Two New 1D Supramolecular Compounds Based on PbI2 for Efficient Iodine Capture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystals Structures of Compounds 1–2
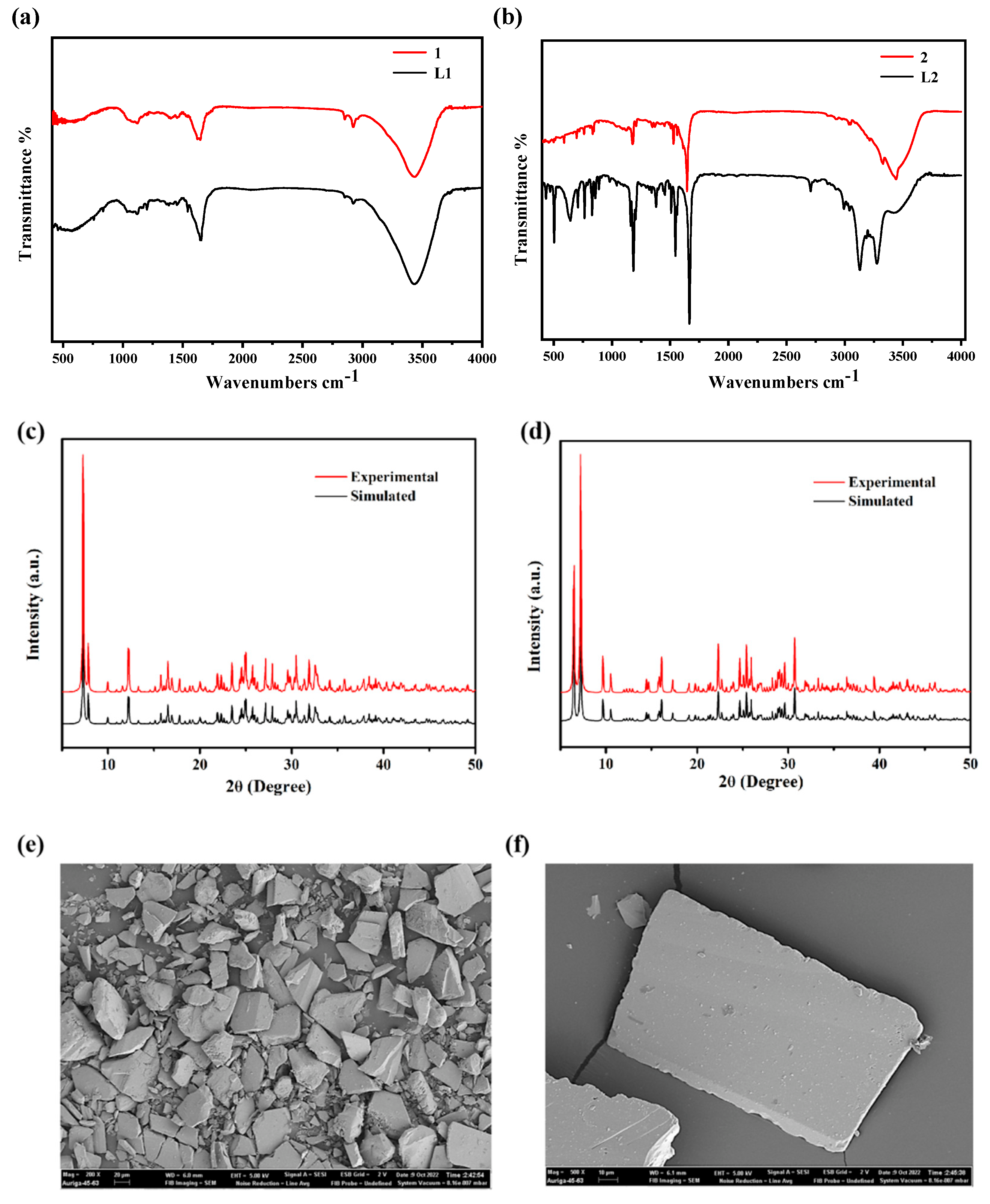
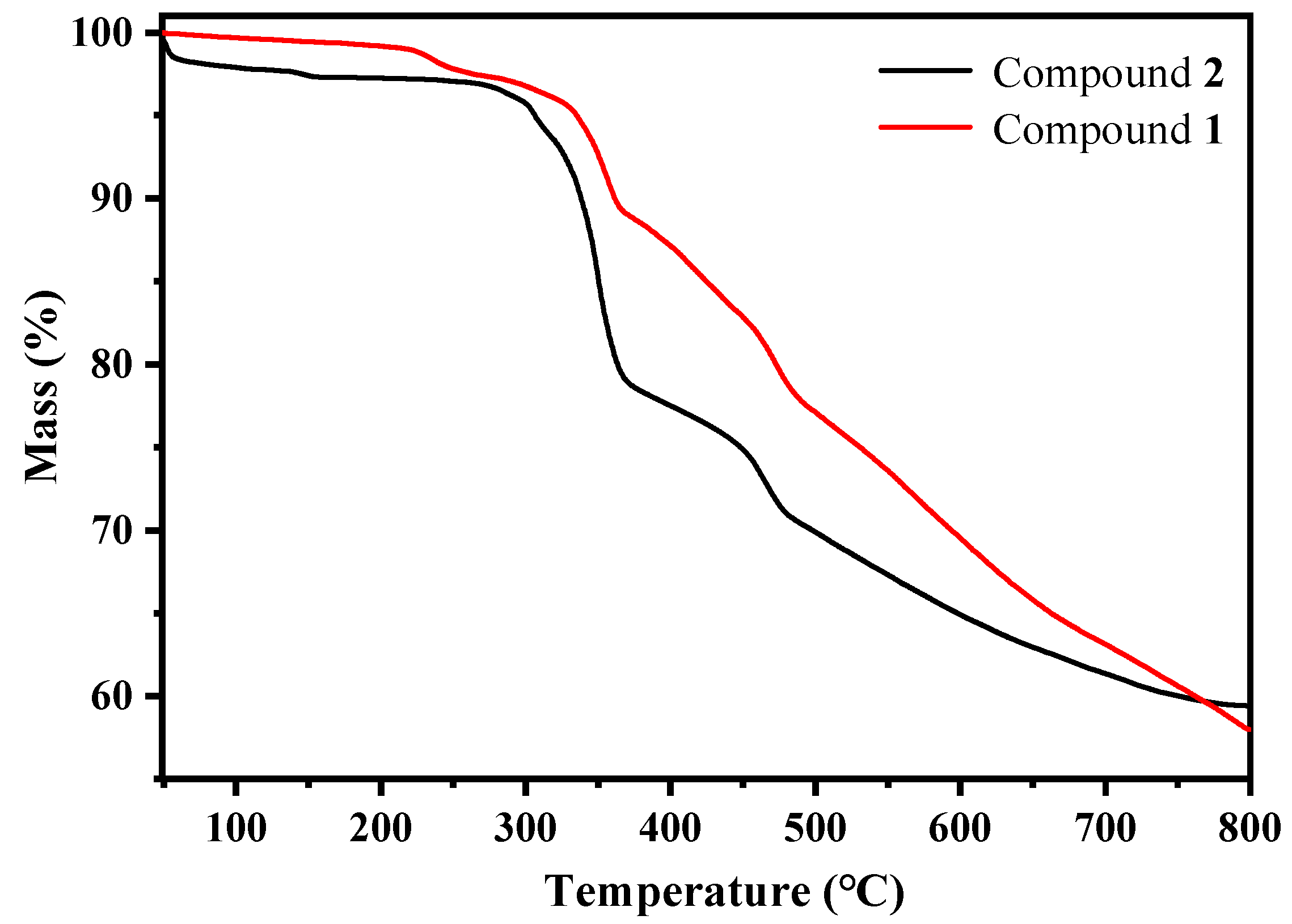
2.2. Structure Characterizations of Compounds 1–2
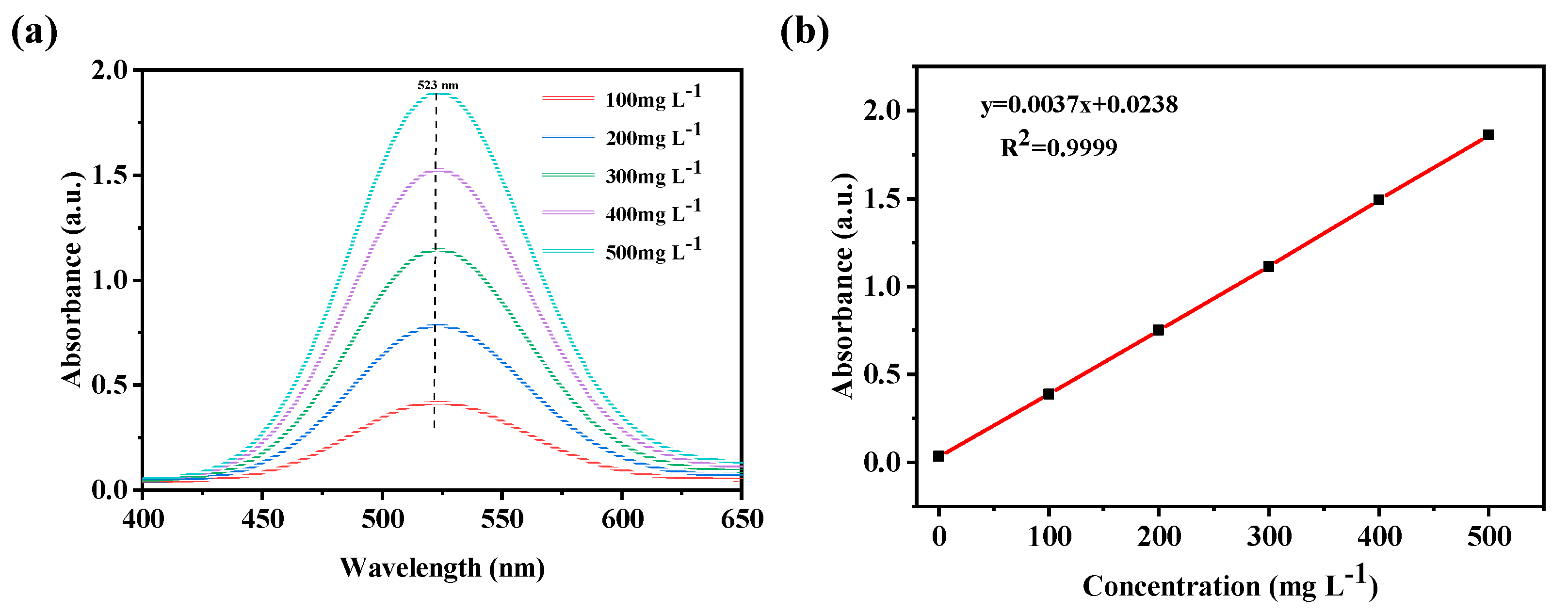
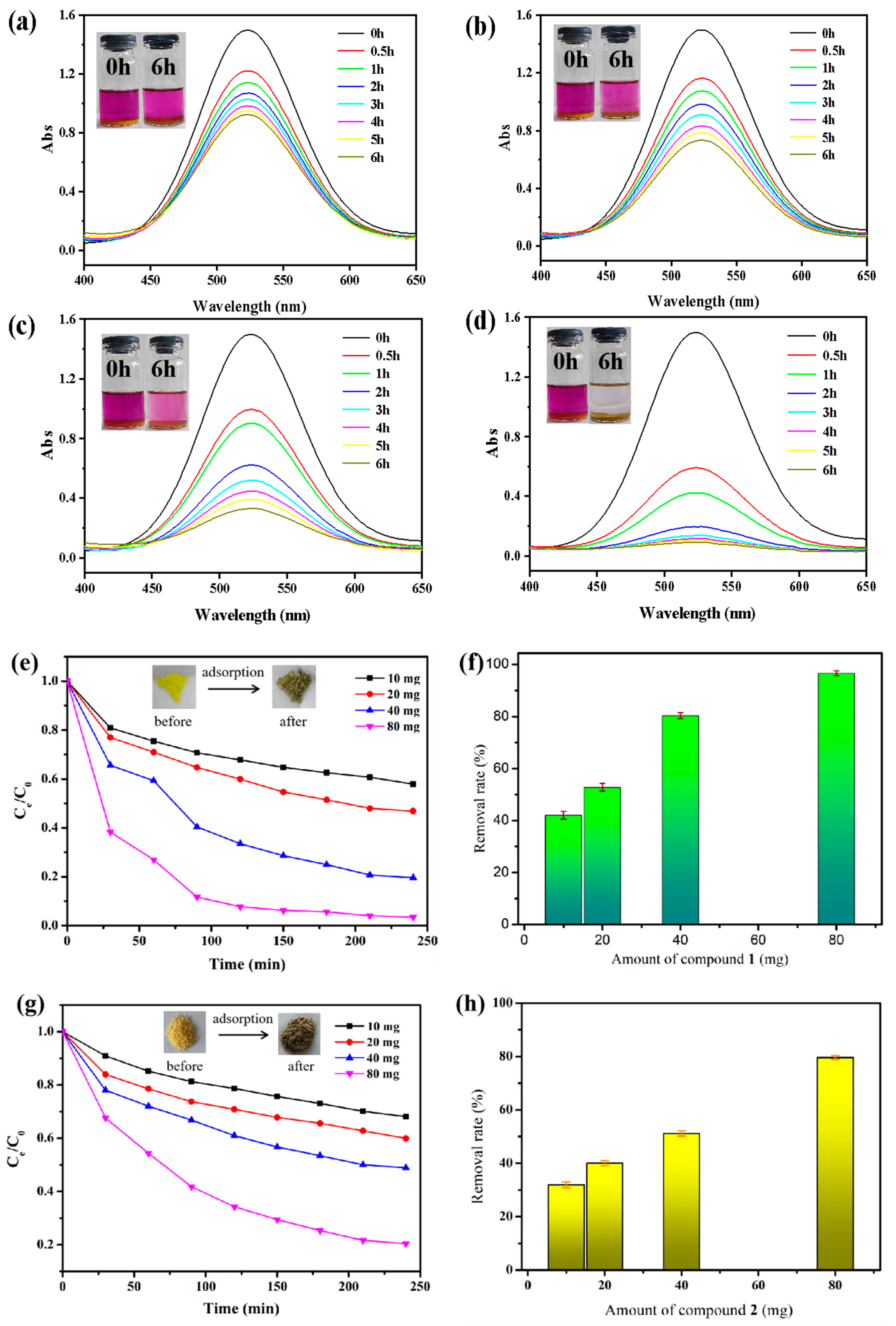
2.3. Capture of Iodine in Cyclohexane Solution
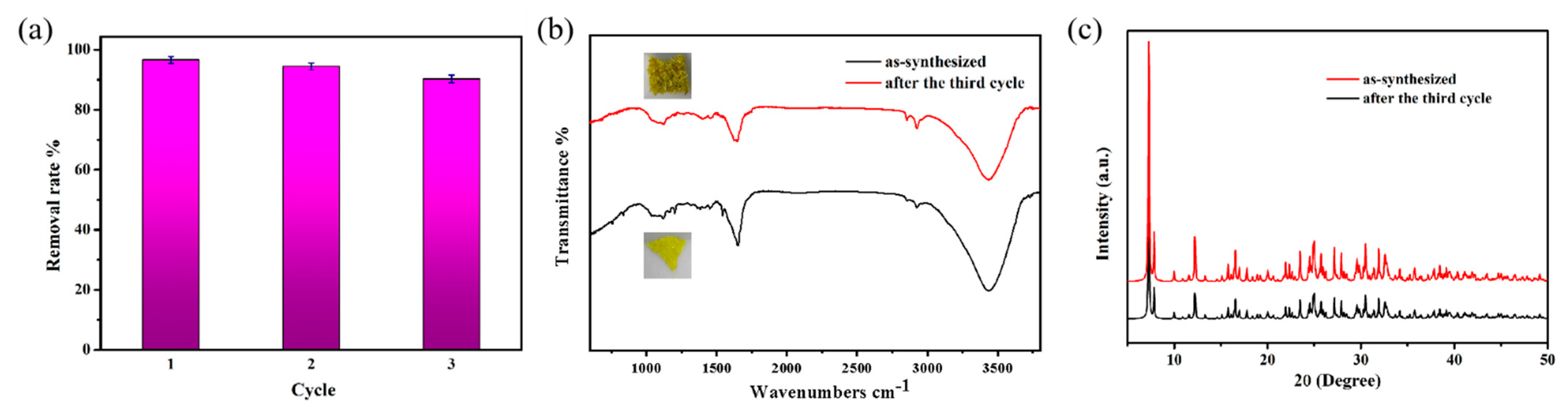
2.4. Cyclic Experiment
3. Materials and Methods
3.1. Materials
3.2. Synthesis Methods
3.2.1. Synthesis of {[L1]·[Pb2I6]}n (1)
3.2.2. Synthesis of {[L2]2[Pb3I10]}n (2)
3.3. Characterization Methods
3.4. Adsorption Experiment
3.4.1. Iodine Sorption Study
3.4.2. Kinetic Studies
3.4.3. Models of Adsorption Isotherm
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Qi, B.B.; Liu, Y.; Zheng, T.; Gao, Q.H.; Yan, X.W.; Jiao, Y.; Yang, Y. Highly efficient capture of iodine by Cu/MIL-101. J. Solid State Chem. 2018, 258, 49–55. [Google Scholar] [CrossRef]
- Zhang, X.; Maddock, J.; Nenoff, T.; Denecke, M.; Yang, S.; Schroder, M. Adsorption of iodine in metal–organic framework materials. Chem. Soc. Rev. 2022, 51, 3243. [Google Scholar] [CrossRef] [PubMed]
- Sava, D.; Rodriguez, M.; Chapman, K.; Chupas, P.; Greathtrouse, J.; Crozier, P.; Nenoff, T. Capture of Volatile Iodine, a Gaseous Fission Product, by Zeolitic Imidazolate Framework—8. J. Am. Chem. Soc. 2011, 133, 12398–12401. [Google Scholar] [CrossRef] [PubMed]
- Subrahmanyam, K.; Sarma, D.; Malliakas, C.; Polychronopoulou, K.; Riley, B.; Pierce, D.; Chun, J.; Kanatzidis, M. Chalcogenide Aerogels as Sorbents for Radioactive Iodine. Chem. Mater. 2015, 27, 2619–2626. [Google Scholar] [CrossRef]
- Shimamoto, Y.; Takahashi, Y.; Terada, Y. Formation of Organic Iodine Supplied as Iodide in a Soil–Water System in Chiba, Japan. Environ. Sci. Technol. 2011, 45, 2086–2092. [Google Scholar] [CrossRef]
- Akram, B.; Lu, Q.C.; Wang, X. Polyoxometalate-Zirconia Coassembled Microdumbbells for Efficient Capture of Iodine. ACS Mater. Lett 2020, 2, 461–465. [Google Scholar] [CrossRef]
