Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders
Abstract
1. Introduction
2. Factors Affecting the Consistency of the Chemical Composition of the Herbal Extracts
2.1. Cultivar and Harvesting Year
2.2. Effect of the Harvesting Year
2.3. Influence of Cultivar on Elderberry Chemical Composition
2.4. Effect of Ripening Stage
2.5. Effect of Source Acquisition: Cultivated Versus Wild-Collected Elderberry
2.6. Effect of Geographic Region
2.7. Effect of Altitude
2.8. Adulteration
3. Polyphenolic Chemical Composition of Black Elderberry Fruit
4. Polyphenolic Chemical Composition of American Elderberry Fruit
5. Stability of Acylated Versus Non-Acylated Anthocyanins
6. Analytical Methods
6.1. Infrared Spectroscopy (IR)
6.2. Nuclear Magnetic Resonance (NMR)
6.3. High-Performance Thin Layer Chromatography (HPTLC)
6.4. Capillary Electrophoresis
6.5. Gas Chromatography/Mass Spectrometry
6.6. Liquid Chromatography/Mass Spectrometry (LC/LC-MS)
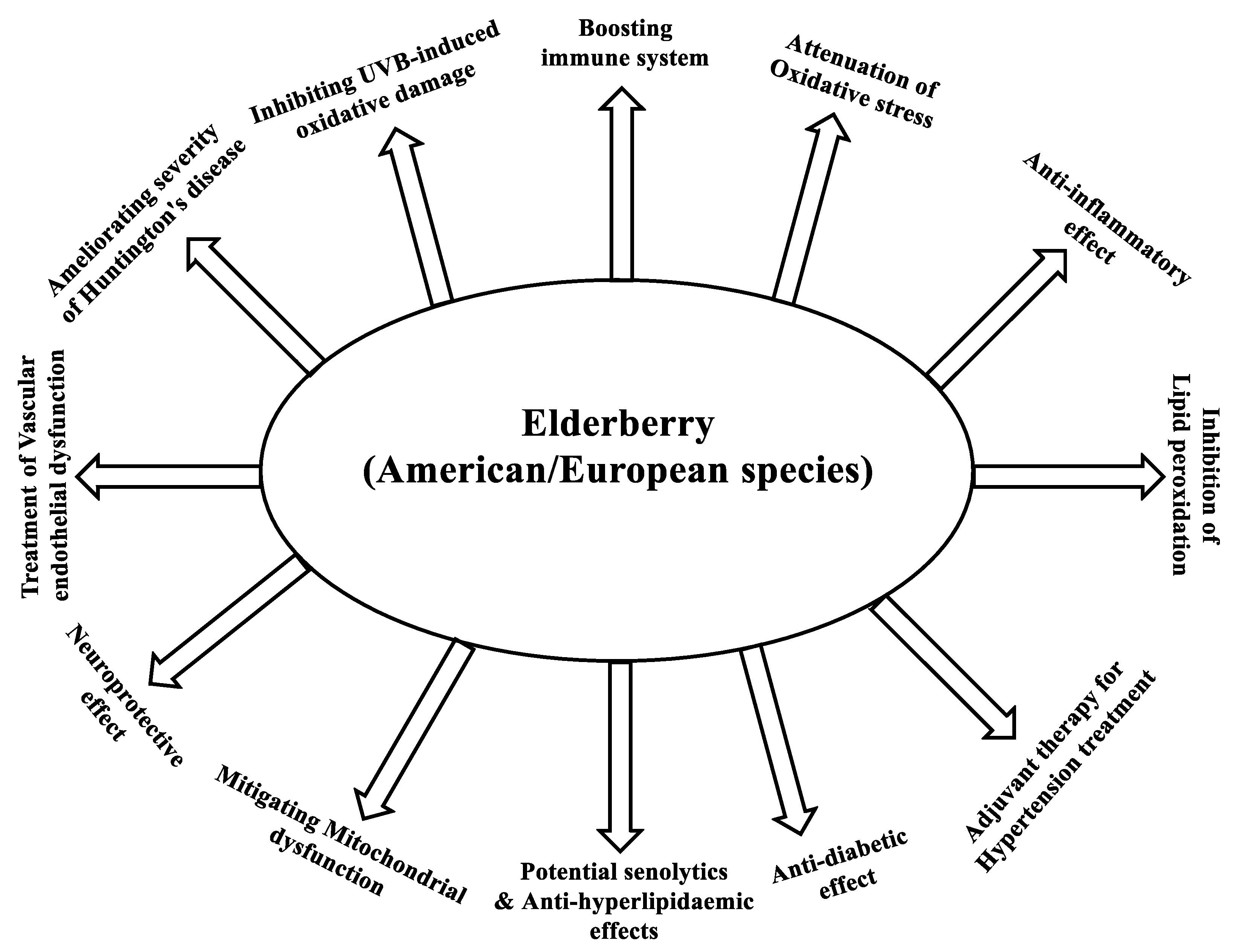
7. Health Effects of European Elderberry and American Elderberry
7.1. Link between Antioxidative Activity and Boosting the Immune System
7.2. Effect of Elderberry Fruit Extracts on Oxidative Stress
7.3. Effect of Elderberry on Huntington’s Disease
7.4. Anti-Inflammatory Effects of Elderberry Extract
7.5. Effects of Elderberry Extract on Diabetes
7.6. Role of Elderberry Anthocyanin in Attenuating Diabetes
7.7. Elderberry Extract as Adjuvant Therapy for the Treatment of Hypertension
7.8. Elderberry Extract as Potential Senolytics
7.9. Role of Elderberry Anthocyanins in Mitigating Mitochondrial Dysfunction
7.10. Role of Elderberry Extract and Cyanidin 3-O-Glucoside in Treatment or Prevention of Vascular Endothelial Dysfunction
7.11. Neuroprotective and Anti-Diabetic Activity of Cyanidin 3-O-Glucoside
7.12. Inhibition of UVB-Induced Oxidative Damage and Inflammation by Cyanidin 3-O-Glucoside
7.13. Anti-Hyperlipidaemic Effect of Elderberry Extract
7.14. Clinical Trials
8. Safety and Potentially Harmful Compounds in Elderberry
9. Stability of Anthocyanins in Elderberry
10. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CAT | Catalase |
| CGE | Cyanidin 3-O-glucoside equivalents |
| CVD | Cardiovascular disease |
| DW | Dry weight |
| FW | Fresh weight |
| GAE | Gallic acid equivalents |
| GPx | Glutathione peroxidase |
| GSH | Glutathione |
| HDL-C | High-density lipoprotein cholesterol |
| HPLC-DAD | High-performance liquid chromatography with diode array detection |
| HPLC-PDA | High-performance liquid chromatography with photodiode-array detection |
| HPTLC-ESI-MS | High-performance thin layer chromatography/electrospray ionization mass spectrometry |
| LDL-C | Low-density lipoprotein cholesterol |
| MEKC | Micellar electrokinetic chromatography |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NIR | Near infrared |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SDS | Sodium dodecyl sulfate |
| SOD | Superoxide dismutase |
| SPME | Solid phase micro-extraction |
| TAC | Total anthocyanin content |
| TNF-α | Tumour Necrosis Factor alpha |
| UHPLC-MWD | Ultra high performance liquid chromatography-multiple wavelenghth detection |
References
- Quideau, S.; Deffieux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef] [PubMed]
- Ozgen, M.; Scheerens, J.C.; Reese, R.N.; Miller, R.A. Total phenolic, anthocyanin contents and antioxidant capacity of selected elderberry (Sambucus canadensis L.) accessions. Pharmacogn. Mag. 2010, 6, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Jahanbakhshi, H.; Irani, S.; Aliaghaei, A. Elderberry diet improves memory function and prevents cell death in rat models of alzheimer’s disease induced by amyloid beta injection. J. Otorhinol. Facial Plast. Surg. 2022, 8, 1–6. [Google Scholar] [CrossRef]
- Zakay-Rones, Z.; Thom, E.; Wollan, T.; Wadstein, J. Randomized study of the efficacy and safety of oral elderberry extract in the treatment of influenza a and b virus infections. J. Int. Med. Res. 2004, 32, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Tiralongo, E.; Wee, S.S.; Lea, R.A. Elderberry supplementation reduces cold duration and symptoms in air-travellers: A randomized, double-blind placebo-controlled clinical trial. Nutrients 2016, 8, 182. [Google Scholar] [CrossRef]
- Murkovic, M.; Abuja, P.; Bergmann, A.; Zirngast, A.; Adam, U.; Winklhofer-Roob, B.; Toplak, H. Effects of elderberry juice on fasting and postprandial serum lipids and low-density lipoprotein oxidation in healthy volunteers: A randomized, double-blind, placebo-controlled study. Eur. J. Clin. Nutr. 2004, 58, 244–249. [Google Scholar] [CrossRef]
- Sidor, A.; Gramza-Michałowska, A. Advanced research on the antioxidant and health benefit of elderberry (Sambucus nigra) in food—A review. J. Funct. Foods 2015, 18, 941–958. [Google Scholar] [CrossRef]
- Torabian, G.; Valtchev, P.; Adil, Q.; Dehghani, F. Anti-influenza activity of elderberry (Sambucus nigra). J. Funct. Foods 2019, 54, 353–360. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, Y.G.; Giusti, M.M. Accumulation of anthocyanins and other phytochemicals in american elderberry cultivars during fruit ripening and its impact on color expression. Plants 2020, 9, 1721. [Google Scholar] [CrossRef] [PubMed]
