Silver(I) Coordination Polymer Ligated by Bipyrazole Me4bpzH2, [Ag(X)(Me4bpzH2)] (X = CF3CO2− and CF3SO3−, Me4bpzH2 = 3,3′,5,5′-Tetramethyl-4,4′-bipyrazole): Anion Dependent Structures and Photoluminescence Properties
Abstract
:1. Introduction
2. Results and Discussion
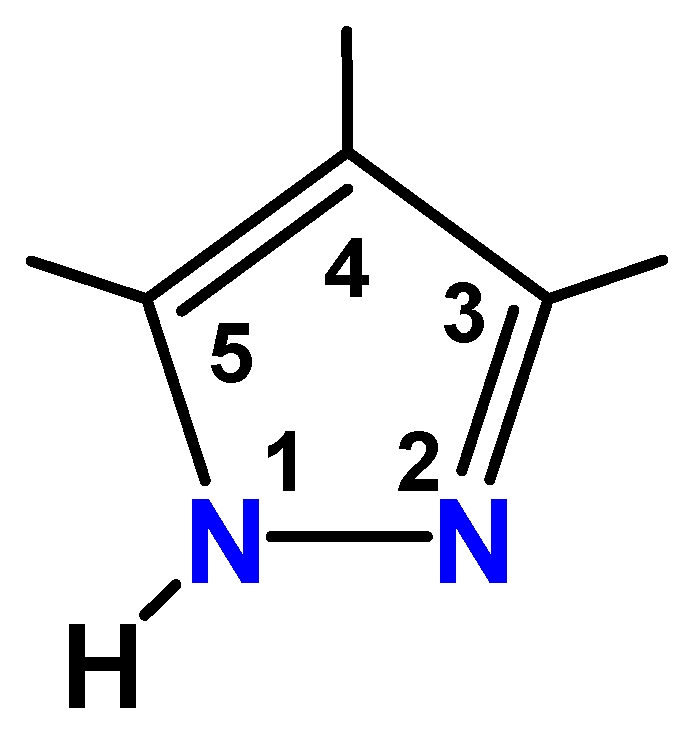
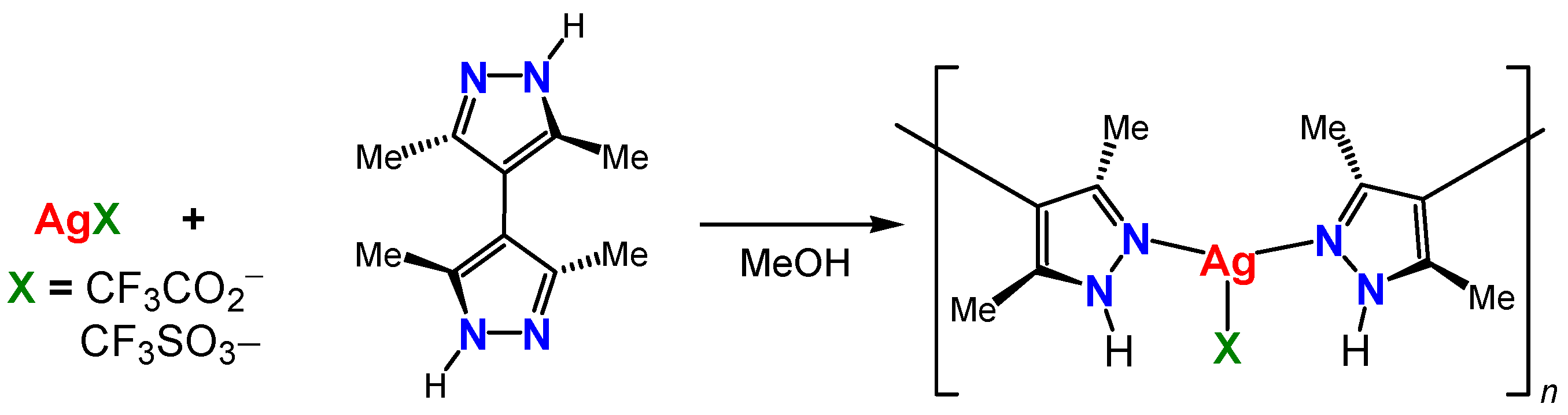
2.1. Synthesis
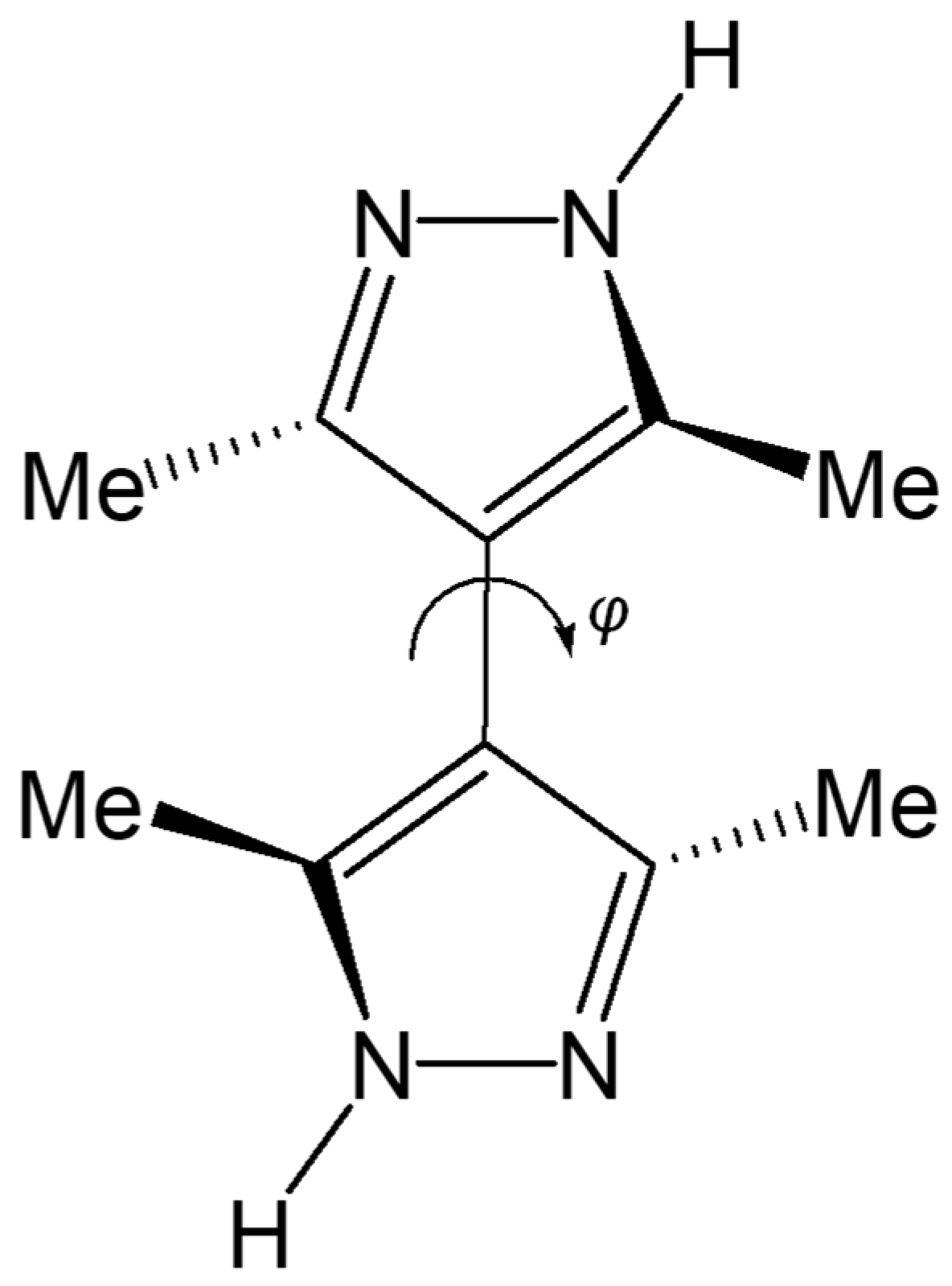
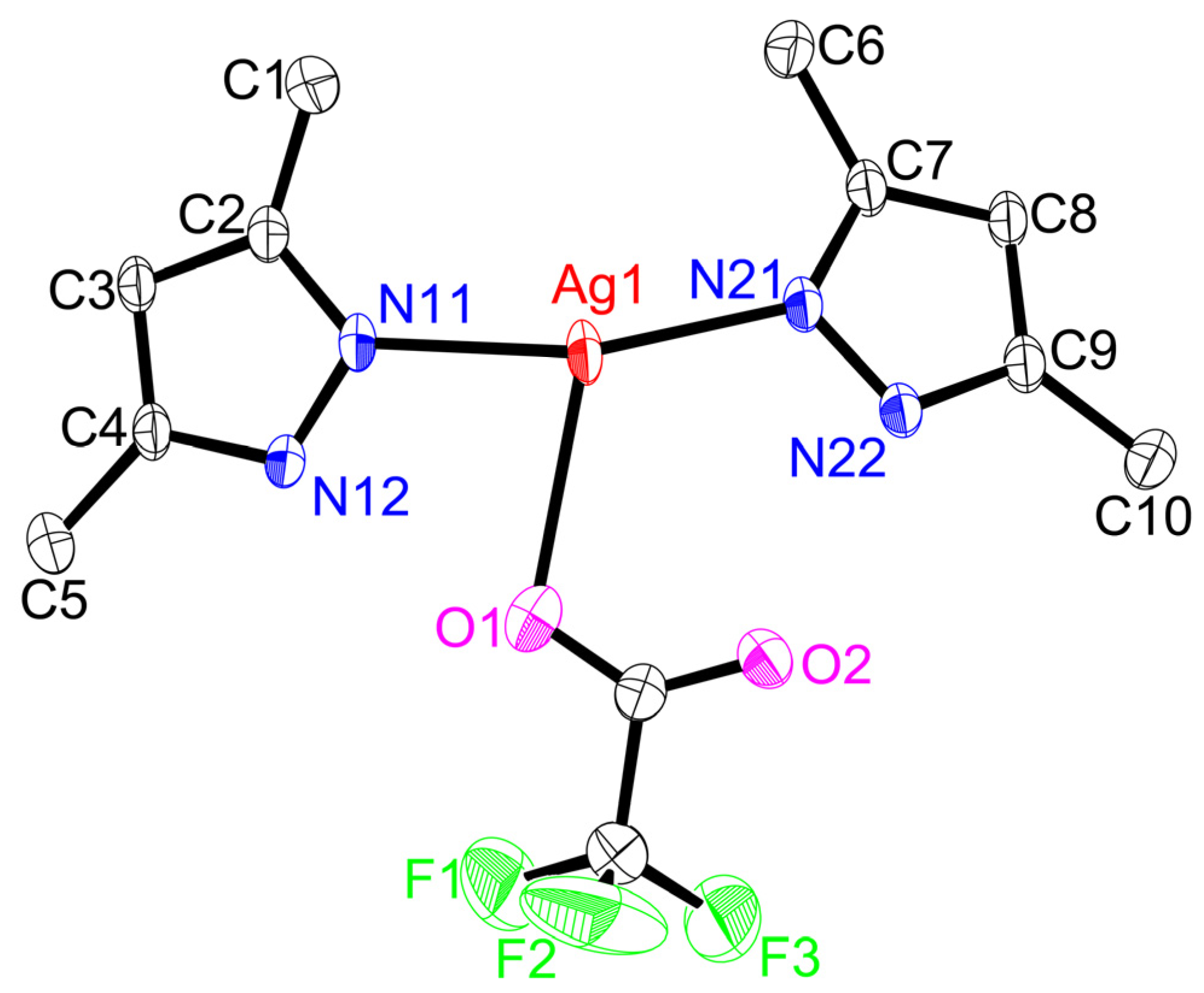
2.2. Structures
2.3. Solution-State Properties
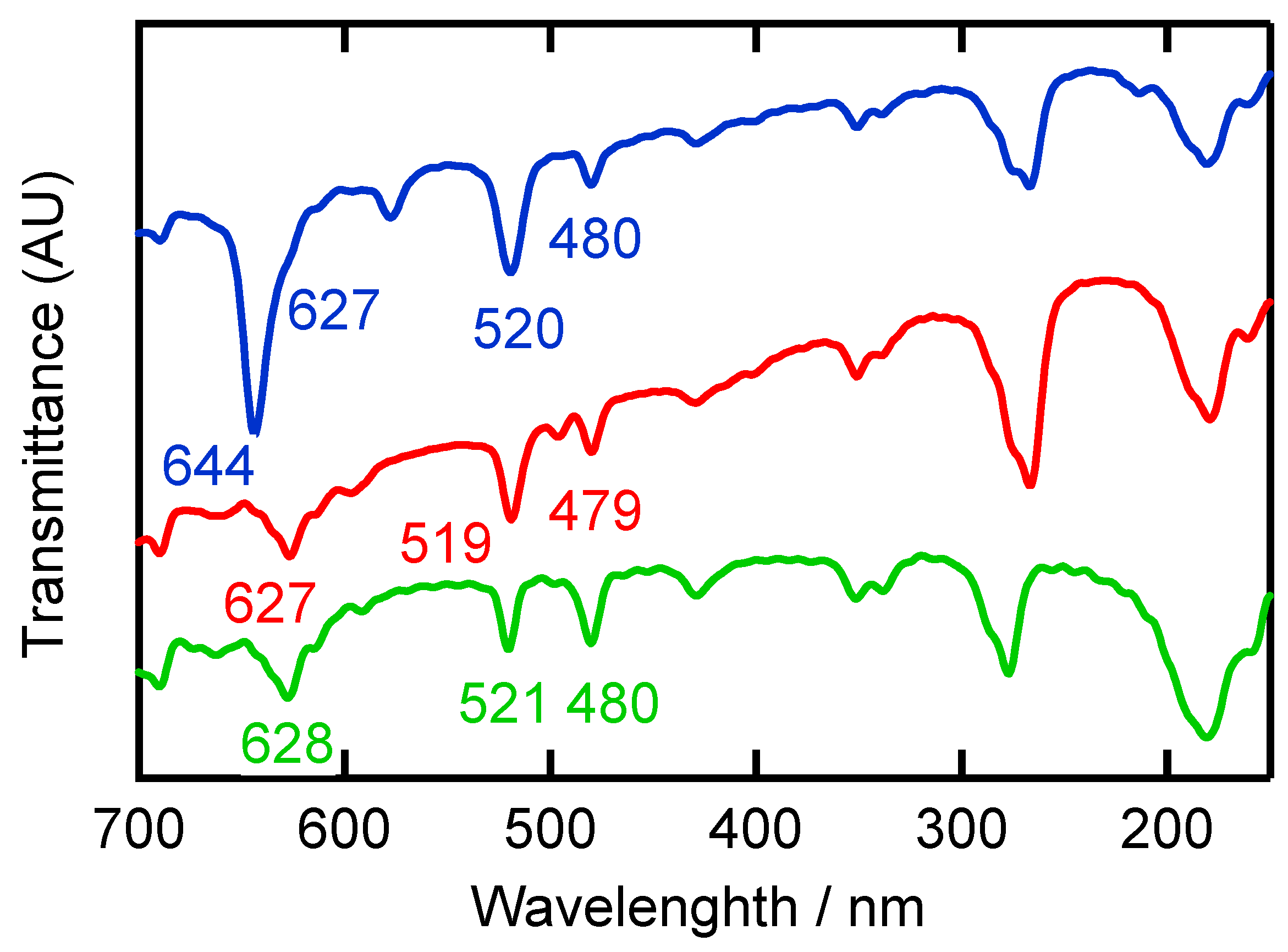
2.4. Solid-State Properties
3. Materials and Methods
3.1. Material and General Techniques
3.2. Instrumentation
3.3. Preparation of Ligand and Complexes
- 3,3′,5,5′-Tetramethyl-4,4′-bipyrazole (Me4bpzH2)
- [Ag(CF3CO2)(Me4bpzH2)]
- [Ag(CF3SO3)(Me4bpzH2)]
3.4. X-ray Crystal Structure Determination
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zheng, J.; Lu, Z.; Wu, K.; Ning, G.-H.; Li, D. Coinage-metal-based cyclic trinuclear complexes with metal−metal interactions: Theories to experiments and structures to functions. Chem. Rev. 2020, 120, 9675–9742. [Google Scholar] [CrossRef] [PubMed]
- Elguero, J.; Alkorta, I. A computational study of metallacycles formed by pyrazolate ligands and the coinage metals M = Cu(I), Ag(I) and Au(I): (pzM)n for n = 2, 3, 4, 5 and 6. Comparison with structures reported in the Cambridge crystallographic data center (CCDC). Molecules 2020, 25, 5108. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.-Y.; Lu, H.-L. From metal-metal bonding to supra-metal-metal bonding directed self-assembly: Supramolecular architectures of group 10 and 11 metals with ligands from mono- to poly-pyrazoles. Isr. J. Chem. 2019, 59, 166–183. [Google Scholar] [CrossRef]
- Fustero, S.; Simón-Fuentes, A.; Sanz-Cervera, J.F. Recent advances in the synthesis of pyrazoles. A Review. Org. Prep. Proced. Int. 2009, 41, 253–290. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Zhang, Y.-B.; Lin, J.-B.; Chen, X.-M. Metal azolate frameworks: From crystal engineering to functional materials. Chem. Rev. 2012, 112, 1001–1033. [Google Scholar] [CrossRef] [PubMed]
- Fustero, S.; Sánchez-Roselló, M.; Barrio, P.; Simón-Fuentes, A. From 2000 to mid-2010: A fruitful decade for the synthesis of pyrazoles. Chem. Rev. 2011, 111, 6984–7034. [Google Scholar] [CrossRef]
- Halcrow, M.A. Pyrazoles and pyrazolides—Flexible synthons in self-assembly. Dalton Trans. 2009, 12, 2059–2073. [Google Scholar] [CrossRef] [PubMed]
- Fujisawa, K. A personal perspective on the discovery of dioxygen adducts of copper and iron by Nobumasa Kitajima. J. Biol. Inorg. Chem. 2017, 22, 237–251. [Google Scholar] [CrossRef]