- Yu, R.L.; Li, Q.F.; Li, Z.L.; Xia, L.Z. Precise regulation of active sites of MOFs for capture of iodine. J. Environ. Chem. Eng 2022, 10, 108779. [Google Scholar] [CrossRef]
- Muhire, C.; Reda, A.T.; Zhang, D.X.; Xu, X.Y.; Cui, C. An overview on metal Oxide-based materials for iodine capture and storage. Chem. Eng. J 2022, 431, 133816. [Google Scholar] [CrossRef]
- Cheng, S.; Chen, W.; Zhao, L.; Wang, X.; Qin, C.; Su, Z. Synthesis, crystal structure and iodine capture of Zr–based metal–organic polyhedron. Inorg. Chim. Acta 2021, 516, 120174. [Google Scholar] [CrossRef]
- Huve, J.; Ryzhikov, A.; Nouali, H.; Lalia, V.; Auge, G.; Daou, T.J. Porous sorbents for the capture of radioactive iodine compounds: A review. RSC Adv. 2018, 8, 29248–29273. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.-J.; Qiao, Y.-M.; Wang, J.-L.; Xie, J.-L.; Cui, B.; Fu, Y.; Lu, J.-W.; Yang, Y.-J.; Bu, N.-S.; Yuan, Y.; et al. An Azo-Group-Functionalized Porous Aromatic Framework for Achieving Highly Efficient Capture of Iodine. Molecules 2022, 27, 6297. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-T.; Liu, Y.-J.; Zhang, C.-Y.; Yang, F.; Fang, W.-H.; Zhang, J. Cluster-Based Crystalline Materials for Iodine Capture. Chem. Eur. J. 2022, 29, e202380262. [Google Scholar]
- Xu, S.; Freeman, S.; Hou, X.; Watanabe, A.; Yamaguchi, K.; Zhang, L. Iodine Isotopes in Precipitation: Temporal Responses to (129)I Emissions from the Fukushima Nuclear Accident. Environ. Sci. Technol. 2013, 47, 10851–10859. [Google Scholar] [CrossRef] [PubMed]
- Shafaei, M.; He, J.; Rothenberger, A.; Kanatzidis, M. Ion–Exchangeable Cobalt Polysulfide Chalcogel. J. Am. Chem. Soc. 2011, 133, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Niu, T.-H.; Feng, C.-C.; Yao, C.; Yang, W.-Y.; Xu, Y.-H. Bisimidazole-Based Conjugated Polymers for Excellent Iodine Capture. ACS Appl. Polym. 2021, 3, 354–361. [Google Scholar] [CrossRef]