- Wieland, L.S.; Piechotta, V.; Feinberg, T.; Ludeman, E.; Hutton, B.; Kanji, S.; Seely, D.; Garritty, C. Elderberry for prevention and treatment of viral respiratory illnesses: A systematic review. BMC Complement. Med. Ther. 2021, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Neves, C.M.B.; Pinto, A.; Gonçalves, F.; Wessel, D.F. Changes in elderberry (Sambucus nigra L.) juice concentrate polyphenols during storage. Appl. Sci. 2021, 11, 6941. [Google Scholar] [CrossRef]
- Csorba, V.; TÓTh, M.; LÁSzlÓ, A.M.; Kardos, L.; KovÁCs, S. Cultivar and year effects on the chemical composition of elderberry (Sambucus nigra L.) fruit. Not. Bot. Horti Agrobot. Cluj-Napoca 2020, 48, 770–782. [Google Scholar] [CrossRef]
- Ferreira, S.S.; Silva, A.M.; Nunes, F.M. Sambucus nigra L. Fruits and flowers: Chemical composition and related bioactivities. Food Rev. Int. 2022, 38, 1237–1265. [Google Scholar] [CrossRef]
- Market, N.A. Natural Antioxidants Market by Product (Vitamins, Carotenoids, Polyphenols) Nature (Organic, Conventional), Source (Fruits & Vegetables, Herbs & Spices) & Region-Forecast 2022–2032. 2022, Natural Antioxidants Market. Available online: https://www.adroitmarketresearch.com/industry-reports/natural-antioxidants-market (accessed on 28 February 2023).
- Tang, P.; Giusti, M.M. Black goji as a potential source of natural color in a wide ph range. Food Chem. 2018, 269, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Avula, B.; Katragunta, K.; Wang, Y.-H.; Ali, Z.; Srivedavyasasri, R.; Gafner, S.; Slimestad, R.; Khan, I.A. Chemical profiling and UHPLC-QToF analysis for the simultaneous determination of anthocyanins and flavonoids in Sambucus berries and authentication and detection of adulteration in elderberry dietary supplements using UHPLC-PDA-MS. J. Food Comp. Anal. 2022, 110, 104584. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Parra, M.D.; Rodríguez, M.C.; Martínez de Morentin, B.E.; Martínez, J.A. A role for fruit content in energy-restricted diets in improving antioxidant status in obese women during weight loss. Nutrition 2006, 22, 593–599. [Google Scholar] [CrossRef]
- Hu, X.; Yang, Y.; Tang, S.; Chen, Q.; Zhang, M.; Ma, J.; Qin, J.; Yu, H. Anti-aging effects of anthocyanin extracts of Sambucus canadensis caused by targeting mitochondrial-induced oxidative stress. Int. J. Mol. Sci. 2023, 24, 1528. [Google Scholar] [CrossRef]
- Nakajima, J.-i.; Tanaka, I.; Seo, S.; Yamazaki, M.; Saito, K. LC/PDA/ESI-MS profiling and radical scavenging activity of anthocyanins in various berries. J. Biomed. Biotechnol. 2004, 2004, 469084. [Google Scholar] [CrossRef]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef]
- Krüger, S.; Mirgos, M.; Morlock, G.E. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. J. Chromatogr. A 2015, 1426, 209–219. [Google Scholar] [CrossRef]
- Salvador, Â.C.; Rocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crops Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Lamy, S.; Muhire, É.; Annabi, B. Antiproliferative efficacy of elderberries and elderflowers (Sambucus canadensis) on glioma and brain endothelial cells under normoxic and hypoxic conditions. J. Funct. Foods 2018, 40, 164–179. [Google Scholar] [CrossRef]
- Santin, J.R.; Benvenutti, L.; Broering, M.F.; Nunes, R.; Goldoni, F.C.; Patel, Y.B.K.; de Souza, J.A.; Kopp, M.A.T.; de Souza, P.; da Silva, R.d.C.V.; et al. Sambucus nigra: A traditional medicine effective in reducing inflammation in mice. J. Ethnopharmacol. 2022, 283, 114736. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.-Q.; Wu, D.-T.; Li, H.-B.; Feng, Y.-B.; Zou, L.; Gan, R.-Y. Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Avula, B.; Chan, M.; Clément, C.; Kreuzer, M.; Khan, I.A. Metabolomic differentiation of maca (Lepidium meyenii) accessions cultivated under different conditions using nmr and chemometric analysis. Planta Med. 2012, 78, 90–101. [Google Scholar] [CrossRef]
- Andola, H.C.; Gaira, K.S.; Rawal, R.S.; Rawat, M.S.M.; Bhatt, I.D. Habitat-dependent variations in berberine content of Berberis asiatica roxb. Ex. Dc. In Kumaon, Western Himalaya. Chem. Biodivers. 2010, 7, 415–420. [Google Scholar] [CrossRef]
- Ghimire, S.K.; McKey, D.; Aumeeruddy-Thomas, Y. Himalayan medicinal plant diversity in an ecologically complex high altitude anthropogenic landscape, Dolpo, Nepal. Environ. Conserv. 2006, 33, 128–140. [Google Scholar] [CrossRef]
- Rawat, P.; Kumar, M.; Srivastava, A.; Kumar, B.; Misra, A.; Pratap Singh, S.; Srivastava, S. Influence of soil variation on diosgenin content profile in Costus speciosus from Indo-gangetic plains. Chem. Biodivers. 2021, 18, e2000977. [Google Scholar] [CrossRef]
- Darfour, B.; Agbenyegah, S.; Ofosu, D.O.; Okyere, A.A.; Asare, I.K. Gamma irradiation of Tetrapleura tetraptera fruit as a post-harvest technique and its subsequent effect on some phytochemicals, free scavenging activity and physicochemical properties. Radiat. Phys. Chem. 2014, 102, 153–158. [Google Scholar] [CrossRef]
- Puranik, V.; Mishra, V. Effect of drying techniques on the physicochemical and bioactive components of selected medicinal herbs. Ann. Phytomed. 2012, 1, 23–29. [Google Scholar]