- Solomon, E.I.; Heppner, D.E.; Johnston, E.M.; Ginsbach, J.W.; Cirera, J.; Qayyum, M.; Kieber-Emmons, M.T.; Kjaergaard, C.H.; Hadt, R.G.; Tian, L. Copper active sites in biology. Chem. Rev. 2014, 114, 3659–3853. [Google Scholar] [CrossRef] [Green Version]
- Kitajima, N.; Fujisawa, K.; Fujimoto, C.; Moro-oka, Y.; Hashimoto, S.; Kitagawa, T.; Toriumi, T.; Tatsumi, K.; Nakamura, A. A new model for dioxygen binding in hemocyanin. Synthesis, characterization, and molecular structure of the μ-η2:η2 peroxo dinuclear copper(II) complexes, [Cu(HB(3,5-R2pz)3)]2(O2) (R = i-Pr and Ph). J. Am. Chem. Soc. 1992, 114, 1277–1291. [Google Scholar] [CrossRef]
- Chen, P.; Fujisawa, K.; Solomon, E.I. Spectroscopic and theoretical studies of mononuclear copper(II) alkyl- and hydroperoxo complexes: Electronic structure contributions to reactivity. J. Am. Chem. Soc. 2000, 122, 10177–10193. [Google Scholar] [CrossRef]
- Randall, D.W.; George, S.D.; Hedman, B.; Hodgson, K.O.; Fujisawa, K.; Solomon, E.I. Spectroscopic and electronic structural studies of blue copper model complexes. 1. Perturbation of the thiolate-Cu bond. J. Am. Chem. Soc. 2000, 122, 11620–11631. [Google Scholar] [CrossRef]
- Fujisawa, K.; Ishikawa, Y.; Miyashita, Y.; Okamoto, K. Pyrazolate-bridged group 11 metal(I) complexes: Substituent effects on the supramolecular structures and physicochemical properties. Inorg. Chim. Acta 2010, 363, 2977–2989. [Google Scholar] [CrossRef]
