Design and Properties of Natural Rosin-Based Phosphoester Functional Surfactants
Abstract
:1. Introduction
2. Results and Discussion
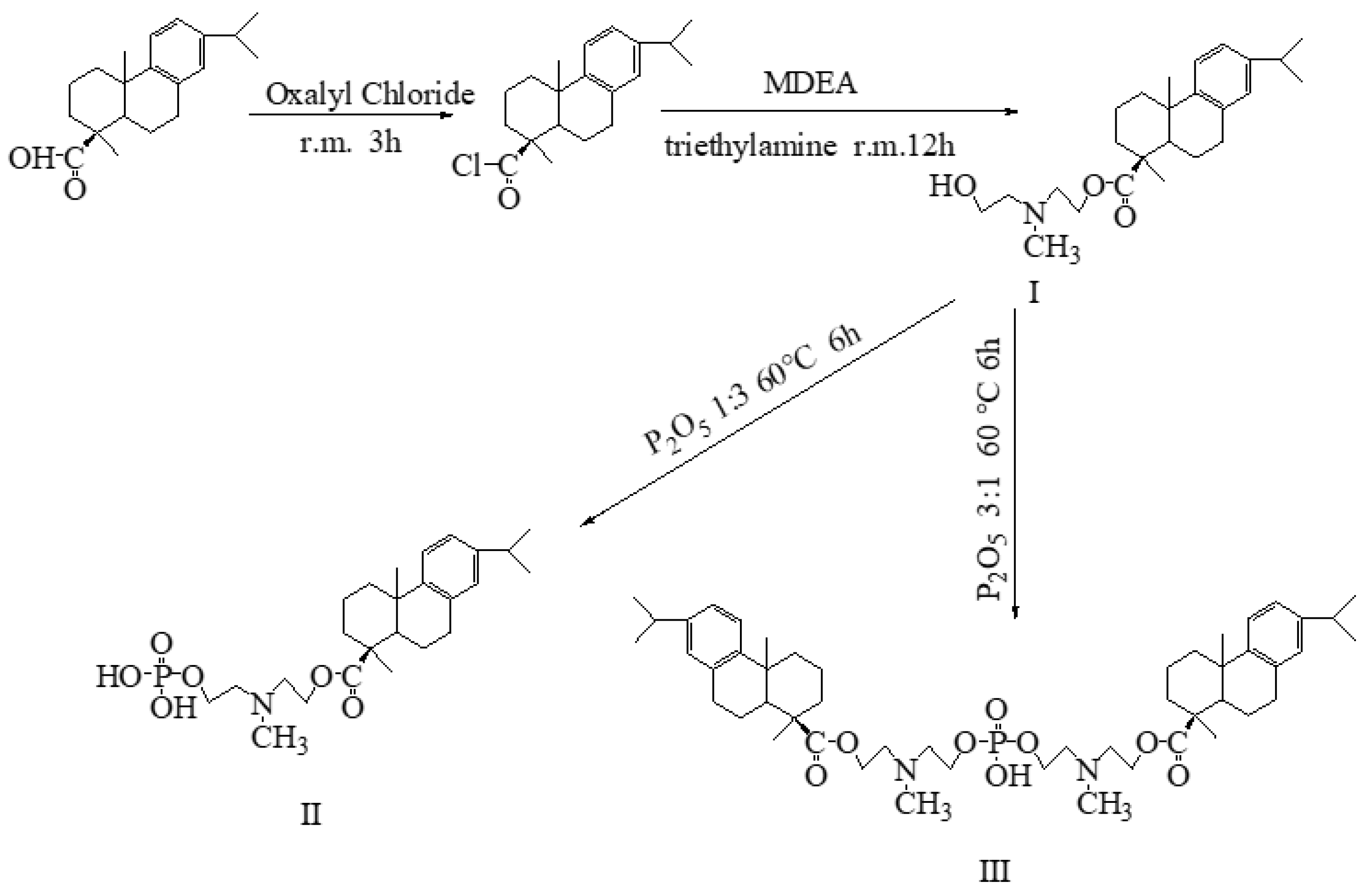
2.1. Chemical Structure
2.2. NMR Analysis
2.2.1. NMR of DPM
2.2.2. NMR of DPD
2.3. Influence of Synthesis Conditions
2.4. Surface Properties
2.5. Self-Assembly Behavior of Micelles in an Aqueous Solution
2.6. Foam Performance
2.7. Emulsification Performance
2.8. Hydrophilic and Lipophilic Balance Value (HLB)
3. Experimental Section
3.1. Main Raw Materials, Reagents and Instruments
3.2. Synthesis Method
3.2.1. Preparation of Rosin-Based Phosphorus Monoester (DPM)
3.2.2. Preparation of Rosin-Based Phosphodiester (DPD)
3.3. Performance Test
3.3.1. FTIR Measurement
3.3.2. NMR Analysis
3.3.3. Hydrophilic and Lipophilic Balance Value (HLB)
3.3.4. Other Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhai, Z.; Ye, S.; Yan, X.; Song, Z.; Shang, S.; Rao, X.; Song, J. pH-Responsive Wormlike Micelles Formed by an Anionic Surfactant Derived from Rosin. J. Agric. Food Chem. 2020, 68, 10063–10070. [Google Scholar] [CrossRef]
- Li, W.; Xie, D.; Song, B.; Feng, L.; Pei, X.; Cui, Z. Synthesis and characterization of ordered mesoporous silica using rosin-based Gemini surfactants. J. Mater. Sci. 2017, 53, 2434–2442. [Google Scholar] [CrossRef]
- Sun, H.; Ma, X.; Fei, L.; Cao, Z.; Zhong, H.; Wang, S. Amide group enhanced self-assembly and adsorption of dicarboxylic amino acid surfactants on a rhodochrosite surface through intermolecular weak interaction. Sep. Purif. Technol. 2023, 309, 122905. [Google Scholar] [CrossRef]
- Gonçalves, R.A.; Holmberg, K.; Lindman, B. Cationic Surfactants: A Review. J. Mol. Liq. 2023, 375, 121335. [Google Scholar] [CrossRef]
- Wang, H.; Tan, B.; Wang, J.; Li, Z.; Zhang, S. Anion-Based pH Responsive Ionic Liquids: Design, Synthesis, and Reversible Self-Assembling Structural Changes in Aqueous Solution. Langmuir 2014, 30, 3971–3978. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhang, L.; Song, J.; Jian, G.; Hirasaki, G.; Johnston, K.; Biswal, S.L. Two-step adsorption of a switchable tertiary amine surfactant measured using a quartz crystal microbalance with dissipation. Langmuir 2019, 35, 695–701. [Google Scholar] [CrossRef]
- Lin, H.X.; Yang, M.S.; Tian, C.; Han, C.R.; Song, J.; Duan, J.F.; Jiang, J.X. Design of diversified self-assembly systems based on a natural rosin-based tertiary amine for doxorubicin delivery and excellent emulsification. Colloids Surf. B Biointerfaces 2018, 165, 191–198. [Google Scholar] [CrossRef]
