Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties
Abstract
1. Introduction
2. Results
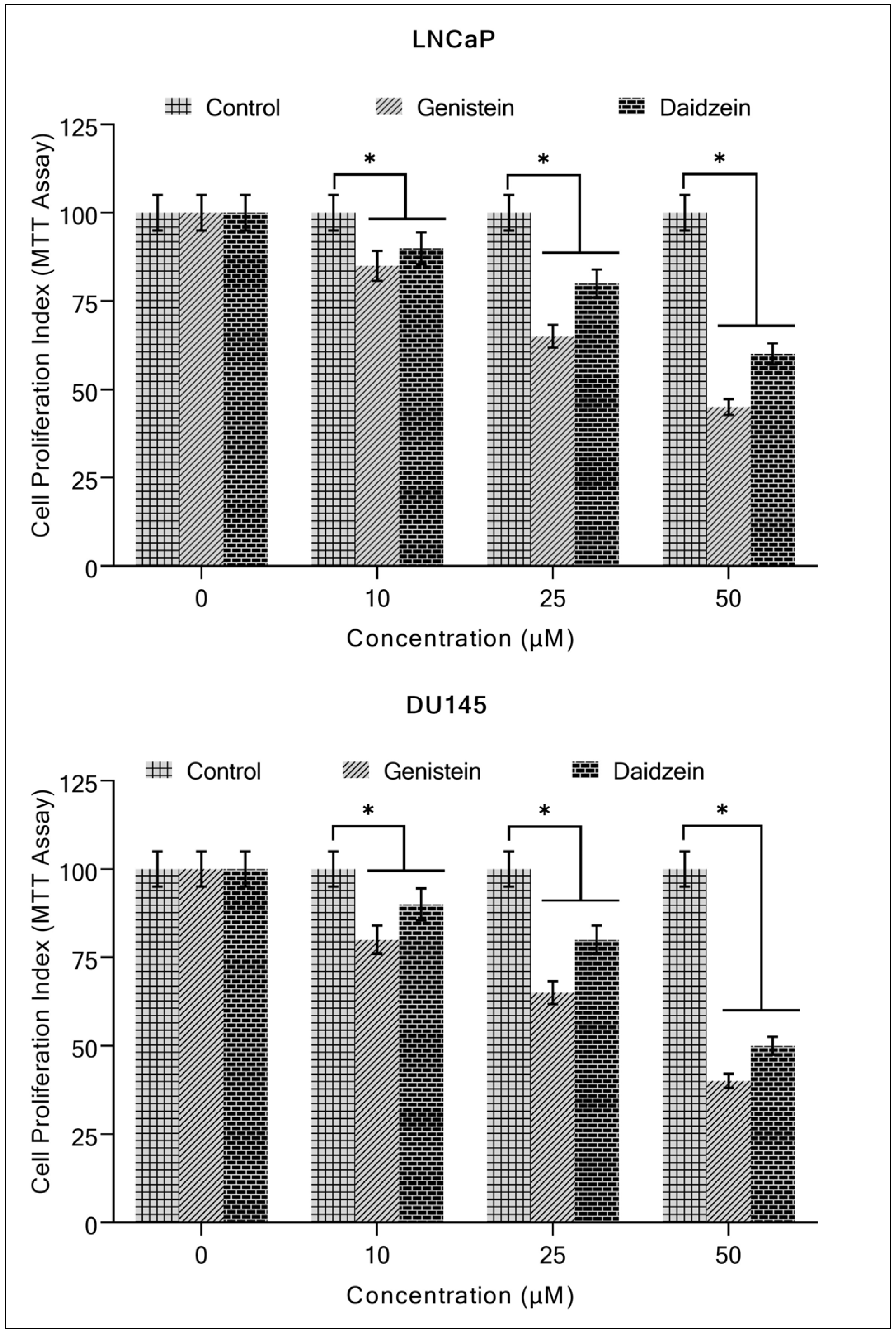
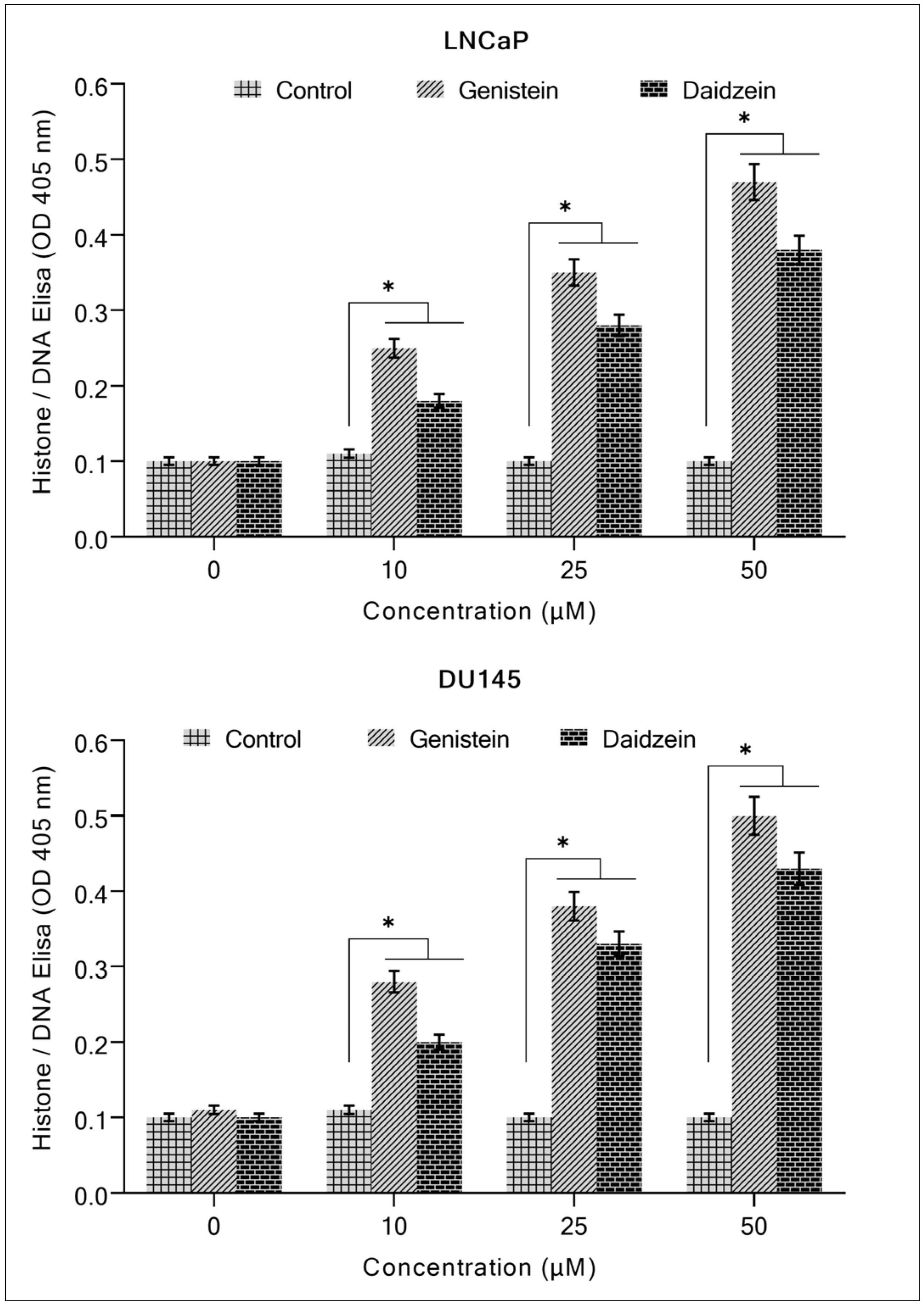
2.1. Isoflavones Inhibit Growth and Induce Apoptosis in Prostate Cancer Cells
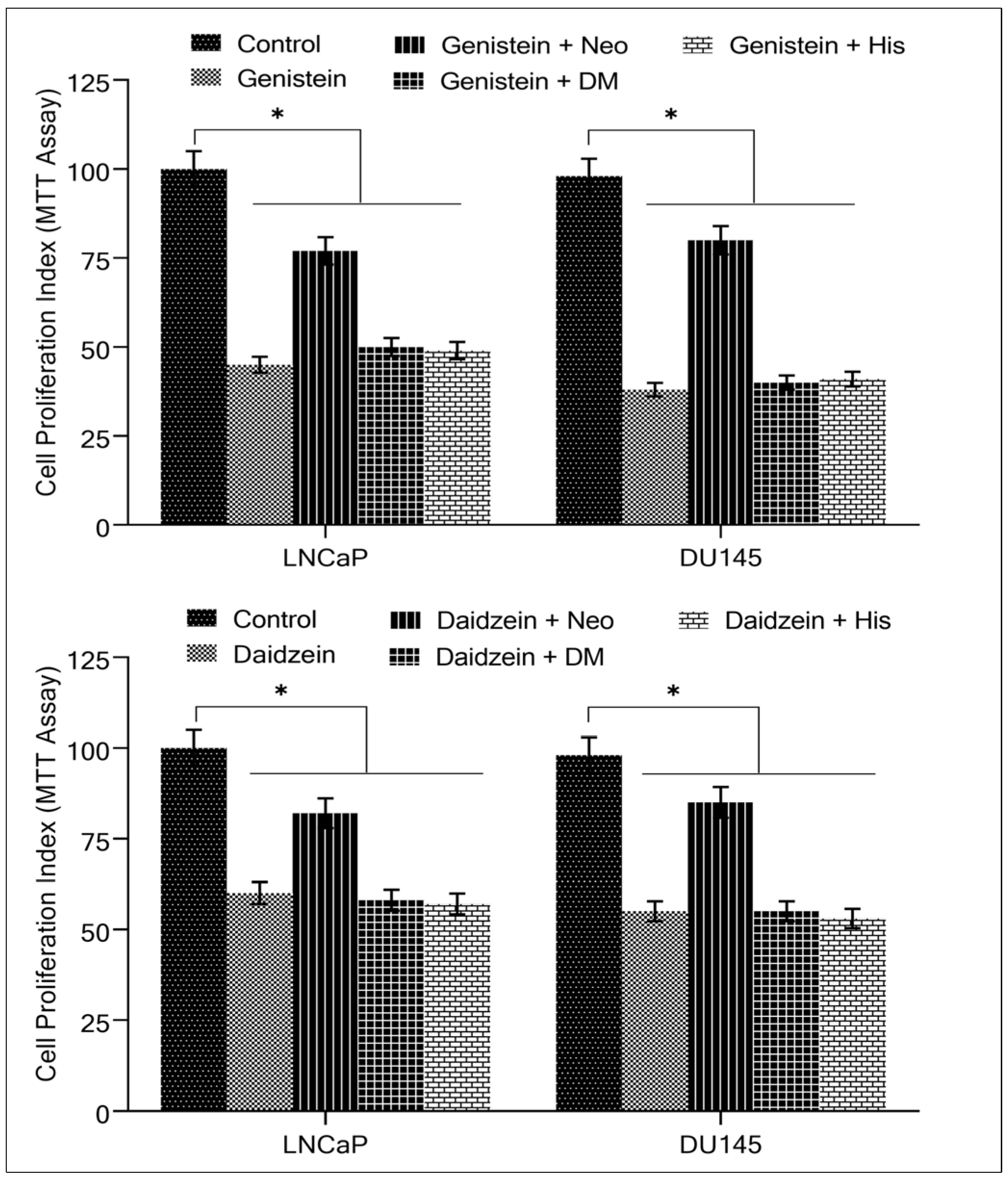
2.2. Copper Chelation Decreases Isoflavone-Induced Growth Inhibition and Apoptosis
2.3. Apoptosis of Cancer Cells Induced by Isoflavones Is Mediated by Reactive Oxygen Species
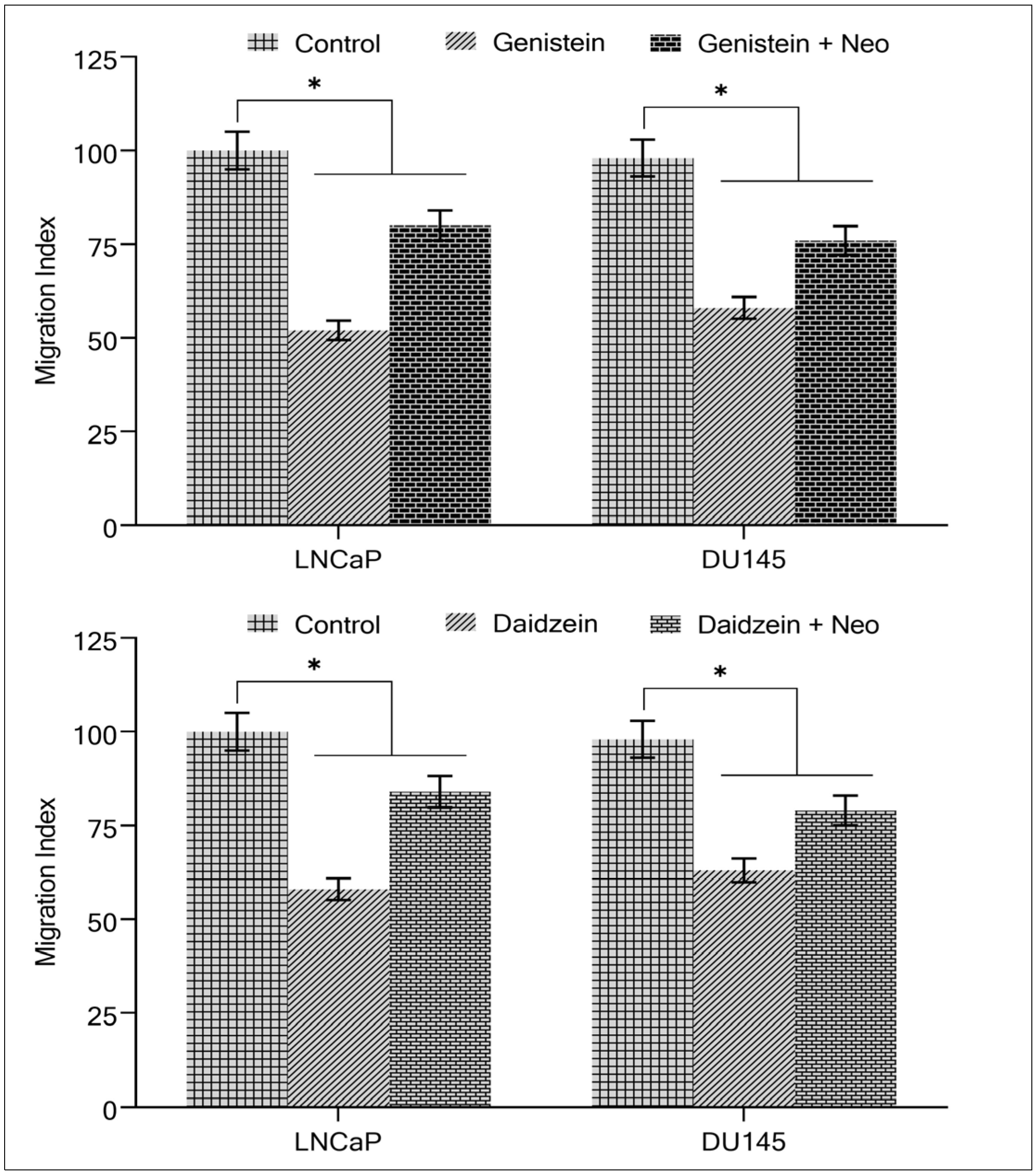
2.4. Copper Chelation Suppresses Isoflavone-Induced Inhibition of Migration by Cancer Cells
2.5. Copper Supplementation Sensitizes Normal Prostate Epithelial Cells to Antiproliferative Action of Isoflavones
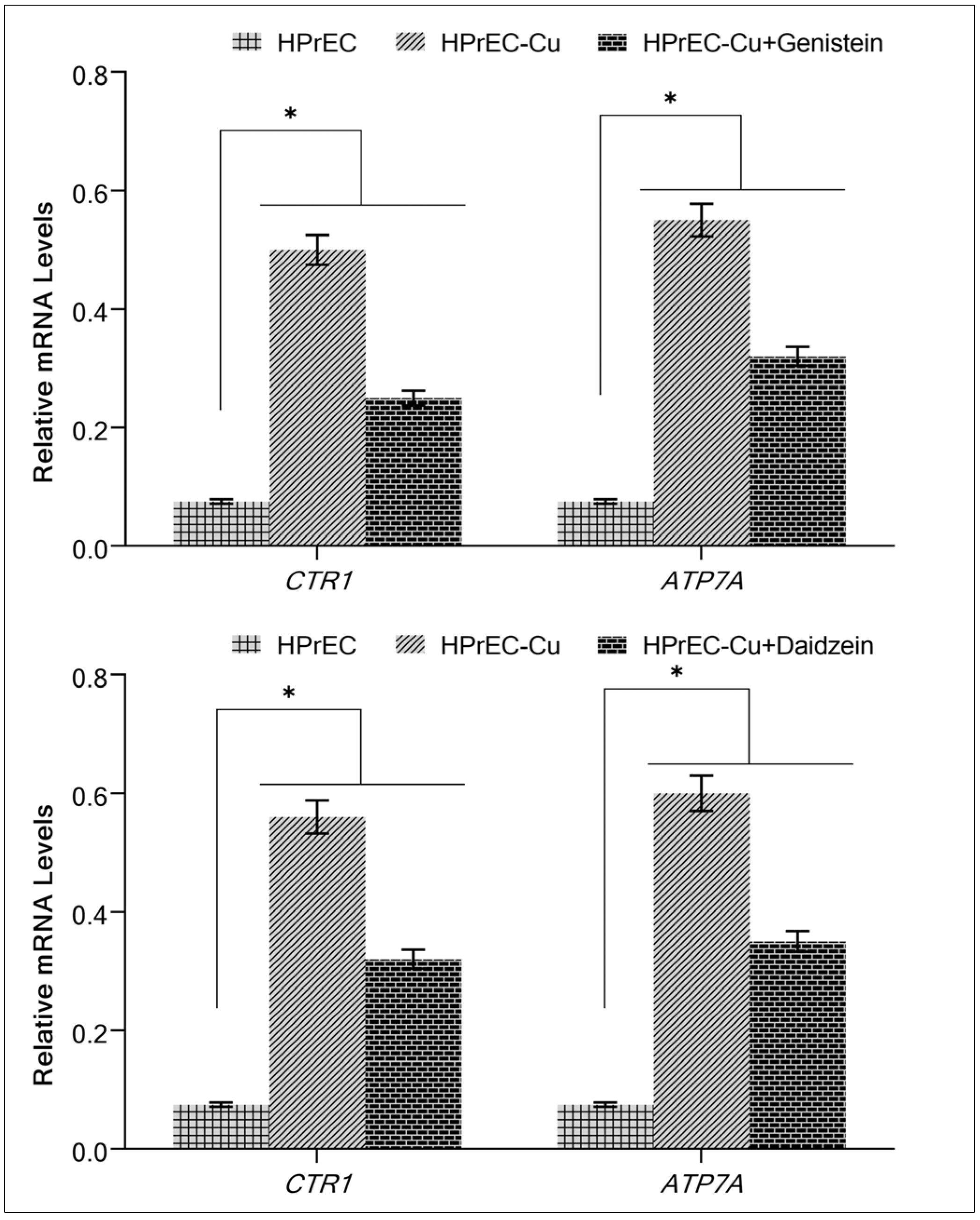
2.6. Isoflavone Suppresses the Expression of Copper Transporters CTR1 and ATP7A in Cancer Cells
2.7. Silencing of CTR1 and ATP7A in HPrEC Cells Grown in Copper-Supplemented Medium Reduces Isoflavone-Induced Inhibition of Proliferation
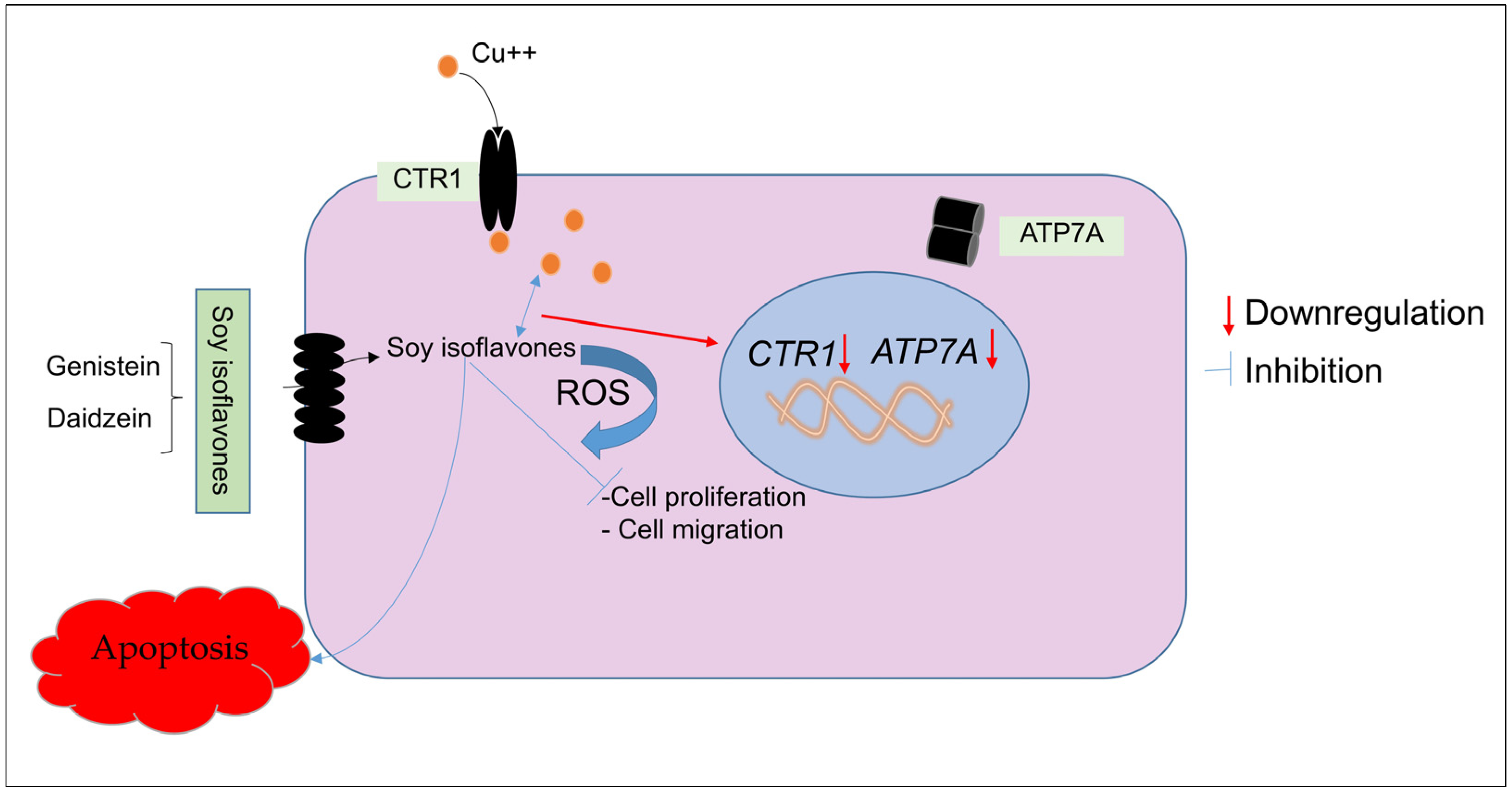
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Lines and Reagents
4.3. Cell Growth Inhibition Studies by 3-(4,5-Dimethylthiazol-2-yl)-2,5 Diphenyl-Tetra-Zolium Bromide (MTT) Assay
4.4. Histone/DNA ELISA for Detection of Apoptosis
4.5. Cell Migration Assay
4.6. Real-Time Reverse Transcriptase PCR
4.7. siRNA (Small Interfering RNA) Transfection
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Fouad, Y.A.; Aanei, C. Revisiting the hallmarks of cancer. Am. J. Cancer Res. 2017, 7, 1016–1036. [Google Scholar]
- Lawson, D.A.; Bhakta, N.R.; Kessenbrock, K.; Prummel, K.D.; Yu, Y.; Takai, K.; Zhou, A.; Eyob, H.; Balakrishnan, S.; Wang, C.Y.; et al. Single-cell analysis reveals a stem-cell program in human metastatic breast cancer cells. Nature 2015, 526, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Zubair, H.; Azim, S.; Khan, H.Y.; Ullah, M.F.; Wu, D.; Singh, A.P.; Hadi, S.M.; Ahmad, A. Mobilization of Intracellular Copper by Gossypol and Apogossypolone Leads to Reactive Oxygen Species-Mediated Cell Death: Putative Anticancer Mechanism. Int. J. Mol. Sci. 2016, 17, 973. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Gu, J.; Wu, H.; Rauf, A.; Emran, T.B.; Khan, Z.; Mitra, S.; Aljohani, A.S.M.; Alhumaydhi, F.A.; Al-Awthan, Y.S.; et al. Phytochemicals, Nutrition, Metabolism, Bioavailability, and Health Benefits in Lettuce—A Comprehensive Review. Antioxidants 2022, 11, 1158. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; El Omari, N.; El Hachlafi, N.; Bakri, Y.; Lee, L.-H.; Bouyahya, A. Dietary Phenolic Compounds as Anticancer Natural Drugs: Recent Update on Molecular Mechanisms and Clinical Trials. Foods 2022, 11, 3323. [Google Scholar] [CrossRef]
- Moreno-Quintero, G.; Castrillon-Lopez, W.; Herrera-Ramirez, A.; Yepes-Perez, A.F.; Quintero-Saumeth, J.; Cardona-Galeano, W. Synthesis and chemopreventive potential of 5-fu/genistein hybrids on colorectal cancer cells. Pharmaceuticals 2022, 15, 1299. [Google Scholar] [CrossRef]
- Park, O.J.; Surh, Y.J. Chemopreventive potential of epigallocatechin gallate and genistein: Evidence from epidemiological and laboratory studies. Toxicol. Lett. 2004, 150, 43–56. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein content of fruits and nuts. J. Nutr. Biochem. 2000, 11, 326–331. [Google Scholar] [CrossRef]
- Liggins, J.; Bluck, L.J.; Runswick, S.; Atkinson, C.; Coward, W.A.; Bingham, S.A. Daidzein and genistein contents of vegetables. Br. J. Nutr. 2000, 84, 717–725. [Google Scholar] [CrossRef]
- Liggins, J.; Mulligan, A.; Runswick, S.; Bingham, S.A. Daidzein and genistein content of cereals. Eur. J. Clin. Nutr. 2002, 56, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Balázs, A.; Faisal, Z.; Csepregi, R.; Kőszegi, T.; Kriszt, B.; Szabó, I.; Poór, M. In Vitro Evaluation of the Individual and Combined Cytotoxic and Estrogenic Effects of Zearalenone, Its Reduced Metabolites, Alternariol, and Genistein. Int. J. Mol. Sci. 2021, 22, 6281. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Qian, M.; Jiang, Q.; Tan, B.; Yin, Y.; Han, X. Evidence of Flavonoids on Disease Prevention. Antioxidants 2023, 12, 527. [Google Scholar] [CrossRef]
- Naeem, A.; Hu, P.; Yang, M.; Zhang, J.; Liu, Y.; Zhu, W.; Zheng, Q. Natural Products as Anticancer Agents: Current Status and Future Perspectives. Molecules 2022, 27, 8367. [Google Scholar] [CrossRef]