- Morishima, Y.; Young, D.J.; Fujisawa, K. Structure and photoluminescence of silver(I) trinuclear halopyrazolato complexes. Dalton Trans. 2014, 43, 15915–19528. [Google Scholar] [CrossRef] [PubMed]
- Saotome, M.; Shimizu, D.; Itagaki, A.; Young, D.J.; Fujisawa, K. Structures and photoluminescence of silver(I) and gold(I) cyclic trinuclear complexes with aryl substituted pyrazolates. Chem. Lett. 2019, 48, 533–536. [Google Scholar] [CrossRef] [Green Version]
- Fujisawa, K.; Saotome, M.; Takeda, S.; Young, D.J. Structures and photoluminescence of coinage metal(I) phenylpyrazolato trinuclear complexes [M(3,5-Et2-4-Ph-pz)]3 and arene sandwich complexes {[Ag(3,5-Et2-4-Ph-pz)]3}2(Ar) (Ar = mesitylene and toluene). Chem. Lett. 2020, 49, 670–673. [Google Scholar] [CrossRef]
- Fujisawa, K.; Nemoto, T.; Morishima, Y.; Leznoff, D.B. Synthesis and structural characterization of a silver(I) pyrazolato coordination polymer. Molecules 2021, 26, 1015. [Google Scholar] [CrossRef]
- Pettinari, C.; Tăbăcaru, A.; Galli, S. Coordination polymers and metal–organic frameworks based on poly(pyrazole)-containing ligands. Coord. Chem. Rev. 2016, 307, 1–31. [Google Scholar] [CrossRef]
- Boldog, I.; Rusanov, E.B.; Chernega, A.N.; Sieler, J.; Domasevitch, K.V. One- and two-dimensional coordination polymers of 3,3′,5,5′-tetramethyl-4,4′-bipyrazolyl, a new perspective crystal engineering module. Polyhedron 2001, 20, 887–897. [Google Scholar] [CrossRef]
