Abstract
Background: Echinophora tenuifolia L. subsp. sibthorpiana is a perennial, aromatic plant used in traditional folk medicine and cuisine of the Mediterranean and the Middle East. However, scholars have not fully studied the pharmacological potential of the herb, and the scientific data on this plant species are limited. This study aimed to evaluate the chemical composition of the essential oil (EO) obtained from the aerial parts of E. tenuifolia subsp. sibthorpiana growing wild in Bulgaria and to perform histochemical analysis. Methods: A microscopic histochemical analysis and gas chromatography with mass spectrometry were performed. Results: The histochemical analysis confirmed the presence of terpenes in the stem and leaf of E. tenuifolia subsp. sibthorpiana. The phenylpropanoid methyleugenol was identified as the main compound in the EO, representing 48.13% of the total oil composition. There were also found considerable amounts of monoterpene hydrocarbons, representing 41.68% of the total EO. Alpha-phellandrene, o-cymene, and β-phellandrene were the most abundant monoterpene hydrocarbons. Conclusion: This is the first histochemical analysis performed on E. tenuifolia subsp. sibthorpiana. This is the first report of the EO composition from Bulgarian E. tenuifolia subsp. sibthorpiana, and our results indicate some future possibilities for evaluating of the biological activity of the EO of E. tenuifolia subsp. sibthorpiana and highlight the potential future use of the EO of this plant species. E. tenuifolia L. subsp. sibthorpiana EO possesses a good potential for use as a biopesticide and repellent an environmentally friendly alternative of synthetic pesticides.
1. Introduction
The genus Echinophora is a small genus within the Apiaceae family composed of about ten species distributed in the Mediterranean and the Middle East, with only three taxa (Echinophora spinosa L., Echinophora tenuifolia L. subsp. sibthorpiana (Guss.) Tutin, and Echinophora tenuifolia L. subsp. tenuifolia) occurring in Europe [1]. Echinophora tenuifolia L. subsp. sibthorpiana (Guss.) (E. tenuifolia subsp. sibthorpiana) is the only representative of the genus in the Bulgarian flora [2,3]. It is a perennial, aromatic plant with a hard stem reaching up to 50 cm in height, branching almost from the base and densely covered with trichomes. The basal leaves have petioles with lobes two to three times deeper than the shoulders. The apical leaves are sessile, and the flowers are yellow, arranged in an inflorescence compound umbel on the terminal branches of the stems [3]. The species grows in other countries, most notably in Turkey, where people use it as a spice in pickles, meatballs, and the traditional fermented cereal food Tarhana [4]. E. tenuifolia subsp. sibthorpiana is also known as Tarhana herb. According to some sources, Tarhana is one of the oldest foods in the cuisine of the Eastern Mediterranean [5,6]. In Greek cuisine, it is known as Trahanas [5]. This fermented cereal-based food is prepared by mixing yoghurt, cereal flours, yeast, different vegetables, herbs, and spices. After mixing the main ingredients, the dough ferments for several days (usually up to 5) [6]. The last step of the process is drying. The specific sour taste and yeast flavor of Tarhana is due to the presence of lactic acid bacteria and yeast [6]. Although E. tenuifolia subsp. sibthorpiana is used as a spice in the production of the Tarhana, it also plays an essential role in the fermentation process [4]. People also use E. tenuifolia L. subsp. sibthorpiana as a folk medicine for its wound-healing properties, its digestive activity, and for gastric ulcer treatment [7].
Plants used in nutrition and folk medicine are particularly rich in many bioactive compounds, possessing various biological effects, and some of them have a promising potential for inclusion in innovative herbal medicines or functional foods. The beneficial effects of these plants are due to the whole, rather than any single nutrient or functional compound [8,9].
However, scholars have not fully studied the pharmacological potential of the herb, and the scientific data on this plant are limited. The authors of several studies have investigated the pharmacological properties of species from the genus Echinophora, and Table 1 summarizes these studies.

Table 1.
Studies investigating the biological and pharmacological activities involving species from the genus Echinophora.
E. tenuifolia L. subsp. sibthorpiana could be considered not only as a plant used in nutrition and traditional medicine but also as a source of essential oil.