- Varinska, L.; Gal, P.; Mojzisova, G.; Mirossay, L.; Mojzis, J. Soy and Breast Cancer: Focus on Angiogenesis. Int. J. Mol. Sci. 2015, 16, 11728–11749. [Google Scholar] [CrossRef]
- Moorehead, R.A. Rodent Models Assessing Mammary Tumor Prevention by Soy or Soy Isoflavones. Genes 2019, 10, 566. [Google Scholar] [CrossRef]
- Said Ahmad, M.; Fazal, F.; Rahman, A.; Hadi, S.M.; Parish, J.H. Activities of flavonoids for the cleavage of DNA in the presence of cu(ii): Correlation with generation of active oxygen species. Carcinogenesis 1992, 13, 605–608. [Google Scholar] [CrossRef]
- Khan, N.S.; Hadi, S.M. Structural features of tannic acid important for DNA degradation in the presence of cu(ii). Mutagenesis 1998, 13, 271–274. [Google Scholar] [CrossRef]
- Ahsan, H.; Hadi, S.M. Strand scission in DNA induced by curcumin in the presence of cu(ii). Cancer Lett. 1998, 124, 23–30. [Google Scholar] [CrossRef]
- Malik, A.; Azam, S.; Hadi, N.; Hadi, S.M. DNA degradation by water extract of green tea in the presence of copper ions: Implications for anticancer properties. Phytother. Res. PTR 2003, 17, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Farhan Asad, S.; Singh, S.; Hadi, S.M. DNA breakage by resveratrol and cu(ii): Reaction mechanism and bacteriophage inactivation. Cancer Lett. 2000, 154, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Kagawa, T.F.; Geierstanger, B.H.; Wang, A.H.; Ho, P.S. Covalent modification of guanine bases in double-stranded DNA. The 1.2-a z-DNA structure of d(cgcgcg) in the presence of cucl2. J. Biol. Chem. 1991, 266, 20175–20184. [Google Scholar] [CrossRef] [PubMed]
- Hadi, S.M.; Asad, S.F.; Singh, S.; Ahmad, A. Putative mechanism for anticancer and apoptosis-inducing properties of plant-derived polyphenolic compounds. IUBMB Life 2000, 50, 167–171. [Google Scholar] [PubMed]
- Hadi, S.; Bhat, S.; Azmi, A.; Hanif, S.; Shamim, U.; Ullah, M. Oxidative breakage of cellular DNA by plant polyphenols: A putative mechanism for anticancer properties. Semin. Cancer Biol. 2007, 17, 370–376. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A. Understanding the prooxidant action of plant polyphenols in the cellular microenvironment of malignant cells: Role of copper and therapeutic implications. Front. Pharmacol. 2022, 13, 929853. [Google Scholar] [CrossRef]