- Reda, A.T.; Pan, M.; Zhang, D.-X.; Xu, X.-Y. Bismuth-based materials for iodine capture and storage: A review. J. Environ. Chem. Eng. 2021, 9, 105279. [Google Scholar] [CrossRef]
- Jiang, M.; Zhu, L.; Zhao, Q.; Chen, G.-Y.; Wang, Z.-R.; Zhang, J.-J.; Zhang, L.; Lei, J.-H.; Duan, T. Novel synthesis of NaY-NH4F-Bi2S3 composite for enhancing iodine capture. Chem. Eng. J. 2022, 443, 136477. [Google Scholar] [CrossRef]
- Nandanwar, S.U.; Coldsnow, K.; Porter, A.; Sabharwall, P.; Aston, D.E.; McIlroy, D.N.; Utgikar, V. Adsorption of radioactive iodine and krypton from off-gas stream using continuous flow adsorption column. Chem. Eng. J. 2017, 320, 222–231. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.H.; Cho, Y.-J.; Shin, J.M.; Yim, M.-S. Bismuth-embedded SBA-15 mesoporous silica for radioactive iodine capture and stable storage. J. Nucl. Mater. 2015, 465, 556–564. [Google Scholar] [CrossRef]
- Kajiya, R.; Sakakibara, S.; Ikawa, H.; Higashiguchi, K.; Matsuda, K.; Wada, H.; Kuroda, K.; Shimojima, A. Inorganic–Organic Hybrid Photomechanical Crystals Consisting of Diarylethenes and Cage Siloxanes. Chem. Mater. 2019, 31, 9372–9378. [Google Scholar] [CrossRef]
- Duan, H.; Yu, S.; Liu, S.; Zhang, H. An inorganic–organic hybrid crystal with a two-step dielectric response and thermochromic luminescence. Dalton Trans. 2017, 46, 2220–2227. [Google Scholar] [CrossRef]
- Roy, S.; Sarkar, S.; Pan, J.; Waghmare, U.; Dhanya, R.; Narayana, C.; Peter, S. Crystal Structure and Band Gap Engineering in Polyoxometalate–Based Inorganic–Organic Hybrids. Inorg. Chem. 2016, 55, 3364–3377. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Kubo, K.; Noro, S.; Akutagawa, T.; Nakamura, T. Self–assembled Structure of Inorganic–Organic Hybrid Crystals Based on Keggin Polyoxometallates [SMo12O402–] and Supramolecular Cations. Cryst. Growth Des. 2016, 16, 800–807. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Shi, T.; Li, C.; Chen, Y.; Wang, H. Double–linked chain in POM–based hybrids. Synthesis, crystal structure and properties of an inorganic–organic compound. Inorg. Chem. Commun. 2016, 65, 49–53. [Google Scholar] [CrossRef]