Essential oils are important sources of compounds with health-beneficial activity in humans, while their main function in plants is to protect them from insects or pathogenic microorganisms [23]. The concentration of the different volatile compounds in essential oils is strongly influenced not only by the plant species but also by the geographical region, the harvesting process, and the soil type [23].
Several previous phytochemical studies have investigated the composition of the E. tenuifolia L. subsp. sibthorpiana EO. The major compounds identified in the EO are the phenylpropanoid methyleugenol (ME) and the monoterpene hydrocarbons α-phellandrene, δ-3-carene, and p-cymene [1,22,24,25,26,27,28]. Different factors including the geographical location, collection time, harvest year, growth stage of the plant, and postharvest handling, specifically the drying method and the EO storage, considerably affect the chemical composition of the EO [7,24,29,30]. When reviewing the literature, no records that discussed the composition of the E. tenuifolia subsp. sibthorpiana EO from Bulgaria were found. Additionally, no record of this aromatic plant exists in any standardization document.
The aim of the present study was to evaluate the chemical composition of the EO from aerial parts of E. tenuifolia subsp. sibthorpiana growing wild in Bulgaria and to perform histochemical analysis. The histochemical analysis of E. tenuifolia subsp. sibthorpiana has been carried out for the first time. Its aim was to trace the accumulation of terpenes, in particular the essential oil (composed mainly of monoterpenes and sesquiterpenes), as a secondary metabolite.
2. Results and Discussion
2.1. Microscopic Histochemical Analysis
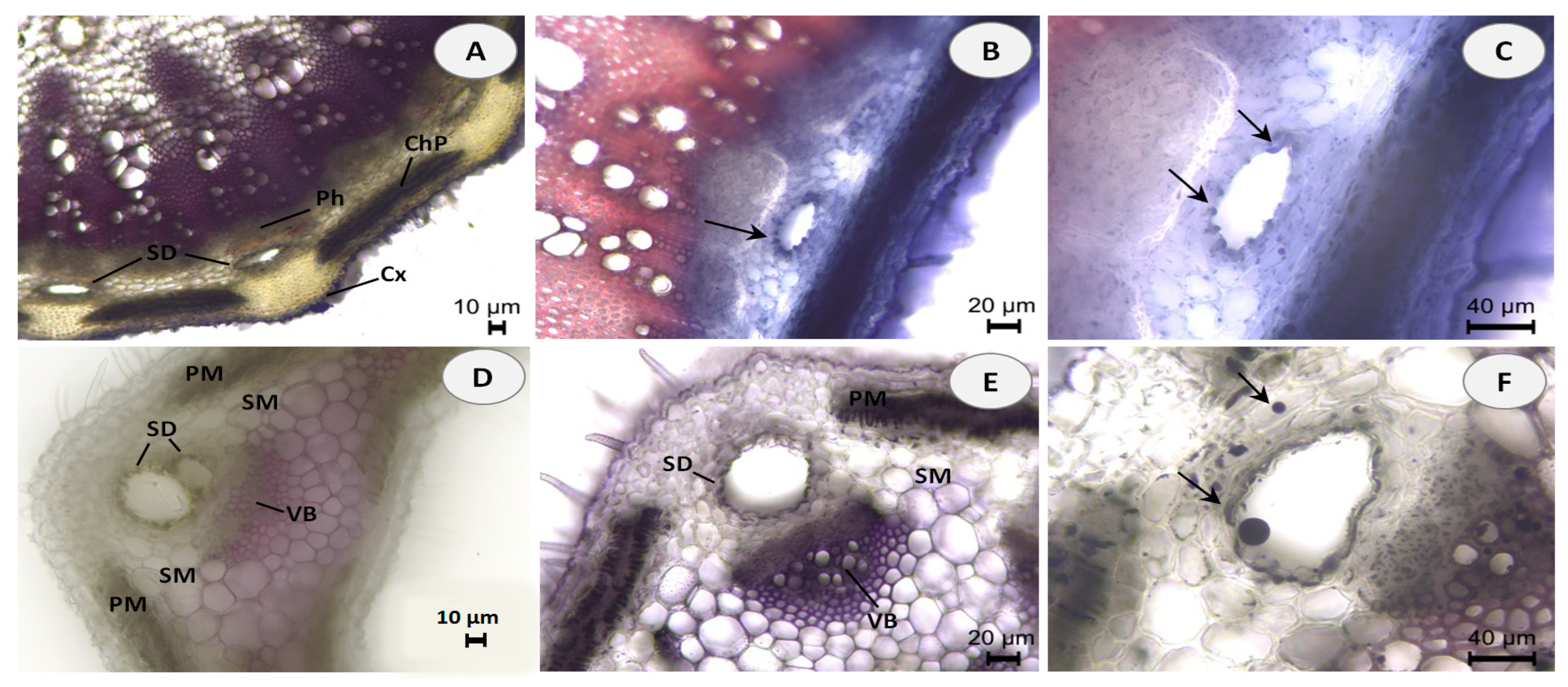
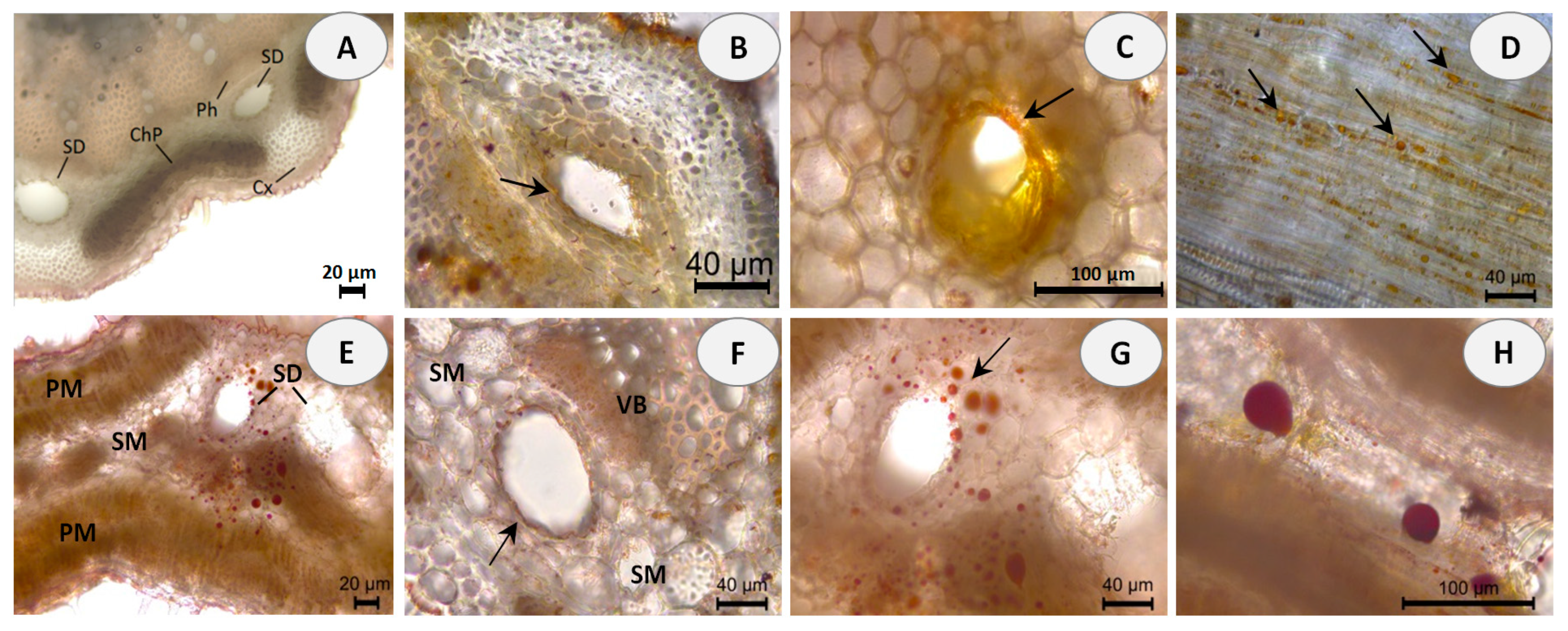
Hand-cut transverse and longitudinal sections of the stem and leaf of E. tenuifolia subsp. sibthorpiana were stained with NADI reagent to detect terpenes and Sudan III to detect lipids. NADI reagent produces differential staining in plant histology to demonstrate various terpene classes. The staining is blue with essential oils and red with resins (diterpenes, triterpenes, and derivatives) [26]. Positive blue staining indicates the presence of essential oil, containing mainly mono- and sesquiterpenes (Figure 1). In the Sudan III test, the presence of red or orange staining demonstrates the presence of lipid compounds (Figure 2).
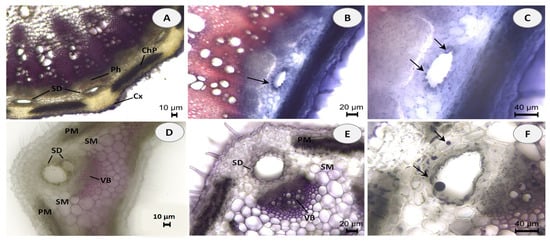
Figure 1.
Histochemical observations of terpenes stained with NADI reagent in stem and leaf cross sections of E. tenuifolia L. subsp. sibthorpiana (Guss.) Tutin. (A) General aspect of stem section, showing the presence of secretory ducts (SD) arranged in a ring between the cortex (Cx), secondary phloem (Ph) and chlorophyllous parenchyma (ChP). (B,C) Details of stem secretory ducts (SD) with positive blue staining droplets. (D) General aspect of leaf, showing palisade mesophyll (PM) and spongy mesophyll (SM) with secretory ducts (SD) adjacent to the vascular bundles (VB). (E,F) Details of leaf secretory ducts (SD) with positive blue staining of the one-layered epithelium and droplets in the intercellular. Scale bars: (A,D) = 10 µm; (B,E) = 20 µm; (C,F) = 40 µm.
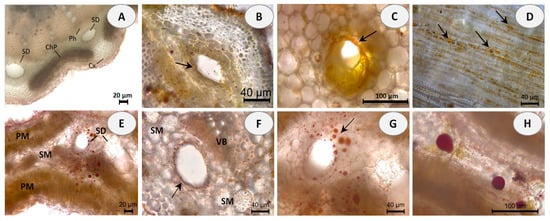
Figure 2.
Histochemical observations of lipids stained with Sudan III in stem and leaf sections of E. tenuifolia L. subsp. sibthorpiana (Guss.) Tutin. (A) General aspect of transverse stem section, showing the presence of secretory ducts (SD) between the cortex (Cx), secondary phloem (Ph) and chlorophyllous parenchyma (ChP). (B,C) Details of stem secretory ducts with positive red-orange staining of the surrounding epithelial cells. (D) Tangential longitudinal section of the cortical region, showing the presence of lipid droplets with positive red-orange staining. (E) Leaf cross section, showing the presence of secretory ducts (SD) with red stained lipid droplets, located in the spongy mesophyll (SM) and palisade mesophyll (PM). (F) Positive red staining of the epithelial cells around the leaf ducts. (G,H) Details of leaf secretory ducts with positive red staining of the lipid deposits. Scale bars: (A,E) = 20 µm; (B,D,F,G) = 40 µm; (C,H) = 100 µm.
Essential oils can be produced in multiple secretory structures, including those that are external, which involve secretory glandular trichomes, and those that are internal, which include secretory cavities, ducts, and individual essential oil cells (parenchyma cells that secrete and store essential oil). These secretory structures can vary depending on the botanical family. The secretory ducts in the plant family Apiaceae are a typical example of schizogenous cavities.