- Margalioth, E.J.; Udassin, R.; Cohen, C.; Maor, J.; Anteby, S.O.; Schenker, J.G. Serum copper level in gynecologic malignancies. Am. J. Obstet. Gynecol. 1987, 157, 93–96. [Google Scholar] [CrossRef]
- Ebadi, M.; Swanson, S. The status of zinc, copper, and metallothionein in cancer patients. Prog. Clin. Biol. Res. 1988, 259, 161–175. [Google Scholar]
- Yoshida, D.; Ikeda, Y.; Nakazawa, S. Quantitative analysis of copper, zinc and copper/zinc ratio in selected human brain tumors. J. Neuro Oncol. 1993, 16, 109–115. [Google Scholar] [CrossRef]
- Ebara, M.; Fukuda, H.; Hatano, R.; Saisho, H.; Nagato, Y.; Suzuki, K.; Nakajima, K.; Yukawa, M.; Kondo, F.; Nakayama, A.; et al. Relationship between copper, zinc and metallothionein in hepatocellular carcinoma and its surrounding liver parenchyma. J. Hepatol. 2000, 33, 415–422. [Google Scholar] [CrossRef]
- Gupte, A.; Mumper, R.J. Elevated copper and oxidative stress in cancer cells as a target for cancer treatment. Cancer Treat. Rev. 2009, 35, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Lowndes, S.A.; Harris, A.L. The role of copper in tumour angiogenesis. J. Mammary Gland Biol. Neoplasia 2005, 10, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Shamim, U.; Hanif, S.; Azmi, A.S.; Hadi, S.M. Cellular DNA breakage by soy isoflavone genistein and its methylated structural analogue biochanin a. Mol. Nutr. Food Res. 2009, 53, 1376–1385. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Zubair, H.; Khan, H.Y.; Wang, Z.; Sarkar, F.H.; Hadi, S.M. Soy isoflavone genistein induces cell death in breast cancer cells through mobilization of endogenous copper ions and generation of reactive oxygen species. Mol. Nutr. Food Res. 2011, 55, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Ullah, M.F.; Ahmad, A.; Bhat, S.H.; Khan, H.Y.; Zubair, H.; Sarkar, F.H.; Hadi, S.M. Simulating hypoxia-induced acidic environment in cancer cells facilitates mobilization and redox-cycling of genomic copper by daidzein leading to pro-oxidant cell death: Implications for the sensitization of resistant hypoxic cancer cells to therapeutic challenges. Biometals 2016, 29, 299–310. [Google Scholar]