- Domasevitch, K.V.; Boldog, I.; Rusanov, E.B.; Hunger, J.; Blaurock, S.; Schröder, M.; Sieler, J. Helical bipyrazole networks conditioned by hydrothermal crystallization. Z. Anorg. Allg. Chem. 2005, 631, 1095–1100. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Horike, S.; Kitagawa, S. A flexible porous coordination polymer functionalized by unsaturated metal clusters. Angew. Chem. Int. Ed. 2007, 46, 889–892. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Kitagawa, S. Supramolecular Isomerism, framework flexibility, unsaturated metal center, and porous property of Ag(I)/Cu(I) 3,3′,5,5′-tetrametyl-4,4′-bipyrazolate. J. Am. Chem. Soc. 2008, 130, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Hunger, J.; Krautscheid, H.; Sieler, J. Hydrothermal synthesis and structure of coordination polymers by combination of bipyrazole and aromatic dicarboxylate ligands. Cryst. Growth Des. 2009, 9, 4613–4625. [Google Scholar] [CrossRef]
- Grzywa, M.; Geβner, C.; Denysenko, D.; Bredenkötter, B.; Gschwind, F.; Fromm, K.M.; Nitek, W.; Klemm, E.; Volkmer, D. CFA-2 and CFA-3 (Coordination framework Augsburg University-2 and -3); novel MOFs assembled from trinuclear Cu(I)/Ag(I) secondary building units and 3,3′,5,5′-tetraphenyl-bipyrazolate ligands. Dalton Trans. 2013, 42, 6909–6921. [Google Scholar] [CrossRef] [PubMed]