- Zhou, K.; Zhang, W.; Luo, Y.; Pan, C. Photoluminescent carbon dots based on a rare 3D inorganic–organic hybrid cadmium borate crystal. Dalton Trans. 2018, 47, 7407. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Chen, W.; Yao, W.; Qin, C.; Su, Z. Steam–assisted assemblies of {Ni6PW9}− based inorganic–organic hybrid chains: Synthesis, crystal structures and properties. Cryst. Eng. Comm. 2018, 20, 7507–7512. [Google Scholar] [CrossRef]
- Ren, Y.; Oswald, W.; Wang, X.; McCandless, G.; Chan, J. Orientation of Organic Cations in Hybrid Inorganic–Organic Perovskite CH3NH3PbI3 from Subatomic Resolution Single Crystal Neutron Diffraction Structural Studies. Cryst. Growth Des. 2016, 16, 2945–2951. [Google Scholar] [CrossRef]
- Yang, Y.-T.; Tu, C.-Z.; Yin, H.-J.; Liu, J.-J.; Cheng, F.-X.; Luo, F. Molecular Iodine Capture by Covalent Organic Frameworks. Molecules 2022, 27, 9045. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.; Liu, Y.; Liu, J.; Li, Y.; Qiao, X.; Huang, W.; Niu, Y. POM–based metal–organic compounds: Assembly, structures and properties. Main Group Chem. 2021, 20, 575–592. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Niu, Y. M-Carboxylic Acid Induced Formation of New Coordination Polymers for Efficient Photocatalytic Degradation of Ciprofloxacin. Molecules 2022, 27, 7731. [Google Scholar] [CrossRef]
- Niu, Y.; Song, Y.; Hou, H.; Zhu, Y. Synthesis, Structure, and Large Optical Limiting Effect of the First Coordination Polymeric Cluster Based on an {I@[AgI(inh)]6} Hexagram Block. Inorg. Chem. 2005, 44, 2553–2559. [Google Scholar] [CrossRef]
- Wang, X.; Qiao, G.; Zhu, G.; Li, J.; Guo, X.; Liang, Y.; Niu, Y. Preparation of 2D supramolecular material doping with TiO2 for degradation of tetracycline. Environ. Res. 2021, 202, 111689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Niu, Y. A Review of Crystalline Multibridged Cyclophane Cages: Synthesis, Their Conformational Behavior, and Properties. Molecules 2022, 27, 7083. [Google Scholar] [CrossRef] [PubMed]