- Rizvi, A.; Hasan, S.S.; Naseem, I. Selective cytotoxic action and DNA damage by Calcitriol-Cu(II) interaction: Putative mechanism of cancer prevention. PLoS ONE 2013, 8, e76191. [Google Scholar] [CrossRef]
- Rizvi, A.; Chibber, S.; Naseem, I. Cu(II)–Vitamin D interaction leads to free radical-mediated cellular DNA damage: A novel putative mechanism for its selective cytotoxic action against malignant cells. Tumor Biol. 2015, 36, 1695–1700. [Google Scholar] [CrossRef]
- Rizvi, A.; Farhan, M.; Naseem, I.; Hadi, S.M. Calcitriol–copper interaction leads to non enzymatic, reactive oxygen species mediated DNA breakage and modulation of cellular redox scavengers in hepatocellular carcinoma. Apoptosis 2016, 21, 997–1007. [Google Scholar] [CrossRef]
- Lelièvre, P.; Sancey, L.; Coll, J.-L.; Deniaud, A.; Busser, B. The Multifaceted Roles of Copper in Cancer: A Trace Metal Element with Dysregulated Metabolism, but Also a Target or a Bullet for Therapy. Cancers 2020, 12, 3594. [Google Scholar] [CrossRef]
- Alhasawi, M.A.I.; Aatif, M.; Muteeb, G.; Alam, M.W.; Oirdi, M.E.; Farhan, M. Curcumin and its derivatives induce apoptosis in human cancer cells by mobilizing and redox cycling genomic copper ions. Molecules 2022, 27, 7410. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Ramesh, V.; Nagaraja, V.; Parish, J.H.; Hadi, S.M. Mode of binding of quercetin to DNA. Mutagenesis 1994, 9, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Asad, S.F.; Ahmad, A.; Khan, N.U.; Hadi, S.M. Oxidative DNA damage by capsaicin and dihydrocapsaicin in the presence of cu(ii). Cancer Lett. 2001, 169, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Ahsan, H.; Parveen, N.; Khan, N.U.; Hadi, S.M. Pro-oxidant, anti-oxidant and cleavage activities on DNA of curcumin and its derivatives demethoxycurcumin and bisdemethoxycurcumin. Chem.-Biol. Interact. 