- Du, L.-Y.; Shi, W.-J.; Hou, L.; Wang, Y.-Y.; Shi, Q.-Z.; Zhu, Z. Solvent or temperature induced diverse coordination polymers of silver(I) sulfate and bipyrazole systems: Syntheses, crystal structures, luminescence, and sorption properties. Inorg. Chem. 2013, 52, 14018–14027. [Google Scholar] [PubMed]
- Ikarugi, R.; Fujisawa, K.; Tiekink, E.R.T. Crystal structure of bis{hydridotris(3-trifluoromethyl-5-methylpyrazolyl-1-yl)borato-κN3}manganese(II), C30H26B2F18MnN12. Z. Krist.—New Cryst. Struct. 2022, 237, 85–87. [Google Scholar]
- Fujisawa, K.; Yoshida, M.; Miyashita, Y.; Okamoto, K. Copper(I) complexes with fluorinated hydrotris(pyrazolyl)borate: Influence of electronic effects on their structure, physicochemical properties, and reactivity. Polyhedron 2009, 28, 1447–1454. [Google Scholar] [CrossRef]
- Alonso, C.; de Marigorta, E.M.; Rubiales, G.; Palacios, F. Carbon trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev. 2015, 115, 1847–1935. [Google Scholar]
- Mosby, W.L. The reactions of some 1:4-dicarbonyl systems with hydrazine. J. Chem. Soc. 1957, 3997–4003. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals volume and radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 6th ed.; John Wiley and Sons, Inc.: New York, NY, USA, 2009. [Google Scholar]