- Qiao, X.; Wang, C.; Niu, Y. N-Benzyl HMTA induced self-assembly of organic–inorganic hybrid materials for efficient photocatalytic degradation of tetracycline. J. Hazard Mater. 2020, 391, 122121. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhu, G.; Wang, C.; Niu, Y. Effective degradation of tetracycline by organic–inorganic hybrid materials induced by triethylenediamine. Environ. Res. 2021, 198, 111253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Huang, W.; Li, Y.; Wu, X.; Niu, Y.; Zang, S. Two Nanometer-Sized High-Nuclearity Homometallic Bromide Clusters (M26Br38)12− (M = Cu, Ag): Syntheses, Crystal Structures, and Efficient Adsorption Properties. Inorg. Chem. 2020, 59, 9579–9586. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Li, Z.; Li, S.; Wang, F. Five novel copper halide/thiocyanate coordination compounds directed by 4-pyridyl dithioether ligands: Syntheses, structures, and photocatalytic properties. J. Mol. Struct. 2018, 1173, 763–769. [Google Scholar] [CrossRef]
- Liu, J.-Q.; Liang, Z.-H.; Xu, B.; Xiang, H.; Xia, Y.-D.; Yin, J.; Liu, Z.-G. Synthesis of PbI2 nanowires for high sensitivity photodetectors. RSC Adv. 2016, 6, 59445–59449. [Google Scholar] [CrossRef]
- Aldawood, S.; AlTalib, O.M.; AlGarawi, M.S.; Turki, S.A.; Yazeed, A.; Nasser, S.; Ahmad Taufek Abdul, R.; Khalid, S.; Syed Mansoor, A. Gamma ray effects on the properties of PbI2 thin films. Radiat. Phys. Chem. 2022, 193, 110003. [Google Scholar] [CrossRef]
- Zhong, M.-Z.; Huang, L.; Deng, H.-X.; Wang, X.-T.; Li, B.; Wei, Z.-M.; Li, J.-B. Flexible photodetectors based on phase dependent PbI2 single crystals. J. Mater. Chem. C 2016, 4, 6492–6499. [Google Scholar] [CrossRef]