1999, 121, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Azam, S.; Hadi, N.; Khan, N.U.; Hadi, S.M. Prooxidant property of green tea polyphenols epicatechin and epigallocatechin-3-gallate: Implications for anticancer properties. Toxicol. In Vitro 2004, 18, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Arif, H.; Rehmani, N.; Farhan, M.; Ahmad, A.; Hadi, S.M. Mobilization of copper ions by flavonoids in human peripheral lymphocytes leads to oxidative DNA breakage: A structure activity study. Int. J. Mol. Sci. 2015, 16, 26754–26769. [Google Scholar] [CrossRef]
- Farhan, M.; Zafar, A.; Chibber, S.; Khan, H.Y.; Arif, H.; Hadi, S.M. Mobilization of copper ions in human peripheral lymphocytes by catechins leading to oxidative DNA breakage: A structure activity study. Arch. Biochem. Biophys. 2015, 580, 31–40. [Google Scholar] [CrossRef]
- Simic, A.; Manojlovic, D.; Segan, D.; Todorovic, M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules 2007, 12, 2327–2340. [Google Scholar] [CrossRef]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting copper in cancer therapy: ‘Copper that cancer’. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Naseem, I.; Hadi, S.M.; Ahmad, A. Targeting increased copper levels in diethylnitrosamine induced hepatocellular carcinoma cells in rats by epigallocatechin-3-gallate. Tumour Biol. 2015, 36, 8861–8867. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Ahmad, A.; Aatif, M.; Alam, M.W.; Hadi, S.M. Structure of Some Green Tea Catechins and the Availability of Intracellular Copper Influence Their Ability to Cause Selective Oxidative DNA Damage in Malignant Cells. Biomedicines 2022, 10, 664. [Google Scholar] [CrossRef]
- Farhan, M.; Rizvi, A.; Ali, F.; Ahmad, A.; Aatif, M.; Malik, A.; Alam, M.W.; Muteeb, G.; Ahmad, S.; Noor, A.; et al. Pomegranate juice anthocyanidins induce cell death in human cancer cells by mobilizing intracellular copper ions and producing reactive oxygen species. Front. Oncol. 2022, 12, 998346. [Google Scholar] [CrossRef] [PubMed]
- Farhan, M. Naringin’s prooxidant effect on tumor cells: Copper’s role and therapeutic implications. Pharmaceuticals 2022, 15, 1431. [Google Scholar] [CrossRef] [PubMed]