- Johnston, D.H.; Shriver, D.F. Vibrational study of the trifluoromethanesulfonate anion: Unambiguous assignment of the asymmetric stretching modes. Inorg. Chem. 1993, 32, 1045–1047. [Google Scholar] [CrossRef]
- Omary, M.A.; Rawashdeh-Omary, M.A.; Gonser, M.W.A.; Elbjeirami, O.; Grimes, T.; Cundari, T.R.; Diyabalanage, H.V.K.; Gamage, C.S.P.; Dias, H.V.R. Metal Effect on the supramolecular structure, photophysics, and acid-base character of trinuclear pyrazolato coinage metal complexes. Inorg. Chem. 2005, 44, 8200–8210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimes, T.; Omary, M.A.; Dias, H.V.R.; Cundari, T.R. Intertrimer and intratrimer metallophilic and excimeric bonding in the ground and phosphorescent states of trinuclear coinage metal pyrazolates: A Computational study. J. Phys. Chem. A 2006, 110, 5823–5830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hettiarachchi, C.V.; Rawashdeh-Omary, M.A.; Korir, D.; Kohistani, J.; Yousufuddin, M.; Dias, H.V.R. Trinuclear copper(I) and silver(I) adducts of 4-chloro-3,5-bis(trifluoromethyl)pyrazolate and 4-bromo-3,5-bis(trifluoromethyl)pyrazolate. Inorg. Chem. 2013, 52, 13576–13583. [Google Scholar] [CrossRef]
- CrystalClear; Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 2001.
- CrysAlisPro; Data Collection and Processing Software; Rigaku Corporation: Tokyo, Japan, 2015.
