CrossRef]
- Liu, H.; Gu, L. Phlorotannins from brown algae (Fucus vesiculosus) inhibited the formation of advanced glycation endproducts by scavenging reactive carbonyls. J. Agric. Food Chem. 2012, 60, 1326–1334. [Google Scholar] [CrossRef]
- Matsuda, H.; Wang, T.; Managi, H.; Yoshikawa, M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorg. Med. Chem. 2003, 11, 5317–5323. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Yang, Z.; He, Y.; Xia, Y.; He, Y.; Liu, J. Multispectroscopic exploration and molecular docking analysis on interaction of eriocitrin with bovine serum albumin. J. Mol. Recognit. 2019, 32, e2779. [Google Scholar] [CrossRef] [PubMed]
- Sarmah, S.; Pahari, S.; Belwal, V.K.; Jana, M.; Roy, A.S. Elucidation of molecular interaction of bioactive flavonoid luteolin with human serum albumin and its glycated analogue using multi-spectroscopic and computational studies. J. Mol. Liq. 2020, 318, 114147. [Google Scholar] [CrossRef]
- Shamsi, A.; Ahmed, A.; Khan, M.S.; Al Shahwan, M.; Husain, F.M.; Bano, B. Understanding the binding between Rosmarinic acid and serum albumin: In vitro and in silico insight. J. Mol. Liq. 2020, 311, 113348. [Google Scholar] [CrossRef]
- Li, D.; Mitsuhashi, S.; Ubukata, M. Protective effects of hesperidin derivatives and their stereoisomers against advanced glycation end-products formation. Pharm. Biol. 2012, 50, 1531–1535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ávila-Gálvez, M.Á.; Giménez-Bastida, J.A.; González-Sarrías, A.; Espín, J.C. New insights into the metabolism of the flavanones eriocitrin and hesperidin: A comparative human pharmacokinetic study. Antioxidants 2021, 10, 435. [Google Scholar] [CrossRef]
