Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin
Abstract
1. Introduction
2. The Biology of Lavandin
2.1. The Taxonomy and Nomenclature of Lavandula × intermedia Emeric ex Loisel
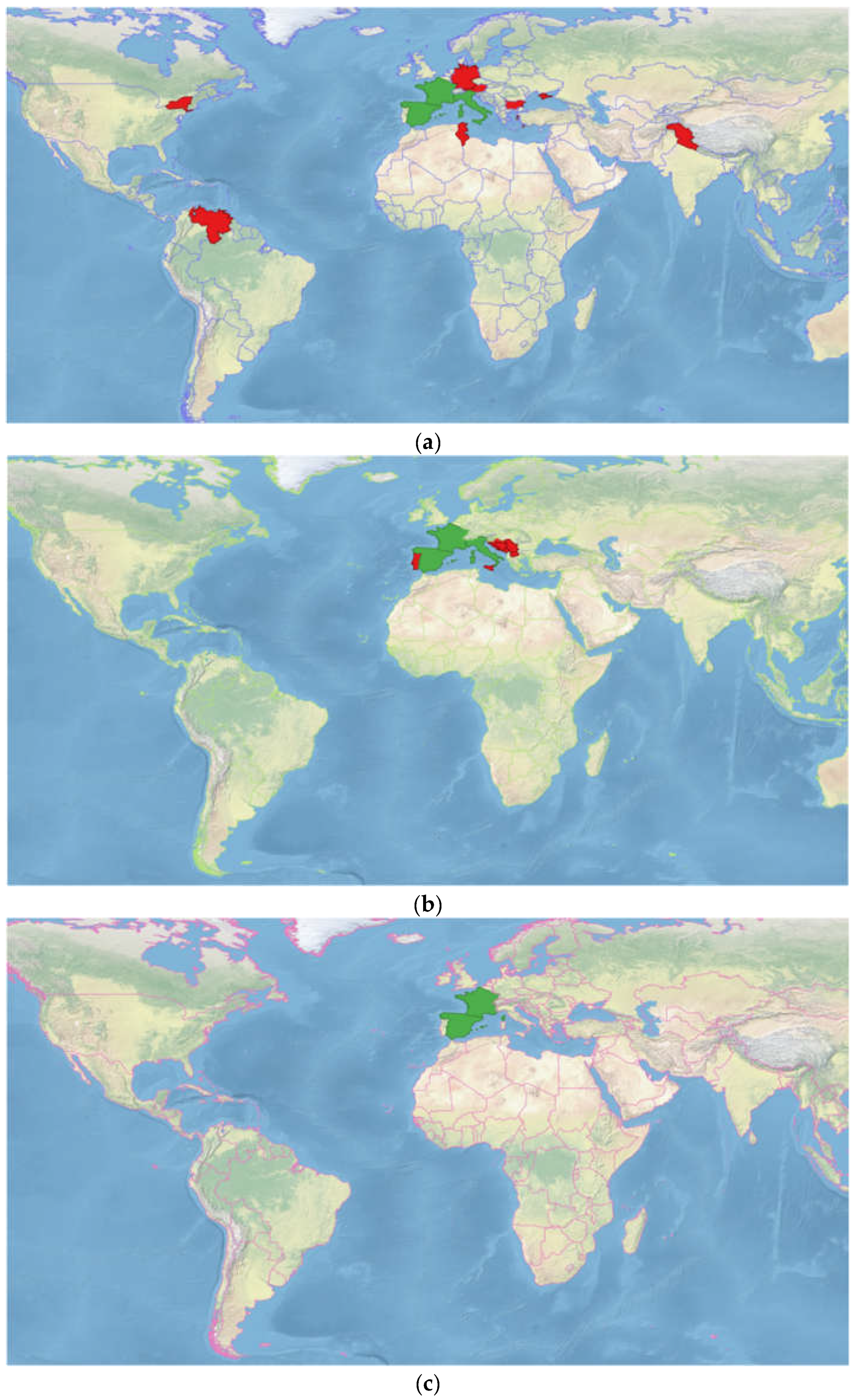
2.2. Geographical Distribution
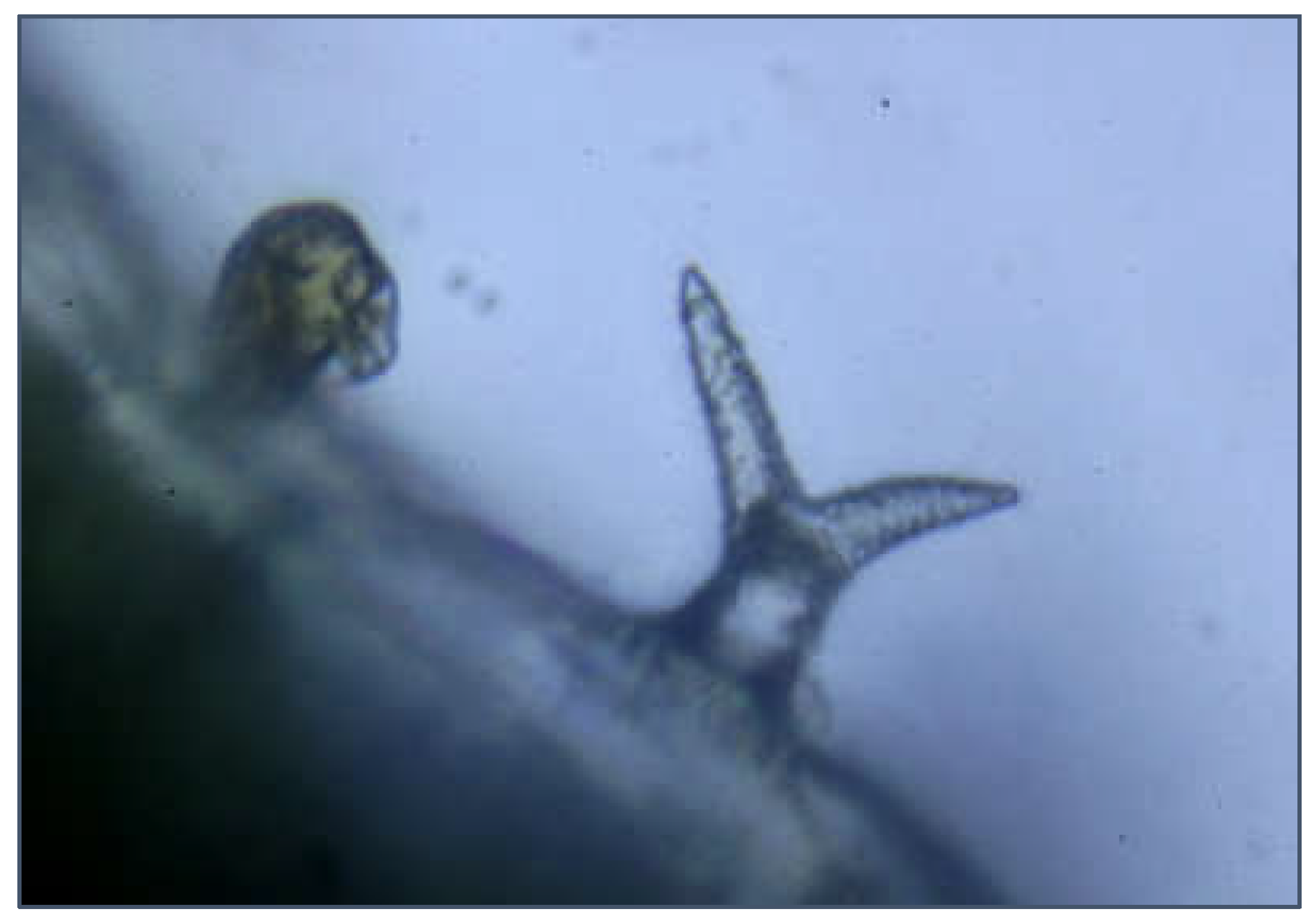
2.3. Species Identification
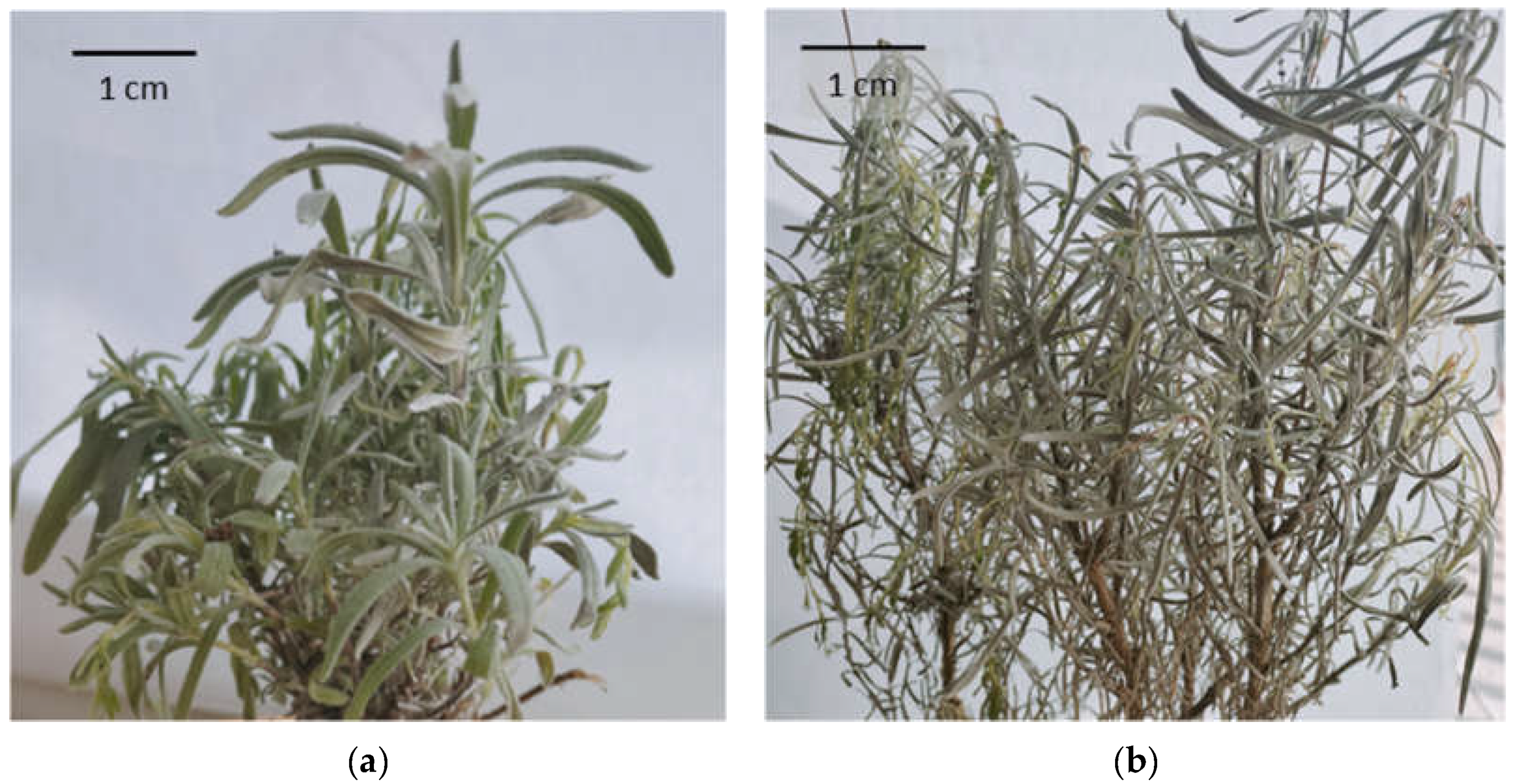
2.4. Cultivars and Cultivation
- Root rot—this is a disease caused by several pathogens such as Fusarium spp., Phytophthora spp., Pythium spp., and Rhizoctonia spp. Its leading cause is moist soil and low temperatures. The effect of the disease is rotting roots, thus slow wilting and the yellowing or browning of the leaves [52].
- Alfalfa Mosaic Virus—a viral disease probably transmitted by aphids and human hands. The disease manifests as yellow leaves and smaller sizes [53].
- Xylella—a bacterial disease caused by Xylella fastidiosa and transmitted by sap-sucking insects. The disease manifests as stunted growth and leaves that look like they have been burned [54].
2.5. Essential Oil Production
3. The Phytochemicals of Lavandin
3.1. Phytochemicals of Essential Oil
3.2. Phytochemicals of Other Lavandin Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| DF | dried flowers |
| EO | essential oil |
| F | furanoid form |
| FF | fresh flowers |
| FS | fresh stems |
| H | hydrolate |
| ISO | International Organization for Standardization |
| LA | Lavandula angustifolia |
| LI | Lavandula × intermedia |
| LL | Lavandula latifolia |
| MT | metric ton |
| ND | not determined |
| NOBANIS | European Network on Invasive Species database |
| Ph. Eur. | European Pharmacopeia |
| t | traces |
| WHO | World Health Organization |
References
- The Plant List. Available online: http://www.theplantlist.org/ (accessed on 4 January 2022).