- Zhang, N.; Sun, L.-X.; Bai, F.-Y.; Xing, Y.-H. Thorium-Organic Framework Constructed with a Semirigid Triazine Hexacarboxylic Acid Ligand: Unique Structure with Thorium Oxide Wheel Clusters and Iodine Adsorption Behavior. Inorg. Chem. 2020, 59, 3964–3973. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xing, Y.; Bai, F. A Uranyl-Organic Framework Featuring Two-Dimensional Graphene-like Layered Topology for Efficient Iodine and Dyes Capture. Inorg. Chem. 2019, 58, 6866–6876. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yue, Z.; Ju, Y.; Wu, X.; Ren, Y.; Wang, S.; Li, Y.; Zhang, Z.; Guo, X.; Lin, J.; et al. Ultrastable Thorium Metal–Organic Frameworks for Efficient Iodine Adsorption. Inorg. Chem. 2020, 59, 4435–4442. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Li, Y.; Zheng, L.; Niu, Y.; Hou, H. Three cation-templated Cu(I) self-assemblies: Synthesis, structures, and photocatalytic properties. New J. Chem. 2016, 40, 6086. [Google Scholar] [CrossRef]
- Wallace, K.; Belcher, W.; Turner, D.; Syed, K.; Steed, J. Slow Anion Exchange, Conformational Equilibria, and Fluorescent Sensing in Venus Flytrap Aminopyridinium-Based Anion Hosts. J. Am. Chem. Soc. 2003, 125, 9699–9715. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Harmalkar, S.; Yadav, R.; Lama, P. Structure directing roles of weak noncovalent interactions and charge–assisted hydrogen bonds in the self-assembly of solvated podands: An example of an anion-assisted dimeric water capsule. CrystEngComm 2022, 24, 4063–4073. [Google Scholar] [CrossRef]
- Venegas-García, D.J.; Wilson, L.D. Utilization of Bioflocculants from Flaxseed Gum and Fenugreek Gum for the Removal of Arsenicals from Water. Materials 2022, 15, 8691. [Google Scholar] [CrossRef]
- Gautam, D.; Lal, S.; Hooda, S. Adsorption of Rhodamine 6G Dye on Binary System of Nanoarchitectonics Composite Magnetic Graphene Oxide Material. J. Nanosci. Nanotechnol. 2020, 20, 2939–2945. [Google Scholar] [CrossRef]
- Tran, Q.T.; Đo, T.H.; Ha, X.L.; Duong, T.T.A.; Chu, M.N.; Vu, V.N.; Chau, H.D.; Tran, T.K.N.; Song, P. Experimental Design, Equilibrium Modeling and Kinetic Studies on the Adsorption of Methylene Blue by Adsorbent: Activated Carbon from Durian Shell Waste. Materials 2022, 15, 8566. [Google Scholar] [CrossRef]



| Sample | Pseudo−First−Order−Model | Pseudo−Second−Order−Model | ||||