- Rustam, M.; Ifora, I.; Fauziah, F. Potential anti-inflammatory effects of eriocitrin: A review. J. Drug Deliv. Ther. 2022, 12, 187–192. [Google Scholar] [CrossRef]
- Liu, J.L.; Kong, Y.C.; Miao, J.Y.; Mei, X.Y.; Wu, S.Y.; Yan, Y.C.; Cao, X.Y. Spectroscopy and molecular docking analysis reveal structural specificity of flavonoids in the inhibition of α-glucosidase activity. Int. J. Biol. Macromol. 2020, 152, 981–989. [Google Scholar] [CrossRef]
- Miyake, Y.; Suzuki, E.; Ohya, S.; Fukumoto, S. Lipid-lowering effect of eriocitrin, the main flavonoid in lemon fruit, in rats on a high-fat and high-cholesterol diet. J. Food Sci. 2006, 71, S633–S637. [Google Scholar] [CrossRef]
- Wan, J.; Feng, Y.; Du, L.; Veeraraghavan, V.P.; Mohan, S.K.; Guo, S. Antiatherosclerotic activity of eriocitrin in high-fat-diet-induced atherosclerosis model rats. J. Environ. Pathol. Toxicol. Oncol. 2020, 39, 61–75. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Choi, M.S. Eriocitrin improves adiposity and related metabolic disorders in high-fat diet-induced obese mice. J. Med. Food 2020, 23, 233–241. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, P.S.; Manthey, J.A.; Nery, M.S.; Spolidorio, L.C.; Cesar, T.B. Low doses of eriocitrin attenuate metabolic impairment of glucose and lipids in ongoing obesogenic diet in mice. J. Nutr. Sci. 2020, 9, e59. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Ma, L.; Fu, P. Eriocitrin attenuates ischemia reperfusion-induced oxidative stress and inflammation in rats with acute kidney injury by regulating the dual-specificity phosphatase 14 (DUSP14)-mediated Nrf2 and nuclear factor-κB (NF-κB) pathways. Ann. Transl. Med. 2021, 9, 350. [Google Scholar] [CrossRef] [PubMed]
- Hiramitsu, M.; Shimada, Y.; Kuroyanagi, J.; Inoue, T.; Katagiri, T.; Zang, L.; Nishimura, Y.; Nishimura, N.; Tanaka, T. Eriocitrin ameliorates diet-induced hepatic steatosis with activation of mitochondrial biogenesis. Sci. Rep. 2014, 4, 3708. [Google Scholar] [CrossRef] [Green Version]
- Fecka, I.; Kowalczyk, A.; Cisowski, W. Optimization of the separation of flavonoid glycosides and rosmarinic acid from Mentha piperita on HPTLC plates. J. Planar Chromatorgr. Mod. TLC 2004, 17, 22–25. [Google Scholar] [CrossRef]
- Fecka, I.; Turek, S. Determination of polyphenolic compounds in commercial herbal drugs and spices from Lamiaceae: Thyme, wild thyme and sweet marjoram by chromatographic techniques. Food Chem. 2008, 108, 1039–1053. [Google Scholar] [CrossRef]

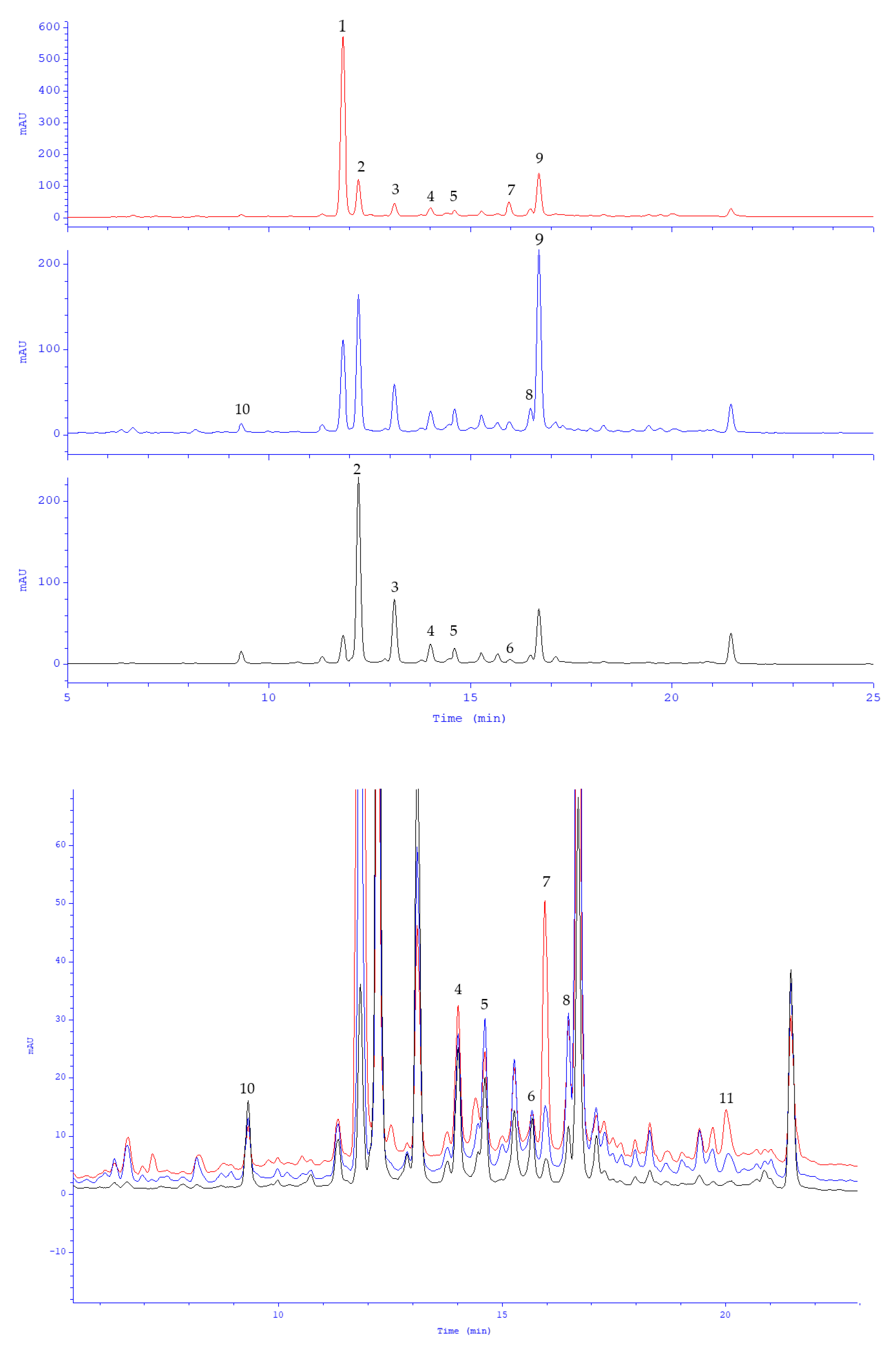


| No. | tR (min) | [M-H]− (m/z) | MS/MS (m/z) | Compound | Reference |
|---|---|---|---|---|---|
| Phenolic acids—caffetannins | |||||
| 1. | 9.23 | 179.0355 | 135 | caffeic acid | Stan. |
| 2. | 11.30 | 537.1044 | 493, 295, 185 | lithospermic acid (lithospermic acid a) | Stan. |