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Hudz, N.; Balwierz, R.; Marciniak, D.; Wieczorek, P.P. Comparative Evaluation of the Essential Oil of the New Ukrainian Lavandula angustifolia and Lavandula × intermedia Cultivars Grown on the Same Plots. Molecules 2022, 27, 2152. [Google Scholar] [CrossRef] [PubMed]
- Giray, F.H. An Analysis of World Lavender Oil Markets and Lessons for Turkey. J. Essent. Oil-Bear. Plants 2018, 21, 1612–1623. [Google Scholar] [CrossRef]
- Pokajewicz, K.; Białoń, M.; Svydenko, L.; Fedin, R.; Hudz, N. Chemical Composition of the Essential Oil of the New Cultivars of Lavandula angustifolia Mill. Bred in Ukraine. Molecules 2021, 26, 5681. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula, 1st ed.; Lis-Balchin, M., Ed.; Taylor & Francis: London, UK; New York, NY, USA, 2002; ISBN 0-415-28486-4. [Google Scholar]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Bejar, E. Adulteration of English Lavender (Lavandula angustifolia) essential oil. In Botanical Adulterants Prevention Bulletin; ABC-AHP-NCNPR Botanical Adulterants Prevention Program: Austin, TX, USA, 2020. [Google Scholar]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula × intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 33, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Beale, D.J.; Morrison, P.D.; Karpe, A.V.; Dunn, M.S. Chemometric analysis of lavender essential oils using targeted and untargeted GC-MS acquired data for the rapid identification and characterization of oil quality. Molecules 2017, 22, 1339. [Google Scholar] [CrossRef] [PubMed]
- Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part II. Biological Activities and Applications of Lavandin. Molecules, 2023; in press. [Google Scholar]
- Chaytor, D.A. A taxonomic study of the genus Lavandula. Bot. J. Linn. Soc. 1937, 51, 153–204. [Google Scholar] [CrossRef]
- Upson, T. The taxonomy of the genus Lavandula L. In Lavender.The Genus Lavandula; Lis-Balchin, M., Ed.; Taylor & Francis: London, UK; New York, NY, USA, 2002; pp. 16–48. ISBN 9780429218590. [Google Scholar]
- Adal, A.M.; Demissie, Z.A.; Mahmoud, S.S. Identification, validation and cross-species transferability of novel Lavandula EST-SSRs. Planta 2015, 241, 987–1004. [Google Scholar] [CrossRef]
- Baydar, H.; Kineci, S. Scent composition of essential oil, concrete, absolute and hydrosol from lavandin (Lavandula × intermedia Emeric ex Loisel.). J. Essent. Oil-Bear. Plants 2009, 12, 131–136. [Google Scholar] [CrossRef]
- Price, L. The genesis of essential oils. In Aromatherapy for Health Professionals; Price, S., Ed.; Churchill Livingstone/Elsevier: London, UK, 2011; pp. 3–18. ISBN 0702035645. [Google Scholar]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Chemical Composition and Antibacterial Activity of Iranian Lavandula × hybrida. Chem. Biodivers 2017, 14, e1700064. [Google Scholar] [CrossRef]
- WFO. Lavandula Intermedia Emeric ex Loisel. Available online: http://www.worldfloraonline.org/taxon/wfo-0000224178 (accessed on 16 January 2023).
- Tucker, A.O. The correct name of lavandin and its cultivars (Labiatae). Baileya 1981, 21, 131–133. [Google Scholar]
- Mateo Sanz, G.; Crespo Villalba, M.B. Novedades taxonómicas y nomenclaturales para la flora del sistema Ibérico, I. Flora Montiberica 2015, 59, 88–96. [Google Scholar]
- Linné, C.V.; Lundmark, J.D. Dissertatio Academica de Lavandula, Upsaliae; Uppsala Universitet: Uppsala, Sweden, 1780. [Google Scholar]
- Loiseleur-Deslongchamps, J.L.A. Flora Gallica, 2nd ed.; Academiae Regiae Medicae Bibliopolam: Paris, France, 1828. [Google Scholar] [CrossRef]
- Andrys, D.; Kulpa, D.; Grzeszczuk, M.; Bihun, M.; Dobrowolska, A. Antioxidant and antimicrobial activities of Lavandula angustifolia Mill. field-grown and propagated in vitro. Folia Hortic. 2017, 29, 161–180. [Google Scholar] [CrossRef]
- Détár, E.; Németh, É.Z.; Gosztola, B.; Demján, I.; Pluhár, Z. Effects of variety and growth year on the essential oil properties of lavender (Lavandula angustifolia Mill.) and lavandin (Lavandula × intermedia Emeric ex Loisel.). Biochem. Syst. Ecol. 2020, 90, 104020. [Google Scholar] [CrossRef]
- Passalacqua, N.G.; Tundis, R.; Upson, T.M. A new species of Lavandula sect. Lavandula (Lamiaceae) and review of species boundaries in Lavandula angustifolia. Phytotaxa 2017, 292, 161–170. [Google Scholar] [CrossRef]
- Gallotte, P.; Fremondière, G.; Gallois, P.; Bernier, J.-P.B.; Buchwalder, A.; Walton, A.; Piasentin, J.; Fopa-Fomeju, B. Lavandula angustifolia Mill. and Lavandula × intermedia Emeric ex Loisel: Lavender and Lavandin. In Medicinal, Aromatic and Stimulant Plants; Novak, J., Blüthner, W.D., Eds.; Springer: Cham, Switzerland, 2020; pp. 303–311. [Google Scholar]
- Herrera, C.M. Variation in mutualisms: The spatiotemporal mosaic of a pollinator assemblage. Biol. J. Linn. Soc. 1988, 35, 95–125. [Google Scholar] [CrossRef]
- Royal Botanic Gardens Kew Plants of the World Online. Available online: http://www.plantsoftheworldonline.org (accessed on 3 January 2023).
- Jug-Dujaković, M.; Ninčević Runjić, T.; Grdiša, M.; Liber, Z.; Šatović, Z. Intra- and Inter-Cultivar Variability of Lavandin (Lavandula × intermedia Emeric ex Loisel.) Landraces from the Island of Hvar, Croatia. Agronomy 2022, 12, 1864. [Google Scholar] [CrossRef]
- NOBANIS NOBANIS—European Network on Invasive Alien Species. Available online: https://www.nobanis.org/ (accessed on 3 January 2023).
- Brailko, V.; Mitrofanova, O.; Leesnikova-Sedoshenko, N.; Chelombit, S.; Mitrofanova, I. Anatomy features of Lavandula angustifolia Mill. and Lavandula hybrida Rev. plants in vitro. Agric. For. 2017, 63, 111–117. [Google Scholar] [CrossRef]
- Paniagua-Zambrana, N.Y.; Bussmann, R.W. Lavandula angustifola Mill.; Lavandula latifolia Medik. In Ethnobotany of Mountain Regions—Ethnobotany of the Andes; Paniagua-Zambrana, N.Y., Bussmann, R.W., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Tucker, A.O.; Hensen, K.J.W. The cultivars of lavender and lavandin (Labiatae). Baileya 1985, 22, 168–177. [Google Scholar]
- Huang, S.S.; Kirchoff, B.K.; Liao, J.P. The capitate and peltate glandular trichomes of Lavandula pinnata L. (Lamiaceae): Histochemistry, ultrastructure, and secretion. J. Torrey Bot. Soc. 2008, 135, 155–167. [Google Scholar] [CrossRef]
- do Rocio Duarte, M.; Carvalho de Souza, D. Microscopic characters of the leaf and stem of Lavandula dentata L. (Lamiaceae). Microsc. Res. Tech. 2014, 77, 647–652. [Google Scholar] [CrossRef]
- Ștefan, G.-A.; Zamfirache, M.M.; Ivănescu, C.L. Histo-anatomical and micromorphological investigations on six Lavandula L. taxa. Analele Ştiinţifice ale Univ. “Al. I. Cuza” Iaşi s. II a. Biol. Veg. 2021, 67, 42–56. [Google Scholar]
- Fakhriddinova, D.K.; Rakhimova, T.R.; Dusmuratova, F.M.; Duschanova, G.M.; Abdinazarov, S.H.; Samadov, I.N. The Anatomical Structure of Vegetative Organs Lavandula officinalis Chaix in the Introduction of Tashkent Botanical Garden. Am. J. Plant Sci. 2020, 11, 578–588. [Google Scholar] [CrossRef]
- Bader, S.B. The Lavender Lover’s Handbook: The 100 Most Beautiful and Fragrant Varieties for Growing, Crafting, and Cooking; Timber Press: Portland, OR, USA, 2012; p. 192. [Google Scholar]
- Adam, K.L. Lavender Production, Products, Markets, and Entertainment Farms; A Publication of ATTRA–National Sustainable Agriculture Information Service: Butte, MT, USA, 2006. [Google Scholar]
- Mountain Valley Growers Lavandula × intermedia “Abrialii”. Available online: https://mountainvalleygrowers.com/organic-plants/lavandula-x-intermedia-abrialii-abrial-lavender/ (accessed on 2 February 2023).