- Tanko, H.; Carrier, D.J.; Duan, L.; Clausen, E. Pre- and post-harvest processing of medicinal plants. Plant Genet. Resour. 2005, 3, 304–313. [Google Scholar] [CrossRef]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, J.; Yin, D.; Zhao, X. Influence of ecological factors on the production of active substances in the anti-cancer plant Sinopodophyllum hexandrum (royle) T.S. Ying. PLoS ONE 2015, 10, e0122981. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Li, L.; Wang, M.; van Wijk, E.; He, M.; van Wijk, R.; Koval, S.; Hankemeier, T.; van der Greef, J.; Wei, S. Effects of growth altitude on chemical constituents and delayed luminescence properties in medicinal rhubarb. J. Photochem. Photobiol. B Biol. 2016, 162, 24–33. [Google Scholar] [CrossRef]
- Mocanu, M.L.; Amariei, S. Elderberries-a source of bioactive compounds with antiviral action. Plants 2022, 11, 740. [Google Scholar] [CrossRef]
- Lee, J.; Finn, C.E. Anthocyanins and other polyphenolics in american elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J. Sci. Food Agric. 2007, 87, 2665–2675. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Todorovic, B.; Veberic, R.; Stampar, F. Fruit phenolic composition of different elderberry species and hybrids. J. Food Sci. 2015, 80, C2180–C2190. [Google Scholar] [CrossRef]
- McCoy, B.; Wang, L.; Zak, M.; Al-Mehmadi, S.; Kabir, N.; Alhadid, K.; McDonald, K.; Zhang, G.; Sharma, R.; Whitney, R.; et al. A prospective open-label trial of a CBD/THC cannabis oil in dravet syndrome. Ann. Clin. Transl. Neurol. 2018, 5, 1077–1088. [Google Scholar] [CrossRef]
- Wu, X.; Gu, L.; Prior, R.L.; McKay, S. Characterization of anthocyanins and proanthocyanidins in some cultivars of ribes, aronia, and sambucus and their antioxidant capacity. J. Agric. Food Chem. 2004, 52, 7846–7856. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Mudge, E.; Applequist, W.L.; Finley, J.; Lister, P.; Townesmith, A.K.; Walker, K.M.; Brown, P.N. Variation of select flavonols and chlorogenic acid content of elderberry collected throughout the Eastern United States. J. Food Comp. Anal. 2016, 47, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Rieger, G.; Müller, M.; Guttenberger, H.; Bucar, F. Influence of altitudinal variation on the content of phenolic compounds in wild populations of Calluna vulgaris, Sambucus nigra, and Vaccinium myrtillus. J. Agric. Food Chem. 2008, 56, 9080–9086. [Google Scholar] [CrossRef] [PubMed]
- Turbitt, J.R.; Colson, K.L.; Killday, K.B.; Milstead, A.; Neto, C.C. Application of 1H-NMR-based metabolomics to the analysis of cranberry (Vaccinium macrocarpon) supplements. Phytochem. Anal. 2020, 31, 68–80. [Google Scholar] [CrossRef] [PubMed]
- Gafner, S.; Blumenthal, M.; Foster, S.; Cardellina, J.H.; Khan, I.A.; Upton, R. Botanical ingredient forensics: Detection of attempts to deceive commonly used analytical methods for authenticating herbal dietary and food ingredients and supplements. J. Nat. Prod. 2023, 86, 460–472. [Google Scholar] [CrossRef]
- Määttä-Riihinen, K.R.; Kamal-Eldin, A.; Mattila, P.H.; González-Paramás, A.M.; Törrönen, A.R. Distribution and contents of phenolic compounds in eighteen Scandinavian berry species. J. Agric. Food Chem. 2004, 52, 4477–4486. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Novak Jovanović, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruit. Dtsch. Lebensm.-Rundsch. Z. Für Lebensm. Lebensm. 2007, 103, 369–377. [Google Scholar]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef]
- Porras-Mija, I.; Chirinos, R.; García-Ríos, D.; Aguilar-Galvez, A.; Huaman-Alvino, C.; Pedreschi, R.; Campos, D. Physico-chemical characterization, metabolomic profile and in vitro antioxidant, antihypertensive, antiobesity and antidiabetic properties of andean elderberry (Sambucus nigra subsp. peruviana). J. Berry Res. 2020, 10, 193–208. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Stintzing, A.S.; Carle, R.; Frei, B.; Wrolstad, R.E. Color and antioxidant properties of cyanidin-based anthocyanin pigments. J. Agric. Food Chem. 2002, 50, 6172–6181. [Google Scholar] [CrossRef]
- Inami, O.; Tamura, I.; Kikuzaki, H.; Nakatani, N. Stability of anthocyanins of Sambucus canadensis and Sambucus nigra. J. Agric. Food Chem. 1996, 44, 3090–3096. [Google Scholar] [CrossRef]
- Avula, B.; Katragunta, K.; Osman, A.G.; Ali, Z.; John Adams, S.; Chittiboyina, A.G.; Khan, I.A. Advances in the chemistry, analysis and adulteration of anthocyanin rich-berries and fruit: 2000–2022. Molecules 2023, 28, 560. [Google Scholar] [CrossRef] [PubMed]
- Stuppner, S.; Mayr, S.; Beganovic, A.; Beć, K.; Grabska, J.; Aufschnaiter, U.; Groeneveld, M.; Rainer, M.; Jakschitz, T.; Bonn, G.K.; et al. Near-Infrared spectroscopy as a rapid screening method for the determination of total anthocyanin content in Sambucus fructus. Sensors 2020, 20, 4983. [Google Scholar] [CrossRef] [PubMed]
- Blunder, M.; Orthaber, A.; Bauer, R.; Bucar, F.; Kunert, O. Efficient identification of flavones, flavanones and their glycosides in routine analysis via off-line combination of sensitive NMR and HPLC experiments. Food Chem. 2017, 218, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Güzelmeriç, E.; Celik, C.; Sen, N.; Oçkun, M.A.; Yesilada, E. Quali/quantitative research on herbal supplements containing black elder (Sambucus nigra L.) fruit. J. Res. Pharm. 2021, 25, 238–248. [Google Scholar] [CrossRef]
- Bridle, P.; García-Viguera, C. Analysis of anthocyanins in strawberries and elderberries. A comparison of capillary zone electrophoresis and HPLC. Food Chem. 1997, 59, 299–304. [Google Scholar] [CrossRef]
- Watanabe, T.; Yamamoto, A.; Nagai, S.; Terabe, S. Analysis of elderberry pigments in commercial food samples by micellar electrokinetic chromatography. Anal. Sci. 1998, 14, 839–844. [Google Scholar] [CrossRef]