- Hebert, J.R.; Hurley, T.G.; Olendzki, B.C.; Teas, J.; Ma, Y.; Hampl, J.S. Nutritional and socioeconomic factors in relation to prostate cancer mortality: A cross-national study. J. Natl. Cancer Inst. 1998, 90, 1637–1647. [Google Scholar] [CrossRef] [PubMed]
- Van Die, M.D.; Bone, K.M.; Williams, S.G.; Pirotta, M.V. Soy and soy isoflavones in prostate cancer: A systematic review and meta-analysis of randomized controlled trials. BJU Int. 2014, 113, E119–E130. [Google Scholar] [CrossRef] [PubMed]
- Messina, M.; Nagata, C.; Wu, A.H. Estimated Asian adult soy protein and isoflavone intakes. Nutr. Cancer 2006, 55, 1–12. [Google Scholar] [CrossRef]
- Jun, S.; Shin, S.; Joung, H. Estimation of dietary flavonoid intake and major food sources of Korean adults. Br. J. Nutr. 2016, 115, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Van Erp-Baart, M.A.; Brants, H.A.; Kiely, M.; Mulligan, A.; Turrini, A.; Sermoneta, C.; Kilkkinen, A.; Valsta, L.M. Isoflavone intake in four different European countries: The VENUS approach. Br. J. Nutr. 2003, 89, S25–S30. [Google Scholar] [CrossRef]
- Valsta, L.M.; Kilkkinen, A.; Mazur, W.; Nurmi, T.; Lampi, A.M.; Ovaskainen, M.L.; Korhonen, T.; Adlercreutz, H.; Pietinen, P. Phyto-oestrogen database of foods and average intake in Finland. Br. J. Nutr. 2003, 89, S31–S38. [Google Scholar] [CrossRef]
- Vladu, A.F.; Ficai, D.; Ene, A.G.; Ficai, A. Combination Therapy Using Polyphenols: An Efficient Way to Improve Antitumoral Activity and Reduce Resistance. Int. J. Mol. Sci. 2022, 23, 10244. [Google Scholar] [CrossRef]
- Aboushanab, S.A.; Khedr, S.M.; Gette, I.F.; Danilova, I.G.; Kolberg, N.A.; Ravishankar, G.A.; Ambati, R.R.; Kovaleva, E.G. Isoflavones derived from plant raw materials: Bioavailability, anti-cancer, anti-aging potentials, and microbiome modulation. Crit. Rev. Food Sci. Nutr. 2021, 63, 261–287. [Google Scholar] [CrossRef]
- Chimento, A.; D’Amico, M.; De Luca, A.; Conforti, F.L.; Pezzi, V.; De Amicis, F. Resveratrol, Epigallocatechin Gallate and Curcumin for Cancer Therapy: Challenges from Their Pro-Apoptotic Properties. Life 2023, 13, 261. [Google Scholar] [CrossRef] [PubMed]