- SMG San Marcos Growers. Available online: https://www.smgrowers.com/ (accessed on 10 January 2023).
- Hendon, J.T.J. Lavandula plant named ‘Bridget Chloe’. US Patent No. US PP27,182 P3, 2016. [Google Scholar]
- Blazekovic, B.; Stanic, G.; Pepeljnjak, S.; Vladimir-Knezevic, S. In vitro antibacterial and antifungal activity of Lavandula × intermedia Emeric ex Loisel. “Budrovka.” Molecules 2011, 16, 4241–4253. [Google Scholar] [CrossRef]
- McNaughton, V. Lavender: The grower’s guide; Timber Press: Portland, OR, USA, 2010; ISBN 1604691255. [Google Scholar]
- RHS Royal Horticultural Society Website. Available online: https://www.rhs.org.uk/ (accessed on 10 January 2023).
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula Essential Oils: A Current Review of Applications in Medicinal, Food, and Cosmetic Industries of Lavender; SAGE Publications: Sage, CA, USA; Los Angeles, CA, USA, 2018; Volume 13, pp. 1403–1417. [Google Scholar]
- Reed, D.; Montague, T.; Simpson, C. Adventitious Rooting of Lavandula × intermedia Cuttings. J. Environ. Hortic. 2021, 39, 150–159. [Google Scholar] [CrossRef]
- Baker, J.; Brown, K.; Rajendiran, E.; Yip, A.; DeCoffe, D.; Dai, C.; Molcan, E.; Chittick, S.A.; Ghosh, S.; Mahmoud, S.; et al. Medicinal lavender modulates the enteric microbiota to protect against Citrobacter rodentium-induced colitis. Am. J. Physiol.—Gastrointest. Liver Physiol. 2012, 303, 825–836. [Google Scholar] [CrossRef]
- Russel Urwin, N.A. Lavender plant named ‘Riverina Margaret’. US Patent No. PP29,077 P3, 2018. [Google Scholar]
- Company, T.P. Lavandula × intermedia “Super”. Available online: https://www.theplantcompany.co.nz/shop/product/shrubs/lavender-super (accessed on 2 February 2023).
- Plantmark Lavandula intermedia Super. Available online: https://www.plantmark.com.au/lavandula-intermedia-super (accessed on 2 February 2023).
- Traven, L.R.; Grazzini, R. Lavandula plant named ‘Tesseract’. US Patent No. US PP31, 786 P2, 2020. [Google Scholar]
- Dlugos, D.; Jeffers, S. Phytophthora nicotianae and P. palmivora: Emerging pathogens of hybrid lavender (Lavandula × intermedia). In Proceedings of the Graduate Research and Discovery Symposium (GRADS), 2019; Available online: https://tigerprints.clemson.edu/grads_symposium/252 (accessed on 19 December 2022).
- Vrandečić, K.; Jurković, D.; Ćosić, J.; Stanković, I.; Vučurović, A.; Bulajić, A.; Krstić, B. First report of alfalfa mosaic virus infecting Lavandula × intermedia in Croatia. Plant Dis. 2013, 97, 1002. [Google Scholar] [CrossRef]
- Cunty, A.; Legendre, B.; de Jerphanion, P.; Dousset, C.; Forveille, A.; Paillard, S.; Olivier, V. Update of the Xylella fastidiosa outbreak in France: Two new variants detected and a new region affected. Eur. J. Plant Pathol. 2022, 163, 505–510. [Google Scholar] [CrossRef]
- Carrasco, A.; Martinez-Gutierrez, R.; Tomas, V.; Tudela, J. Lavandin (Lavandula × intermedia Emeric ex Loiseleur) essential oil from Spain: Determination of aromatic profile by gas chromatography-mass spectrometry, antioxidant and lipoxygenase inhibitory bioactivities. Nat. Prod. Res. 2016, 30, 1123–1130. [Google Scholar] [CrossRef]
- Garlick, B.K. Quality factors in Iavandin. Perfum. Flavorist 1977, 2, 25–26. [Google Scholar]
- Sort, J.; Calonge, J.; Ventós, E. Spanish Essential Oils. Red thyme, lavandin, spike lavender, rosemary and more. Perfum. Flavorist 2012, 37, 40–52. [Google Scholar]
- PERSiSTENCE Market Research. Lavender Oil Market—Global Market Study on Lavender Oil: Rising Awareness about Benefits of Lavender Oils on Human Health to Gain Momentum in the Upcoming Years; PERSiSTENCE Market Research: New York, NY, USA, 2023. Available online: https://www.persistencemarketresearch.com/market-research/lavender-oil-market.asp (accessed on 15 December 2022).