- Duymus Agalar, H.; Demirci, B.; Baser, K.H.C. The volatile compounds of elderberries (Sambucus nigra L.). Nat. Volatiles Essent. Oils 2014, 1, 51–54. [Google Scholar]
- Salvador, Â.C.; Rudnitskaya, A.; Silvestre, A.J.D.; Rocha, S.M. Metabolomic-based strategy for fingerprinting of Sambucus nigra L. Berry volatile terpenoids and norisoprenoids: Influence of ripening and cultivar. J. Agric. Food Chem. 2016, 64, 5428–5438. [Google Scholar] [CrossRef]
- Najar, B.; Ferri, B.; Cioni, P.L.; Pistelli, L. Volatile emission and essential oil composition of Sambucus nigra L. Organs during different developmental stages. Plant Biosyst. 2021, 155, 721–729. [Google Scholar] [CrossRef]
- Jensen, K.; Christensen, L.P.; Hansen, M.; Jørgensen, U.; Kaack, K. Olfactory and quantitative analysis of volatiles in elderberry (Sambucus nigra L.) juice processed from seven cultivars. J. Sci. Food Agric. 2001, 81, 237–244. [Google Scholar] [CrossRef]
- Vujanovic, M.; Djurovic, S.; Radojkovic, M. Chemical composition of essential oils of elderberry (Sambucus nigra L.) flowers and fruit. Acta Period. Technol. 2021, 52, 229–237. [Google Scholar] [CrossRef]
- Vitova, E.; Divišová, R.; Sůkalová, K.; Matějíček, A. Determination and quantification of volatile compounds in fruit of selected elderberry cultivars grown in Czech Republic. J. Food Nutr. Res. 2013, 52, 1–11. [Google Scholar]
- Brønnum-Hansen, K.; Honoré Hansen, S. High-performance liquid chromatographic separation of anthocyanins of Sambucus nigra L. J. Chromatogr. A 1983, 262, 385–392. [Google Scholar] [CrossRef]
- Nagl, M.; Eder, R.; Wendelin, S.; Reich, G.; Sontag, G. Qualitative and quantitative analysis of phenolic constituents in elderberry juices. Ernährung/Nutr. 2006, 30, 409. [Google Scholar]
- Wu, H.; Johnson, M.C.; Lu, C.H.; Fritsche, K.L.; Thomas, A.L.; Cai, Z.; Greenlief, C.M. Determination of anthocyanins and total polyphenols in a variety of elderberry juices by UHPLC-MS/MS and other methods. Acta Hortic. 2015, 1061, 43–51. [Google Scholar] [CrossRef]
- Da Silva, R.F.R.; Barreira, J.C.M.; Heleno, S.A.; Barros, L.; Calhelha, R.C.; Ferreira, I.C.F.R. Anthocyanin profile of elderberry juice: A natural-based bioactive colouring ingredient with potential food application. Molecules 2019, 24, 2359. [Google Scholar] [CrossRef]
- Chandra, A.; Rana, J.; Li, Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J. Agric. Food Chem. 2001, 49, 3515–3521. [Google Scholar] [CrossRef]
- Fejer, J.; Salamon, I.; Grulova, D.; Michalek, S.; Zvalova, M. Elderberry (Sambucus nigra) cultivation in Slovak Republic and identification and quantification of anthocyanins. In Proceedings of the I International Symposium on Elderberry, Columbia, MO, USA, 9–14 June 2015; pp. 253–258. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, J.M. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef]
- Anton, A.M.; Pintea, A.; Rugină, D.; Diaconeasa, Z.; Hanganu, D.; Vlase, L.; Benedec, D. Preliminary studies on the chemical characterization and antioxidant capacity of polyphenols from Sambucus sp. Dig. J. Nanomater. Biostruct. 2013, 8, 973–980. [Google Scholar]
- Mandrone, M.; Lorenzi, B.; Maggio, A.; La Mantia, T.; Scordino, M.; Bruno, M.; Poli, F. Polyphenols pattern and correlation with antioxidant activities of berries extracts from four different populations of sicilian Sambucus nigra L. Nat. Prod. Res. 2014, 28, 1246–1253. [Google Scholar] [CrossRef]
- Zorzi, M.; Gai, F.; Medana, C.; Aigotti, R.; Peiretti, P.G. Identification of polyphenolic compounds in edible wild fruit grown in the North-West of italy by means of HPLC-DAD-ESI-HRMS. Plant Foods Hum. Nutr. 2020, 75, 420–426. [Google Scholar] [CrossRef]
- Biller-Takahashi, J.D.; Takahashi, L.S.; Mingatto, F.E.; Urbinati, E.C. The immune system is limited by oxidative stress: Dietary selenium promotes optimal antioxidative status and greatest immune defense in pacu piaractus mesopotamicus. Fish Shellfish Immunol. 2015, 47, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Ciliberti, M.G.; Albenzio, M.; De Palo, P.; Santillo, A.; Caroprese, M. Nexus between immune responses and oxidative stress: The role of dietary hydrolyzed lignin in ex vivo bovine peripheral blood mononuclear cell response. Front. Vet. Sci. 2020, 7, 9. [Google Scholar] [CrossRef]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, K.; Khurana, S.; Tai, T.C. Oxidative stress in aging-matters of the heart and mind. Int. J. Mol. Sci. 2013, 14, 17897–17925. [Google Scholar] [CrossRef] [PubMed]
- Genestra, M. Oxyl radicals, redox-sensitive signalling cascades and antioxidants. Cell. Signal. 2007, 19, 1807–1819. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Salisbury, D.; Bronas, U. Reactive oxygen and nitrogen species: Impact on endothelial dysfunction. Nurs. Res. 2015, 64, 53–66. [Google Scholar] [CrossRef]
- Birben, E.; Sahiner, U.M.; Sackesen, C.; Erzurum, S.; Kalayci, O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012, 5, 9–19. [Google Scholar] [CrossRef]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef]
- Cordaro, M.; D’Amico, R.; Morabito, R.; Fusco, R.; Siracusa, R.; Peritore, A.F.; Impellizzeri, D.; Genovese, T.; Crupi, R.; Gugliandolo, E.; et al. Physiological and biochemical changes in nrf2 pathway in aged animals subjected to brain injury. Cell. Physiol. Biochem. 2021, 55, 160–179. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- Stadler, K. Oxidative stress in diabetes. In Diabetes: An Old Disease, a New Insight; Ahmad, S.I., Ed.; Springer: New York, NY, USA, 2013; pp. 272–287. [Google Scholar]
