Structural Insights and Intermolecular Energy for Some Medium and Long-Chain Testosterone Esters
Abstract
:1. Introduction
- (i)
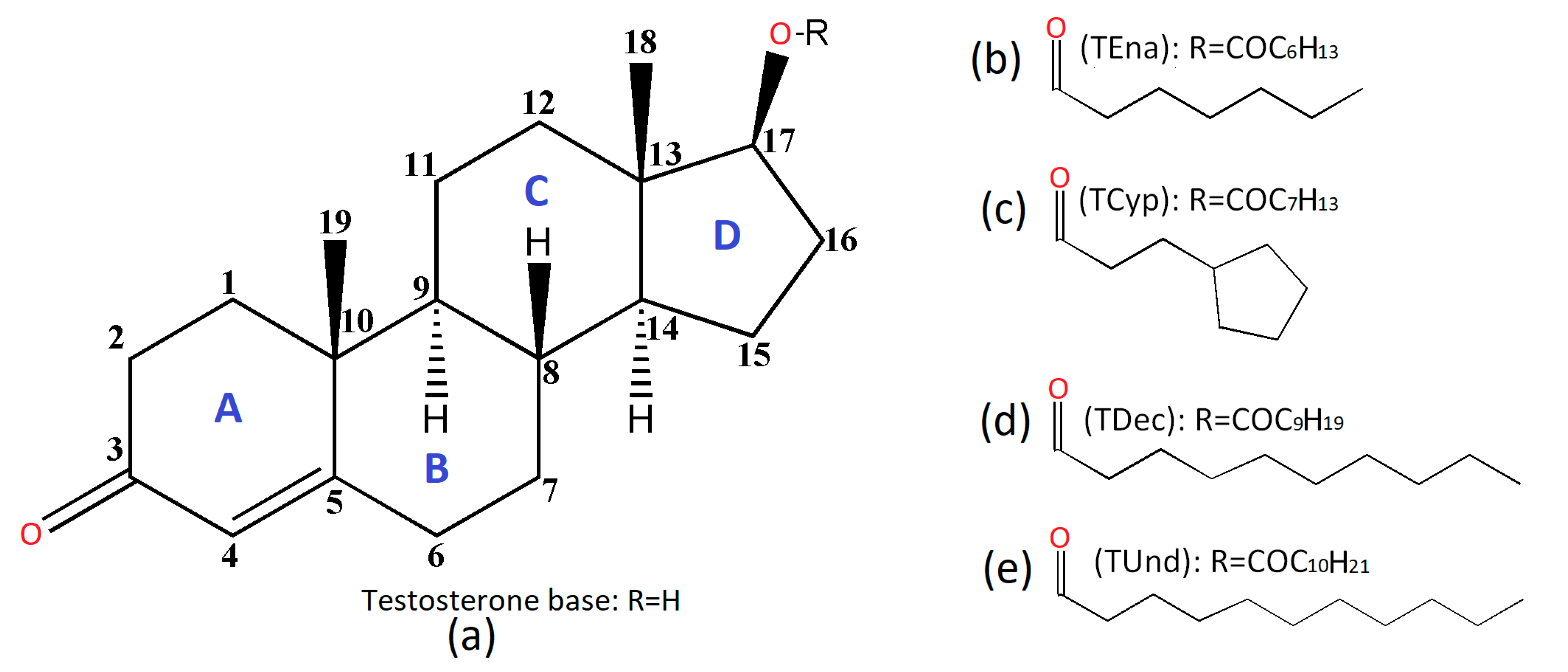
- Testosterone enanthate: Androst-4-en-17β-ol-3-one 17β-heptanoate, (TEna, Figure 1b);
- (ii)
- Testosterone cypionate: Androst-4-en-17β-ol-3-one 17β-cyclopentylpropionate, (TCyp, Figure 1c);
- (iii)
- Testosterone decanoate: Androst-4-en-17β-ol-3-one 17β-decanoate, (TDec, Figure 1d);
- (iv)
- Testosterone undecanoate: Androst-4-en-17β-ol-3-one 17β-undecanoate, (TUnd, Figure 1e).