|---|---|---|---|---|---|---|
| qe (cal) (mg·g−1) | Kf (1·min−1) | R2 | qe (cal) (mg·g−1) | Ks (g·mg−1·min−1) | R2 | |
| Compound 1 | 15.96 | 0.0176 | 0.9155 | 24.96 | 0.0022 | 0.9997 |
| Sample | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm (mg·g−1) | K1 (L·mg−1) | R2 | 1/n | K2 (g·mg−1·min−1) | R2 | |
| Compound 1 | 15.22 | 0.0043 | 0.9984 | 0.0992 | 466.33 | 0.3625 |
| Compounds | 1 | 2 |
|---|---|---|
| Formula | C18H20I6N4Pb2 | C36H40I10N8Pb3 |
| Formula Weight | 1468.16 | 2475.33 |
| Temperature/K | 299.00 | 300.00 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/n | P21/c |
| a/Å | 9.4089 (2) | 13.724 (3) |
| b/Å | 22.4783 (5) | 24.503 (5) |
| c/Å | 14.4369 (4) | 8.9479 (18) |
| α/° | 90 | 90 |
| β/° | 93.4570(10) | 94.487 (7) |
| γ/° | 90 | 90 |
| Volume/Å3 | 3047.79 (13) | 2999.8 (10) |
| Z | 4 | 2 |
| ρ calc, g/cm3 | 3.200 | 2.740 |
| μ/mm−1 | 17.124 | 13.574 |
| F(000) | 2552.0 | 2176.0 |
| Crystal size/mm3 | 0.26 × 0.23 × 0.14 | 0.23 × 0.22 × 0.21 |
| Reflections collected | 76,647 | 78,864 |
| Independent reflections | 7001[Rint = 0.0831, Rsigma = 0.0397] | 6848[Rint = 0.0841, Rsigma = 0.0359] |
| Data/restraints/parameters | 7001/0/272 | 6848/0/261 |
| Goodness-of-fit on F2 | 1.027 | 1.077 |
| Final R indexes (I ≥ 2σ (I)) | R1 = 0.0302, wR2 = 0.0629 | R1 = 0.0301, wR2 = 0.0638 |
| Final R indexes (all data) | R1 = 0.0423, wR2 = 0.0662 | R1 = 0.0454, wR2 = 0.0672 |
| Largest diff. peak/hole/e Å−3 | 0.95/−1.10 | 1.09/−1.05 |
| Compound 1 | |||||
| Pb1-I1 | 3.3015 (5) | Pb1-I2 | 2.9931 (5) | Pb1-I3 | 3.2378 (5) |
| Pb1-I4 | 3.1758 (5) | Pb1-I61 | 3.3566 (5) | Pb2-I12 | 3.3261 (5) |
| Pb2-I33 | 3.2566 (5) | Pb2-I4 | 3.2066 (5) | Pb2-I5 | 2.9998 (5) |
| Pb2-I6 | 3.3275 (5) | I1-Pb1-I61 | 94.147 (13) | I2-Pb1-I1 | 90.717 (13) |
| I2-Pb1-I3 | 91.864 (15) | I2-Pb1-I4 | 94.913 (14) | I2-Pb1-I61 | 88.327 (14) |
| I3-Pb1-I1 | 85.429 (13) | I3-Pb1-I61 | 179.537 (15) | I4-Pb1-I1 | 174.300 (14) |
| I4-Pb1-I3 | 95.263 (15) | I4-Pb1-I61 | 85.140 (14) | I12-Pb2-I6 | 91.846 (13) |