- Blažeković, B.; Yang, W.; Wang, Y.; Li, C.; Kindl, M.; Pepeljnjak, S.; Vladimir-Knežević, S. Chemical composition, antimicrobial and antioxidant activities of essential oils of Lavandula × intermedia ‘Budrovka’ and L. angustifolia cultivated in Croatia. Ind. Crops Prod. 2018, 123, 173–182. [Google Scholar] [CrossRef]
- Chakopoulou, P.S.; Goliaris, A.H.; Katsiotis, S.T. Contribution to the analysis of the volatile constituents from some lavender and Lavandin cultivars grown in Greece. Sci. Pharm. 2003, 71, 229–234. [Google Scholar] [CrossRef]
- Renaud, E.N.C.; Charles, D.J.; Simon, J.E. Essential oil quantity and composition from 10 cultivars of organically grown lavender and lavandin. J. Essent. Oil Res. 2001, 13, 269–273. [Google Scholar] [CrossRef]
- Soltanbeigi, A. Qualitative Variations of Lavandin Essential Oil under Various Storage Conditions. J. Essent. Oil-Bearing Plants 2020, 23, 1237–1252. [Google Scholar] [CrossRef]
- Kaloustian, J.; Pauli, A.M.; Pastor, J. Evolution of camphor and others components in the essential oils of two labiate species during the biological cycle. Analusis 2000, 28, 308–315. [Google Scholar] [CrossRef]
- Walasek-Janusz, M.; Grzegorczyk, A.; Zalewski, D.; Malm, A.; Gajcy, S.; Gruszecki, R. Variation in the Antimicrobial Activity of Essential Oils from Cultivars of Lavandula angustifolia and L. × intermedia. Agronomy 2022, 12, 2955. [Google Scholar] [CrossRef]
- Bajalan, I.; Pirbalouti, A.G. Variation in chemical composition of essential oil of populations of Lavandula × intermedia collected from Western Iran. Ind. Crops Prod. 2015, 69, 344–347. [Google Scholar] [CrossRef]
- Jianu, C.; Pop, G.; Gruia, A.T.; Horhat, F.G. Chemical Composition and Antimicrobial Activity of Essential Oils of Lavender (Lavandula angustifolia) and Lavandin (Lavandula × intermedia) Grown in Chemical Composition and Antimicrobial Activity of Essential Oils of Lavender (Lavandula angustifolia). Int. J. Agric. Biol. 2013, 15, 772–776. [Google Scholar]
- Robu, S.; Aprotosoaie, A.C.; Spac, A.; Cioanca, O.; Hancianu, M.; Stanescu, U. Studies regarding chemical composition of lavender volatile oils. Rev. Med. Chir. Soc. Med. Nat. Iasi 2011, 115, 584–589. [Google Scholar]
- Kara, N.; Baydar, H. Essential oil contents and composition of lavenders and lavandins cultivated in Turkey. Res. Crops 2012, 13, 675–681. [Google Scholar]
- Kara, N.; Baydar, H. Determination of lavender and lavandin cultivars (Lavandula sp.) containing high quality essential oil in Isparta, Turkey. Turkish J. F. Crops 2013, 18, 58–65. [Google Scholar]
- Varona, S.; Rodríguez Rojo, S.; Martín, Á.; Cocero, M.J.; Serra, A.T.; Crespo, T.; Duarte, C.M.M. Antimicrobial activity of lavandin essential oil formulations against three pathogenic food-borne bacteria. Ind. Crops Prod. 2013, 42, 243–250. [Google Scholar] [CrossRef]
- Bombarda, I.; Dupuy, N.; Da, J.P.L.V.; Gaydou, E.M. Comparative chemometric analyses of geographic origins and compositions of lavandin var. Grosso essential oils by mid-infrared spectroscopy and gas chromatography. Anal. Chim. Acta 2008, 613, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.M. Progress in essential oils. Perfum. Flavorist 1993, 38, 41–43. [Google Scholar]
- Moon, T.; Cavanagh, H.M.A.; Wilkinson, J.M. Antifungal activity of Australian grown Lavandula spp. essential oils against Aspergillus nidulans, Trichophyton mentagrophytes, Leptosphaeria maculans and Sclerotinia sclerotiorum. J. Essent. Oil Res. 2007, 19, 171–175. [Google Scholar] [CrossRef]
- Morgan, T.J.; Morden, W.E.; Al-Muhareb, E.; Herod, A.A.; Kandiyoti, R. Essential oils investigated by size exclusion chromatography and gas chromatography—Mass spectrometry. Energy Fuels 2006, 20, 734–737. [Google Scholar] [CrossRef]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Liquid and vapour phase of lavandin (Lavandula × intermedia) Essential Oil: Chemical composition and antimicrobial activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef]
- Politi, M.; Menghini, L.; Conti, B.; Bedini, S.; Farina, P.; Cioni, P.L.; Braca, A.; De Leo, M. Reconsidering hydrosols as main products of aromatic plants manufactory: The lavandin (Lavandula × intermedia) case study in Tuscany. Molecules 2020, 25, 2225. [Google Scholar] [CrossRef]
- Plotto, A.; Roberts, D. Aroma quality of lavender water: A comparative study. Perfum. Flavorist 2001, 26, 44–64. [Google Scholar]
- Robu, S.; Chesaru, B.I.; Diaconu, C.; Dumitriubuzia, O.; Tutunaru, D.; Stanescu, U.; Lisa, E.L. Lavandula hybrida: Microscopic characterization and the evaluation of the essential oil. Farmacia 2016, 64, 914–917. [Google Scholar]
- Lesage-Meessen, L.; Bou, M.; Sigoilliot, J.-C.; Faulds, C.B.; Lomascolo, A. Essential oils and distilled straws of lavender and lavandin: Current use and potential application in white biotechnology. Appl. Microbiol. Biotechnol. 2015, 99, 3375–3385. [Google Scholar] [CrossRef] [PubMed]
- Détár, E.; Zámbori-Németh, E.; Gosztola, B.; Harmath, A.; Ladányi, M.; Pluhár, Z. Ontogenesis and harvest time are crucial for high quality lavender—Role of the flower development in essential oil properties. Ind. Crops Prod. 2021, 163, 113334. [Google Scholar] [CrossRef]
- Steltenkamp, R.J.; Casazza, W.T. Composition of the Essential Oil of Lavandin. J. Agric. Food Chem. 1967, 15, 1063–1069. [Google Scholar] [CrossRef]
- European Directorate for the Quality of Medicines & HealthCare. European Pharmacopoeia (Ph. Eur.), 11th ed.; European Directorate for the Quality of Medicines & HealthCare: Strasbourg, France, 2020. [Google Scholar]
- WHO. Selected Medicinal Plants. In WHO Monographs on Selected Medicinal Plants; WHO: Geneva, Switzerland, 2007; Volume 3, ISBN 978924154702 4. [Google Scholar]
- ISO 3515:2002; Oil of Lavender (Lavandula angustifolia Mill.). ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/36253.html (accessed on 4 January 2022).
- ISO 4719:2012; Essential Oil of Spike Lavender (Lavandula latifolia Medikus), Spanish Type. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/55964.html (accessed on 4 January 2022).
- ISO 8902:2009; Oil of lavandin Grosso (Lavandula angustifolia Mill. × Lavandula latifolia Medik.), French Type. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/45332.html (accessed on 4 January 2022).
- ISO 3054:2017; Essential oil of lavandin Abrial (Lavandula angustifolia Mill. × Lavandula latifolia Medik.), French Type. ISO: Geneva, Switzerland. Available online: https://www.iso.org/standard/67961.html (accessed on 4 January 2022).