2. Results
2.1. Crystal Structures Analysis
2.1.1. TEna (Testosterone Enanthate)
2.1.2. TCyp (Testosterone Cypionate)
2.1.3. TDec (Testosterone Decanoate)
2.1.4. TUnd (Testosterone Undecanoate)
- (i)
- All four esters crystallize in various non-centrosymmetric monoclinic and orthorhombic space groups;
- (ii)
- (iii)
- Although C-H⋯O interactions participate in the formation of supramolecular 3D assemblies, their weight is quite small compared to dispersion effects (see the crystal energies analysis section);
- (iv)
- C-H⋯O bonds are characterized by hydrogen⋯carbonyl donor-acceptor distances (Table S3, Supplementary Materials), which fall into the same range as other structures of the steroid class [26,27,28,29];
- (v)
- The six membered A rings depict an intermediate sofa-half-chair conformation, B and C exhibit chair geometry, and the five membered D backbone rings display an intermediate envelope-half-chair geometry. The C17 methylated form [30] and other short esters have been shown to possess similar geometries [16,18].
2.2. Crystal Lattice Energies Evaluation
2.3. Intermolecular Energies Evaluation
- (i)
- Since the crystalline structures do not possess classic strong hydrogen bonds but C-H⋯O interactions, the electrostatic terms have small values in all cases;
- (ii)
- The values of the polarization energies are very low, which indicates that the molecules are not polarized;
- (iii)
- The values of the total energies are relatively low and vary in a wide range (−10.4 to −60.8 kJ/mol) due to the different orientations of the molecules relative to each other; between the neighboring molecules, there are rather weak interactions due to the lack of strong hydrogen bonds;
- (iv)
- Table S4 (Supplementary Materials) shows that the dispersion energy is dominant.
2.4. Hirshfeld Surfaces and Fingerprint Plots Analysis
- (i)
- Fingerprint plots of esterified forms (Figure S3, Supporting Information) are asymmetric, which is characteristic of crystals with two or more molecules in the asymmetric unit and appears as a result of a distinct molecular environment in crystal;
- (ii)
- The plots are illustrating H⋯O/O⋯H spikes, which denote the existence of C-H⋯O hydrogen bonds, but for TDec the H⋯O/O⋯H spikes are less protruding as a consequence of longer distances in the C-H⋯O bonds, which are closer to the sum of vdW radii;
- (iii)
- A quantitative breakdown of fingerprint diagrams (Table S2, Supplementary Materials) shows the similarities in individual contributions in all four esters, with the highest percentage in H⋯H contacts, followed by a medium percentage of O⋯H/H⋯O intercontacts and a smaller one for C⋯H/H⋯C, respectively;
- (iv)
- Based on the large percentages of H⋯H contacts for all crystals (fingerprint breakdown in Table S2, Supplementary Materials) corroborated crystal and intermolecular energies (Table 2 and Table S4 from Supplementary Materials) validate that dispersion components play the major role in overall packing.
2.5. Solubility Evaluation
2.6. FT-IR Spectroscopy Analysis
2.7. DTA/TG Analysis
3. Materials and Methods
3.1. Materials and Recrystallization Experiments
3.2. Powder X-ray Diffraction
3.3. Single Crystal X-ray Diffraction and Structures Refinement
3.4. Computational Methods
3.5. Solubility Evaluation
3.6. FT-IR Spectroscopy