In the present histochemical analysis of E. tenuifolia subsp. sibthorpiana, such internal secretory ducts were observed in both of the plant organs examined. In the stem, these oil secretory structures were arranged in a ring between the phloem parenchyma and the surrounding cortex (Figure 1A and Figure 2A). In the leaf, the ducts were located among the mesophyll cells, accompanying the vascular bundles (Figure 1D,E and Figure 2E,F). The NADI reaction resulted in blue staining of the secreted droplets in the ducts and related surrounding cells in the samples of both plant organs analyzed, which confirmed the presence of essential oil (Figure 1B,C,E,F). Regarding the Sudan III test, the lipid nature of the secretory ducts was confirmed by the positive red-orange staining of the epithelial cells, grouped as a layer around the duct and responsible for the oil secretion (Figure 2B,C,F). In addition, numerous red-orange stained lipid droplets were observed in the leaf mesophyll cells adjacent to the ducts (Figure 2G,H), as well as in the tangential stem section of the cortical region (Figure 2D). Due to the lipophilic nature of essential oil compounds, their presence was confirmed in both analyses.
2.2. Volatile Constituents of the E. tenuifolia ssp. sibthorpiana Essential Oil
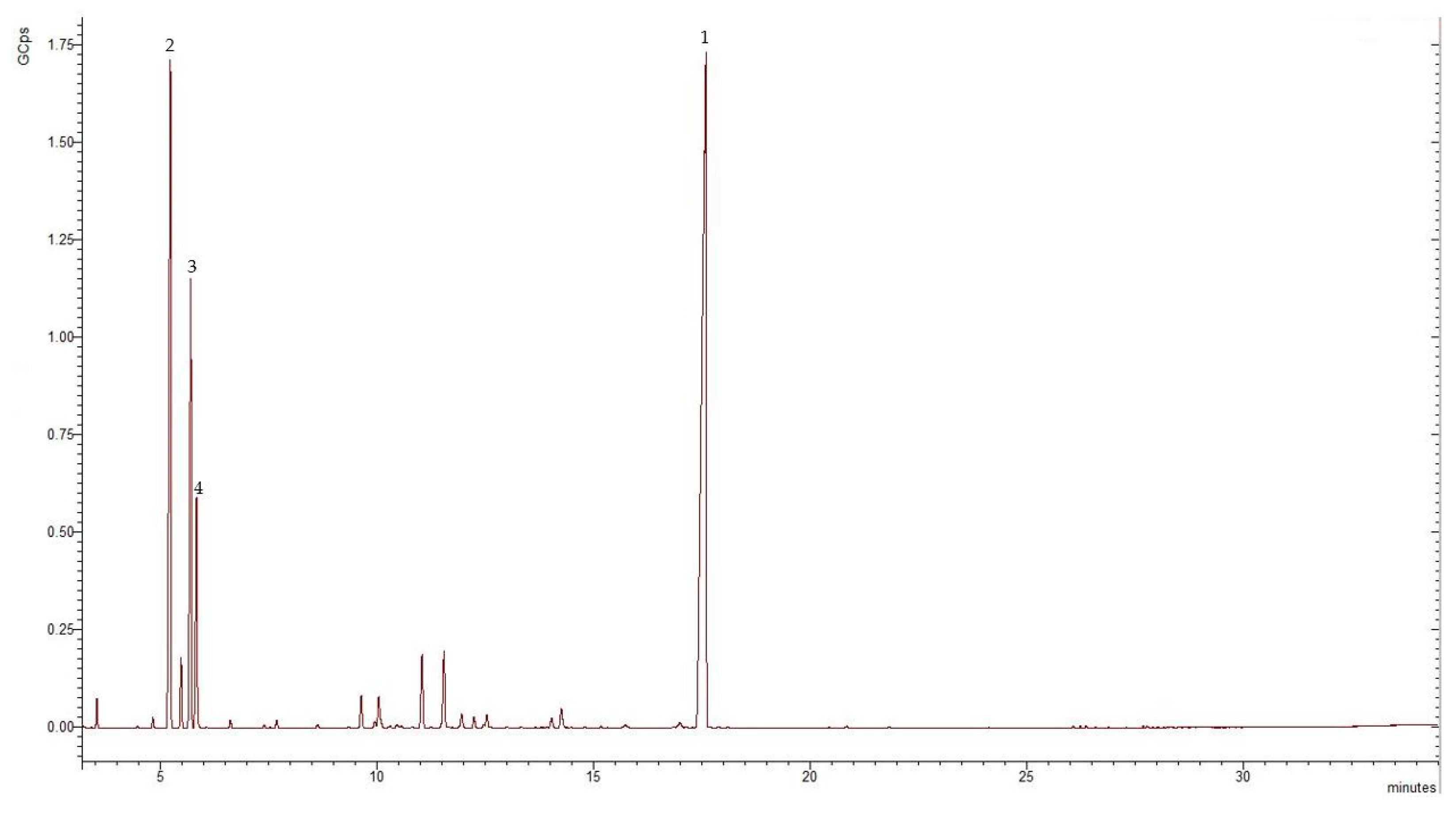
The essential oil obtained from the aerial parts of E. tenuifolia subsp. sibthorpiana was pale yellow in color with a 1.3% yield. The results of the GC-MS analyses of the essential oil showed that 16 constituents representing 92% of the total oil content were tentatively present. Figure 3 and Table 2 show the chromatogram and the chemical composition of the EO.
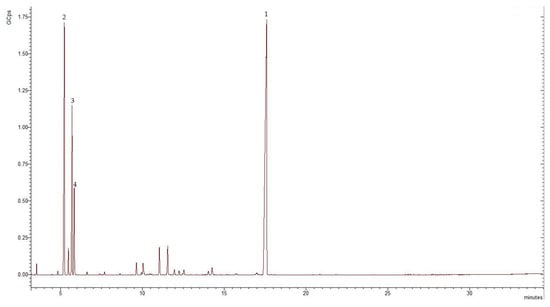
Figure 3.
GC-MS chromatogram of the E. tenuifolia subsp. sibthorpiana EO, where GCps—Giga Counts per second, and the numbers refer to the following compounds: 1—methyleugenol, 2—α-phellandrene, 3—o-cymene, and 4—β-phellandrene.

Table 2.
Volatile constituents of the EO of E. tenuifolia subsp. sibthorpiana from Bulgaria as a percentage of total EO; where tr—trace (less than 0.05%).
The identified compounds belonged to the phenylpropanoids, the monoterpene hydrocarbon, and the oxygenated monoterpene classes. Phenylpropanoid methyleugenol was the main compound identified in the analyzed EO, representing 48.13% of the total oil composition. Considerable amounts of monoterpene hydrocarbons were also found, representing 41.68% of the total EO. Alpha-phellandrene, o-cymene, and β-phellandrene were the most abundant monoterpene hydrocarbons, representing 20.51%, 12.29%, and 6% of the total oil, respectively. The oxygenated monoterpenes represented 2.22% of the total EO.
Our results complied with the results of earlier studies on the chemical composition of E. tenuifolia subsp. sibthorpiana EO from different geographical regions (Table 3).

Table 3.
Comparison of the main volatile constituents of the EO of E. tenuifolia subsp. sibthorpiana from different regions.
The data suggest that the main volatile compounds identified in E. sibthorpiana from some regions of Turkey were methyleugenol and α-phelandrene. The amount of methyleugenol ranges from 9.61% to 90.16%, and that of α-phellandrene ranges from 6.10% to 66.39% [22,24,25,28,29,30,32,33] which corresponds with the results of the current study. Methyleugenol has also been identified as a major compound in the EO derived from the plant species studied, distributed in some regions in Iran (50.40%) and the Republic of North Macedonia (60.40%) [26,27]. Additionally, in previous reported studies, α-phellandrene (10.23–43.80%) was among the major volatile compounds detected in E. tenuifolia subsp. sibthorpiana EO from regions of Iran, Macedonia, and Greece [1,26,27,31]. Despite the different factors such as harvest period, environmental conditions, and geographical variations affecting the chemical composition of EO and the observed quantitative differences, the main constituent detected in EOs from Bulgaria, Turkey, and Macedonia was methyleugenol [1,24,25,26,34]. The monoterpene hydrocarbon δ-3-carene has been identified as a major constituent of EO from Turkey [22]; however, this compound was not detected in the presented EO analysis. Sesquiterpenes were also not detected. While in small amounts the sesquiterpenes germacrene D, β-selinene, β-ionone, and caryophyllene oxide were identified in previous studies [25,26].