- Tian, C.; Liang, Y.; Lin, H.; Song, J.; Li, Q.; Li, R.; Han, C. Surface properties and doxorubicin delivery in mixed systems comprising a natural rosin-based ester tertiary amine and an anionic surfactant. J. Dispers. Sci. Technol. 2018, 40, 892–900. [Google Scholar]
- Andrada, H.E.; Silva, O.F.; Morales, G.M.; Correa, N.M.; Falcone, R.D. Spontaneous formation of unilamellar vesicles based on the surfactant 1-methylimidazolium bis-(2-ethylhexyl) phosphate, evaluated as a function of pH and in saline solution. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606, 125435. [Google Scholar] [CrossRef]
- Ma, G.; Wang, C.; Du, C.; Li, X.; Wang, X. A corrosion-resistance waterborne polyacrylate coatings based on novel phosphate esters polymeric surfactant. J. Appl. Polym. Sci. 2022, 139, 52267. [Google Scholar] [CrossRef]
- Li, J.; Lin, H.X.; Chen, X.Y.; Zhu, J.R.; Yang, M.S.; Yang, J.; Han, C.R. Self-assembled structures and excellent surface properties of a novel anionic phosphate diester surfactant derived from natural rosin acids. J. Colloid Interface Sci. 2017, 486, 67–74. [Google Scholar] [CrossRef]
- Yang, M.S.; Tian, C.; Han, C.R.; Zhao, G.Z. Hierarchical self-assembled hollow hydroxyapatite flower microspheres containing terpene functional groups for efficient drug loading and pH-responsive drug release. Ceram. Int. 2018, 44, 20913–20920. [Google Scholar] [CrossRef]
- Meng, X.; Ye, Q.; Pan, Q.; Ding, Y.; Wei, M.; Liu, Y.; van de Voort, F.R. Total Phospholipids in Edible Oils by In-Vial Solvent Extraction Coupled with FTIR Analysis. J. Agric. Food Chem. 2014, 62, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Font, J.; Salvadó, N.; Butí, S.; Enrich, J. Fourier transform infrared spectroscopy as a suitable technique in the study of the materials used in waterproofing of archaeological amphorae. Anal. Chim. Acta 2007, 598, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Jin, Y.; Fan, B.; Qi, R.; Cheng, X.; Peng, S. Synthesis and surface activity of mono- and diphosphate ester mixture with different alkyl chain length. J. Dispers. Sci. Technol. 2016, 38, 704–711. [Google Scholar] [CrossRef]
