Abstract
In the current paper, we present the results of Kazakh propolis investigations. Due to limited data about propolis from this country, research was focused mainly on phytochemical analysis and evaluation of propolis antimicrobial activity. uHPLC-DAD (ultra-high-pressure-liquid chromatography coupled with diode array detection, UV/VIS) and uHPLC-MS/MS (ultra-high-pressure-liquid chromatography coupled with tandem mass spectrometry) were used to phytochemical characteristics while antimicrobial activity was evaluated in the serial dilution method (MIC, minimal inhibitory concentration, and MBC/MFC, minimal bactericidal/fungicidal concentration measurements). In the study, Kazakh propolis exhibited a strong presence of markers characteristic of poplar-type propolis—flavonoid aglycones (pinocembrin, galangin, pinobanksin and pinobanskin-3-O-acetate) and hydroxycinnamic acid monoesters (mainly caffeic acid phenethyl ester and different isomers of caffeic acid prenyl ester). The second plant precursor of Kazakh propolis was aspen–poplar with 2-acetyl-1,3-di-p-coumaroyl glycerol as the main marker. Regarding antimicrobial activity, Kazakh propolis revealed stronger activity against reference Gram-positive strains (MIC from 31.3 to above 4000 mg/L) and yeasts (MIC from 62.5 to 1000 mg/L) than against reference Gram-negative strains (MIC ≥ 4000 mg/L). Moreover, Kazakh propolis showed good anti-Helicobacter pylori activity (MIC and MBC were from 31.3 to 62.5 mg/L). All propolis samples were also tested for H. pylori urease inhibitory activity (IC50, half-maximal inhibitory concentration, ranged from 440.73 to 11,177.24 µg/mL). In summary Kazakh propolis are potent antimicrobial agents and may be considered as a medicament in the future.
1. Introduction
Propolis, also called “bee glue” is a natural product of different bee species. Its viscous form is due to the fact that exudates collected from the buds and flowers of different plant species growing in the vicinity of the bee hive are used to produce propolis [1]. These exudates of botanical origin are chewed by the honeybees and mixed later with pollen and the bee’s saliva (containing several enzymes, among them β-glucosidase) and, finally, after the addition of bee wax the raw propolis is formed [1]. Propolis is used as building and sealing material for protecting the hive entrance of the hive and plugging the holes in hive construction as well as covering intruders (insects and small rodents) who died inside the hive in order to avoid their decomposition [2,3].
The characteristic of physiochemical properties (density, color, and odor), as well as the chemical profile and bioactivity of particular propolis samples, is dependent on numerous factors such as plant source (precursor), climate and weather conditions in the year of harvest, and sometimes the harvest time [3,4].
The different main types of propolis are mentioned in the literature [5,6], poplar type, where plant precursor belongs to some Populus spp. (e.g., Populus nigra or Populus species about similar exudates composition such as P. balsamifera) and aspen type (P. tremula and similar Populus species). Both types are characteristic for central Europe, non-tropic regions of Asia, New Zealand and North America; birch type (exudates are collected from Betula verrucosa and B. pendula, which occurs in Russia and the northern part of Europe); green type (characteristic for Brazil, where the main plant source of propolis is genus Baccharis, e.g., B. dracunculifolia); red type (characteristic for Brazil, Mexico, Cuba and Nepal, where plants from Dalbergia spp. occur, e.g., Dalbergia ecastaphyllum or D. sisoo); Pacific type (plant precursor of propolis is Macaranga tanarius from Indonesia, Taiwan, and Okinawa Prefecture of Japan); Mediterranean type (mainly Plants from Cupresaceae family occurring in Greece, Sicily, Malta and other islands). Apart from ‘pure’ types of propolis, there are also observed mixed types of propolis, e.g., aspen–poplar or aspen–birch–poplar.
Propolis has been used in traditional medicine since ancient times. There is evidence that it was used for medicinal purposes in 3000 BC in Egypt [7]. Nowadays, propolis is widely and often used in cosmetology, the food industry, beverages and nutritional supplements, as well as an ingredient in functional food [7]. Herbalists have recommended propolis according to its antibacterial, antiviral and anti-inflammatory activity, which can be used in the treatment of different types of infections as well as duodenal and gastric ulcers [1,8]. Many biological activities of propolis have been confirmed by modern studies, including anticancer [9], antioxidant [10], antileishmanial [11], wound healing [12], anti-inflammatory [13] and immunomodulatory [14,15].
Propolis in different preparations is used worldwide as a potent antimicrobial agent and is active against numerous bacterial strains, but is especially effective against Gram-positive bacteria, and less effective against Gram-negative ones, with Helicobacter pylorian as exception [16]. Most of the bioactivities of propolis are related to the presence of polyphenolic compounds in the chemical composition of this natural mixture. It is noteworthy that polyphenols present in propolis, e.g., chrysin, will not only exhibit an antibacterial effect against microorganisms, e.g., H. pylori, but also potentiate the activity of antibiotics [17].
Kazakhstan, as a country with a large area and high biodiversity, is an excellent place to collect various natural products. Surprisingly, almost no research has been undertaken so far on propolis from Kazakhstan—in the same Scopus database we found only our previous research [18,19]. In this investigation, one sample of Kazakhstan propolis was analyzed among the rest Eurasian propolis extracts. Therefore, in the research Kazakhstan propolis exhibited strong antibacterial and further research was justified.
For this purpose, we performed (ultra-high-pressure-liquid chromatography coupled with diode array detection, UV/VIS) and uHPLC-MS/MS (ultra-high-pressure-liquid chromatography coupled with tandem mass spectrometry) profiling of 70% ethanol in water extracts (70EEP, ethanol: water, 70:30, V/V) of ten propolis samples from Kazakhstan as well as evaluated their antimicrobial potential-evaluation of MIC (minimal inhibitory concentration) and MBC/MFC (minimal bactericidal/fungicidal concentration measurements) in serial dilution technique. According to our previous results [18] and expected strong activity against H. pylori, we also investigated the potential of extracts as urease inhibitors (one of the most important virulence factors of H. pylori). These measurements were based on the evaluation of IC50 (half-maximal inhibitory concentration). It is worth adding that the urease inhibition potential of Kazakh propolis extracts was not tested before.
2. Results and Discussion
2.1. Chemical Composition of Propolis Extracts from Kazakhstan
Results of uHPLC analyses were presented in Table 1 (component identification), Table 2 (relatively presence of components in mass chromatograms) and Table 3 (relatively presence of main components in UV chromatograms). Representative UV chromatograms (280 nm) were also presented in Figure 1.

Table 1.
Identification of propolis components in 70EEP by uHPLC-MS/MS analysis.

Table 2.
Relatively presence of main propolis components in UHPLC-MS/MS chromatograms of 70EEP from Kazakhstan.

Table 3.
Matrix of main components in UHPLC-DAD profile of Kazakh propolis 70% ethanol in water extract in 280 nm *.
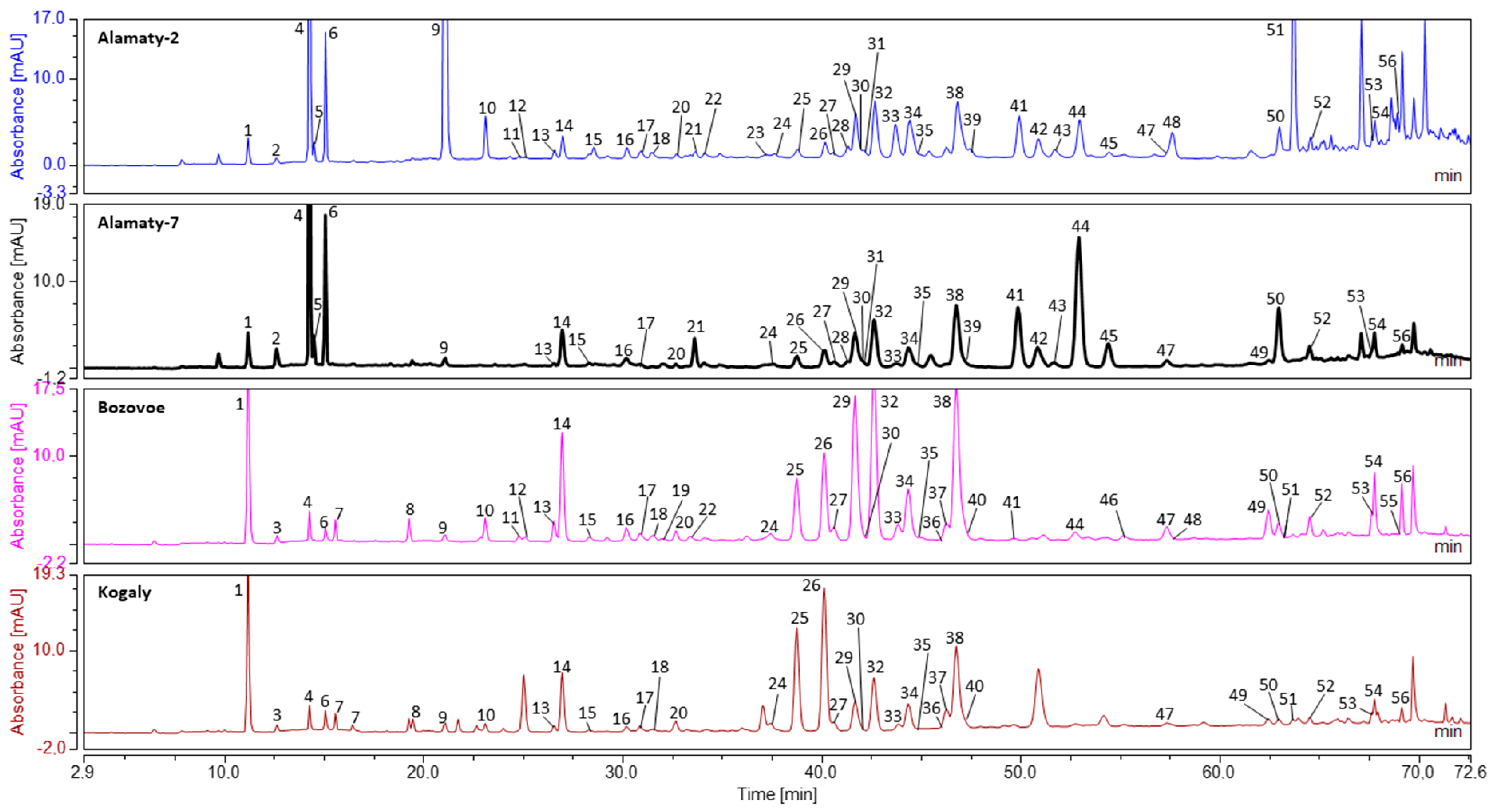
Figure 1.