3.7. Differential Thermal Analysis (DTA) and Thermogravimetric Analysis (TG)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sinha-Hikim, I.; Taylor, W.E.; Gonzalez-Cadavid, N.F.; Zheng, W.; Bhasin, S. Androgen Receptor in Human Skeletal Muscle and Clutured Muscle Satellite Cells: Up-Regulation by Androgen Treatment. JCEM 2004, 89, 5245–5255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamad, N.V.; Soelaiman, I.N.; Chin, K.Y. A concise review of testosterone and bone health. Clin. Interv. Aging 2016, 11, 1317–1324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katznelson, L. Terapeutic role of androgens in the treatment of osteoporosis in men. Baillieres Clin. Endocrinol. Metab. 1998, 12, 453–470. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in women-the clinical significance. Lancet Diabetes Endocrionl. 2015, 3, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Lavrovsky, Y.; Song, C.S.; Jung, M.H.; Velu, N.K.; Bi, B.Y.; Chatterjee, B. Regulation of androgen action. Vitam. Horm. 1999, 55, 309–352. [Google Scholar] [CrossRef] [PubMed]
- Irwig, M.S. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. Metab. 2017, 5, 301–311. [Google Scholar] [CrossRef]
- Zuloaga, D.G.; Puts, D.A.; Jordan, C.L.; Breedlove, S.M. The role of androgen receptors in the masculinization of brain and behavior: What we’ve learned from the testicular feminization mutation. Horm. Behav. 2008, 53, 613–626. [Google Scholar] [CrossRef] [Green Version]
- Kelly, D.M.; Jones, T.H. Testosterone: A metabolic hormone in health and disease. J. Endocrionol. 2013, 217, 25–45. [Google Scholar] [CrossRef] [Green Version]
- Vlahopoulos, S.; Zimmer, W.E.; Jenster, G.; Belaguli, N.S.; Balk, S.P.; Brinkmann, A.O.; Lanz, R.B.; Zoumpourlis, V.C.; Schwartz, R.J. Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene. JBC 2005, 280, 7786–7792. [Google Scholar] [CrossRef] [Green Version]
- Braunstein, G.D. The influence of anabolic steroids on muscular strength. Princ. Med. Biol. 1997, 8, 465–474. [Google Scholar] [CrossRef]
- Vermeulen, A. Longacting steroid preparations. Acta Clin. Belg. 1975, 30, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Forsdahl, G.; Erced, D.; Geisendorfer, T.; Turkalj, M.; Plavec, D.; Thevis, M.; Tretzele, L.; Gmeinera, G. Detection of testosterone esters in blood. Drug Test. Anal. 2015, 7, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Behre, H.M.; Abshagen, K.; Oettel, M.; Hubler, F.; Nieschlag, E. Intramuscular injection of testosterone undecanoate for the treatment of male hypogonadism: Phase I studies. Eur. J. Endocrinol. 1999, 140, 414–419. [Google Scholar] [CrossRef] [Green Version]
- Elks, J.; Ganellin, C.R. The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies, 1st ed.; Springer: Easton, PA, USA, 1990; p. 652. [Google Scholar]
- Reisch, J.; Eki-Gucer, N.; Takacs, M.; Henkel, G. Photochemische Studien, 54. Photodimerisierung von Testosteronpropionat in kristallinem Zustand und Kristallstruktur von Testosteronpropionat. LACHDL 1989, 6, 595–597. [Google Scholar] [CrossRef]
- Alcock, N.W.; Sanders, K.J.; Rodger, A. Potential injectable contraceptive steroids: Testosterone buciclate. Acta Cryst. 2004, E60, 348–349. [Google Scholar] [CrossRef]
- Roberts, P.J.; Pettersen, R.C.; Sheldrick, G.M.; Isaacs, N.W.; Kennard, O. Crystal and molecular structure of 17β-hydroxyandrost-4-en-3-one (testosterone). J.Chem. Soc. Perkin Trans. 2 1973, 14, 1978–1984. [Google Scholar] [CrossRef]
- Turza, A.; Popescu, V.; Mare, L.; Borodi, G. Structural Aspects and Intermolecular Energy for Some Short Testosterone Esters. Materials 2022, 15, 7245. [Google Scholar] [CrossRef]
- Böcskei, Z.; Gérczei, T.; Bodor, A.; Schwartz, R.; Náray-Szabó, G. Three Testosterone Derivatives. Acta Cryst. 1996, C52, 2899–2903. [Google Scholar] [CrossRef]
- Silva, M.R.; Beja, A.M.; Moreira, V.M.; Santos, R.C.; Salvador, A.R. 3-Oxoandrost-4-en-17β-yl iH-imidazole-1-carboxylate. Acta Cryst. 2007, E63, o4824. [Google Scholar] [CrossRef]