- Madamanchi, N.R.; Vendrov, A.; Runge, M.S. Oxidative stress and vascular disease. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lefer, D.J.; Granger, D.N. Oxidative stress and cardiac disease. Am. J. Med. 2000, 109, 315–323. [Google Scholar] [CrossRef]
- Csányi, G.; Miller, F.J., Jr. Oxidative stress in cardiovascular disease. Int. J. Mol. Sci. 2014, 15, 6002–6008. [Google Scholar] [CrossRef] [PubMed]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative stress in cardiovascular diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, S.V.; Padmaja, G.; Kuppusamy, P.; Kutala, V.K. Oxidative stress in cardiovascular disease. Indian J. Biochem. Biophys. 2009, 46, 421–440. [Google Scholar]
- Durkar, A.M.; Patil, R.R.; Naik, S.R. Hypolipidemic and antioxidant activity of ethanolic extract of Symplocos racemosa Roxb. In hyperlipidemic rats: An evidence of participation of oxidative stress in hyperlipidemia. Indian J. Exp. Biol. 2014, 52, 36–45. [Google Scholar]
- Chen, X.; Guo, C.; Kong, J. Oxidative stress in neurodegenerative diseases. Neural Regen. Res. 2012, 7, 376–385. [Google Scholar] [CrossRef]
- Roberts, R.A.; Laskin, D.L.; Smith, C.V.; Robertson, F.M.; Allen, E.M.; Doorn, J.A.; Slikker, W. Nitrative and oxidative stress in toxicology and disease. Toxicol. Sci. 2009, 112, 4–16. [Google Scholar] [CrossRef]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic diseases. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Ash, S.L.; Catignani, G.L. Antioxidants and prevention of chronic disease. Crit. Rev. Food Sci. Nutr. 2004, 44, 275–295. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Stampfer, M.J. Antioxidants for vascular disease. Med. Clin. N. Am. 2000, 84, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Albarracin, S.L.; Stab, B.; Casas, Z.; Sutachan, J.J.; Samudio, I.; Gonzalez, J.; Gonzalo, L.; Capani, F.; Morales, L.; Barreto, G.E. Effects of natural antioxidants in neurodegenerative disease. Nutr. Neurosci. 2012, 15, 1–9. [Google Scholar] [CrossRef]
- Obrenovich, M.E.; Li, Y.; Parvathaneni, K.; Yendluri, B.B.; Palacios, H.H.; Leszek, J.; Aliev, G. Antioxidants in health, disease and aging. CNS Neurol. Disord. Drug Targets 2011, 10, 192–207. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; López, V. Anthocyanins: Plant pigments, food ingredients or therapeutic agents for the cns? A mini-review focused on clinical trials. Curr. Pharm. Des. 2020, 26, 1790–1798. [Google Scholar] [CrossRef]
- Fang, J. Classification of fruit based on anthocyanin types and relevance to their health effects. Nutrition 2015, 31, 1301–1306. [Google Scholar] [CrossRef]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of plant secondary metabolites to environmental factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 1, 163–187. [Google Scholar] [CrossRef]
- Shimizu, S.; Matsushita, H.; Morii, Y.; Ohyama, Y.; Morita, N.; Tachibana, R.; Watanabe, K.; Wakatsuki, A. Effect of anthocyanin-rich bilberry extract on bone metabolism in ovariectomized rats. Biomed. Rep. 2018, 8, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Meng, X.; Yan, C.; Wang, C. Effect of purple sweet potato anthocyanins on β-amyloid-mediated pc-12 cells death by inhibition of oxidative stress. Neurochem. Res. 2010, 35, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Speer, H.; D’Cunha, N.M.; Alexopoulos, N.I.; McKune, A.J.; Naumovski, N. Anthocyanins and human health-a focus on oxidative stress, inflammation and disease. Antioxidants 2020, 9, 366. [Google Scholar] [CrossRef]
- Alam, M.A.; Islam, P.; Subhan, N.; Rahman, M.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential health benefits of anthocyanins in oxidative stress related disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Neves, D.; Valentão, P.; Bernardo, J.; Oliveira, M.C.; Ferreira, J.M.G.; Pereira, D.M.; Andrade, P.B.; Videira, R.A. A new insight on elderberry anthocyanins bioactivity: Modulation of mitochondrial redox chain functionality and cell redox state. J. Funct. Foods 2019, 56, 145–155. [Google Scholar] [CrossRef]
- Ciocoiu, M.; Badescu, M.; Badulescu, O.; Badescu, L. The beneficial effects on blood pressure, dyslipidemia and oxidative stress of Sambucus nigra extract associated with renin inhibitors. Pharm. Biol. 2016, 54, 3063–3067. [Google Scholar] [CrossRef]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016, 30, 1265–1286. [Google Scholar] [CrossRef]
- Mauray, A.; Felgines, C.; Morand, C.; Mazur, A.; Scalbert, A.; Milenkovic, D. Nutrigenomic analysis of the protective effects of bilberry anthocyanin-rich extract in apo e-deficient mice. Genes Nutr. 2010, 5, 343–353. [Google Scholar] [CrossRef]