Representative uHPLC-DAD chromatograms of Kazakhstan propolis. Figure Legend: 1—Caffeic acid; 2—Vanilline; 3—Caffeoylglycerol; 4—p-Coumaric acid; 5—Benzoic acid; 6—Ferulic acid; 7—Isoferulic acid; 8—Caffeoylmalic acid (Phaseolic acid) isomer; 9—Cinnamic acid; 10—Pinobanksin-5-methyl ether; 11—Quercetin; 12—luteloin; 13—Quercetin-3-methyl-ether; 14—Pinobanksin; 15—Naringenin; 16—Apigenin; 17—Kaempferol; 18—Isorhamnetin; 19—Quercetin-methyl-ether; 20—Luteolin-5-methyl-ether; 21—1,3-di-p-Coumaroylglycerol; 22—Quercetin-dimethyl-ether; 23—2-Acetyl-1,3-di-caffeoylglycerol; 24—Rhamnetin; 25—Caffeic acid 2-methyl-2-butenyl ester; 26—Caffeic acid 3-methyl-2-butenyl ester; 27—Caffeic acid 3-methyl-3-butenyl ester; 28—(R/S) 2-Acetyl-1-caffeoyl-3-p-coumaroylglycerol; 29—Chrysin; 30—Caffeic acid benzyl ester; 31—(R/S) 2-Acetyl-1-caffeoyl-3-feruloylglycerol; 32—Pinocembrin; 33—Sakuranetin; 34—Galangin; 35—Genkwanin; 36—Caffeic acid pentyl or isopentylester ester; 37—Caffeic acid phenethyl ester (CAPE); 38—Pinobanksin 3-O-acetate; 39—Kaempferide (Kaempferol 4′-methyl ether); 40—Methoxychrysin; 41—2-Acetyl-1,3-di-p-coumaroylglycerol; 42—(R/S) 2-Acetyl-3-p-coumaroyl-1-feruloylglycerol; 43—2-Acetyl-1,3-di-feruloylglycerol; 44—p-Coumaric acid benzyl ester; 45—Ferulic acid benzyl ester; 46—Caffeic acid cinnamyl ester; 47—Pinobanksin-3-O-propanoate; 48—p-Coumaric acid phenethyl ester; 49—Tectochrysin; 50—Pinostrobin; 51—p-Coumaric acid cinnamyl ester; 52—Pinobanksin 3-O-butanoate or isobutanoate; 53—Pinobanksin 3-O-pentanoate or isopentenoate isomer I; 54—Pinobanksin 3-O-pentanoate or isopentenoate isomer II; 55—Pinobanksin-3-O-hydroxycinnamate; 56—p-Methoxy cinnamic acid cinnamyl ester.
Components were identified by comparison with previous research [19,20]. Most of the substances belonged to one of four main groups: free cinnamic and hydroxycinnamic acids, hydroxycinnamic acids monoesters, hydroxycinnamic acids glycerides, flavonoids and others. Among all groups of components, in most samples, the highest peaks belonged to hydroxycinnamic acids monoester and flavonoids.
Generally, most of the components were presented in uHPLC-MS/MS chromatograms in negative ionization mode (Table 2). uHPLC-MS/MS is a very sensitive technique, which allows to track a lot of substances. In the same propolis extracts, we observed above 200 singular peaks. However, most of them remained as unidentified traces. For this reason, in the current paper, we presented 155 main components detected in uHPLC-MS/MS chromatograms (Table 1 and Table 2).
Despite the high sensitivity of uHPLC-MS/MS, there were some specific substances, which did not produce ions in negative mode or produce trace amounts of ions in experimental conditions. These components included some known substances (benzoic acid, caffeic acid ethyl ester, ferulic acid benzyl ester, tectochrysin and pinostrobin) and 14 unidentified compounds. Among these components, benzoic acid, caffeic acid ethyl ester and ferulic acid benzyl ester produced ions in negative mode, but, in our experience, their production strongly depends on experimental conditions. Usually, too low concentration and close proximity of easy ionized component on the chromatogram suppress ion production by these components. For this reason, their presence was exhibited only in uHPLC-DAD chromatograms (Table 3) and they were identified by comparison of UV spectra with previous research [19,20,21]. Therefore, most of the components were detected in the uHPLC-MS/MS analysis (Table 1 and Table 2), Table 3 was limited to major substances which were further used in statistical analysis.
The main components of the hydroxycinnamic acids monoesters group included caffeic acid 2-methyl-2-butenyl ester, caffeic acid 3-methyl-2-butenyl ester, caffeic acid 3-methyl-3-butenyl ester and p-methoxy cinnamic acid cinnamyl ester. These components were presented in all samples. Apart from them, high peaks were also observed for p-coumaric acid benzyl ester but not for all samples.
In the flavonoid group, most of the components were flavonoid aglycones or their ester derivatives (mainly pinobanksin). The most abundant component was pinobanksin-3-O-acetate. The rest of the common flavonoid aglycones included pinobanksin, chrysin, pinocembrin and galangin.
Among free cinnamic and hydroxycinnamic acids, the most often observed in uHPLC-MS/MS chromatograms were caffeic acid, p-coumaric and ferulic acid. The same caffeic acid was observed in all the samples, while the rest of the components were not. The last group included mainly unidentified components and some known, such as benzoic acid. This group of components was significant only in Alamaty-1. The chemical composition of analyzed propolis types as well as plant distribution maps [22,23] and our previous research [18,19] suggested poplar trees as the main plant precursors of Kazakh propolis. Main poplar markers included flavonoid aglycones (pinobanksin, pinbankisn-3-O-actetate, galangin, chrysin, pinocembrin) and methylbutenyl esters of caffeic acid (2-methyl-2-butenyl ester, 3-methyl-2-butenyl and 3-methyl-3-butenyl).
Apart from poplar, the presence of 2-acetyl-1,3-di-p-coumaroyl glycerol suggested aspen origin of some samples. This substance is specific marker of P. tremula (aspen) as well as some other Populus species (e.g., P. lasiocarpa [24]). Aspen is widely spread across whole Eurasia [23] while P. lasiocarpa is naturally present in China. Outside China, it is rather planted in botanical gardens and parks than easily spreading in the natural environment. For these reasons, presence of 2-acetyl-1,3-di-p-coumaroyl glycerol in propolis rather proves the presence of P. tremula exudates more than another species.
Previous research exhibited mixed aspen–poplar origin of Kazakh propolis sample [19]. In the current research, most samples exhibited a strong presence of poplars markers (Almaty 1, 4, 6, Bozovoe and Kogaly and Kegen) and lower (Almaty 2, 3, 5) or a strong of aspen ones (Almaty 7). However, there were some unidentified components, which may not be connected with Populus genus origin, especially in Almaty-1 (see Table 2 and Table 3). Moreover, a high occurrence of cinnamic acid is also not usual for black poplar trees, but hydroxycinnamic acids are presented in its place [25,26]. According to the plant distribution map [22], P. nigra should be rather present in northern Kazakhstan. Therefore, the sample from Bozovoe may exhibit black poplar origin. Propolis from the southern part (Almaty, Kegen and Kogaly) should be rather originated from another balsamic Populus tree (e.g., P. laurifolia or P. euphratica). However, literature studies exhibited that the situation of Populus genus distribution in Kazakhstan is complex. During the Soviet Union time in the central Asia region, there were introduced many Populus species such as P. nigra, P. bolleana and P. deltoides and other cultivars [27]. Moreover, many Populus cultivars are known for the easy creation of crossbreed species. In a result in the same Almaty, many crossbreed species were observed (e.g., P. nigra × P. maximowiczii, P. nigra × deltoides or P. laurifolia × P. canadensis) [28]. Dependences between Populus bud exudate compositions and their genetic origin were not well investigated and, for this reason, accurate tracking of plant precursors of Kazakhstani poplar type propolis may be very difficult.