- Hoyama, H.; Shiro, M.; Sato, T.; Tsukuda, Y. Crystal Structures of 8β-Methyltestosterone 17 β-Monobromoacetate and Testosterone 17 β-p-Bromobenzoate. J. Chem. Soc. B 1970, 2, 443–452. [Google Scholar] [CrossRef]
- Ohrt, J.M.; Haner, B.A.; Norton, D.A. Crystal data (I) for some halogenated steroids. Acta Cryst. 1965, 19, 280. [Google Scholar] [CrossRef]
- Land, L.M.; Li, P.; Baummer, P.M. The Influence of Water Content of Triglyceride Oils on the Solubility. Pharm. Res. 2005, 5, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Remington, J.P. Remington: The Science and Practice of Pharmacy, 23rd ed.; Adejare, A., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2020. [Google Scholar]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. (Eds.) Handbook of Pharmaceutical Excipients, 5th ed.; Libros Digitales-Pharmaceutical Press: London, Great Britain, 2006. [Google Scholar]
- Rendle, D.F.; Trotter, J. Crystal and molecular structure of 17β-hydroxy-17α-methyl-2oxa-5α-androstan-3-one. J. Chem. Soc. Perkin Trans. 1975, 2, 1361–1365. [Google Scholar] [CrossRef]
- Turza, A.; Borodi, G.; Pop, M.M.; Ulici, A. Polymorphism and β-cyclodextrin complexation of methyldrostanolone. J. Mol. Struct. 2022, 1250, 131852. [Google Scholar] [CrossRef]
- Turza, A.; Ulici, A.; Pop, M.M.; Borodi, G. Solid forms and β-cyclodextrin complexation of turinabol. Acta Cryst. 2022, C78, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Courseille, C.; Precigoux, G.; Leroy, F.; Busetta, B. 5α-Androstan-17β-ol-3one, C19H30O2. Cryst. Struct. Commun. 1973, 2, 441. [Google Scholar]
- Gaedecki, Z.; Grochulski, P.; Wawrzak, Z. Structure of 17α-methyl-testosterone semihydrate, C20H30O2·1/2H2O. J. Crystallogr. Spectrosc. Res. 1989, 19, 577–587. [Google Scholar] [CrossRef]
- Turza, A.; Miclaus, M.O.; Pop, A.; Borodi, G. Crystal and molecular structures of boldenone and four boldenone steroid esters. Z. Kristallogr. Cryst. Mater. 2019, 234, 671–683. [Google Scholar] [CrossRef]
- Borodi, G.; Turza, A.; Camarasan, P.A.; Ulici, A. Structural studies of Trenbolone, Trenbolone Acetate, Hexahydrobenzylcarbonate and Enanthate esters. J. Mol. Struct. 2020, 1212, 128127. [Google Scholar] [CrossRef]
- Borodi, G.; Turza, A.; Bende, A. Exploring the Polymorphism of Drostanolone Propionate. Molecules 2020, 25, 1436. [Google Scholar] [CrossRef] [Green Version]
- Turza, A.; Borodi, G.; Pop, A.; David, M. Structural studies of some androstane based prodrugs. J. Mol. Struct. 2022, 1248, 131440. [Google Scholar] [CrossRef]
- Caplette, J.; Frigo, T.; Jozwiakowski, M.; Shea, H.; Mirmehrabi, M.; Müller, P. Characterization of new crystalline forms of hydroxyprogesterone caproate. Int. J. Pharm. 2017, 527, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.T.; Alarcon, R.T.; Perpetuo, G.L.; Bannach, G. Investigation and characterization by TG/DTG-DTA and DSC of the fusion of Riboflavin, and its interaction with the antibiotic norfloxacin in the screening of cocrystal. J. Therm. Anal. Calorim. 2019, 136, 581–588. [Google Scholar] [CrossRef] [Green Version]
- Rigaku, O.D. CrysAlis PRO; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2015. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Gavezzotti, A. Efficient computer modeling of organic materials. The atom–atom, Coulomb–London–Pauli (AA-CLP) model for intermolecular electrostatic-polarization, dispersion and repulsion energies. New J. Chem. 2011, 35, 1360–1368. [Google Scholar] [CrossRef]
- Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. Spackman, CrystalExplorer17; University of Western Australia: The Nedlands, Australia, 2017. [Google Scholar]