- SIR2008: Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Siliqi, D.; Spagna, R. IL MILIONE: A suite of computer programs for crystal structure solution of proteins. J. Appl. Cryst. 2007, 40, 609–613. [Google Scholar] [CrossRef]
- SIR2004: Burla, M.C.; Caliandro, R.; Camalli, M.; Carrozzini, B.; Cascarano, G.L.; De Caro, L.; Giacovazzo, C.; Polidori, G.; Spagna, R. SIR2004: An improved tool for crystal structure determination and refinement. J. Appl. Cryst. 2005, 38, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Crystal Structure 4.3; Crystal Structure Analysis Package; Rigaku Corporation: Tokyo, Japan, 2003.
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]





| Complex | [Ag(CF3CO2)(Me4bpzH2)] | [Ag(CF3SO3)(Me4bpzH2)] |
|---|---|---|
| CCDC number | 2,227,168 | 2,227,169 |
| Empirical formula | C12H14AgF3N4O2 | C11H14AgF3N4O3S |
| Formula weight | 411.13 | 447.18 |
| Crystal system | Monoclinic | Triclinic |
| Space group | P21/n (#14) | P (#2) |
| a/Å | 13.2724(2) | 8.66665(15) |
| b/Å | 8.67316(15) | 9.91576(18) |
| c/Å | 13.3047(2) | 10.2913(2) |
| α/° | 90 | 111.6690(19) |
| β/° | 91.3059(17) | 102.4538(17) |
| γ/° | 90 | 90.8501(14) |
| V/Å3 | 1531.15(4) | 798.20(3) |
| Z | 4 | 2 |
| Dcalc/g cm−3 | 1.783 | 1.860 |
| μ(MoKα)/cm−1 | 13.560 | 14.391 |
| 2θ range, ° | 6–55 | 6–55 |
| Reflections collected | 23895 | 25699 |
| Unique reflections | 3516 | 3666 |
| Rint | 0.0304 | 0.0270 |
| Number of variables | 199 | 208 |
| Refls./Para. ratio | 17.67 | 17.63 |
| Residuals: R1 (I > 2 σ (I)) | 0.0337 | 0.0320 |
| Residuals: R (All refl.) | 0.0359 | 0.0351 |
| Residuals: wR2 (All refl.) | 0.0999 | 0.0965 |
| Goodness of fit ind. | 1.054 | 1.078 |
| Max/min peak,/e Å−3 | 1.27/–0.73 | 1.21/–0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujisawa, K.; Kobayashi, Y.; Okano, M.; Iwabuchi, R.; Kondo, S.; Young, D.J. Silver(I) Coordination Polymer Ligated by Bipyrazole Me4bpzH2, [Ag(X)(Me4bpzH2)] (X = CF3CO2− and CF3SO3−, Me4bpzH2 = 3,3′,5,5′-Tetramethyl-4,4′-bipyrazole): Anion Dependent Structures and Photoluminescence Properties. Molecules 2023, 28, 2936. https://doi.org/10.3390/molecules28072936
Fujisawa K, Kobayashi Y, Okano M, Iwabuchi R, Kondo S, Young DJ. Silver(I) Coordination Polymer Ligated by Bipyrazole Me4bpzH2, [Ag(X)(Me4bpzH2)] (X = CF3CO2− and CF3SO3−, Me4bpzH2 = 3,3′,5,5′-Tetramethyl-4,4′-bipyrazole): Anion Dependent Structures and Photoluminescence Properties. Molecules. 2023; 28(7):2936. https://doi.org/10.3390/molecules28072936
Chicago/Turabian StyleFujisawa, Kiyoshi, Yui Kobayashi, Mitsuki Okano, Ryota Iwabuchi, Shiori Kondo, and David James Young. 2023. "Silver(I) Coordination Polymer Ligated by Bipyrazole Me4bpzH2, [Ag(X)(Me4bpzH2)] (X = CF3CO2− and CF3SO3−, Me4bpzH2 = 3,3′,5,5′-Tetramethyl-4,4′-bipyrazole): Anion Dependent Structures and Photoluminescence Properties" Molecules 28, no. 7: 2936. https://doi.org/10.3390/molecules28072936