Compared to other European species of the genus Echinophora, the main constituents found in E. spinosa from Montenegro were δ-3-carene, α-phellandrene, and p-cymene [35]. Beta-phellandrene was found as the major constituent of flowering aerial parts of E. spinosa from Italy, with myristicin, p-cymene, and δ-3-carene also being present in significant amounts. The major volatile constituent of EO obtained from the ripe fruit of the same plant was p-cymen [18]. Para-cymen, α-phellandrene, and α-pinene were the principal constituents of EO of the aerial parts of E. spinosa from Corsica Island, France. Methyleugenol was presented in small amounts in the root EO of the same plant, while the most abundant compound was the phenylpropanoid myristicin [36].
The major volatile compounds identified in EO from Iranian E. platyloba were trans-β-ocimene, β-cymene spathulenol, β-myrcene, DL-limonene, α-pinene, β-phellandrene, and α- phellandrene [37,38,39]. A study on the chemical composition of EO isolated from Echinophora lamondiana B. Yildiz et Z. Bahcecioglu showed that the major volatile compounds were δ-3-carene, α-phellandrene, and p-cymene [40,41]. In a previous study, it was reported that the EO of E. tournefortii contained α-pinene and caryophyllene oxide as the major volatile components, while the EO of E. chrysantha contained α-phellandrene and β-phellandrene [42]. Moreover, it has been reported that the E. trichophylla EO contained sabinene and 2,6-dimethyl-1,3(E),5(E),7-octatetraene as main constituents, that the EO of E. carvifolia contained β-caryophyllene and germacrene D, and that the EO of E. orientalis contained myrcene and p-cymene as major volatile components [42].
When comparing EOs isolated from different Echinophora species, the main volatile compounds detected were α-phellandrene, β-phellandrene, δ-3-carene, myrcene, and p-cymene. The difference between EO isolated from E. tenuifolia subsp. sibthorpiana and EO isolated from other Echinophora species was the detected methyleugenol as the main volatile compound in the E. tenuifolia subsp. sibthorpiana EO.
Methyleugenol represents a colorless to pale yellow liquid with a clove odor and a bitter taste [43]. It could be produced by methylation of eugenol or isolated from the EO of different plants such as citronella (Cymbopogon spp.), basil (Ocimum spp.), and tea tree (Melaleuca spp.) [43]. Nowadays, it is mainly used as repellent [43]. In 1975, ME was classified by the Food and Drug Administration (FDA) as a safe compound under the conditions of use. It was approved for use in foods as a flavoring agent and as a fragrance in cosmetics. However, the safety of ME is a matter of some controversy [44]. The biological activity of ME is quite diverse and has been assessed by various in vitro and in vivo studies [45,46,47,48,49,50,51,52,53,54,55]. Methyleugenol exerted antiallergic activity in IgE-activated RBL-2H3 cells through various mechanisms [45,46]. It showed neuroprotective effects due to its radical scavenging activity and inhibition of the production of NO and proinflammatory cytokines [47]. Methyleugenol possessed counter-anorexigenic effects related to satiety [50]. The central effects of ME may be attributed to its agonism toward inhibitory neuronal γ-aminobutyric acid (GABA) receptors [48,49,51]. Another study reported the potential of ME to lower blood pressure, most likely by relaxation of the vascular smooth muscles [53]. It has been reported that methyleugenol has significant antifungal activity [54,55]. Methyleugenol also demonstrated antibacterial activity against Staphylococcus aureus, Streptococcus pneumoniae, and Haemophilus influenzae [54]. Methyleugenol intake was also associated with anesthetic, hypothermic, myorelaxant, and anticonvulsant effects [52]. However, it should be noted that cytotoxicity, genotoxicity, and hepatotoxicity have also been reported in animal studies [56,57,58]. The lipophilicity of phenylpropanoids had an influence on their toxicity [54]. Additionally, the toxic effects are dose-dependent. The cancerogenic doses of methyleugenol in rodents following chronic exposure are approximately 1000-fold higher than the usual dietary intake of methyleugenol as a flavoring constituent in food [59]. According to the European Medicine Agency (EMA) report, the acute toxicity of this compound has not been fully investigated [44]. EMA reports LD50 values for ME from 850 to 1560 mg/kg for rats and from 540 mg/kg for mice.
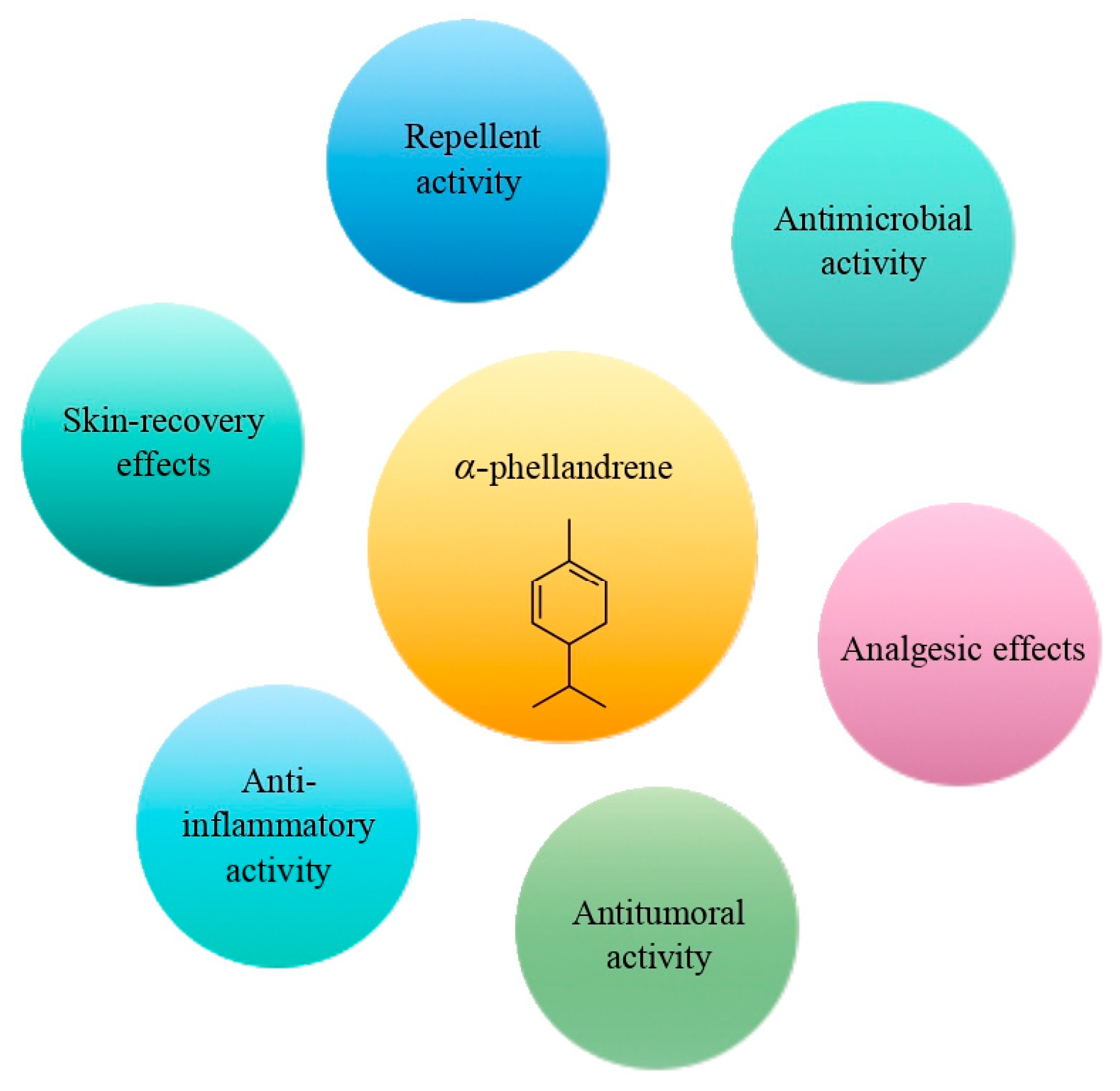
The cyclic monoterpene α-phellandrene was introduced for public use in the 1940s [60]. It represents a colorless liquid, characterized by a peppery, woody, and herbaceous aroma [60]. It could be found in cosmetics, food, and EOs. Nowadays, α-phellandrene has been characterized by a broad spectrum of pharmacological activities, including antimicrobial, anti-inflammatory, antitumoral, analgesic, and insecticidal activities (Figure 4) [60,61,62,63]. Alpha-phellandrene accelerated wound closure due to collagen deposition and demonstrated good wound-healing properties [64,65,66]. In addition, α-phellandrene is associated with enhancement of the proliferation of vascular endothelial growth factor in dermal papilla cells, which indicates that α-phellandrene has a strong potential to prevent hair loss [67]. According to recent studies, α-phellandrene could be used as a preservant in the food industry [68,69].