| 3. | 12.85 | 359.0778 | 197, 179, 161, 135 | rosmarinic acid | Stan. |
| 4. | 13.32 | 717.1457 | 519, 339, 321, 295 | lithospermic acid b (salvianolic acid b) | [15] |
| Flavonoids | |||||
| 5. | 11.12 | 595.1673 | 287 | eriodictyol-7-O-rutinoside (eriocitrin) | Stan. |
| 6. | 11.44 | 593.1508 | 285 | luteolin-7-O-rutinoside (scolymoside) | Stan. |
| 7. | 11.45 | 449.1089 | 287 | eriodictyol-7-O-β-glucoside | [15] |
| 8. | 11.73 | 447.0943 | 285 | luteolin-7-O-β-glucoside | Stan. |
| 9. | 11.76 | 463.0863 | 287 | eriodictyol-7-O-β-glucuronoside | [15] |
| 10. | 11.79 | 461.0734 | 285 | luteolin-7-O-β-glucuronoside | Stan. |
| 11. | 11.98 | 579.1710 | 271 | naringenin-7-O-rutinoside (narirutin) | Stan. |
| 12. | 12.16 | 577.1556 | 269 | apigenin-7-O-rutinoside (isorhoifolin) | Stan. |
| 13. | 12.45 | 609.1816 | 301 | hesperetin-7-O-rutinoside (hesperidin) | Stan. |
| 14. | 12.48 | 607.1667 | 299 | diosmetin-7-O-rutinoside (diosmin) | Stan. |
| 15. | 12.67 | 445.0775 | 269 | apigenin-7-O-β-glucuronoside | [15] |
| 16. | 14.43 | 287.0565 | - | eriodictyol | Stan. |
| 17. | 14.82 | 285.0406 | - | luteolin | Stan. |
| 18. | 16.13 | 269.0449 | - | apigenin | Stan. |
| 19. | 16.37 | 301.0723 | - | hesperetin | Stan. |
| Compound | M.W. | Content | SD | Percentage of Compound | ||
|---|---|---|---|---|---|---|
| g/mol | mg/g | μM/g | % | % (for mg/g) | % (for μM/g) | |
| Caffeic acid | 180.16 | 0.52 | 2.89 | 1.9 | 0.1 | 0.3 |
| Rosmarinic acid | 360.31 | 57.81 | 160.45 | 3.3 | 11.8 | 17.7 |
| Lithospermic acid | 538.46 | 8.29 | 15.40 | 2.4 | 1.7 | 1.7 |