- Hirschmann, R.; Yager, K.M.; Taylor, C.M.; Witherington, J.; Sprengeler, P.A.; Phillips, B.W.; Moore, W.; Smith, A.B. Phosphonate Diester and Phosphonamide Synthesis. Reaction Coordinate Analysis by 31P NMR Spectroscopy: Identification of Pyrophosphonate Anhydrides and Highly Reactive Phosphonylammonium Salts1. J. Am. Chem. Soc. 1997, 119, 8177–8190. [Google Scholar]
- O’Day, P.A.; Nwosu, U.G.; Barnes, M.E.; Hart, S.C.; Berhe, A.A.; Christensen, J.N.; Williams, K.H. Phosphorus Speciation in Atmospherically Deposited Particulate Matter and Implications for Terrestrial Ecosystem Productivity. Environ. Sci. Technol. 2020, 54, 4984–4994. [Google Scholar] [CrossRef]
- Jarlbring, M.; Sandström, D.E.; Antzutkin, O.N.; Forsling, W. Characterization of active phosphorus surface sites at synthetic carbonate-free fluorapatite using single-pulse 1H, 31P, and 31P CP MAS NMR. Langmuir 2006, 22, 4787–4792. [Google Scholar] [CrossRef] [PubMed]
- Tu, F.; Lee, D. Shape-Changing and Amphiphilicity-Reversing Janus Particles with pH-Responsive Surfactant Properties. J. Am. Chem. Soc. 2014, 136, 9999–10006. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Sanada, Y.; Nishimura, T.; Akiba, I.; Sakurai, K.; Yagi, N.; Mylonas, E. A Stimulus-Responsive Shape-Persistent Micelle Bearing a Calix 4 arene Building Block: Reversible pH-Dependent Transition between Spherical and Cylindrical Forms. Langmuir 2012, 28, 3092–3101. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Kang, W.; Yang, H.; Yin, X.; Zhao, Y.; Zhu, Z.; Zhang, X. pH-Responsive wormlike micelles based on microstructural transition in a C22-tailed cationic surfactan” aromatic dibasic acid system. RSC Adv. 2017, 7, 37699–37705. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.K.; Mitra, S.; Mukhopadhyay, R. Dynamics of Molecular Species in Confined Geometry. J. Phys. Soc. Jpn. 2013, 82, SA006. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kondo, Y.; Schmidt, J.; Talmon, Y. Self-assembly of a fluorocarbon-hydrocarbon hybrid surfactant: Dependence of morphology on surfactant concentration and time. J. Phys. Chem. B 2010, 114, 13319–13325. [Google Scholar] [CrossRef] [PubMed]
- Fuller, S.; Shinde, N.N.; Tiddy, G.J.; Attard, G.S.; Howell, O. Thermotropic and Lyotropic Mesophase Behavior of Amphitropic Diammonium Surfactants. Langmuir 1996, 12, 1117–1123. [Google Scholar] [CrossRef]
- Patist, A.; Huibers, P.D.T.; Deneka, B.; Shah, D.O. Effect of Tetraalkylammonium Chlorides on Foaming Properties of Sodium Dodecyl Sulfate Solutions. Langmuir 1998, 14, 4471–4474. [Google Scholar] [CrossRef]