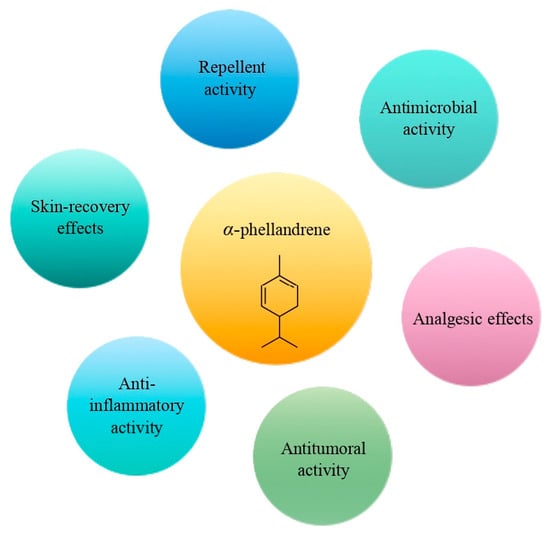
Figure 4.
The main effects of α-phellandrene.
Scholars have mainly investigated the biological activity of o-cymene and β-phellandrene as components of different essential oils [67,70,71,72,73,74]. No randomized double-blind studies involving methyleugenol, α-phellandrene, o-cymene, and β-phellandrene exist. Such studies would be useful to confirm the safety and future applications of these compounds as medicines. In our view, the EO isolated from E. tenuifolia subsp. sibthorpiana has a good potential to be included in innovative herbal medicinal products, functional foods, and food supplements aimed at improving chronic mood disorders, cognitive impairment, skin recovery, etc. However, studies on this EO are limited; in vitro and in vivo studies would be especially beneficial for evaluation of its real potential. The acute and the sub-chronic toxicity of this EO should also be well investigated.
Nowadays, there is a great interest in EOs as products for pest management and repellents [75] because of their much safer potential than synthetic pesticides. Something more, EOs are a green alternative tool for insect management that is less environmentally damaging [75,76]. E. tenuifolia L. subsp. sibthorpiana EO could be a promising species for use as a biopesticide and a biorepellent because of the concentrations of its active compounds: methyleugenol and α-phellandrene.
3. Materials and Methods
3.1. Plant Material
The aerial parts of flowering E. tenuifolia L. subsp. sibthorpiana were collected in August 2022 near the village of Raykova Mogila (41°50′14.9″ N 26°18′17.3″ E), Thracian Lowland floristic region, Bulgaria. A voucher specimen of the species (No: 063306) was deposited in the Herbarium of the Agricultural University—Plovdiv (SOA).
3.2. Chemicals and Reagents
To determine the retention indices (RI), the following hydrocarbons were used: nonane (≥99%), decane (≥99%), undecane (≥99%), dodecane (99%), tridecane (≥99%), tetradecane (≥99%), and hexadecane (≥99%) purchased from Merck KGaA (Darmstadt, Germany). Dichloromethane (Sigma Aldrich, Steinheim, Germany) was used for the dilution of the EO.
3.3. Microscopic Histochemical Analysis
The plant material had been fixed in a solution of 50% ethanol and glycerol in a ratio of 7:3 for 24 h to soften the tissues. Hand-cut transverse and tangential longitudinal sections were made from the stem and leaf of the plant. Terpene histochemistry was examined using NADI reagent (0.5 mL of 0.1% α-naphthol (Merck KGaA, Darmstadt, Germany), 0.5 mL of 1% N,N-dimethyl-p-phenylenediamine (Merck KGaA, Darmstadt, Germany), and 49 mL of 0.1 M sodium phosphate buffer, pH 7.2 (Merck KGaA, Darmstadt, Germany) [77]. Cross sections were incubated with NADI reagent for 1 h in the dark, washed in sodium phosphate buffer (0.1 M, pH 7.2) for 2 min, and mounted in the same buffer. Lipid histochemistry was examined using Sudan III (Merck KGaA, Darmstadt, Germany). The sections were treated with Sudan III in 70% ethanol for 30 min, rinsed briefly in 80% ethanol, and mounted in 50% glycerol [77]. The sections were observed with a light microscope (Leica DM 2000 LED, Leica Microsystems, Wetzlar, Germany) equipped with a digital camera (Leica DMC 2900) and image processing software (Leica Application Suite, LAS).
3.4. Isolation of the Essential Oil
The air-dried flowering aerial parts (100 g) of the plant were subjected to hydrodistillation for 4 h using a Clevenger-type apparatus to obtain the essential oil. The collected oil was dried over anhydrous sodium sulfate and stored in dark glass vials at 4 °C until the GC-MS analysis. The oil yield (%) was measured based on the dry weight of the plant.
3.5. Chromatographic Conditions
The analysis of the EO was performed using gas chromatography with mass spectrometry (GC-MS). For the GC-MS analysis a Bruker Scion 436-GC SQ MS (Bremen, Germany) equipped with a Bruker BR-5ms fused silica capillary column (0.25 µm film thickness and 15 m × 0.25 mm i.d.) was used. Helium was used as a carrier gas with a constant flow rate of 1mL/min. The injection volume was 1 µL. The split ratio of the injector was 1:50. The oven temperature was set at 50 °C for 1 min, then increased to 140 °C at a rate of 4 °C/min, and then increased to 290 °C at a rate of 15 °C/min and held for 1 min. The temperature of the injector was set to 250 °C, and the detector temperature was set to 300 °C. The mass spectra were in full scan mode with a mass range of 50–350 m/z. The identification of the separated essential oil constituents was achieved by comparing their MS spectra and retention indices (RI) with spectral data within the Wiley NIST11 Mass Spectral Library (NIST11/2011/EPA/NIH) and the literature data. The RI values were calculated from the retention times of the C8–C30 n-alkane series injected under the same conditions described above.
4. Conclusions
This is the first report describing the histochemical localization of Echinophora tenuifolia subsp. sibthorpiana EO and the first study which investigated the chemical profile of this EO from Bulgarian E. tenuifolia L. subsp. sibthorpiana. The presented histochemical analysis confirmed the presence of lipids and terpenes in the stem and leaf of E. tenuifolia subsp. sibthorpiana. The conducted GC-MS analysis indicated the presence of monoterpene hydrocarbons, oxygenated monoterpenes, and phenylpropanoid. Sixteen volatile constituents representing 92.03% of the total oil were tentatively detected. The major component of the isolated EO was methyleugenol, representing 48.13%. The results obtained in this study indicated that future possibilities to evaluate the biological activity of the EO of E. tenuifolia L. subsp. sibthorpiana exist, and they highlight the potential future use of the EO of this plant species. Further investigations in this area could be especially useful for the future development of novel medicines for use in cardiology, neurology, rheumatology, and psychiatry. E. tenuifolia L. subsp. sibthorpiana EO also has a prominent potential for use as a biopesticide and repellent as well.