| Eriodictyol-7-O-rutinoside | 596.54 | 285.37 | 478.38 | 3.4 | 58.1 | 52.9 |
| Luteolin-7-O-rutinoside | 594.52 | 78.52 | 132.07 | 3.5 | 16.0 | 14.6 |
| Luteolin-7-O-β-glucoside | 448.38 | 0.03 | 0.00 | 0.0 | 0.0 | 0.0 |
| Luteolin-7-O-β-glucuronoside | 462.36 | 27.56 | 59.61 | 3.7 | 5.6 | 6.6 |
| Naringenin-7-O-rutinoside | 580.54 | 1.24 | 2.14 | 1.1 | 0.3 | 0.2 |
| Apigenin-7-O-rutinoside | 578.52 | 3.38 | 5.84 | 2.1 | 0.7 | 0.6 |
| Diosmin | 608.55 | 4.74 | 7.79 | 2.3 | 1.0 | 0.9 |
| Hesperidin | 610.56 | 22.86 | 37.44 | 1.2 | 4.7 | 4.1 |
| Eriodictyol | 288.25 | 0.61 | 2.12 | 2.5 | 0.1 | 0.2 |
| Luteolin | 286.24 | 0.11 | 0.38 | 3.7 | 0.0 | 0.0 |
| Apigenin | 270.05 | <LOQ | <LOQ | - | 0.0 | 0.0 |
| Hesperetin | 302.28 | <LOQ | <LOQ | - | 0.0 | 0.0 |
| Sum of phenolic acids | 66.62 | 178.73 | 2.4 | 13.6 | 19.8 | |
| Sum of flavonoids | 424.42 | 725.76 | 4.3 | 86.4 | 80.2 | |
| Sum of polyphenols | 491.04 | 904.49 | 4.2 | 100.0 | 100.0 | |
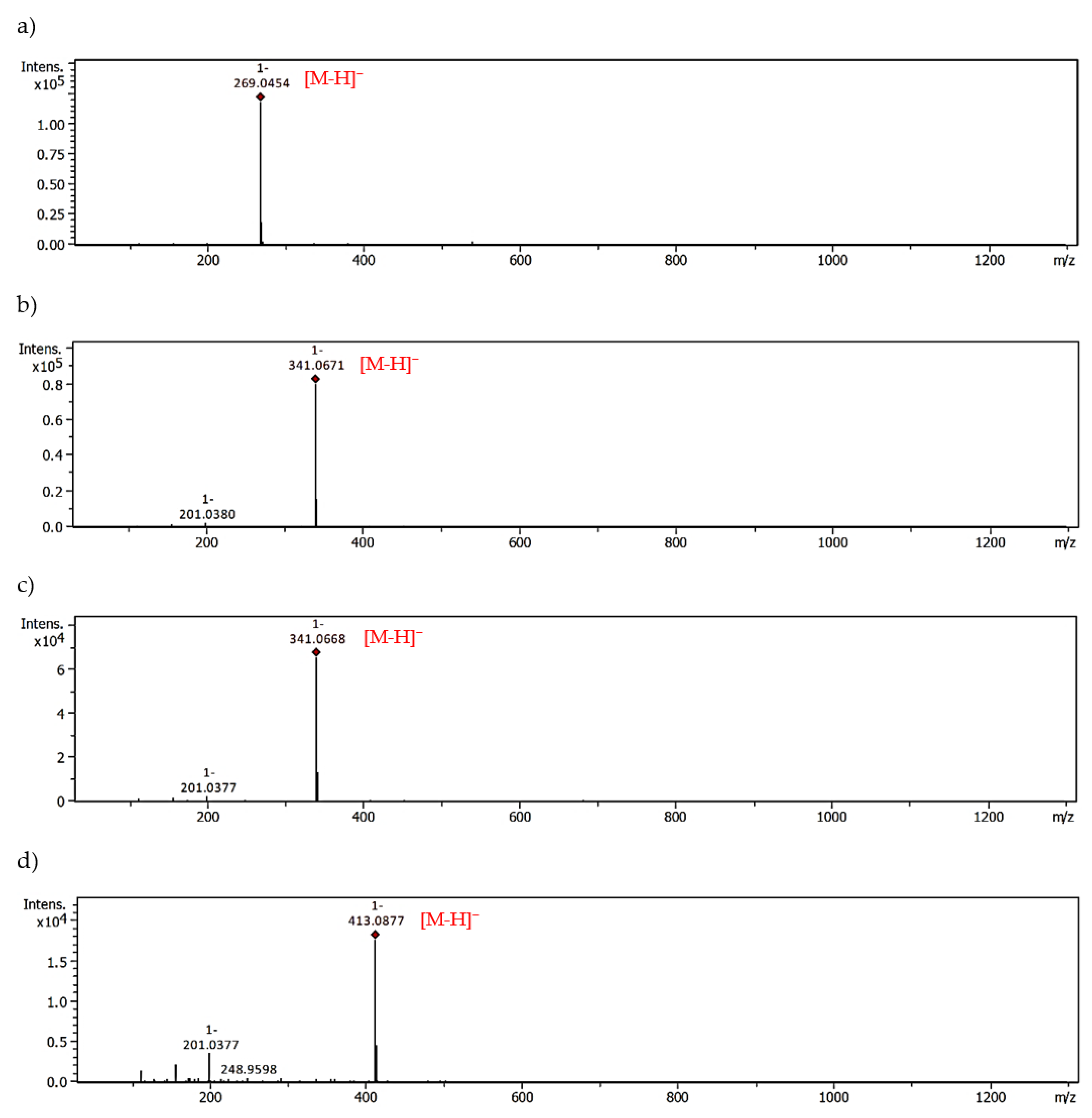
| Compound | Pseudomolecular Ions of Adducts, m/z [Da] | |||
|---|---|---|---|---|
| Mono-MGO | tR [min] | Di-MGO | tR [min] | |
| [M+72-H]− | [M+144-H]− | |||
| Apigenin | 341.0671 | 28.57 | 413.0877 | 25.98 |
| 341.0668 | 28.03 | |||
| Luteolin | 357.0615 | 26.23 | 429.0826 | 24.22 |
| 357.0615 | 25.97 | |||
| Luteolin-7-O-rutinoside | - | - | - | - |
| Luteolin-7-O-β-glucuronoside | - | - | - | - |
| Luteolin-7-O-β-glucoside | - | - | - | - |
| Hesperetin | 373.0942 | 28.70 | 445.1126 | 25.33 |