Apart from the Populus genus, it is worth to add that Kazakhstani propolis may have some other plant sources. In the previous research [19], we observed also the presence of flavonoid ermanin which may be connected with birch origin. However, in the present research, ermanin was only a trace component and the same Betula genus may be a marginal plant precursor. Moreover, there are possibly more minor or marginal plant precursors. Confirmation of birch presence requires further research with additional techniques such as GC-MS [25].
The possibility of non-Populus plant precursors suggested also caffeoylmalic acid (phaseolic acid) isomer and some unidentified components, especially described in Table 2. They were strongly presented in the mainly two samples (Kogaly and Almaty-1) which may suggest additional unknown plant precursors.
In the end, it is worth adding that not all components presented in propolis have natural origin. Sometimes, there may be traces of different beekeeping techniques (e.g., treatment of honeybees to American and the European foulbrood [29]) or even pollutants occurring in the environment [30]. For this reason, the presence of an uncommon component in propolis should be analyzed in detail.
2.2. Comparative Analysis of Chemical Composition of Extracts for Kazakh Propolis Samples
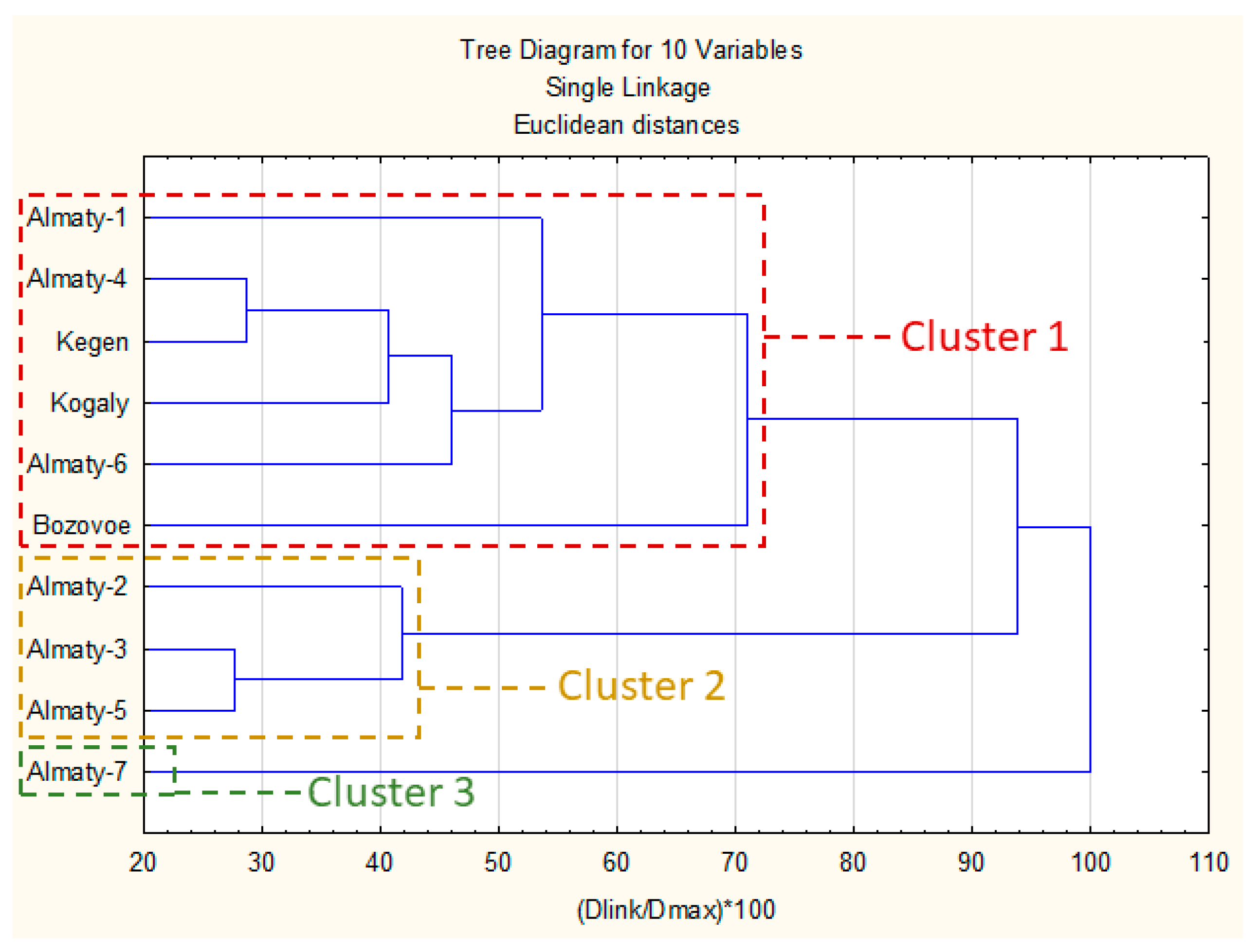
The results of comparative analysis of chemical composition are presented in Figure 2. An investigation based on the uHPLC-DAD matrix (Table 3) with the spectral properties of polyphenols allowed to group of the samples into three main clusters.
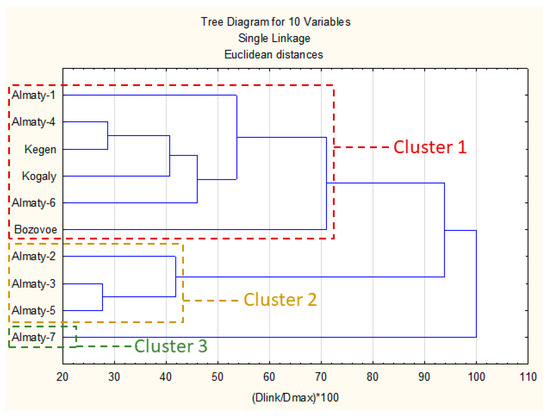
Figure 2.
Dendrogram of Kazakh propolis chemical composition. Figure legend: Dlink—Linkage Distance; D max—Maximal distance.
The first cluster was composed of six propolis samples with a high presence of different prenyl (methylbutenyl) esters of caffeic acid (mainly 2-methyl-2-butenyl ester, 3-methyl-2-butenyl and 3-methyl-3-butenyl) and pinobanksin-3-O-acetate. In this cluster Bozovoe was less similar to the other samples due to a higher concentration of flavonoid aglycones and lower prenyl esters of caffeic acid. Samples in this cluster exhibited a strong presence of P. nigra and similar poplars resins. There were possible other plant precursors; however, they are rather minor or even marginal in most cases.
The second cluster contained three samples with strong presence of p-coumaric and cinnamic acids and a lower amount of prenyl esters of caffeic acid than cluster 1. Moreover, in this cluster, there were also some of hydroxycinnamic acid glycerides present. Generally, this cluster represented mixed, aspen–poplar type of propolis.
The last cluster (3) had only one sample (Almaty-7). Its main components were p-coumaric, 2-acetyl-1,3-di-p-coumaroyl glycerol and p-coumaric acid benzyl ester. The composition of Almaty-7 suggested a strong aspen origin with lower amount of poplar markers.
In summary clusters presented in dendrogram reflected presence of poplar and aspen markers described in Section 2.1. Generally, presence of unidentified components in some samples (Kogaly and Almaty 1, 2 and 6) exhibited rather low impact on their clusters grouping—all these samples were presented in two main clusters. For this reason, we may suspect that Populus genus was main plant precursor of these samples.
2.3. Antimicrobial Activity of Propolis Samples from Kazakhstan
The results of the antimicrobial assays are presented in Table 4. The main goal of our study was a general screening of antimicrobial properties of propolis from Kazakhstan. In our study, we evaluated activity of 70EEP against the following reference microorganisms: six strains of Gram-positive and six strains of Gram-negative bacteria as well as three strains of yeasts.

Table 4.
Antimicrobial activity of Kazakh propolis *.
Among Gram-positive bacteria, Kazakh propolis samples were more active against Micrococcus luteus with MIC values of 31.3 µg/mL; however, some samples also showed weak activity (>4000 µg/mL). According to criterium of bioactivity presented by O’Donnell [31], it is defined as good activity. Against two strains of Staphylococcus aureus tested, 70EEPs exerted activity 62.5 – > 4000 µg/mL. It is worth mentioning that most of propolis samples presented good bioactivity (62.5–125 µg/mL), and only samples from Kogaly were inactive. Distinguished from the rest of the samples, propolis from Kogaly had its own specific markers in uHPLC-DAD (Table 3) analysis which suggested the presence of an additional unknown plant precursor. It is possible, that its presence may cause strongly lower activity of this sample.