Author Contributions
Conceptualization, S.D., S.I. and K.I.; methodology, S.D., S.I. and K.I.; software S.D., S.I. and K.I.; formal analysis, S.D., S.I., D.K.-B., V.T., Y.G., N.B. and K.I.; investigation, S.D., V.T., S.I., D.K.-B., Y.G., N.B. and K.I.; resources, S.D., S.I. and K.I.; data curation, S.D., S.I. and K.I.; writing—original draft preparation, S.D., S.I., K.I. and D.K.-B.; writing—review and editing, S.D., S.I., D.K.-B. and K.I.; visualization, S.D., S.I., D.K.-B., V.T., N.B. and K.I.; supervision, S.I., D.K.-B., N.B. and K.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the essential oil are not available.
References
- Georgiou, C.; Koutsaviti, A.; Bazos, I.; Tzakou, O. Chemical Composition of Echinophora tenuifolia subsp. sibthorpiana Essential Oil from Greece. Rec. Nat. Prod. 2010, 4, 167. [Google Scholar]
- Stoyanov, K.; Raycheva, T.; Cheschmedzhiev, I. Key to the Native and Foreign Vascular Plants in Bulgaria; Agricultural University Plovdiv Academic Press: Plovdiv, Bulgaria, 2021. [Google Scholar]
- Flora Republicae Popularis Bulgaricae. In Serdicae; Jordanov, D., Ed.; Aedibus Academiae Scientiarum Bulgaricae: Sofia, Bulgaria, 1982; Volume 8. [Google Scholar]
- Deghirmencioghlu, N.; Göçmen, D.; Daghdelen, A.; Daghdelen, F. Influence of Tarhana Herb (Echinophora sibthorpiana) on Fermentation of Tarhana, Turkish Traditional Fermented Food. Food Technol. Biotechnol. 2005, 43, 175–179. [Google Scholar]
- Georgala, A. The Nutritional Value of Two Fermented Milk/Cereal Foods Named ‘Greek Trahanas and Turkish Tarhana: A Review. J. Nutr. Disord. Ther. 2013, 3, 1–4. [Google Scholar] [CrossRef]
- Ozdemir, S.; Gocmen, D.; Yildirim Kumral, A. A Traditional Turkish Fermented Cereal Food: Tarhana. Food Rev. Int. 2007, 23, 107–121. [Google Scholar] [CrossRef]
- Şanli, A.; Karadoğan, T.; Tosun, B.; Tonguç, M.; Erbaş, S. Growth Stage and Drying Methods Affect Essential Oil Content and Composition of Pickling Herb (Echinophora tenuifolia subsp. sibthorpiana Tutin). SDÜ Fen Bilim. Enstitüsü Dergisi. 2016, 20, 43–49. [Google Scholar] [CrossRef]
- Spano, M.; Di Matteo, G.; Ingallina, C.; Ambroselli, D.; Carradori, S.; Gallorini, M.; Giusti, A.M.; Salvo, A.; Grosso, M.; Mannina, L. Modulatory Properties of Food and Nutraceutical Components Targeting NLRP3 Inflammasome Activation. Nutrients 2022, 14, 490. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and Combined Antioxidant Activity of Spices and Spice Phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef]
- Avijgan, M.; Hafizi, M.; Saadat, M.; Nilforoushzadeh, M. Antifungal Effect of Echinophora Platyloba’s Extract against Candida Albicans. Iran. J. Pharm. Res. 2006, 5, 285–289. [Google Scholar] [CrossRef]
- Avijgan, M.; Mirzadeh, F.; Nia, E. The Comparative Study of Anti-Fungal Effect of Pharmaceutical Products Containing Hydroalcoholic Extract of Echinophora Platyloba DC and Flucona- Zole in Women with Chronic Recurrent Vaginitis Caused by Candida Albicans. Res. J. Med. Sci. 2012, 17, S103–S107. [Google Scholar]
- Delaram, M.; Kheiri, S.; Hodjati, M.R. Comparing the Effects of Echinophora-platyloba, Fennel and Placebo on Pre-Menstrual Syndrome. J. Reprod. Infertil. 2011, 12, 221–226. [Google Scholar] [PubMed]
- Heidarian, E.; Saffari, J.; Jafari-Dehkordi, E. Hepatoprotective Action of Echinophora Platyloba DC Leaves Against Acute Toxicity of Acetaminophen in Rats. J. Diet. Suppl. 2014, 11, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Shahneh, F.Z.; Baradaran, B.; Majidi, J.; Babaloo, Z. Echinophora Platyloba DC (Apiaceae) Crude Extract Induces Apoptosis in Human Prostate Adenocarcinoma Cells (PC 3). Biomed. J. 2014, 37, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Shahneh, F.Z.; Valiyari, S.; Azadmehr, A.; Hajiaghaee, R.; Yaripour, S.; Bandehagh, A.; Baradaran, B. Inhibition of Growth and Induction of Apoptosis in Fibrosarcoma Cell Lines by Echinophora Platyloba DC: In Vitro Analysis. Adv. Pharmacol. Sci. 2013, 2013, 512931. [Google Scholar] [CrossRef] [PubMed]
- Ezatpour, B.; Bahmani, M.; Azami, M.; Kheirandish, F.; Rafieian-Kopaei, M. The in Vitro Effects of Echinophora Cinerea on Cell Line, Giardia Lamblia Cyst, and Giardia Muris. Herb. Med. J. 2018, 3, 70–76. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Khajouei, S.; Ahmadi, F.; Ghiasvand, N.; Hosseinzadeh, L. Protective Effect of Bioactive Compounds from Echinophora Cinerea against Cisplatin-Induced Oxidative Stress and Apoptosis in the PC12 Cell Line. Iran. J. Basic Med. Sci. 2017, 20, 438–445. [Google Scholar] [CrossRef]
- Fraternale, D.; Genovese, S.; Ricci, D. Essential Oil Composition and Antimicrobial Activity of Aerial Parts and Ripe Fruits of Echinophora Spinosa (Apiaceae) from Italy. Nat. Prod. Commun. 2013, 8, 527–530. [Google Scholar] [CrossRef]
- Kaska, A.; Mammadov, R. Antioxidant Properties, Proximate Content and Cytotoxic Activity of Echinophora Tournefortii Jaub. & Spach. Food Sci. Technol. 2019, 39, 875–880. [Google Scholar] [CrossRef]
- Marrelli, M.; Statti, G.A.; Menichini, F.; Conforti, F. Echinophora Tenuifolia L. Inflorescences: Phytochemistry and in Vitro Antioxidant and Anti-Inflammatory Properties in LPS-Stimulated RAW 264.7 Macrophages. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2017, 151, 1073–1081. [Google Scholar] [CrossRef]
- Marrelli, M.; Pisani, F.; Amodeo, V.; Duez, P.; Conforti, F. Echinophora Tenuifolia L. Branches Phytochemical Profile and Antiproliferative Activity on Human Cancer Cell Lines. Nat. Prod. Res. 2020, 34, 2664–2667. [Google Scholar] [CrossRef]