- Mackenzie, C.F.; Spackman, P.R.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer model energies and energy frameworks: Extension to metal coordination compounds, organic salts, solvates and open-shell systems. IUCrJ 2017, 4, 575–587. [Google Scholar] [CrossRef] [Green Version]





| Identification Code | TEna | TCyp | TDec | TUnd |
|---|---|---|---|---|
| Empirical formula | C26H40O3 | C27H40O3 | C29H46O3 | C30H48O3 |
| Formula weight | 400.58 | 411.58 | 442.66 | 456.68 |
| Temperature/K | 293(2) | 293(2) | 293(2) | 293(2) |
| Crystal system | monoclinic | orthorhombic | orthorhombic | monoclinic |
| Space group | P21 | P21212 | P212121 | P21 |
| a/Å | 10.5644(6) | 20.9920(6) | 8.3367(3) | 10.5820(2) |
| b/Å | 8.2875(6) | 32.0578(8) | 19.0481(6) | 8.23950(10) |
| c/Å | 27.6918(15) | 7.2292(2) | 33.7169(12) | 32.3890(4) |
| α/° | 90 | 90 | 90 | 90 |
| β/° | 94.652(5) | 90 | 90 | 99.3530(10) |
| γ/° | 90 | 90 | 90 | 90 |
| Volume/Å3 | 2416.5(3) | 4864.9(2) | 5354.2(3) | 2786.47(7) |
| Z | 4 | 8 | 8 | 4 |
| ρcalc g/cm3 | 1.101 | 1.124 | 1.098 | 1.089 |
| μ/mm−1 | 0.542 | 0.553 | 0.531 | 0.523 |
| F(000) | 880.0 | 1800.0 | 1952.0 | 1008.0 |
| Radiation | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) | CuKα (λ = 1.54184) |
| 2Θ range/° | 6.404 to 141.12 | 6.938 to 145.1 | 7.002 to 148.164 | 8.3 to 141.722 |
| Index ranges | −12 ≤ h ≤ 12, −10 ≤ k ≤ 9, −33 ≤ l ≤ 26 | −25 ≤ h ≤ 25, −38 ≤ k ≤ 36, −7 ≤ l ≤ 8 | −8 ≤ h ≤ 10, −23 ≤ k ≤ 23, −41 ≤ l ≤ 41 | −12 ≤ h ≤ 12, −10 ≤ k ≤ 9, −39 ≤ l ≤ 39 |
| Reflections collected | 15,913 | 32,749 | 75,177 | 40,701 |
| Independent reflections | 8094 [Rint = 0.0422, Rsigma = 0.0471] | 9217 [Rint = 0.0620, Rsigma = 0.0556] | 10,249 [Rint = 0.1805, Rsigma = 0.0748] | 10,192 [Rint = 0.0333, Rsigma = 0.0213] |
| Data/restraints/parameters | 8094/1/529 | 9217/0/545 | 10,249/0/583 | 10,192/1/601 |
| Goodness-of-fit on F2 | 1.001 | 1.031 | 1.085 | 1.044 |
| Final R indexes [I>=2σ (I)] | R1 = 0.0549, wR2 = 0.1372 | R1 = 0.0706, wR2 = 0.1833 | R1 = 0.0938, wR2 = 0.1762 | R1 = 0.0428, wR2 = 0.1141 |
| Final R indexes [all data] | R1 = 0.0896, wR2 = 0.1652 | R1 = 0.1199, wR2 = 0.2265 | R1 = 0.1406, wR2 = 0.2100 | R1 = 0.0462, wR2 = 0.1186 |
| Largest diff. peak/hole/e Å−3 | 0.15/−0.18 | 0.25/−0.17 | 0.16/−0.22 | 0.17/−0.21 |
| Flack parameter | −0.21(17) | 0.04(18) | −0.02(16) | −0.09(8) |
| Structure | Molar Mass g/mol | Ecoul (kJ/mol) | Epol (kJ/mol) | Edisp (kJ/mol) | Erep (kJ/mol) | Elatt (kJ/mol) |
|---|---|---|---|---|---|---|
| TBas | 288.43 | −33/3 | −47.5 | −130.9 | 60.9 | −150.8 |
| TAce | 330.46 | −15.5 | −55.0 | −126.1 | 37.1 | −159.5 |
| TPro | 344.49 | −18.3 | −55.5 | −126.4 | 33.9 | −166.3 |
| TIso | 386.57 | −21.3 | −57.7 | −141.9 | 52.7 | −168.2 |
| TPhp | 420.59 | −19.3 | −52.3 | −149.0 | 36.1 | −184.5 |
| TEna | 400.60 | −22.5 | −60.4 | −157.4 | 64.6 | −175.7 |
| TCyp | 412.61 | −17.6 | −58.5 | −142.8 | 37.0 | −181.9 |
| TDec | 442.68 | −17.4 | −70.4 | −167.3 | 40.1 | −215.1 |
| TUnd | 456.71 | −19.6 | −73.0 | −175.4 | 47.3 | −220.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turza, A.; Pascuta, P.; Mare, L.; Borodi, G.; Popescu, V. Structural Insights and Intermolecular Energy for Some Medium and Long-Chain Testosterone Esters. Molecules 2023, 28, 3097. https://doi.org/10.3390/molecules28073097
Turza A, Pascuta P, Mare L, Borodi G, Popescu V. Structural Insights and Intermolecular Energy for Some Medium and Long-Chain Testosterone Esters. Molecules. 2023; 28(7):3097. https://doi.org/10.3390/molecules28073097
Chicago/Turabian StyleTurza, Alexandru, Petru Pascuta, Liviu Mare, Gheorghe Borodi, and Violeta Popescu. 2023. "Structural Insights and Intermolecular Energy for Some Medium and Long-Chain Testosterone Esters" Molecules 28, no. 7: 3097. https://doi.org/10.3390/molecules28073097