- De Pascual-Teresa, S. Molecular mechanisms involved in the cardiovascular and neuroprotective effects of anthocyanins. Arch. Biochem. Biophys. 2014, 559, 68–74. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.L.; Simon, J.E.; Lila, M.A.; Rochet, J.C. Neuroprotective effects of anthocyanin- and proanthocyanidin-rich extracts in cellular models of parkinson׳s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.C.; Agostinho, P.; Marisco, P.C.; Vieira, J.M.; Rosa, M.M.; Bohnert, C.; Rubin, M.A.; Morsch, V.M.; et al. Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int. J. Develop. Neurosci. 2014, 33, 88–97. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Dong, Z.; Yuan, Y. Accelerated inflammation and oxidative stress induced by lps in acute lung injury: Ιnhibition by st1926. Int. J. Mol. Med. 2018, 41, 3405–3421. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative stress in neurodegenerative diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.H.; Bayat, A.-H.; Eskandari, N.; Abdollahifar, M.-a.; Fotouhi, F.; Forouzannia, A.; Rafiei, R.; Hatari, S.; Seraj, A.; Shahidi, A.M.E.J.; et al. Elderberry diet ameliorates motor function and prevents oxidative stress-induced cell death in rat models of huntington disease. Brain Res. 2021, 1762, 147444. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.S.; Martins-Gomes, C.; Nunes, F.M.; Silva, A.M. Elderberry (Sambucus nigra L.) extracts promote anti-inflammatory and cellular antioxidant activity. Food Chem. X 2022, 15, 100437. [Google Scholar] [CrossRef] [PubMed]
- Ciocoiu, M.; Mirón, A.; Mares, L.; Tutunaru, D.; Pohaci, C.; Groza, M.; Badescu, M. The effects of Sambucus nigra polyphenols on oxidative stress and metabolic disorders in experimental diabetes mellitus. J Physiol. Biochem. 2009, 65, 297–304. [Google Scholar] [CrossRef]
- Liu, Y.; Li, D.; Zhang, Y.; Sun, R.; Xia, M. Anthocyanin increases adiponectin secretion and protects against diabetes-related endothelial dysfunction. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E975–E988. [Google Scholar] [CrossRef]
- Al-Awwadi, N.A.; Araiz, C.; Bornet, A.; Delbosc, S.; Cristol, J.-P.; Linck, N.; Azay, J.; Teissedre, P.-L.; Cros, G. Extracts enriched in different polyphenolic families normalize increased cardiac nadph oxidase expression while having differential effects on insulin resistance, hypertension, and cardiac hypertrophy in high-fructose-fed rats. J. Agric. Food Chem. 2005, 53, 151–157. [Google Scholar] [CrossRef]
- Cao, H.; Ou, J.; Chen, L.; Zhang, Y.; Szkudelski, T.; Delmas, D.; Daglia, M.; Xiao, J. Dietary polyphenols and type 2 diabetes: Human study and clinical trial. Crit. Rev. Food Sci. Nutr. 2019, 59, 3371–3379. [Google Scholar] [CrossRef]
- Waswa, E.N.; Li, J.; Mkala, E.M.; Wanga, V.O.; Mutinda, E.S.; Nanjala, C.; Odago, W.O.; Katumo, D.M.; Gichua, M.K.; Gituru, R.W.; et al. Ethnobotany, phytochemistry, pharmacology, and toxicology of the genus Sambucus L. (Viburnaceae). J. Ethnopharmacol. 2022, 292, 115102. [Google Scholar] [CrossRef] [PubMed]
- Colavitti, R.; Finkel, T. Reactive oxygen species as mediators of cellular senescence. IUBMB Life 2005, 57, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Baar, M.; Brandt, R.; Putavet, D.; Klein, J.; Derks, K.; Bourgeois, B.; Stryeck, S.; Rijksen, Y.; van Willigenburg, H.; Feijtel, D.; et al. Targeted apoptosis of senescent cells restores tissue homeostasis in response to chemotoxicity and aging. Cell 2017, 169, 132–147.e116. [Google Scholar] [CrossRef] [PubMed]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.L.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. Mtor at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Tan, P.; Wang, Y.-J.; Li, S.; Wang, Y.; He, J.-Y.; Chen, Y.-Y.; Deng, H.-Q.; Huang, W.; Zhan, J.-K.; Liu, Y.-S. The pi3k/akt/mtor pathway regulates the replicative senescence of human vsmcs. Mol. Cell. Biochem. 2016, 422, 1–10. [Google Scholar] [CrossRef]
- Monteiro-Cardoso, V.F.; Oliveira, M.M.; Melo, T.; Domingues, M.R.; Moreira, P.I.; Ferreiro, E.; Peixoto, F.; Videira, R.A. Cardiolipin profile changes are associated to the early synaptic mitochondrial dysfunction in Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 43, 1375–1392. [Google Scholar] [CrossRef]
- Youdim, K.A.; Martin, A.; Joseph, J.A. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic. Biol. Med. 2000, 29, 51–60. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Bagchi, M.; Bagchi, D. Health benefits of edible berry anthocyanins: Novel antioxidant and anti-angiogenic properties. ACS Symp. Ser. 2007, 956, 337–351. [Google Scholar] [CrossRef]
- Olivas-Aguirre, F.J.; Rodrigo-García, J.; Martínez-Ruiz, N.d.R.; Cárdenas-Robles, A.I.; Mendoza-Díaz, S.O.; Álvarez-Parrilla, E.; González-Aguilar, G.A.; De la Rosa, L.A.; Ramos-Jiménez, A.; Wall-Medrano, A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules 2016, 21, 1264. [Google Scholar] [CrossRef]
- Cásedas, G.; Les, F.; González-Burgos, E.; Gómez-Serranillos, M.P.; Smith, C.; López, V. Cyanidin-3-O-glucoside inhibits different enzymes involved in central nervous system pathologies and type-2 diabetes. S. Afr. J. Bot. 2019, 120, 241–246. [Google Scholar] [CrossRef]