- Gokbulut, I.; Bilenler, T.; Karabulut, I. Determination of Chemical Composition, Total Phenolic, Antimicrobial, and Antioxidant Activities of Echinophora Tenuifolia Essential Oil. Int. J. Food Prop. 2013, 16, 1442–1451. [Google Scholar] [CrossRef]
- Najmi, Z.; Scalia, A.C.; De Giglio, E.; Cometa, S.; Cochis, A.; Colasanto, A.; Locatelli, M.; Coisson, J.D.; Iriti, M.; Vallone, L.; et al. Screening of Different Essential Oils Based on Their Physicochemical and Microbiological Properties to Preserve Red Fruits and Improve Their Shelf Life. Foods 2023, 12, 332. [Google Scholar] [CrossRef]
- Özcan, M.; Akgül, A. Essential Oil Composition of Turkish Pickling Herb (Echinophora Tenuifolia L. Subsp. sibthorpiana (Guss.) Tutin). Acta Bot. Hung. 2003, 45, 163–167. [Google Scholar] [CrossRef]
- Telci, I.; Hisil, Y. Essential Oil Composition of the Spice Plant Echinophora Tenuifolia L. Subsp. sibthorpiana Tutin from Turkey. Chem. Nat. Compd. 2008, 44, 534–536. [Google Scholar] [CrossRef]
- Mileski, K.; Dzamic, A.; Ciric, A.; Grujic, S.; Ristic, M.; Matevski, V.; Marin, P.D. Radical Scavenging and Antimicrobial Activity of Essential Oil and Extracts of Echinophora sibthorpiana Guss. from Macedonia. Arch. Biol. Sci. 2014, 66, 401–413. [Google Scholar] [CrossRef]
- Ahmad, V.U.; Jassbi, A.R.; Pannahi, M.S.C. Analysis of the Essential Oil of Echinophora sibthorpiana Guss. by Means of GC, GC/MS and 13C-NMR Techniques. J. Essent. Oil Res. 1999, 11, 107–108. [Google Scholar] [CrossRef]
- Akgül, A.; Chialva, F. Constituents of the Essential Oil of Echinophora Tenuifolia L. Subsp. sibthorpiana (Guss.) Tutin from Turkey. Flavour Fragr. J. 1989, 4, 67–68. [Google Scholar] [CrossRef]
- Chalchat, J.C.; Ozcan, M.M.; Dagdelen, A.; Akgul, A. Variability of Essential Oil Composition of Echinophora Tenuifolia Subsp. sibthorpiana Tutin by Harvest Location and Year and Oil Storage. Chem. Nat. Compd. 2007, 43, 225–227. [Google Scholar] [CrossRef]
- Chalchat, J.; Özcan, M.; Figueredo, G.; Chalard, P. The Effect of Harvest Years on Chemical Composition of Essential Oil of Pickling Herb (Echinophora Tenuifolia Subsp. sibthorpiana) Leaves Used as Medicinal Plant. Acta Bot. Hung. 2011, 53, 73–77. [Google Scholar] [CrossRef]
- Sefidkon, F. Extraction and Identification of Volatile Components of Echinophora sibthorpiana Guss. Iran. J. Med. Aromat. Plants Res. 2004, 20, 149–158. [Google Scholar]
- Baser, K.H.C.; Erdemgil, F.Z.; Özek, T. Essential Oil of Echinophora Tenuifolia L. Subsp. sibthorpiana (Guss.) Tutin. J. Essent. Oil Res. 1994, 6, 399–400. [Google Scholar] [CrossRef]
- Sanli, A.; Ok, F.Z. Chemical Composition and Antimicrobial Activity against Phytopathogenic Fungi of Essential Oils Obtained from Echinophora tenuifolia subsp. sibthorpiana Grown in Wild and Cultivated Conditions in Turkey. Molecules 2023, 28, 585. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors Affecting Secondary Metabolite Production in Plants: Volatile Components and Essential Oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Glamoclija, J.M.; Sokovic, M.D.; Siljegovic, J.D.; Ristic, M.S.; Ciric, A.; Grubisic, D.V. Chemical Composition and Anti-Microbial Activity of Echinophora Spinosa L. (Apiaceae) Essential Oil. Rec. Nat. Prod. 2011, 5, 319. [Google Scholar]
- Pavela, R.; Maggi, F.; Cianfaglione, K.; Canale, A.; Benelli, G. Promising Insecticidal Efficacy of the Essential Oils from the Halophyte Echinophora Spinosa (Apiaceae) Growing in Corsica Island, France. Environ. Sci. Pollut. Res. 2020, 27, 14454–14464. [Google Scholar] [CrossRef]
- Sodeifian, G.; Sajadian, S.A. Investigation of Essential Oil Extraction and Antioxidant Activity of Echinophora Platyloba DC. Using Supercritical Carbon Dioxide. J. Supercrit. Fluids 2017, 121, 52–62. [Google Scholar] [CrossRef]
- Moghaddam, M.; Taheri, P.; Pirbalouti, A.G.; Mehdizadeh, L. Chemical Composition and Antifungal Activity of Essential Oil from the Seed of Echinophora Platyloba DC. against Phytopathogens Fungi by Two Different Screening Methods. LWT Food Sci. Technol. 2015, 61, 536–542. [Google Scholar] [CrossRef]
- Asghari, G.; Abedi, D.; Jalali, M.; Farsi, S. Antimicrobial Activities and Phytochemical Composition of Echinophora Platyloba DC. Essential Oils from Isfahan. J. Essent. Oil Bear. Plants 2007, 10, 76–82. [Google Scholar] [CrossRef]
- Baser, K.H.C.; Biçakçi, A.; Malyer, H. Composition of the Essential Oil of Echinophora Lamondiana B.Yildiz et Z.Bahçecioglu. J. Essent. Oil Res. 2000, 12, 147–148. [Google Scholar] [CrossRef]
- Ali, A.; Tabanca, N.; Ozek, G.; Ozek, T.; Aytac, Z.; Bernier, U.R.; Agramonte, N.M.; Baser, K.H.C.; Khan, I.A. Essential Oils of Echinophora Lamondiana (Apiales: Umbelliferae): A Relationship Between Chemical Profile and Biting Deterrence and Larvicidal Activity Against Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Baser, K.H.C.; Kürkcüoglu, M.; Malyer, H.; Bicakci, A. Essential Oils of Six Echinophora Species from Turkey. J. Essent. Oil Res. 1998, 10, 345–351. [Google Scholar] [CrossRef]
- Humans, I.W.G. On the E. of C.R. to Methyleugenol; International Agency for Research on Cancer: Lyon, France, 2013. [Google Scholar]
- Public Statement on the Use of Herbal Medicinal Products Containing Methyleugenol; European Medicines Agency: Amsterdam, The Netherlands, 2005; Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/public-statement-use-herbal-medicinal-products-containing-methyleugenol_en.pdf (accessed on 25 February 2023).