Antibacterial activity of Kazakh propolis against Staphylococcus epidermidis and Bacillus cereus can be described as good as 31.3–125 µg/mL and 62.5–125 µg/mL, respectively, except the samples obtained from Kogaly, which were inactive (MIC > 4000 µg/mL). Against Enterococcus faecalis, propolis from Kazakhstan expressed good or moderate antibacterial activity (62.5–250 µg/mL), which deserves attention. Gram-negative bacteria were insensitive to propolis collected in Kazakhstan (MIC = 4000 µg/mL or more). This may be related to the structure of the bacterial cells and the double cell membrane, which, when exposed to a fraction of surface-active compounds, can stiffen and remodel, increasing its resistance [32]. The presence of the periplasmic space may also cause compounds that have already penetrated the cell to be cut by hydrolytic enzymes, losing their activity [33]. H. pylori was the only exception of the Gram-negative bacteria which was inhibited by all propolis samples showing good antibacterial activity against this pathogen (MIC = 31.3–62.5 µg/mL). This high activity can often be associated with impaired urease activity and may affect stick adhesion and cell viability [34]. Moreover, MIC values in all 70EEPs were equal to MBCs. The results presented by the other authors [18,19,35], as well as our own research, clearly indicate that Gram-positive bacteria are susceptible to lower propolis concentrations than Gram-negative ones. S. aureus and B. cereus are well-known because of their involvement in the gastrointestinal and respiratory tract diseases [15]. Since propolis is usually administered orally, its antimicrobial activity against these pathogens is of great practical importance in its possible therapeutic use [15].
The results of this study showed higher activity of 70EEPs obtained from Kazakhstan in comparison to green and brown propolis ethanolic extracts from Brazil (MIC = 125 and 250 µg/mL, respectively) [15]. Interestingly, partitioning in dichloromethane has enhanced the extraction of antibacterial compounds from Brazilian propolis samples, as it can be inferred from the lower MIC values observed for green (MIC = 7.8 µg/mL) and brown propolis (MIC = 62.5 µg/mL), what was correlated with enhanced levels of phenolic compounds in the extracts [15]. Better activity against M. luteus was reported for propolis samples collected in Anatolia (Turkey). Four samples from a different locations, and characterized by the presence of flavonoid compounds (pinocembrin, pinostrobin, isalpinin, pinobanksin, quercetin, naringenin, chrysin and galangin) showed MIC values from 4 to 16 µg/mL [36]. The different species of staphylococci are the microorganisms most often used as models for antimicrobial activity of propolis. This is probably due to their high importance for human morbidity. Staphylococci colonize about 30% of humans (usually asymptomatically) and are responsible for a wide spectrum of difficult-to-treat infections (eye inflammation, pneumonia, meningitis and others) [37]. The anti-staphylococcal potential of tested Kazakh propolis samples against the three reference strains: S. aureus ATCC 25923, S. aureus ATCC 43300 and S. epidermidis ATCC 12228 were better (31.3–250 µg/mL) than results obtained by Grecka et al. (32–4096 µg/mL) [37] and our studies concerning Georgian propolis samples (64–512 µg/mL) [20] and similar to our other result presented in paper about antimicrobial activity of poplar-type propolis (10–2500 µg/mL) [18]. It should be underlined that the MIC is equal to the MBC for most of the tested propolis samples from Kazakhstan. Similar antimicrobial properties (similar ranges of MIC values) of the propolis in question correlate with data on the chemical composition of propolis samples. All samples are characterized by a phytochemical profile (flavonoids and derivatives of phenolic acids) indicating different species of Populus as the plant precursor of propolis. The biological activity of propolis is related to time (plant source of exudate), harvest time and geographic origin. Due to these considerations, propolis from a particular geographic region should exhibit similar physicochemical characteristics and other properties, e.g., antimicrobial activity. Several independent studies have shown high susceptibility of S. aureus and S. epidermidis to different types of propolis originating from Brazil. For example, Reguiera et al. revealed the good bioactivity of Brazilian red propolis hydroalcoholic extracts against S. aureus ATCC 6538 and clinical isolates with MIC in the range of concentration 64 to 1024 µg/mL [3]. Another study confirmed the antimicrobial efficacy of ethanolic extracts of three different types of propolis from Brazil: brown, red and green against S. aureus ATCC 25923 and S. aureus ATCC 25923. The lowest MIC values characterized red type of propolis (25–50 µg/mL), higher in green ones (200–400 µg/mL) and highest (lowest antimicrobial activity) in brown propolis (200–800 µg/mL) [38]. In the same study, the authors compared two methods of raw propolis extraction methods: classical low-pressure extraction with ethanol and supercritical fluid extraction (SFE). Taking into consideration the antibacterial activity, the most potent were ethanolic extracts of propolis, characterized by the highest content of phenolic compounds and high values of flavonoids [38]. These findings confirmed the method we used for the extraction of propolis samples from Kazakhstan due to the increased amount of polar compounds in the extract (phenolic acids and their derivatives and flavonoids) that determine antimicrobial activity. Interestingly, anti-staphylococcal activity of Brazilian red propolis was presented by Regueira et al. [3]. Samples of propolis were collected in the rainy and the dry season. Hydroethanolic extracts showed MIC values for S. aureus ATCC 6538 and clinical isolate ≥1024 and 101.6 µg/mL as well 512 and 64 µg/mL for the rainy and the dry season samples, respectively [3]. Comparison of two extracts demonstrated two-times higher concentration of phenolic compounds in the dry season sample, which had the crucial influence on the antibacterial activity [3]. The study reported on the inhibitory and bactericidal properties of 39 South African and three propolis samples from Brazil and was conducted by Suleman et al. [1]. Some samples of African propolis displayed substantial antimicrobial activity with MIC and MBC values at a very low level of 6 µg/mL against S. aureus ATCC 25923 [1]; however, the remaining samples had weaker bioactivity (24 up to 1563 µg/mL). The main bioactive constituents of propolis were identified as chrysin, pinocembrin, galangin or 3-pinobankin-3-O-acetate, ingredients, that are also found in poplar type of propolis [1]. The activity of tested Kazakh propolis against the rest of Gram-positive bacteria was similar to results presented in the literature and our own studies [18,20,35,36].
Among all tested Gram-negative bacteria, only H. pylori was sensitive for 70EEPs form samples collected in Kazakhstan. To our best knowledge, it is the first communication on the anti-Helicobacter activity of Kazakh propolis (except two papers reported one propolis sample from Kazakhstan from different origin). Our group assessed ten samples of 70EEP obtained from different propolis samples derived from various parts of Kazakhstan. The highest bioactivity (31.3 µg/mL) against reference H. pylori strain was expressed by 70EEP from Kogaly, Bozove, and four samples from Almaty (Almaty-2, Almaty-3, Almaty-5 and Almaty-6). The rest of samples were characterized by MIC values 62.5 µg/mL. Generally, the antibacterial activity of Kazakh propolis against H. pylori and, according to O’Donell criterium, is regarded as good [31]. Moreover, the PE from the sample obtained in Kolgaly has one of the highest activities, despite its inactivity against all Gram-positive bacteria. Surprisingly, for all 70EEPs evaluated against H. pylori, MBC/MIC ratio was 1, which confirmed the bactericidal activity of tested propolis extracts [39]. In the frame of our studies, we tried to combine the results of the microbiological evaluation of the inhibition of H. pylori growth by propolis extracts from several locations in Kazakhstan with qualitative analysis of their composition by using chromatographic and spectral analysis (uHPLC-DAD and uHPLC-MS/MS). The antibacterial activity of Kazakh 70EEPs were similar to our previous studies focused on the activity propolis from Georgia (MIC = 31.3–125 µg/mL) [40] or different European propolis samples (MIC = 20–30 µg/mL) [18]. Similar results were obtained in the study performed by Santiago et al. [41] with hydroalcoholic extracts of Brazilian propolis against H. pylori ATCC 43526 (MIC = MBC = 50.0 µg/mL) and clinical isolate of H. pylori (MIC = MBC = 100.0 µg/mL) [41]. The weaker activity against H. pylori was presented by 19 propolis samples from Northern Spain (Basque Country) extracted with ethanol and propylene glycol (MIC from 6 to 14 mg/mL) [42]. Indonesian propolis produced by a stingless bee, belonging to Trigona spp. was tested against ten clinical isolates of H. pylori (from dyspeptic patients) [43]. The results of experiments indicate very weak activity of ethanolic extracts of propolis (MIC = 1024–8192); however, there were promising results of an additive effect against H. pylori when used together with clarithromycin and metronidazole [43]. The chemical composition of tested Kazakh propolis, showed a similar phytochemical profile, which is a good explanation of the activity of 70EEPs from Kazakhstan against H. pylori. However, results presented by Romero and coauthors showed that activity of complex natural mixtures as propolis is more than just simple sum properties of all constituents and interaction among them, which should be taken into consideration [44]. Finally, analyses by transmission electron microscopy at sub-inhibitory concentration showed vesicle formation and bacterial cell lysis after exposition to individual polyphenols and in the mixture, suggesting a potential bactericidal activity of propolis [44].