- Krajewska, A.; Mietlińska, K. Determining the Parameters of the Stinging Nettle (Urtica dioica L.) Hydrolate Distillation Process. Molecules 2022, 27, 3912. [Google Scholar] [CrossRef]
- Maciąg, A.; Kalemba, D. Composition of rugosa rose (Rosa rugosa thunb.) hydrolate according to the time of distillation. Phytochem. Lett. 2015, 11, 373–377. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Sienkiewicz, M.; Stobiecka, A.; Macia, A.; Szoka, Ł.; Karna, E. Chemical Composition and Biological Activity of Abies alba and A. koreana Seed and Cone Essential Oils and Characterization of Their Seed Hydrolates. Chem. Biodyversity 2015, 12, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.S.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Hydrolates: A review on their volatiles composition, biological properties and potential uses. Phytochem. Rev. 2022, 21, 1661–1737. [Google Scholar] [CrossRef]
- Andrés, M.F.; González-Coloma, A.; Muñoz, R.; De la Peña, F.; Julio, L.F.; Burillo, J. Nematicidal potential of hydrolates from the semi industrial vapor-pressure extraction of Spanish aromatic plants. Environ. Sci. Pollut. Res. 2018, 25, 29834–29840. [Google Scholar] [CrossRef]
- Dobros, N.; Zawada, K.; Paradowska, K. Phytochemical Profile and Antioxidant Activity of Lavandula angustifolia and Lavandula × intermedia Cultivars Extracted with Different Methods. Antioxidants 2022, 11, 711. [Google Scholar] [CrossRef]
- Torras-Claveria, L.; Jauregui, O.; Bastida, J.; Codina, C.; Viladomat, F. Antioxidant activity and phenolic composition of lavandin (Lavandula × intermedia Emeric ex Loiseleur) waste. J. Agric. Food Chem. 2007, 55, 8436–8443. [Google Scholar] [CrossRef] [PubMed]

| Domain | Latin Name |
|---|---|
| Kingdom | Plantae |
| Division | Tracheophyta |
| Class | Magnoliopsida |
| Order | Lamiales Bromhead |
| Family | Lamiaceae Martinov |
| Genus | Lavandula L. |
| Section | Lavandula (=Spica Ging.) |
| Species | Lavandula angustifolia Mill. |
| Species | Lavandula latifolia Medik. |
| Hybrids | Lavandula × intermedia Emeric ex Loisel |
| Infraspecies | Lavandula × intermedia nothosubsp. intermedia |
| Infraspecies | Lavandula × intermedia nothosubsp. leptostachya (Pau) Mateo & M. B. Crespo |
| Morphological Feature | Lavandula angustifolia | Lavandula latifolia | Lavandula × intermedia |
|---|---|---|---|
| Mill | Medik. | Emeric ex Loisel | |
| Plant habit | Shrub to 50 cm high | Shrub up to 50–70 (100) cm high | Shrub up to 60–150 cm high |
| Leaves | Shape: narrow, linear-lanceolate | Shape: linear-lanceolate to spathulate | Shape: linear-lanceolate to spathulate |
| Color: in younger plants, they are grey tomentose; in older plants, they are green | Color: grey, with silvery-grey indumentum | Color: often grey tomentose | |
| Inflorescences | Stalk: unbranched, length approx. 10–25 cm | Stalk: branched, length to approx. 25 cm | Stalk: branched |
| Spike: compact in outline, length approx. 4–5(8) cm, sometimes there are small clusters of flowers below the main spike | Spike: trident-shaped in outline, length approx. 5–8 cm, often interrupted | Spike: lax in outline, sometimes interrupted | |
| Bracts: broad, ovate-rhombic to obovate in outline | Bracts: narrow, linear-lanceolate in outline | Bracts: ovate-rhombic in outline, but varied in shape and size | |
| Bracteoles: present but minute | Bracteoles: present but small, length to approx. 4 mm | Bracteoles: present but small, length approx. 1–4 mm | |
| Flowers | Calyx: thirteen-nerved, has a small circular appendage | Calyx: thirteen-nerved, has a circular appendage | Calyx: thirteen-nerved, has a rotund to elliptic appendage |
| Corolla: bilaterally symmetrical, two times longer than calyx, prominent lobes with colorings of blue/mauve, white, infrequently violet to pink | Corolla: bilaterally symmetrical, lobes with colorings of blue to mauve | Corolla: bilaterally symmetrical, lobes are shades of lilac-purple to white | |
| Flowering time: mid-June to July | Flowering time: from mid-July | Flowering time: from late June to July |
| Variety | Description | Literature |
|---|---|---|
| ‘Abrial’ | ‘Abrial’ was introduced by Professor Claude Abrial in 1920 and was more vigorous and adaptable than the other lavender taxa cultivars. It is an evergreen shrub up to 60 cm high. The flowers have a violet color. The leaves are linear green-grey. | [39] |
| ‘Alba’ | ‘Alba’ has been known in Europe since 1880. It is an evergreen shrub with a habit of forming a dome up to 75 m high. The name of the cultivar comes from its white flowers collected in long spikes. Its linear leaves have a grey-green color. | [5,8,32,40] |
| ‘Bridget Chloe’ | ‘Bridget Chloe’ was created in 2014 by John Thomas Hendon from Georgia (US). It is an evergreen shrub up to 75 cm high, with light green to silver-gray leaves. The flowers have dark purple colors. The cultivar is resistant to Lavender Leaf Spot and the Alfalfa Mosaic Virus. | [41] |
| ‘Budrovka’ | ‘Budrovka’ is a cultivar produced in Croatia and grown for EO. The extract of ‘Budrovka’ has medicinal features—it inhibits bacterial or fungal growth. | [42] |
| ‘Dutch Group’ | ‘Dutch Group’ is a widely grown cultivar of lavandin introduced in 1920 and historically called ‘Vera.’ Cultivars in the Dutch Group are evergreen shrubs up to 40 cm high; the inflorescence stalk can grow to 91 cm high. Flowers have a dark aster, a violet corolla, and a light green calyx. The leaves are grey. | [5,32,43] |