| 373.0941 | 28.76 | |||
| Eriodictyol | 359.0772 | 21.48 | - | - |
| 359.0771 | 21.57 | |||
| 359.0777 | 22.46 | |||
| 359.0775 | 24.67 | |||
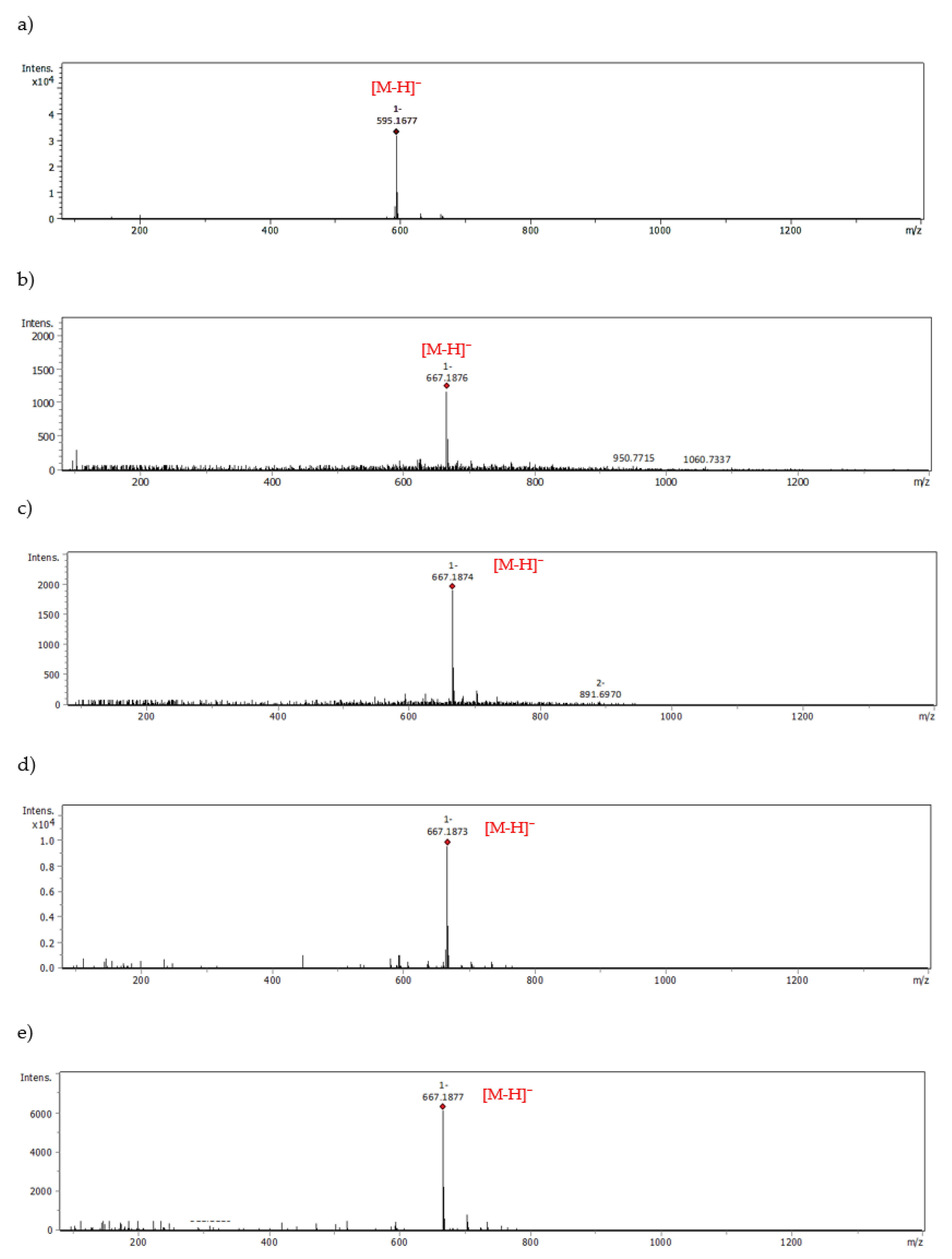
| Eriocitrin | 667.1876 | 19.21 | - | - |
| 667.1874 | 20.59 | |||
| 667.1873 | 21.40 | |||
| 667.1877 | 24.23 | |||
| Rosmarinic acid | - | - | - | - |
| Peppermint leaf dry extract—Eriocitrin | 667.1876 | 19.21 | - | - |
| 667.1874 | 20.59 | |||
| 667.1873 | 21.40 | |||
| 667.1877 | 24.23 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fecka, I.; Bednarska, K.; Kowalczyk, A. In Vitro Antiglycation and Methylglyoxal Trapping Effect of Peppermint Leaf (Mentha × piperita L.) and Its Polyphenols. Molecules 2023, 28, 2865. https://doi.org/10.3390/molecules28062865
Fecka I, Bednarska K, Kowalczyk A. In Vitro Antiglycation and Methylglyoxal Trapping Effect of Peppermint Leaf (Mentha × piperita L.) and Its Polyphenols. Molecules. 2023; 28(6):2865. https://doi.org/10.3390/molecules28062865
Chicago/Turabian StyleFecka, Izabela, Katarzyna Bednarska, and Adam Kowalczyk. 2023. "In Vitro Antiglycation and Methylglyoxal Trapping Effect of Peppermint Leaf (Mentha × piperita L.) and Its Polyphenols" Molecules 28, no. 6: 2865. https://doi.org/10.3390/molecules28062865
APA StyleFecka, I., Bednarska, K., & Kowalczyk, A. (2023). In Vitro Antiglycation and Methylglyoxal Trapping Effect of Peppermint Leaf (Mentha × piperita L.) and Its Polyphenols. Molecules, 28(6), 2865. https://doi.org/10.3390/molecules28062865