Antifungal activity of 70EEPs was tested against three Candida species. Propolis exhibited moderate antifungal activity (125–500 µg/mL) against C. glabrata and moderate-to-mild bioactivity (125–1000 µg/mL) against C. albicans and C. parapsilosis. There are numerous experimental works on the antimicrobial activity of various kinds of propolis collected from different geographical locations [1,15,36,45]. Depending on the content of the samples, propolis may inhibit the process of filamentation and yeast adhesion and increase intracellular oxidative stress [46]. The bioactivity of tested samples against pathogenic yeasts (62.5–1000 µg/mL) is weaker than our research concerning propolis for samples obtained from Georgia and central Europe, but similar to the activity of propolis samples from South Africa (MIC between 98–1563 µg/mL] [1] and Cretan propolis (370–1560 µg/mL) [45]. Better activity against C. albicans was exerted by Anatolian propolis (MIC range 4–32 µg/mL) [36]. Propolis owes its antimicrobial activity mainly to the presence of polyphenolic compounds (phenolic acids and flavonoids).
The mechanism underlying the antimicrobial activity of propolis involves the flavonoid and phenolic acids present in propolis. The literature data, among them some reviews confirmed and summarized these mechanisms of activity [3]. Polyphenols are responsible for the inhibition of nucleic acid synthesis (DNA and RNA) and the inhibitory mechanism on DNA gyrase (procaryotic enzyme plays an important role in processes of replication, transcription and recombination) [47,48,49]. Galangin (flavonol) and derivatives of caffeic acids derivatives (polyphenolic acids) have the ability to uncouple the energy-transducing cytoplasmic membrane and inhibit bacterial motility. Moreover, these effects on the bioenergetic status of the membrane may contribute to the antimicrobial action of propolis and its observed synergism with selected antibiotics [50]. Flavonoids, among other phenolic compounds, interfere with the energy metabolism of the bacterial cell due to the damage to the cytoplasmatic membranes, their permeability alteration and the perturbance in the exchange of nutrients and metabolites [35,47,48,49]. Additionally, flavonoids from propolis inhibit adhesion and biofilm formation [3,35,47,48,49].
In summary observed differences of the antimicrobial activity of Kazakh propolis may bresultesult of different factors. The basic one may be differences between Kazakh propolis plant precursors. Usually, propolis with a stronger presence of poplar markers is expected to be stronger than propolis with aspen–poplar origin [18,40]. However, P. treumula exudates sometimes exhibited stronger activity than P. nigra resins [51,52,53]. Moreover, the same propolis may exhibit lower or stronger activity than its plant precursor [51,52,53]. Propolis as well as Populus genus bud exudates are a complex matrix and their antimicrobial activity is an effect of interaction between many components. The same exudates of this same Populus species may be observed in different chemotypes [26,51,52] that should also exhibit an impact on propolis antimicrobial activity. In results, total effect may also be connected with presence of minor and even marginal plant precursors as well as specific chemical composition of plant precursor.
2.4. Urease Inhibitory Activity and Anti-Helicobacter Activity of Tested Kazakh Propolis Extracts
The results for the assessment of selected 70EEPs from Kazakh propolis are listed in Table 5.

Table 5.
Antibacterial activity (MIC) against reference H. pylori strain and inhibition of H. pylori urease activity (IC50) by tested 70EEP.
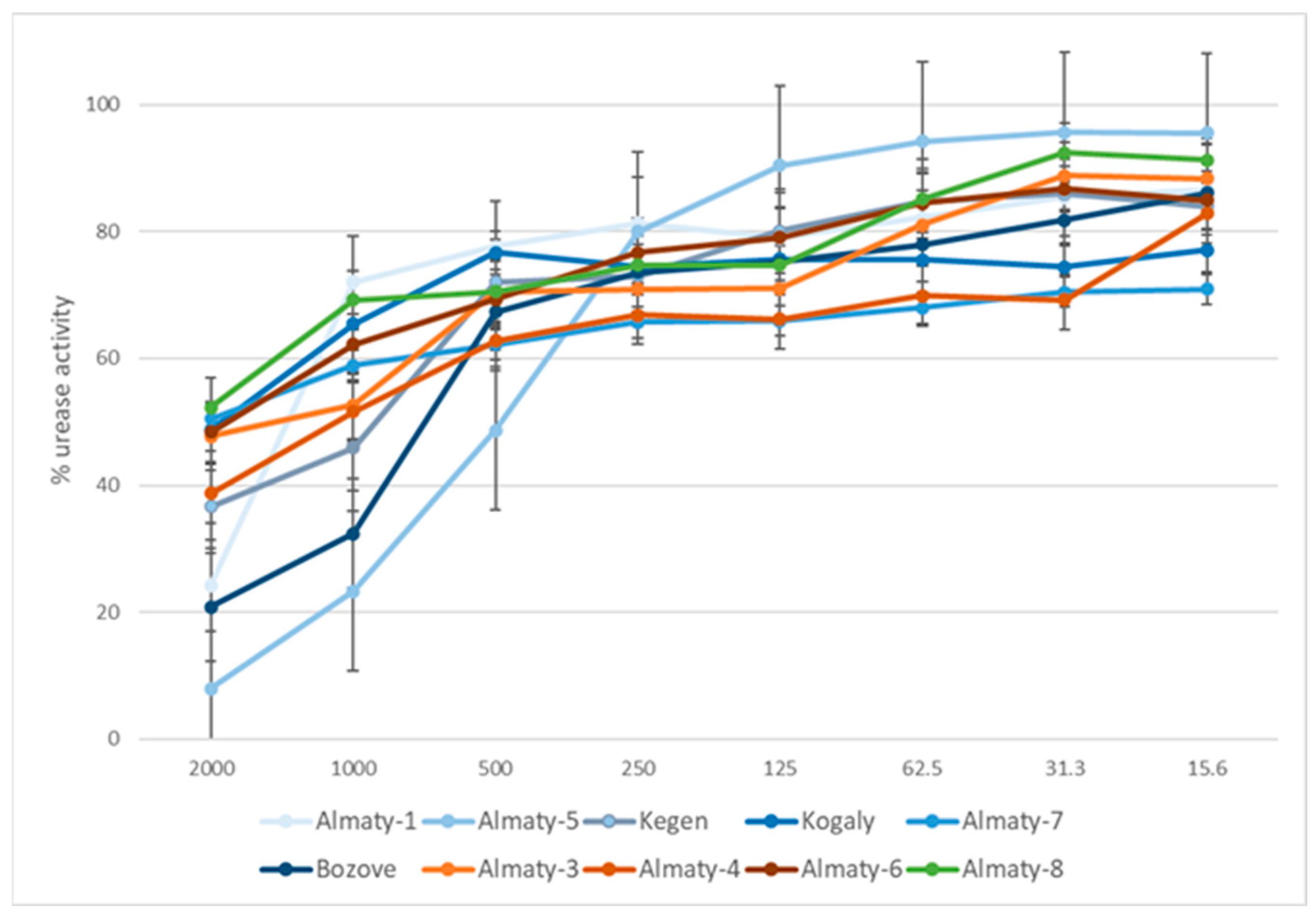
The presented study is the first attempt to evaluate the effects of 70EEPs obtained from propolis sample collected in Kazakhstan, a natural bee product used in the treatment of gastric diseases, on H. pylori growth in vitro as well as the activity of its enzyme urease which is crucial for the ability of the pathogen to colonize the stomach. Results of bioassay shows IC50 values for 70EEPs ranging from 440.73 to 11,177.24 µg/mL and IC50 = 92.7 µg/mL for thiourea (reference inhibitor) (Table 5 and Figure 3). Obtained results suggested the same plant origin of Kazakhstan propolis. Presented results suggested that inhibition of urease is not directly connected with bactericidal activity against H. pylori. Moreover, variability of activity inside clusters also suggested that the same type of propolis may not be directly connected with urease inhibition activity. It is more possible that the obtained effect is a result of interaction between components in complex natural matrix of propolis. For this reason, some samples may exhibit better activity inside this same cluster.
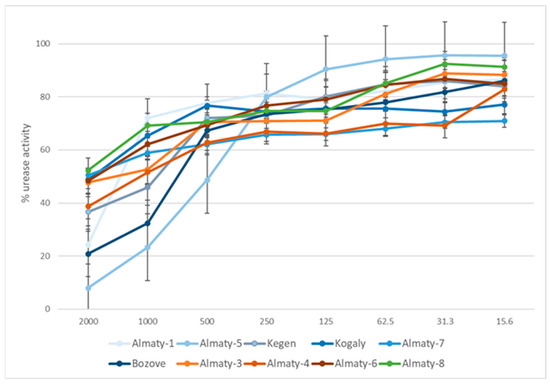
Figure 3.
Urease activity inhibition by tested 70EEP of Kazakh propolis.
Results showed in described experiments are similar to the other research of 70EEPs inhibitory activity and indicate that searching for a novel, natural urease inhibitors among bee products is the proper direction. For example, Baltsas et al. [54] tested 15 PEs of Turkish propolis samples for urease inhibitory activity. The tested propolis samples exerted IC50 in the range of 0.260 to 1.525 mg per mL, similar to the results exhibited in this study. Inhibition activity of 70EEPs from Kazakhstan is distinctly weaker than other natural products, e.g., essential oils. For example, essential oil from Origanum vulgare (MIC = 31.3 µg/mL) presented IC50 against H. pylori urease equal to 208.3 µg/mL [55]. Moreover, the most active essential oil (cedarwood essential oil) has IC50 = 5.3 µg/mL (MIC = 15.6 µg/mL) [55].