- Pratheeshkumar, P.; Son, Y.-O.; Wang, X.; Divya, S.P.; Joseph, B.; Hitron, J.A.; Wang, L.; Kim, D.; Yin, Y.; Roy, R.V.; et al. Cyanidin-3-glucoside inhibits UVB-induced oxidative damage and inflammation by regulating map kinase and nf-κb signaling pathways in skh-1 hairless mice skin. Toxicol. Appl. Pharmacol. 2014, 280, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Farrell, N.; Norris, G.; Lee, S.G.; Chun, O.K.; Blesso, C.N. Anthocyanin-rich black elderberry extract improves markers of HDL function and reduces aortic cholesterol in hyperlipidemic mice. Food Funct. 2015, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Castro-Acosta, M.L.; Smith, L.; Miller, R.J.; McCarthy, D.I.; Farrimond, J.A.; Hall, W.L. Drinks containing anthocyanin-rich blackcurrant extract decrease postprandial blood glucose, insulin and incretin concentrations. J. Nutr. Biochem. 2016, 38, 154–161. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Jafari, T. Effect of dietary anthocyanins on biomarkers of glycemic control and glucose metabolism: A systematic review and meta-analysis of randomized clinical trials. Food Res. Int. 2020, 137, 109379. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.-A.; Catalán, Ú.; Llauradó, E.; Valls, R.-M.; Salamanca, P.; Rubió, L.; Yuste, S.; Solà, R. The health benefits of anthocyanins: An umbrella review of systematic reviews and meta-analyses of observational studies and controlled clinical trials. Nutr. Rev. 2022, 80, 1515–1530. [Google Scholar] [CrossRef]
- Daneshzad, E.; Shab-Bidar, S.; Mohammadpour, Z.; Djafarian, K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1153–1165. [Google Scholar] [CrossRef]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef]
- Do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Paterson, A.; Roodenrys, S.; Thomas, S.; Bliokas, V.; Potter, J.; Walton, K.; Weston–Green, K. Food anthocyanins decrease concentrations of TNF-α in older adults with mild cognitive impairment: A randomized, controlled, double blind clinical trial. Nutr. Metabol. Cardiovasc. Dis. 2021, 31, 950–960. [Google Scholar] [CrossRef]
- Xu, Z.; Xie, J.; Zhang, H.; Pang, J.; Li, Q.; Wang, X.; Xu, H.; Sun, X.; Zhao, H.; Yang, Y. Anthocyanin supplementation at different doses improves cholesterol efflux capacity in subjects with dyslipidemia—A randomized controlled trial. Eur. J. Clin. Nutr. 2021, 75, 345–354. [Google Scholar] [CrossRef]
- Curtis, P.J.; Kroon, P.A.; Hollands, W.J.; Walls, R.; Jenkins, G.; Kay, C.D.; Cassidy, A. Cardiovascular disease risk biomarkers and liver and kidney function are not altered in postmenopausal women after ingesting an elderberry extract rich in anthocyanins for 12 weeks. J. Nutr. 2009, 139, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Determination of amygdalin in apple seeds, fresh apples and processed apple juices. Food Chem. 2015, 170, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Mazza, G.; Cottrell, T. Carotenoids and cyanogenic glucosides in Saskatoon berries (Amelanchier alnifolia nutt.). J. Food Comp. Anal. 2008, 21, 249–254. [Google Scholar] [CrossRef]
- Bolarinwa, I.F.; Orfila, C.; Morgan, M.R.A. Amygdalin content of seeds, kernels and food products commercially-available in the UK. Food Chem. 2014, 152, 133–139. [Google Scholar] [CrossRef]
- Lee, J.; Zhang, G.; Wood, E.; Rogel Castillo, C.; Mitchell, A.E. Quantification of amygdalin in nonbitter, semibitter, and bitter almonds (Prunus dulcis) by UHPLC-(ESI)QQQ-MS/MS. J. Agric. Food Chem. 2013, 61, 7754–7759. [Google Scholar] [CrossRef] [PubMed]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. Transition of phenolics and cyanogenic glycosides from apricot and cherry fruit kernels into liqueur. Food Chem. 2016, 203, 483–490. [Google Scholar] [CrossRef]
- Bohm, B.A.; Glennie, C.W. A chemosystematic study of the Caprifoliaceae. Can. J. Bot. 1971, 49, 1799–1807. [Google Scholar] [CrossRef]
- Gibbs, R.D. Chemotaxonomy of Flowering Plants—V. 1: Constituents.—V. 2: Families.—V. 3: Orders.—V. 4: Bibliography (and) Index; McGill-Queen’s Univ. Press: London, UK, 1974. [Google Scholar]
- Hegnauer, R. Chemotaxonomie der Pflanzen; BirkhaKuser Verlag: Basel, Switzerland, 1989; Volume 8, pp. 1–847. [Google Scholar]
- Dellagreca, M.; Fiorentino, A.; Monaco, P.; Previtera, L.; Simonet, A.M. Cyanogenic glycosides from Sambucus nigra. Nat. Prod. Lett. 2000, 14, 175–182. [Google Scholar] [CrossRef]
- Buhrmester, R.A.; Ebinger, J.E.; Seigler, D.S. Sambunigrin and cyanogenic variability in populations of Sambucus canadensis L. (Caprifoliaceae). Biochem. Syst. Ecol. 2000, 28, 689–695. [Google Scholar] [CrossRef]
- Jensen, S.; Nielsen, B. Cyanogenic glucosides in Sambucus nigra L. Acta Chem. Scand. 1973, 27, 2661–2662. [Google Scholar] [CrossRef] [PubMed]
- Bruneton, J. Plantas tóxicas. Vegetales Peligrosos para el Hombre y los Animales; Acriba Editorial: Zaragoza, Spain, 2001. [Google Scholar]
- Senica, M.; Stampar, F.; Veberic, R.; Mikulic-Petkovsek, M. The higher the better? Differences in phenolics and cyanogenic glycosides in Sambucus nigra leaves, flowers and berries from different altitudes. J. Sci. Food Agric. 2017, 97, 2623–2632. [Google Scholar] [CrossRef] [PubMed]
- Appenteng, M.K.; Krueger, R.; Johnson, M.C.; Ingold, H.; Bell, R.; Thomas, A.L.; Greenlief, C.M. Cyanogenic glycoside analysis in american elderberry. Molecules 2021, 26, 1384. [Google Scholar] [CrossRef] [PubMed]
- Stirpe, F. Ribosome-inactivating proteins. Toxicon 2004, 44, 371–383. [Google Scholar] [CrossRef] [PubMed]
- Tejero, J.; Jiménez, P.; Quinto, E.J.; Cordoba-Diaz, D.; Garrosa, M.; Cordoba-Diaz, M.; Gayoso, M.J.; Girbés, T. Elderberries: A source of ribosome-inactivating proteins with lectin activity. Molecules 2015, 20, 2364–2387. [Google Scholar] [CrossRef]
- Förster-Waldl, E.; Marchetti, M.; Schöll, I.; Focke, M.; Radauer, C.; Kinaciyan, T.; Nentwich, I.; Jäger, S.; Schmid, E.R.; Boltz-Nitulescu, G.; et al. Type i allergy to elderberry (Sambucus nigra) is elicited by a 33.2 KDa allergen with significant homology to ribosomal inactivating proteins. Clin. Exp. Allergy 2003, 33, 1703–1710. [Google Scholar] [CrossRef]