| ‘Grappenhall’ | ‘Grappenhall’ is one of the oldest cultivars of lavandin introduced in 1902 and historically called ‘Gigantea’ or ‘Giant Grappenhall’. It is an evergreen shrub up to 36 cm high; the inflorescence stalk can grow to 101 cm high. Flowers have a dark aster, a violet corolla, and a light green calyx. The leaves are broad green-grey. | [5,32] |
| ‘Grosso’ | ‘Grosso’ is an aromatic French cultivar of lavandin introduced in 1972. It is an evergreen shrub up to 20 cm high; the inflorescence stalk can grow to 76 cm high. Flowers have a violet corolla and a light green calyx. The leaves are narrow, grey-green, and long-stemmed. It is the most commonly cultivated lavender, characterized by many flowers, a high biomass production, and a large amount of extracted EO. This cultivar is resistant to mycoplasmas that threaten the crops of ‘Abrial’. | [5,32,40,44,45] |
| ‘Hidcote Giant’ | ‘Hidcote Giant’ was introduced in 1958 by L. Johnson. It is an evergreen shrub up to 20 cm high; the inflorescence stalk can grow to 76 cm high. Flowers have a dark aster, a violet corolla, and a light green calyx. The leaves are narrow, grey-green. The advantage of the plant is its stout stems, which is why it is often grown for cut flowers. | [5,32,44,46] |
| ‘Okanagan’ | ‘Okanagan’ is a cultivar produced for medical reasons. The plant contains an EO enriched with two therapeutic components: 1,8-cineole and borneol, and was used to test the efficacy of these compounds in a murine model of acute colitis. | [47] |
| ‘Old English’ | ‘Old English’ was introduced in the 1930s and is historically known as L. spica. It is an evergreen shrub up to 43 cm high; the inflorescence stalk can grow to 114–117 cm. The flowers are pale-purple. The leaves are narrow, grey-green. This cultivar is ideal for cottage gardens. | [5,32,43,44] |
| ‘Provence’ | ‘Provence’ has flowers with a dark violet corolla and a light-green calyx. | [32] |
| ‘Riverina Margaret’ | ‘Riverina Margaret’ was created in 2017 by Nigel Alexander Russell from Australia. It is an evergreen shrub up to 20 cm high with green leaves. Purple flowers form a cylindrical spike. | [48] |
| ‘Seal’ | ‘Seal’ was introduced in 1955 in England. It is an evergreen shrub up to 33 cm high; the inflorescence stalk can grow to 84 cm high. Flowers have a dark violet corolla and a light green calyx. | [32] |
| ‘Super’ | ‘Super’ is one of the most common plants cultivated for essential oil with a delightful aroma. It is an evergreen shrub up to 80 cm high. Flowers have a light bluish-purple corolla and a light violet-green calyx. The leaves are narrow green-grey. | [49,50] |
| ‘Sussex’ | ‘Sussex’ is an evergreen shrub up to 90 cm high with grey-green leaves. This cultivar has the longest blue flowers in the lavender group, making it particularly interesting. | [5,44] |
| ‘Tesseract’ | ‘Tesseract’ was created in 2019 by Lloyd R. Traven and Richard Grazzini from Pennsylvania. It is an evergreen shrub up to 45 cm high with broad and bright silver leaves. Purple flowers form dense spikes. This cultivar is resistant to leaf spotting and root disease. | [51] |
| Essential Oil Origin | Fresh | Dried Flowers/ | Dried Leaves |
|---|---|---|---|
| Flowers | Flowering Tops | ||
| Oil Yield [%] | |||
| Croatia | 3.3 [59] | ||
| France | 4.5–9.7 [6] | 0.4–0.8 [63] | |
| Greece | 7.5–8.5 [60] | ||
| Iran | 0.5–1.5 [65] | ||
| Norway | 7.1–9.9 [61] | ||
| Poland | 4.4–8.1 [64] | ||
| Romania | 2.75 [66] | 3.0 [67] | |
| Spain | 0.2–1.3 [55] | ||
| Turkey | 0.9–1.7 [68] | 3.6–8.4 [68,69] | |
| Ukraine | 0.9–2.0 [2] | ||
| Compound | Greece [60] | Norway [61] | Spain [81] | Spain [55] | Turkey [14] | Turkey [68] |
|---|---|---|---|---|---|---|
| DF | DF | DF | ND | FF | FF | |
| Percentage (%) | ||||||
| α-Thujene | 0.1 | |||||
| Camphene | 0.3 | |||||
| Octan-3-one | 3.4 | |||||
| Sabinene | 0.4 | 1.3 | ||||
| α-Pinene | 0.7 | 1.4 | ||||
| β-Pinene | 1.0 | |||||
| Myrcene | 1.0 | 0.5 | 1.5 | |||
| α-Phellandrene | t | |||||
| Hexyl acetate | 0.2 | |||||
| 3- Carene | 0.1 | |||||
| α-Terpinene | 0.1 | |||||
| p-Cymene | 0.1 | 5.0 | ||||
| Limonene | 0.7 | 0.9 | ||||
| 1,8-Cineole | 15.9 | 6.8 | 4.3–5.4 | 7.6 | 2.6 | |
| (Z)-β-Ocimene | 2.7 | 1.7–1.8 | 0.4 | |||
| (E)-β-Ocimene | 1.9 | 2.3–2.2 | 0.4 | |||
| γ-Terpinene | 0.3 | |||||
| trans-Sabinene hydrate | 0.3 | |||||
| Terpinolene | 0.3 | |||||
| Linalool | 23.0 | 38.5 | 31.4–37.4 | 33.2 | 34.0 | 33.8 |
| Camphor | 11.4 | 3.5 | 5.2–8.6 | 7.1 | 4.8 | 3.8 |
| Limonene dioxide | 0.3 | |||||
| Linalool oxide | 0.4 | |||||
| Borneol | 1.3 | 2.5–3.1 | 2.7 | 4.2 | 3.4 | |
| Lavandulol | 0.2–0.9 | |||||
| Terpinen-4-ol | 6.7 | 0.4–1.7 | 3.3 | 0.6 | ||
| Cryptone | 0.3 | |||||
| Hexyl butyrate | 0.7 | |||||
| α-Terpineol | 3.8 | 1.5 | 1.8 | |||
| Table continuation… | ||||||
| Cumin aldehyde | 0.1 | |||||
| Nerol | 0.4 | |||||
| Hexyl isovalerate | 0.1 | |||||
| Geraniol | 0.3 | 0.8 | ||||
| Linalyl acetate | 20.4 | 17.7 | 22.4–28.1 | 29.7 | 47.7 | 34.2 |
| Bornyl acetate | 0.1 | |||||
| Lavandulyl acetate | 0.4 | 2.2 | 1.3 | 2.6 | ||
| Neryl acetate | 0.7 | 2.4 | 2.2 | |||
| Geranyl acetate | 1.3 | |||||
| (E)-β-Farnesene | 0.3 | 1.9 | 0.5 | 0.8 | ||
| Germacrene D | 0.3 | 0.7 | ||||
| (E)-β-Caryophyllene | 0.9 | 1.1 | 1.4 | 1.0 | ||
| Caryophyllene oxide | 0.1 | 0.3 | ||||
| α-Santalene | 0.1 | |||||
| α-Bisabolol | 1.6 | 0.4 | 0.8 | |||
| t-Cadinol | 0.1 | |||||
| Compound | Australia [73] | France [72] | France [71] | Norway [61] | Spain [55] |
|---|---|---|---|---|---|
| ND | ND | ND | DF | FF | |