In research performed by Can [56], 11 propolis samples from the Marmara region of Turkey were tested. Their activity concerning urease inhibition was in the range from 1.110 to 5.870 mg/mL and authors suggested that it indicates good bioactivity of tested propolis extracts [56]. In another experiment done by Can [57], where enzyme inhibition of urease was examined by different bee products—honey, pollen and propolis. The IC50 values were changed from 7.02 to 33.25 mg/mL, 5.00 to 8.78 and 0.16 to 1.98 mg/mL in the honey, pollen and propolis samples, respectively [57].
Urease is a crucial enzyme for H. pylori to survive in an acidic environment of the stomach. Propolis extracts, which contain numerous polyphenolic compounds that have the ability to inhibit urease, can be considered a useful component of H. pylori eradication therapy.
3. Materials and Methods
3.1. Propolis and Chemicals
Propolis samples from the following region of Kazakhstan were collected in 2021: Almaty region (7 samples), Kogaly, Kegen and Bozone. Obtained raw propolis samples were frozen in liquid nitrogen and crushed in a mortar. Procedures were repeated three times. Before extraction, ground propolis was stored in sealed containers under −20 °C.
LiChrosolv® hyper grade eluents for uHPLC-MS/MS and uHPLC-DAD analysis (acetonitrile, water and methanol) were purchased from Merck company (Darmstad, Germany). Mueller-Hinton agar and Sabouraud agar were obtained from Oxoid (Hampshire, UK).
3.2. Preparation of Propolis Extracts (70EEPs)
Grounded research material was extracted by ethanol in water (70:30; v/v) in proportion 1:10 (1.0 g of propolis sample per 10 mL of solution). Extraction was performed in an ultrasonic bath (Sonorex, Bandelin, Berlin, Germany). Extraction conditions were set on 20 °C for 45 min and 756 W (90% of ultrasound bath power). Obtained extracts were stored at room temperature for 12 h and then filtered through Whatman No. 10 paper (Cytiva, Marlborough, MA, USA).
3.3. UHPLC-DAD-MS/MS Profile of Propolis Extracts
uHPLC analyses were performed as the previously described [20] with a Thermo Scientific UltiMate 3000 system (Thermo Scientific™ Dionex™, Sunnyvale, CA, USA), coupled with an autosampler and DAD detector recording spectral data in the 200–600 nm range and monitoring at 280, 320 and 360 nm. uHPLC-MS/MS was carried out using Compact QqTOF MS/MS detector (Bruker, Darmstadt, Germany). MS detector was used in electrospray negative mode. Conditions of analysis were: ion source temperature was set to210 °C, nebulizer gas pressure to 2.0 bar, dry gas (nitrogen) flow 8.01 L/min and temperature to 210 °C. The capillary voltage was programmed to 4.5 kV. The collision energy was set to 8.0 eV. Internal calibration was obtained with a 10 mM solution of sodium formate. For ESI-MS/MS experiments, collision energy was set at 35.0 eV and nitrogen was used as collision gas. Scan range was set from 30 to 1300 m/z.
3.4. Determination of Antimicrobial Activity
The propolis extracts dissolved in dimethylo-sulfoxide (DMSO) were screened for antibacterial and antifungal activities by microdilution broth method according to both the European Committee on Antimicrobial Susceptibility Testing (EUCAST) (www.eucast.org (accessed on 3 January 2023) using Mueller–Hinton broth or RPMI with MOPS for growth of fungi as we described elsewhere [58,59]. Minimal inhibitory concentration (MIC) of the tested extracts were evaluated for the wide panel of the reference microorganisms, including Gram-negative bacteria (Escherichia coli ATCC 25922, Salmonella typhimurium ATCC14028, Klebsiella pneumoniae ATCC 13883, Pseudomonas aeruginosa ATCC 9027), Gram-positive bacteria (Staphylococcus aureus ATCC 25923, Staphylococcus aureus ATCC 43300, Staphylococcus epidermidis ATCC 12228, Micrococcus luteus ATCC 10240, Enterococcus faecalis ATCC 29212, Bacillus cereus ATCC 10876) and fungi (Candida albicans ATCC 10231, Candida parapsilosis ATCC 22019, Candida glabrata ATCC 90030). The sterile 96-well polystyrene microtitrate plates (Nunc, Roskilde, Denmark) were prepared by dispensing 100 µL of appropriate dilution of the tested extracts in broth medium per well by serial two-fold dilutions in order to obtain final concentrations of the tested extracts ranged from 0.0195 to 10 mg/mL The inocula were prepared with fresh microbial cultures in sterile 0.85% NaCl to match the turbidity of 0.5 McFarland standard were added to wells to obtain final density of 5 × 105 CFU/mL for bacteria and 5 × 104 CFU/mL for yeasts (CFU, colony forming units). After incubation (35 °C for 24 h), the MICs were assessed visually as the lowest concentration of the extracts showing complete growth inhibition of the reference microbial strains. Appropriate DMSO control (at a final concentration of 10%), a positive control (containing inoculum without the tested derivatives) and negative control (containing the tested derivatives without inoculum) were included on each microplate.
The MIC for H. pylori ATCC 43504 was determined using a two-fold microdilution method in MH broth with 7% of lysed horse blood at extract concentration ranging from 1000 to 1.95 mg/L with bacterial inocula of 3 McFarland standard. After incubation at 35 °C for 72 h under microaerophilic conditions (5% O2, 15% CO2, and 80% N2) the growth of H. pylori was visualized with the addition 10 µL of 0.04% resazurin. The MIC endpoint was recorded after 4 h incubation as the lowest concentration of extract that completely inhibits growth [55].
Minimal bactericidal concentration (MBC) or minimal fungicidal concentration (MFC) was obtained by culture of 5 mL from each well that showed through growth inhibition, from the last positive one, and from the growth control onto recommended agar plates. The plates were incubated at 35° for 24 h for all microorganisms but H. pylori which were incubated for 72 h in microaerophilic conditions.
The MBC/MFC was defined as the lowest concentration of extract without the growth of microorganisms. The MBC/MIC ratios were calculated to determine the bactericidal or bacteriostatic effect of the assayed extract. Vancomycin, clarithromycin, ciprofloxacin and nystatin were used as the reference drugs appropriate for different group of microorganisms.
The experiments were repeated in triplicate. Representative data are presented.
3.5. Urease Inhibitory Assay
In short, H. pylori were incubated for 72 h in the MH broth with 7% of horse serum (Sigma-Merk, Saint Louis, Missouri, USA) in microaerophilic conditions. Bacterial biomass was collected by centrifugation at 4000× g at 4 °C for 10 min, then the cells were dissolved in ice-cold phosphate buffer (pH 7.3) with a protease inhibitor cocktail (Sigma). The urease enzyme was prepared by disturbing H. pylori cells by sonication, followed by centrifugation at 12,000× g at 4 °C for 10 min.
Initial urease inhibitory activity of all the obtained extracts were evaluated at the concentration of 2 mg/mL with the modified Berthelot spectrophotometric method with phenol–hypochlorite reaction at the absorbance of 570 nm. The enzyme reaction was activated in 96-well plates by mixing the appropriate volume of 2% urea, sodium phosphate buffer solution (100 µL), different concentrations (2000–3.9 µg/mL) of propolis extract, and the reaction mixture was incubated for 15 min at 37 °C, then the concentration of ammonia was determined using the Berthelot method. The amount of the ammonia is equivalent to the hydrolysis of urea using the urease enzyme. The experiments were performed in triplicate. Activity of uninhibited urease was chosen as the control activity of 100% [60]. Inhibition rate (%) was calculated following the formula: I% = (1 − average with inhibitors/average activity without inhibitors) × 100%. The IC50 was expressed as the concentration of inhibitor that decreased urease activity by 50% and calculated by plotting the percent of inhibition using the internet IC50 Calculator (AAT Bioquest).
3.6. Statistical Analysis
Statistical analysis was performed by Statistica 14.0.0.5 software (Tibco Sofware Inc., Palo Alto, CA, USA). Analysis included hierarchical fuzzy clustering trees (dendrogram) from the prepared matrix. It was composed of % of UV chromatograms (280 nm) relatively peak area. Substances about at least 1% of relatively area (in any sample) were used to construct matrix.
4. Conclusions
The phytochemical profile and activity against 15 microorganisms 70EEP from ten propolis samples collected in Kazakhstan were evaluated. This is the first wider study on Kazakh propolis extracts phytochemical composition and the antimicrobial potential. Tested extracts exhibited good activity against Gram-positive bacteria, fungal species (yeasts) and H. pylori (the only Gram-negative bacterium sensitive to the tested propolis). In addition, bioactivity tests were conducted for urease inhibition. Propolis from Kazakhstan seems to belong to the poplar type, but analysis of the chemical composition showed the presence of polyphenolic compounds from other plant sources (especially aspen) which requires further research. Dependences between their plant origin and activity was ambiguous. This may be caused be specific chemotype of Kazakstani Populus species or presence additional, unknown plant precursors. An attempt of the isolation the active components from tested propolis samples should be performed in the future, in order to study more the origin of propolis and its various biological activities.