- Chimento, A.; De Luca, A.; D’Amico, M.; De Amicis, F.; Pezzi, V. The Involvement of Natural Polyphenols in Molecular Mechanisms Inducing Apoptosis in Tumor Cells: A Promising Adjuvant in Cancer Therapy. Int. J. Mol. Sci. 2023, 24, 1680. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Zhu, L.; Zhu, F.; Sun, J.; Zhu, Z. Effects of different sources of copper on ctr1, atp7a, atp7b, mt and dmt1 protein and gene expression in caco-2 cells. J. Trace Elem. Med. Biol. 2014, 28, 344–350. [Google Scholar] [CrossRef] [PubMed]



| Cancer Cell Line | Dose | Apoptosis (Folds) | Effect of Scavengers |
|---|---|---|---|
| LNCaP | Untreated | - | - |
| Genistein (50 µM) | 4.42 * | - | |
| Thiourea | 2.48 * | 43.89 | |
| Catalase | 3.15 * | 28.73 | |
| Superoxide dismutase | 3.43 * | 22.39 | |
| LNCaP | Untreated | - | - |
| Daidzein (50 µM) | 4.62 * | - | |
| Thiourea | 2.86 * | 38.09 | |
| Catalase | 3.38 * | 26.83 | |
| Superoxide dismutase | 3.64 * | 21.21 | |
| DU145 | Untreated | - | - |
| Genistein (50 µM) | 5.81 * | - | |
| Thiourea | 3.79 * | 34.76 | |
| Catalase | 4.23 * | 27.19 | |
| Superoxide dismutase | 4.55 * | 21.68 | |
| DU145 | Untreated | - | - |
| Daidzein (50 µM) | 5.34 * | - | |
| Thiourea | 3.48 * | 34.83 | |
| Catalase | 3.73 * | 30.14 | |
| Superoxide dismutase | 4.06 * | 23.97 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhan, M.; El Oirdi, M.; Aatif, M.; Nahvi, I.; Muteeb, G.; Alam, M.W. Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties. Molecules 2023, 28, 2925. https://doi.org/10.3390/molecules28072925
Farhan M, El Oirdi M, Aatif M, Nahvi I, Muteeb G, Alam MW. Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties. Molecules. 2023; 28(7):2925. https://doi.org/10.3390/molecules28072925
Chicago/Turabian StyleFarhan, Mohd, Mohamed El Oirdi, Mohammad Aatif, Insha Nahvi, Ghazala Muteeb, and Mir Waqas Alam. 2023. "Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties" Molecules 28, no. 7: 2925. https://doi.org/10.3390/molecules28072925
APA StyleFarhan, M., El Oirdi, M., Aatif, M., Nahvi, I., Muteeb, G., & Alam, M. W. (2023). Soy Isoflavones Induce Cell Death by Copper-Mediated Mechanism: Understanding Its Anticancer Properties. Molecules, 28(7), 2925. https://doi.org/10.3390/molecules28072925