- Berton, C.; Genot, C.; Ropers, M.-H. Quantification of unadsorbed protein and surfactant emulsifiers in oil-in-water emulsions. J. Colloid Interface Sci. 2011, 354, 739–748. [Google Scholar] [CrossRef]
- Berton, C.; Ropers, M.-H.; Bertrand, D.; Viau, M.; Genot, C. Oxidative stability of oil-in-water emulsions stabilised with protein or surfactant emulsifiers in various oxidation conditions. Food Chem. 2011, 131, 1360–1369. [Google Scholar] [CrossRef]
- Björkegren, S.M.S.; Dias, M.C.A.F.; Lundahl, K.; Nordstierna, L.; Palmqvist, A.E.C. Phase inversions observed in thermo-responsive Pickering emulsions stabilized by surface functionalized, colloidal silica. Langmuir 2020, 36, 2357–2367. [Google Scholar] [CrossRef]
- Speranza, A.; Corradini, M.G.; Hartman, T.G.; Ribnicky, D.; Oren, A.; Rogers, M.A. Influence of emulsifier structure on lipid bioaccessibility in oil-water nanoemulsions. J. Agric. Food Chem. 2013, 61, 6505–6515. [Google Scholar] [CrossRef]
- Pasquali, R.C.; Taurozzi, M.P.; Bregni, C. Some considerations about the hydrophilic–lipophilic balance system. Int. J. Pharm. 2008, 356, 44–51. [Google Scholar] [CrossRef]






| Sample Number | P2O5 (mol) | DDAM (mol) | Temperature Reflex (°C) | Reaction Time (h) |
|---|---|---|---|---|
| 1 | 1 | 3 | 60 | 6 |
| 2 | 1 | 3 | 70 | 6 |
| 3 | 1 | 3 | 80 | 6 |
| 4 | 3 | 1 | 70 | 6 |
| 5 | 3 | 1 | 60 | 6 |
| pH | 1.42 | 3.04 | 6.04 | 9.06 | 10.08 |
|---|---|---|---|---|---|
| γCMC−DPM (mN·m−1) | 37.782 | 39.57 | 40.987 | 44.21 | 43.104 |
| CMC−DPM (mmol·L−1) | 2.166 | 1.25 | 1.657 | 1.202 | 5.964 |
| γCMC−DPD (mN·m−1) | 36.536 | 37.412 | 44.379 | 41.805 | 41.911 |
| CMC−DPD (mmol·L−1) | 0.631 | 0.498 | 0.612 | 0.199 | 0.321 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Yang, X.; Han, B.; Zhang, S.; Han, C.; Xia, C. Design and Properties of Natural Rosin-Based Phosphoester Functional Surfactants. Molecules 2023, 28, 3091. https://doi.org/10.3390/molecules28073091
Wang M, Yang X, Han B, Zhang S, Han C, Xia C. Design and Properties of Natural Rosin-Based Phosphoester Functional Surfactants. Molecules. 2023; 28(7):3091. https://doi.org/10.3390/molecules28073091
Chicago/Turabian StyleWang, Maogong, Xiaofang Yang, Bing Han, Shifeng Zhang, Chunrui Han, and Changlei Xia. 2023. "Design and Properties of Natural Rosin-Based Phosphoester Functional Surfactants" Molecules 28, no. 7: 3091. https://doi.org/10.3390/molecules28073091