- Tang, F.; Chen, F.; Ling, X.; Huang, Y.; Zheng, X.; Tang, Q.; Tan, X. Inhibitory Effect of Methyleugenol on IgE-Mediated Allergic Inflammation in RBL-2H3 Cells. Mediat. Inflamm. 2015, 2015, 463530. [Google Scholar] [CrossRef]
- Shin, B.-K.; Lee, E.-H.; Kim, H.-M. Suppression Ofl-Histidine Decarboxylase MRNA Expression by Methyleugenol. Biochem. Biophys. Res. Commun. 1997, 232, 188–191. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.K.; Cho, G.-S.; Hwang, S.; Kim, B.W.; Lim, J.H.; Lee, J.-C.; Kim, H.C.; Kim, W.-K.; Kim, Y.S. Methyleugenol Reduces Cerebral Ischemic Injury by Suppression of Oxidative Injury and Inflammation. Free Radic. Res. 2010, 44, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Norte, M.C.B.; Cosentino, R.M.; Lazarini, C.A. Effects of Methyl-Eugenol Administration on Behavioral Models Related to Depression and Anxiety, in Rats. Phytomedicine 2005, 12, 294–298. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Fan, H.-R.; Deng, S.; Zhu, T.; Yan, Y.; Ge, W.-H.; Li, W.-G.; Li, F. Methyleugenol Potentiates Central Amygdala GABAergic Inhibition and Reduces Anxiety. J. Pharmacol. Exp. Ther. 2019, 368, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Yan, Y.; Deng, S.; Liu, Y.-M.; Fan, H.-R.; Ma, B.; Meng, B.; Mei, B.; Li, W.-G.; Li, F. Methyleugenol Counteracts Anorexigenic Signals in Association with GABAergic Inhibition in the Central Amygdala. Neuropharmacology 2018, 141, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Huang, C.; Peng, Z.; Xie, Y.; Deng, S.; Nie, Y.-Z.; Xu, T.-L.; Ge, W.-H.; Li, W.-G.; Li, F. Electrophysiological Characterization of Methyleugenol: A Novel Agonist of GABA(A) Receptors. ACS Chem. Neurosci. 2014, 5, 803–811. [Google Scholar] [CrossRef]
- Lima, C.C.; Criddle, D.N.; Coelho-de-Souza, A.N.; Monte, F.J.Q.; Jaffar, M.; Leal-Cardoso, J.H. Relaxant and Antispasmodic Actions of Methyleugenol on Guinea-Pig Isolated Ileum. Planta Med. 2000, 66, 408–411. [Google Scholar] [CrossRef]
- Lahlou, S.; Figueiredo, A.F.; Magalhães, P.J.C.; Leal-Cardoso, J.H.; Gloria, P.D. Cardiovascular Effects of Methyleugenol, a Natural Constituent of Many Plant Essential Oils, in Normotensive Rats. Life Sci. 2004, 74, 2401–2412. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, Q.; Wang, Z.; Cao, H.; Zhang, D. Structure-Activity Relationships of Cinnamaldehyde and Eugenol Derivatives against Plant Pathogenic Fungi. Ind. Crops Prod. 2017, 97, 388–394. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Khan, L.A.; Manzoor, N. In Vitro Synergy of Eugenol and Methyleugenol with Fluconazole against Clinical Candida Isolates. J. Med. Microbiol. 2010, 59, 1178–1184. [Google Scholar] [CrossRef]
- Götz, M.E.; Sachse, B.; Schäfer, B.; Eisenreich, A. Myristicin and Elemicin: Potentially Toxic Alkenylbenzenes in Food. Foods 2022, 11, 1988. [Google Scholar] [CrossRef]
- Quan, N.V.; Dang Xuan, T.; Teschke, R. Potential Hepatotoxins Found in Herbal Medicinal Products: A Systematic Review. Int. J. Mol. Sci. 2020, 21, 5011. [Google Scholar] [CrossRef] [PubMed]
- Eisenreich, A.; Götz, M.E.; Sachse, B.; Monien, B.H.; Herrmann, K.; Schäfer, B. Alkenylbenzenes in Foods: Aspects Impeding the Evaluation of Adverse Health Effects. Foods 2021, 10, 2139. [Google Scholar] [CrossRef] [PubMed]
- Smith, F. Therapeutics within a Naturopathic Approach. In Naturopathic Medicine; Springer International Publishing: Cham, Switzerland, 2022; pp. 129–202. ISBN 978-3-031-13387-9. [Google Scholar]
- Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life 2022, 12, 1602. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B.S.; Kesika, P.; Tiyajamorn, T.; Bharathi, M.; Chaiyasut, C. A Narrative Review on the Bioactivity and Health Benefits of Alpha-Phellandrene. Sci. Pharm. 2022, 90, 57. [Google Scholar] [CrossRef]
- Evergetis, E.; Michaelakis, A.; Haroutounian, S.A. Exploitation of Apiaceae Family Essential Oils as Potent Biopesticides and Rich Source of Phellandrenes. Ind. Crops Prod. 2013, 41, 365–370. [Google Scholar] [CrossRef]
- Dalli, M.; Azizi, S.; Benouda, H.; Azghar, A.; Tahri, M.; Bouammali, B.; Maleb, A.; Gseyra, N. Molecular Composition and Antibacterial Effect of Five Essential Oils Extracted from Nigella Sativa L. Seeds against Multidrug-Resistant Bacteria: A Comparative Study. Evid.-Based Complement. Altern. Med. 2021, 2021, 6643765. [Google Scholar] [CrossRef]
- Salas-Oropeza, J.; Jimenez-Estrada, M.; Perez-Torres, A.; Castell-Rodriguez, A.E.; Becerril-Millan, R.; Rodriguez-Monroy, M.A.; Jarquin-Yañez, K.; Canales-Martinez, M.M. Wound Healing Activity of α-Pinene and α-Phellandrene. Molecules 2021, 26, 2488. [Google Scholar] [CrossRef]
- de Christo Scherer, M.M.; Marques, F.M.; Figueira, M.M.; Peisino, M.C.O.; Schmitt, E.F.P.; Kondratyuk, T.P.; Endringer, D.C.; Scherer, R.; Fronza, M. Wound Healing Activity of Terpinolene and α-Phellandrene by Attenuating Inflammation and Oxidative Stress in Vitro. J. Tissue Viability 2019, 28, 94–99. [Google Scholar] [CrossRef]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Silva, M.A.N.; Deschamps, C.; Molento, M.B. Insecticide Activity of Curcuma Longa (Leaves) Essential Oil and Its Major Compound α-Phellandrene against Lucilia Cuprina Larvae (Diptera: Calliphoridae): Histological and Ultrastructural Biomarkers Assessment. Pestic. Biochem. Physiol. 2019, 153, 17–27. [Google Scholar] [CrossRef]
- Kang, W.; Park, S.; Choi, D.; Son, B.; Park, T. Activation of CAMP Signaling in Response to α-Phellandrene Promotes Vascular Endothelial Growth Factor Levels and Proliferation in Human Dermal Papilla Cells. Int. J. Mol. Sci. 2022, 23, 8959. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Lin, C.L.; Huang, C.Y.; Hsieh, S.; Liu, C.H.; Hsieh, S.L. α-Phellandrene Enhances the Immune Response and Resistance against Vibrio Alginolyticus in White Shrimp (Litopenaeus Vannamei). Fish Shellfish Immunol. 2019, 84, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.-Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia Officinalis L. and Schinus Molle L. Essential Oils: Their Chemical Compositions and Their Preservative Effects against Salmonella Inoculated in Minced Beef Meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahluwalia, V.; Singh, P.; Kumar, N.; Prakash Sati, O.; Sati, N. Antifungal and Phytotoxic Activity of Essential Oil from Root of Senecio Amplexicaulis Kunth. (Asteraceae) Growing Wild in High Altitude-Himalayan Region. Nat. Prod. Res. 2016, 30, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Magwa, M.L.; Gundidza, M.; Gweru, N.; Humphrey, G. Chemical Composition and Biological Activities of Essential Oil from the Leaves of Sesuvium portulacastrum. J. Ethnopharmacol. 2006, 103, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Al-Burtamani, S.K.S.; Fatope, M.O.; Marwah, R.G.; Onifade, A.K.; Al-Saidi, S.H. Chemical Composition, Antibacterial and Antifungal Activities of the Essential Oil of Haplophyllum tuberculatum from Oman. J. Ethnopharmacol. 2005, 96, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Tchoumbougnang, F.; Zollo, P.H.; Dagne, E.; Mekonnen, Y. In Vivo Antimalarial Activity of Essential Oils from Cymbopogon citratus and Ocimum gratissimum on Mice Infected with Plasmodium berghei. Planta Med. 2005, 71, 20–23. [Google Scholar] [CrossRef]
- Bonesi, M.; Menichini, F.; Tundis, R.; Loizzo, M.R.; Conforti, F.; Passalacqua, N.G.; Statti, G.A.; Menichini, F. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activity of Pinus Species Essential Oils and Their Constituents. J. Enzym. Inhib. Med. Chem. 2010, 25, 622–628. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Fuentes-Lopez, K.; Stashenko, E.E.; Olivero-Verbel, J. Chemical Composition, Repellent Action, and Toxicity of Essential Oils from Lippia Origanoide, Lippia. alba Chemotypes, and Pogostemon cablin on Adults of Ulomoides dermestoides (Coleoptera: Tenebrionidae). Insects 2022, 14, 41. [Google Scholar] [CrossRef]
- Alcala-Orozco, M.; Caballero-Gallardo, K.; Stashenko, E.E.; Olivero-Verbel, J. Repellent and Fumigant Actions of the Essential Oils from Elettaria Cardamomum (L.) Maton, Salvia Officinalis (L.) Linnaeus, and Lippia Origanoides (V.) Kunth Against Tribolium Castaneum and Ulomoides dermestoides. J. Essent. Oil Bear. Plants 2019, 22, 18–30. [Google Scholar] [CrossRef]
- Pellicciari, C.; Biggiogera, M. (Eds.) Histochemistry of Single Molecules: Methods and Protocols; Springer New York: New York, NY, USA, 2017; Volume 1560. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).