| Percentage (%) | |||||
| α-Pinene | 1.0 | 0.6 | |||
| β-Pinene | 1.1 | 0.4 | |||
| Camphene | 0.3 | ||||
| Myrcene | 1.5 | 0.7–0.8 | |||
| p-Cymene | |||||
| Limonene | 0.9 | 0.5–0.6 | 0.8–1.0 | ||
| 1,8-Cineole | 10.9 | 10.2 | 5.4–7.4 | 10.7 | 4.8–6.6 |
| (Z)-β-Ocimene | 1.9 | 1.1 | 0.8–1.1 | 0.5–1.3 | |
| (E)-β-Ocimene | 0.5 | 0.3–0.5 | 0.4–8.0 | ||
| Linalool | 34.3 | 22.5 | 28.7–32.1 | 27.9 | 37.7–51.3 |
| Camphor | 7.3 | 12.2 | 6.8–7.7 | 8.1 | 7.7–7.8 |
| cis-Linalool oxide | 0.1 | ||||
| Borneol | 1.6 | 2.9 | 2.1–2.4 | 2.3–4.3 | |
| Lavandulol | 0.8 | 0.3–0.5 | 0.4–1.5 | ||
| Terpinen-4-ol | 2.3 | 2.7 | 3.4–5.3 | ||
| Cryptone + p-Cymen-8-ol | 0.6 | ||||
| Hexyl butyrate | 0.3–0.5 | ||||
| α-Terpineol | 0.9 | 1.2 | 2.1–2.6 | 1.5–1.6 | |
| γ-Terpineol | |||||
| Linalyl acetate | 23.6 | 26.2 | 29.1–32.3 | 17.8 | 18.6–34.2 |
| Lavandulyl acetate | 2.2 | 2.3 | 2.4–2.7 | 3.1 | 1.5–2.6 |
| Geranyl acetate | 1.2 | ||||
| (E)-β-Farnesene | 1.1 | ||||
| α-Santalene | 0.2 | ||||
| Germacrene D | 1.1 | ||||
| (E)-β-Caryophyllene | 1.7–1.9 | 1.9 | |||
| Compound | France [72] | France [81] | Norway [61] | Spain [55] |
|---|---|---|---|---|
| ND | ND | DF | DF | |
| Percentage (%) | ||||
| α-Pinene | 0.9 | 0.4 | ||
| Camphene | 0.6 | 0.3 | ||
| Octan-3-one | 1.0 | |||
| Sabinene | 0.1 | |||
| Oct-1-en-3-ol | 0.3 | |||
| β-Pinene | 0.9 | 0.3 | ||
| Myrcene | 1.2 | 0.3 | ||
| Limonene | 1.5 | 0.7 | 1.1 | |
| 1,8-Cineole | 10.3 | 7.6 | 8.8 | 8.4 |
| (Z)-β-Ocimene | 2.6 | 2.6 | 3.5 | |
| (E)-β-Ocimene | 4.2 | 3.0 | ||
| Terpinolene | 0.2 | |||
| Linalool | 19.6 | 35.0 | 31.1 | 41.9 |
| Camphor | 12.2 | 8.9 | 7.5 | 10.3 |
| trans-Linalool oxide | 0.2 | |||
| cis-Linalool oxide | 0.1 | |||
| Borneol | 3.7 | 2.9 | 2.2 | |
| Lavandulol | 0.6 | 0.6 | 0.8 | |
| Terpinen-4-ol | 1.2 | 1.1 | ||
| α-Terpineol | 1.0 | 0.5 | ||
| Linalyl acetate | 18.6 | 27.0 | 17.2 | 22.0 |
| Lavandulyl acetate | 2.6 | 1.0 | 2.9 | 1.7 |
| Hexyl tiglate | 0.3 | |||
| Neryl acetate | 0.7 | |||
| Geranyl acetate | 1.2 | 0.3 | ||
| (E)-β-Farnesene | 1.2 | 0.3 | ||
| (E)-β-Caryophyllene | 0.7 | 1.9 | ||
| α-Santalene | 0.2 | |||
| Lavandulyl butyrate | 0.2 | |||
| Germacrene D | 1.2 | |||
| Caryophyllene oxide | 0.3 | |||
| Compound | Content of Regulated Components [%] | ||
|---|---|---|---|
| Ph. Eur. | WHO | Ph. Eur. | |
| LA [82] | LA or LI [83] | LL [82] | |
| Linalool | 20–45 | 20–45 | 34–50 |
| Linalyl acetate | 25–47 | 25–46 | <1.6 |
| 1,8-Cineole | <2.5 | <2.5 | 16–39 |
| Camphor | <1.2 | <1.2 | 8–16 |
| Limonene | <1 | <1 | 0.5–3 |
| Terpinen-4-ol | 0.1–8 | 1.2–6.0 | |
| α-Terpineol | <2 | <2.0 | 0.2–2 |
| Lavandulyl acetate | >0.2 | >0.1 | |
| Lavandulol | >0.1 | ||
| Octan-3-one | 0.1–5 | <2.5 | |
| trans-α-Bisabolene | 0.4–2.5 | ||
| Plant Material Origin Reference | Australia [73] | Australia [73] | California [77] | Italy [76] | Spain [92] | Turkey [14] | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Material | EO | H | EO | H | EO | H | H | H | H | EO | H |
| Compound | Percentage (%) | ||||||||||
| β-Pinene | 1.3 | 0.9 | 0.1 | ||||||||
| Myrcene | 0.9 | 0.1 | 1.7 | 1.4 | |||||||
| β-Phellandrene | 7.9 | ||||||||||
| Limonene | 1.1 | 0.6 | |||||||||
| 1,8-Cineole | 15.3 | t | 11.6 | 1.7 | 7.4 | 1.6 | 25.4 | 28.9 | 5.0 | 2.6 | 9.8 |
| (Z)-β-Ocimene | 6.5 | ||||||||||
| γ-Terpinene | 1.6 | 0.1 | |||||||||
| Linalool | 36.1 | 19.0 | 12.1 | 19.9 | 29.6 | 68.5 | 43.8 | 34.4 | 14.6 | 34.0 | 55.6 |
| cis-Linalool oxide (f) | 0.1 | 2.8 | 4.6 | t | 0.8 | 0.1 | t | 7.8 | 0.4 | 6.0 | |
| trans-Linalool oxide (f) | 3.2 | 4.2 | t | 0.6 | 7.4 | ||||||
| Camphor | 0.7 | 2.4 | 20.3 | 17.5 | 8.1 | 0.8 | 12.8 | 15.4 | 9.9 | 4.8 | 13.4 |
| Borneol | 0.7 | 5.2 | 6.0 | 31.8 | 2.5 | 1.4 | 4.3 | 4.0 | 9.3 | 4.2 | 13.5 |
| Lavandulol | 0.5 | 1.4 | |||||||||
| Terpinen-4-ol | 3.5 | 14.0 | 2.3 | 1.9 | 4.4 | 4.5 | 2.7 | 0.2 | |||
| Cryptone | 7.1 | 7.5 | 1.3 | 0.1 | |||||||
| (Z)-Hex-3-enyl butyrate | 1.9 | ||||||||||
| α-Terpineol | 1.0 | 24.0 | 10.1 | 1.6 | 9.0 | 1.8 | 2.2 | 14.6 | |||
| Nerol | 1.7 | ||||||||||
| Geraniol | 0.7 | 5.3 | 0.2 | 1.4 | 0.3 | 1.6 | |||||
| Linalyl acetate | 5.8 | 9.3 | 2.1 | 0.4 | 47.7 | t | |||||
| Lavandulyl acetate | 0.8 | 0.2 | 0.5 | 1.0 | |||||||
| Neryl acetate | 0.6 | 0.1 | 0.2 | 0.4 | 2.4 | t | |||||
| Geranyl acetate | 1.5 | 0.1 | 0.3 | 1.1 | |||||||
| (E)-β-Caryophyllene | 1.8 | 0.1 | 0.2 | 1.3 | 1.0 | t | |||||
| (E)-β-Farnesene | 0.2 | 0.2 | 1.4 | 0.5 | t | ||||||
| Caryophyllene oxide | 1.1 | t | 0.2 | 0.3 | t | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pokajewicz, K.; Czarniecka-Wiera, M.; Krajewska, A.; Maciejczyk, E.; Wieczorek, P.P. Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin. Molecules 2023, 28, 2943. https://doi.org/10.3390/molecules28072943
Pokajewicz K, Czarniecka-Wiera M, Krajewska A, Maciejczyk E, Wieczorek PP. Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin. Molecules. 2023; 28(7):2943. https://doi.org/10.3390/molecules28072943
Chicago/Turabian StylePokajewicz, Katarzyna, Marta Czarniecka-Wiera, Agnieszka Krajewska, Ewa Maciejczyk, and Piotr P. Wieczorek. 2023. "Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin" Molecules 28, no. 7: 2943. https://doi.org/10.3390/molecules28072943
APA StylePokajewicz, K., Czarniecka-Wiera, M., Krajewska, A., Maciejczyk, E., & Wieczorek, P. P. (2023). Lavandula × intermedia—A Bastard Lavender or a Plant of Many Values? Part I. Biology and Chemical Composition of Lavandin. Molecules, 28(7), 2943. https://doi.org/10.3390/molecules28072943