| I33-Pb2-I12 | 178.799 (15) | I33-Pb2-I6 | 88.579 (14) | I4-Pb2-I12 | 82.350 (13) |
| I4-Pb2-I33 | 97.253 (14) | I4-Pb2-I6 | 174.008 (16) | I5-I2-I12 | 91.964 (14) |
| I5-Pb2-I33 | 89.166 (14) | I5-Pb2-I4 | 89.512 (14) | I5-I2-I6 | 89.201 (14) |
| Pb1-I1-Pb22 | 137.703 (16) | Pb1-I3-Pb24 | 128.609 (17) | Pb1-I4-Pb2 | 87.013 (12) |
| Pb2-I6-Pb11 | 135.438 (16) | ||||
| Compound 2 | |||||
| Pb1-I1 | 3.1687 (6) | Pb1-I11 | 3.1688 (6) | Pb1-I31 | 3.3441 (6) |
| Pb1-I3 | 3.3441 (6) | Pb1-I21 | 3.2365 (6) | Pb1-I2 | 3.2364 (6) |
| Pb2-I11 | 3.3841 (7) | Pb2-I3 | 3.2696 (7) | Pb2-I22 | 3.3502 (7) |
| Pb2-I4 | 2.9499 (7) | Pb2-I5 | 3.0913 (6) | I1-Pb1-I11 | 180.0 |
| I1-Pb1-I31 | 84.097 (15) | I11-Pb1-I31 | 95.901 (16) | I1-Pb1-I3 | 95.903 (16) |
| I11-Pb1-I3 | 84.098 (15) | I1-Pb1-I2 | 92.692 (19) | I1-Pb1-I21 | 87.309 (19) |
| I11-Pb1-I21 | 92.690 (19) | I11-Pb1-I2 | 87.309 (19) | I3-Pb1-I31 | 180.0 |
| I21-Pb1-I31 | 79.946 (16) | I21-Pb1-I3 | 100.054 (16) | I2-Pb1-I31 | 100.054 (16) |
| I2-Pb1-I3 | 79.946 (16) | I2-Pb1-I21 | 180.0 | I3-Pb2-I11 | 81.954 (17) |
| I3-Pb2-I22 | 163.585 (14) | I22-Pb2-I11 | 81.683 (18) | I4-Pb2-I11 | 88.270 (17) |
| I4-Pb2-I3 | 88.450 (15) | I4-Pb2-I22 | 89.767 (16) | I4-Pb2-I5 | 85.572 (19) |
| I5-Pb2-I11 | 173.831 (14) | I5-Pb2-I3 | 98.223 (19) | I5-Pb2-I22 | 97.91 (2) |
| Pb1-I1-I21 | 88.903 (19) | Pb2-I3-Pb1 | 87.927 (15) | Pb1-I2-Pb22 | 109.55 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, J.; Niu, Y. Two New 1D Supramolecular Compounds Based on PbI2 for Efficient Iodine Capture. Molecules 2023, 28, 2934. https://doi.org/10.3390/molecules28072934
Zhang X, Li J, Niu Y. Two New 1D Supramolecular Compounds Based on PbI2 for Efficient Iodine Capture. Molecules. 2023; 28(7):2934. https://doi.org/10.3390/molecules28072934
Chicago/Turabian StyleZhang, Xingxing, Jian Li, and Yunyin Niu. 2023. "Two New 1D Supramolecular Compounds Based on PbI2 for Efficient Iodine Capture" Molecules 28, no. 7: 2934. https://doi.org/10.3390/molecules28072934