Author Contributions
Conceptualization, J.W., P.O., A.M. and I.K.-G.; methodology, J.W., P.O. and I.K.-G.; software, J.W. and P.O.; validation, J.W., P.O. and I.K.-G.; formal analysis, J.W., P.O. and K.S.; investigation, J.W., K.S. and P.O.; resources, A.S., G.Z., G.I. and Z.S.; data curation, P.O. and I.K.-G.; writing—original draft preparation, J.W. and P.O.; writing—review and editing, P.O., E.P., A.M., Z.S. and I.K.-G.; visualization, J.W. and P.O.; supervision, A.M. and I.K.-G.; project administration, J.W. and P.O.; funding acquisition, P.O., I.K.-G. and A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Medical University of Lublin (DS no. 29 and DS no. 27) and Wroclaw Medical University (grant number SUBZ.D110.23.029 and SUBZ.A130.23.070).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the authors.
Acknowledgments
uHPLC-DAD and uHPLC-MS/MS analyses were carried out in the Laboratory of Elemental Analysis and Structural Research, Faculty of Pharmacy, Wroclaw Medical University. We thank Hanna Czapor-Irzabek from Laboratory for Elemental Analysis and Structural Research for assistance with uHPLC-MS/MS analyses.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Suleman, T.; van Vuuren, S.; Sandasi, M.; Viljoen, A.M. Antimicrobial activity and chemometric modelling of South African propolis. J. Appl. Microbiol. 2015, 119, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Righi, A.A.; Alves, T.R.; Negri, G.; Marques, L.M.; Breyer, H.; Salatino, A. Brazilian red propolis: Unreported substances, antioxidant and antimicrobial activities. J. Sci. Food Agric. 2011, 91, 2363–2370. [Google Scholar] [CrossRef] [PubMed]
- Regueira, M.S.; Tintino, S.R.; da Silva, A.R.P.; Costa, M.D.S.; Boligon, A.A.; Matias, E.F.F.; de Queiroz Balbino, V.; Menezes, I.R.A.; Melo Coutinho, H.D. Seasonal variation of Brazilian red propolis: Antibacterial activity, synergistic effect and phytochemical screening. Food Chem. Toxicol. 2017, 107, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.O.; Andrade, V.M.; Bullé Rêgo, E.S.; Azevedo Dória, G.A.; Santos Lima, B.D.; da Silva, F.A.; de Souza Araújo, A.A.; de Albuquerque Júnior, R.L.C.; Cordeiro Cardoso, J.; Zanardo Gomes, M. Acute and sub-acute oral toxicity of Brazilian red propolis in rats. J. Ethnopharmacol. 2015, 170, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Sforcin, J.M.; Bankova, V. Propolis: Is there a potential for the development of new drugs? J. Ethnopharmacol. 2011, 133, 253–260. [Google Scholar] [CrossRef]
- Graikou, K.; Popova, M.; Gortzi, O.; Bankova, V.; Chinou, I. Characterization and biological evaluation of selected Mediterranean propolis samples. Is it a new type? LWT Food Sci. Technol. 2016, 65, 261–267. [Google Scholar] [CrossRef]
- Melliou, E.; Chinou, I. Chemical analysis and antimicrobial activity of Greek propolis. Planta Med. 2004, 70, 515–519. [Google Scholar] [CrossRef]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid. Based Complement. Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef]
- Mouse, H.A.; Tilaoui, M.; Jaafari, A.; M’barek, L.A.; Aboufatima, R.; Chait, A.; Zyad, A. Evaluation of the in vitro and in vivo anticancer properties of Moroccan propolis extracts. Rev. Bras. Farmacogn. 2012, 22, 558–567. [Google Scholar] [CrossRef]
- Popova, M.P.; Bankova, V.S.; Bogdanov, S.; Tsvetkova, I.; Naydenski, C.; Marcazzan, G.L.; Sabatini, A.G. Chemical characteristics of poplar type propolis of different geographic origin. Apidologie 2007, 38, 306. [Google Scholar] [CrossRef]
- Cavalcante, G.M.; Camara, C.A.; Da Silva, E.M.S.; Santos, M.S.; Leite, A.B.; Queiroz, A.C.; Evelyn Da Silva, A.; Araújo, M.V.; Alexandre-Moreira, M.S.; Silva, T.M.S. Leismanicidal Activity of Propolis Collected in the Semiarid Region of Brazil. Front. Pharmacol. 2021, 12, 702032. [Google Scholar] [CrossRef]
- Da Rosa, C.; Bueno, I.L.; Quaresma, A.C.M.; Longato, G.B. Healing Potential of Propolis in Skin Wounds Evidenced by Clinical Studies. Pharmaceuticals 2022, 15, 1143. [Google Scholar] [CrossRef]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent Update on the Anti-Inflammatory Activities of Propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef]
- Araujo, M.A.R.; Libério, S.A.; Guerra, R.N.M.; Ribeiro, M.N.S.; Nascimento, F.R.F. Mechanisms of action underlying the anti-inflammatory and immunomodulatory effects of propolis: A brief review. Rev. Bras. Farmacogn. 2012, 22, 208–219. [Google Scholar] [CrossRef]
- Bittencourt, M.L.F.; Ribeiro, P.R.; Franco, R.L.P.; Hilhorst, H.W.M.; de Castro, R.D.; Fernandez, L.G. Metabolite profiling, antioxidant and antibacterial activities of Brazilian propolis: Use of correlation and multivariate analyses to identify potential bioactive compounds. Food Res. Int. 2015, 76, 449–457. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A Complex Natural Product with a Plethora of Biological Activities That Can Be Explored for Drug Development. Evid. Based Complement. Altern. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Krzyżek, P.; Paluch, E.; Gościniak, G. Synergistic Therapies as a Promising Option for the Treatment of Antibiotic-Resistant Helicobacter pylori. Antibiotics 2020, 9, 658. [Google Scholar] [CrossRef]
- Widelski, J.; Okińczyc, P.; Paluch, E.; Mroczek, T.; Szperlik, J.; Żuk, M.; Sroka, Z.; Sakipova, Z.; Chinou, I.; Skalicka-Woźniak, K.; et al. The antimicrobial properties of poplar and aspen–poplar propolises and their active components against selected microorganisms, including Helicobacter pylori. Pathogens 2022, 11, 191. [Google Scholar] [CrossRef]
- Okińczyc, P.; Widelski, J.; Szperlik, J.; Żuk, M.; Mroczek, T.; Skalicka-Woźniak, K.; Sakipova, Z.; Widelska, G.; Kuś, P.M. Impact of plant origin on Eurasian propolis on phenolic profile and classical antioxidant activity. Biomolecules 2021, 11, 68. [Google Scholar] [CrossRef]
- Okińczyc, P.; Widelski, J.; Ciochoń, M.; Paluch, E.; Bozhadze, A.; Jokhadze, M.; Mtvarelishvili, G.; Korona-Głowniak, I.; Krzyżanowska, B.; Kuś, P.M. Phytochemical profile, plant precursors and some properties of Georgian propolis. Molecules 2022, 27, 7714. [Google Scholar] [CrossRef] [PubMed]
- Svečnjak, L.; Marijanović, Z.; Okińczyc, P.; Kuś, P.M.; Jerković, I. Mediterranean propolis from the adriatic sea islands as a source of natural antioxidants: Comprehensive chemical biodiversity determined by GC-MS, ftiratr, UHPLC-DAD-QQTOF-MS, DPPH and FRAP assay. Antioxidants 2020, 9, 337. [Google Scholar] [CrossRef] [PubMed]
- EUFORGEN—The European Forest Genetic Resources Programme. Populus nigra European Black Poplar. Available online: https://www.euforgen.org/species/populus-nigra/ (accessed on 11 January 2023).
- EUFORGEN—The European Forest Genetic Resources Programme. Populus tremula Eurasian Aspen. Available online: https://www.euforgen.org/species/populus-tremula/ (accessed on 11 January 2023).
- Asakawa, Y.; Takemoto, T.; Wollenweber, E.; Aratani, T. Lasiocarpin A, B and C, three novel phenolic triglycerides from Populus lasiocarpa. Phytochemistry 1977, 16, 1791–1795. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Szczepaniak, L.; Bakier, S. Rapid gc/ms determination of botanical precursors of eurasian propolis. Food Chem. 2014, 142, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Okinczyc, P.; Szumny, A.; Szperlik, J.; Kulma, A.; Franiczek, R.; Zbikowska, B.; Krzyzanowska, B.; Sroka, Z. Profile of polyphenolic and essential oil composition of polish propolis, black poplar and aspens buds. Molecules 2018, 23, 1262. [Google Scholar] [CrossRef] [PubMed]
- Ozolin, G.P. Cultivation of Poplars under Conditions of Artificial Irrigatio; Lesnaya Promyslennost: Moscow, Russia, 1966. [Google Scholar]
- Thevs, N.; Fehrenz, S.; Aliev, K.; Emileva, B.; Fazylbekov, R.; Kentbaev, Y.; Qonunov, Y.; Qurbonbekova, Y.; Raissova, N.; Razhapbaev, M.; et al. Growth Rates of Poplar Cultivars across Central Asia. Forests 2021, 12, 373. [Google Scholar] [CrossRef]
- Zhou, J.; Xue, X.; Li, Y.; Zhang, J.; Chen, F.; Wu, L.; Chen, L.; Zhao, J. Multiresidue determination of tetracycline antibiotics in propolis by using HPLC-UV detection with ultrasonic-assisted extraction and two-step solid phase extraction. Food Chem. 2009, 115, 1074–1080. [Google Scholar] [CrossRef]
- Roman, A.; Madras-Majewska, B.; Popiela, E. Comparative study of selected toxic elements in propolis and honey. J. Apic. Sci. 2011, 55, 97–106. [Google Scholar]
- O’Donnell, F.; Smyth, T.J.P.; Ramachandran, V.N.; Smyth, W.F. A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents 2010, 35, 30–38. [Google Scholar] [CrossRef]
- Rewak-Soroczyńska, J.; Paluch, E.; Siebert, A.; Szałkiewicz, K.; Obłąk, E. Biological activity of glycine and alanine derivatives of quaternary ammonium salts (QASs) against micro-organisms. Lett. Appl. Microbiol. 2019, 69, 212–220. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- Shapla, U.M.; Raihan, J.; Islam, A.; Alam, F.; Solayman, N.; Gan, S.H.; Hossen, S.; Khalil, I. Propolis: The future therapy against Helicobacter pylori-mediated gastrointestinal diseases. J. Appl. Biomed. 2018, 16, 81–99. [Google Scholar] [CrossRef]
- Popova, M.; Giannopoulou, E.; Skalicka-Wózniak, K.; Graikou, K.; Widelski, J.; Bankova, V.; Kalofonos, H.; Sivolapenko, G.; Gaweł-Bȩben, K.; Antosiewicz, B.; et al. Characterization and biological evaluation of propolis from Poland. Molecules 2017, 22, 1159. [Google Scholar] [CrossRef]
- Uzel, A.; Sorkun, K.; Önçağ, Ö.; Çoğulu, D.; Gençay, Ö.; Sali, H.B. Chemical compositions and antimicrobial activities of four different Anatolian propolis samples. Microbiol. Res. 2005, 160, 189–195. [Google Scholar] [CrossRef]
- Grecka, K.; Kuś, P.M.; Okińczyc, P.; Worobo, R.W.; Walkusz, J.; Szweda, P. The anti-staphylococcal potential of ethanolic Polish propolis extracts. Molecules 2019, 24, 1732. [Google Scholar] [CrossRef]
- Machado, B.A.S.; Silva, R.P.D.; de Barreto, G.A.; Costa, S.S.; da Silva, D.F.; Brandão, H.N.; da Rocha, J.L.C.; Dellagostin, O.A.; Henriques, J.A.P.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Coll, F.V.; Jordà, R.E. The composition, active components and bacteriostatic activity of propolis in dietetics. J. Am. Oil Chem. Soc. 1994, 71, 529–532. [Google Scholar] [CrossRef]
- Widelski, J.; Okińczyc, P.; Suśniak, K.; Malm, A.; Bozhadze, A.; Jokhadze, M.; Korona-Głowniak, I. Correlation between Chemical Profile of Georgian Propolis Extracts and Their Activity against Helicobacter pylori. Molecules 2023, 28, 1374. [Google Scholar] [CrossRef]
- Santiago, M.B.; Leandro, L.F.; Rosa, R.B.; Silva, M.V.; Teixeira, S.C.; Servato, J.P.S.; Ambrósio, S.R.; Veneziani, R.C.S.; Aldana-Mejía, J.A.; Bastos, J.K.; et al. Brazilian Red Propolis Presents Promising Anti-H. pylori Activity in In Vitro and In Vivo Assays with the Ability to Modulate the Immune Response. Molecules 2022, 27, 7310. [Google Scholar] [CrossRef]
- Bonvehí, J.S.; Gutiérrez, A.L. The antimicrobial effects of propolis collected in different regions in the Basque Country (Northern Spain). World J. Microbiol. Biotechnol. 2012, 28, 1351–1358. [Google Scholar] [CrossRef]
- Ratnasari, N.; Rezkitha, Y.A.A.; Adnyana, I.K.; Alfaray, R.I.; Fauzia, K.A.; Doohan, D.; Panjaitan, A.; Priskila, Y.; Yulinah, E.; Khomsan, A.; et al. Anti-Helicobacter pylori effects of propolis ethanol extract on clarithromycin and metronidazole resistant strains. Sys. Rev. Pharm. 2020, 11, 429–434. [Google Scholar]
- Romero, M.; Freire, J.; Pastene, E.; García, A.; Aranda, M.; González, C. Propolis polyphenolic compounds affect the viability and structure of Helicobacter pylori in vitro. Rev. Bras. Farmacogn. 2019, 29, 325–332. [Google Scholar] [CrossRef]
- Popova, M.P.; Chinou, I.B.; Marekov, I.N.; Bankova, V.S. Terpenes with antimicrobial activity from Cretan propolis. Phytochemistry 2009, 70, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Okińczyc, P.; Paluch, E.; Franiczek, R.; Widelski, J.; Wojtanowski, K.K.; Mroczek, T.; Krzyżanowska, B.; Skalicka-Woźniak, K.; Sroka, Z. Antimicrobial activity of Apis mellifera L. and Trigona sp. propolis from Nepal and its phytochemical analysis. Biomed. Pharmacother. 2020, 129, 110435. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, W.; Tang, F.; Chen, X.; Ren, L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr. Med. Chem. 2014, 22, 132–149. [Google Scholar] [CrossRef] [PubMed]
- Freires, I.A.; de Alencar, S.M.; Rosalen, P.L. A pharmacological perspective on the use of Brazilian red propolis and its isolated compounds against human diseases. Eur. J. Med. Chem. 2016, 110, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Isidorov, V.A.; Bakier, S.; Pirożnikow, E.; Zambrzycka, M.; Swiecicka, I. Selective behaviour of honeybees in acquiring European propolis plant precursors. J. Chem. Ecol. 2016, 42, 475–485. [Google Scholar] [CrossRef]
- Okińczyc, P. Phytochemical Characteristics and Biological Activity of Different Kinds of Propolis and Their Plant Sources [Title in Polish: Charakterystyka Fitochemiczna Oraz Aktywność Biologiczna Różnych Rodzajów Propolisów Oraz ich Źródeł Roślinnych]. Ph.D. Thesis, Uniwersytet Medyczny we Wrocławiu, Wrocław, Poland, 2017. [Google Scholar]
- Vardar-Ünlü, G.; Silici, S.; Ünlü, M. Composition and in vitro antimicrobial activity of Populus buds and poplar-type propolis. World J. Microbiol. Biotechnol. 2008, 24, 1011–1017. [Google Scholar] [CrossRef]
- Baltas, N.; Karaoglu, S.A.; Tarakci, C.; Kolayli, S. Effect of propolis in gastric disorders: Inhibition studies on the growth of Helicobacter pylori and production of its urease. J. Enzym. Inhib. Med. Chem. 2016, 31, 46–50. [Google Scholar] [CrossRef]
- Korona-Glowniak, I.; Glowniak-Lipa, A.; Ludwiczuk, A.; Baj, T.; Malm, A. The in vitro activity of essential oils against Helicobacter Pylori growth and urease activity. Molecules 2020, 25, 586. [Google Scholar] [CrossRef] [PubMed]
- Can, Z.; Kara, Y.; Kolayli, S.; Çakmak, İ. Determination of anti-urease activity of propolis from the Marmara region of Turkey. Uludağ Arıcılık Derg. 2022, 22, 25–30. [Google Scholar] [CrossRef]
- Can, Z. Determination of in-vitro antioxidant, anti-urease, anti-hyaluronidase activities by phenolic rich bee products from different region of turkey. Fresenius Environ. Bull. 2018, 27, 6858–6866. [Google Scholar]
- The European Committee on Antimicrobial Susceptibility Testing—EUCAST. Available online: https://www.eucast.org/ (accessed on 10 October 2022).
- Paluch, E.; Okińczyc, P.; Zwyrzykowska-Wodzińska, A.; Szperlik, J.; Żarowska, B.; Duda-Madej, A.; Bąbelewski, P.; Włodarczyk, M.; Wojtasik, W.; Kupczyński, R.; et al. Composition and Antimicrobial Activity of Ilex Leaves Water Extracts. Molecules 2021, 26, 7442. [Google Scholar] [CrossRef]
- Mahernia, S.; Bagherzadeh, K.; Mojab, F.; Amanlou, M. Urease inhibitory activities of some commonly consumed herbal medicines. Iran. J. Pharm. Res. IJPR 2015, 14, 943–947. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).