- Jimenez, P.; Tejero, J.; Cabrero, P.; Cordoba-Diaz, D.; Girbes, T. Differential sensitivity of D-galactose-binding lectins from fruit of dwarf elder (Sambucus ebulus L.) to a simulated gastric fluid. Food Chem. 2013, 136, 794–802. [Google Scholar] [CrossRef]
- Jiménez, P.; Cabrero, P.; Cordoba-Diaz, D.; Cordoba-Diaz, M.; Garrosa, M.; Girbés, T. Lectin digestibility and stability of elderberry antioxidants to heat treatment in vitro. Molecules 2017, 22, 95. [Google Scholar] [CrossRef]
- Atkinson, M.D.; Atkinson, E. Sambucus nigra L. J. Ecol. 2002, 90, 895–923. [Google Scholar] [CrossRef]
- Leja, M.; Mareczek, A.; Nanaszko, B. Annals of the Agricultural University in Poznań. Gardening; Wydawnictwo Akademii Rolniczej im. Augusta Cieszkowskiego: Poznaniu, Poland, 2007; Volume 41, pp. 327–331. [Google Scholar]
- Kaack, K.; Austed, T. Interaction of vitamin c and flavonoids in elderberry (Sambucus nigra L.) during juice processing. Plant Foods Hum. Nutr. 1998, 52, 187–198. [Google Scholar] [CrossRef]
- Schmitzer, V.; Veberic, R.; Slatnar, A.; Stampar, F. Elderberry (Sambucus nigra L.) wine: A product rich in health promoting compounds. J. Agric. Food Chem. 2010, 58, 10143–10146. [Google Scholar] [CrossRef]
- Vlachojannis, C.; Zimmermann, B.F.; Chrubasik-Hausmann, S. Quantification of anthocyanins in elderberry and chokeberry dietary supplements. Phytother. Res. 2015, 29, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, J.; Tomás-Barberán, A. Evaluation of commercial red fruit juice concentrates as ingredients for antioxidant functional juices. Eur. Food Res. Technol. 2004, 219, 133–141. [Google Scholar] [CrossRef]
- Kaack, K.; Fretté, X.C.; Christensen, L.P.; Landbo, A.-K.; Meyer, A.S. Selection of elderberry (Sambucus nigra L.) genotypes best suited for the preparation of juice. Eur. Food Res. Technol. 2008, 226, 843–855. [Google Scholar] [CrossRef]
- Gafner, S.; Bush, M.; Sudberg, S.; Feuillere, N.; Tenon, M.; Jolibois, J.; Bellenger, P.; You, H.; Adams, R.; Stewart, J. Tales from the elder: Adulteration issues of elder berry. HerbalGram 2021, 130, 24–32. [Google Scholar]
- Walkowiak-Tomczak, D.; Czapski, J.; Młynarczyk, K. Assessment of colour changes during storage of elderberry juice concentrate solutions using the optimization method. Acta Sci. Pol. Technol. Aliment. 2016, 15, 299–309. [Google Scholar] [CrossRef]
- Wang, W.-D.; Xu, S.-Y. Degradation kinetics of anthocyanins in blackberry juice and concentrate. J. Food Eng. 2007, 82, 271–275. [Google Scholar] [CrossRef]
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- BrØNnum-Hansen, K.; Jacobsen, F.; Flink, J.M. Anthocyanin colourants from elderberry (Sambucus nigra L.). 1. Process considerations for production of the liquid extract. Int. J. Food Sci. Technol. 1985, 20, 703–711. [Google Scholar] [CrossRef]
- Seabra, I.J.; Braga, M.E.M.; Batista, M.T.P.; de Sousa, H.C. Fractioned high pressure extraction of anthocyanins from elderberry (Sambucus nigra L.) pomace. Food Bioprocess Technol. 2010, 3, 674–683. [Google Scholar] [CrossRef]

| Type of Effect | Values | Total Polyphenols | Total Anthocyanins |
|---|---|---|---|
| Cultivar | Minimum | 852.6 mg/100 g FW | 443.6 mg/100 g FW |
| Maximum | 2541.6 mg/100 g FW | 1413.8 mg/100 g FW | |
| Harvesting Year | Minimum | 1456.9 mg GAE/100 g | 710.5 mg Cy-3-glc/100 g |
| Maximum | 1740 mg GAE/100 g | 895.4 mg Cy-3-glc/100 g |
| Cultivar “Bastardeira” | TPC | ODPC | TAC |
|---|---|---|---|
| Year 1 | 820 mg GAE/100 g FW | 704 mg GAE/100 g FW | 510 mg Cy-3-glc/100 g FW |
| Year 2 | 1177 mg GAE/100 g FW | 703 mg GAE/100 g FW | 820 mg Cy-3-glc/100 g FW |
| Year 3 | 1016 mg GAE/100 g FW | 2009 mg GAE/100 g FW | 744 mg Cy-3-glc/100 g FW |
| No. | Source (Year) | Anthocyanins | Extraction Solvent | % Yield | Conditions | Detection Method | Purpose of Analysis | Pharmacological Activity | [Ref] | |
|---|---|---|---|---|---|---|---|---|---|---|
| Stationary Phase | Mobile Phase | |||||||||
| 1 | S. nigra berries (1983) | Cy-3-O-sam-5-O-glc; Cy-3,5-O-diglc; Cy-3-O-sam; Cy-3-glc; | 5 g of sample pomace macerated for 2 min in blender with 100 mL of 0.1 M HCl | - | Nucleosil C18 (150 × 4.6 mm, 5 µm) | A: THF; B: 0.05 M Phosphoric acid; 0–50 min, 1–40% A; 1.2 mL/min | HPLC-UV/Vis | HPLC separation of anthocyanins | - | [64] |
| 2 | S. nigra and S. canadensis berries (1996) | S. nigra Cy-3-O-sam-5-O-glc; Cy-3,5-O-diglc; Cy-3-O-sam; Cy-3-O-glc; S. canadensis Cy-3-O-sam-5-O-glc; Cy-3,5-O-diglc; Cy-3-O-sam; Cy-3-O-glc; Cy-3-O-(6-O-E/Z-p-cou-2-O-β-D-xylopyranosyl)- β -D-glcpyr-5-O- β-D-glcpyr; Cy-3-O-(6-O-E-p-cou-2-O-b-D-xylopyranosyl)-O-b-D-glcpyr | 500 g of ripe fruit extracted with 2 L of 0.1% HCl in MeOH at room temp. (five repeated extractions) | - | Capcell Pak C18 (250 × 4.6 mm, 5 µm) | A: 0.5% Phosphoric acid in water; B: 0.5% Phosphoric acid in 60% THF 0–30 min, 10–100% B; 1.0 mL/min | HPLC-PDA 520 nm | To study the stability of anthocyanins | - | [51] |
| 3 | Elderberry powder (2001) | Cy-3-O-sam-5-O-glc; Cy-3-O-sam; Cy-3-O-glc; Cy | Commercial powder containing anthocyanins | - | ODS Hypersil (125 × 4.0 mm, 5 µm) | A: 0.5% Phosphoric acid in water; B: Water/ACN/AA/Phosphoric acid (50:48.5:1:0.5 v/v) 0–20 min, 20% B; 20–26 min, 20–60% B; 26–30 min, 60–20% B; 30–35 min, 20% B | HPLC-PDA-MS 520 nm | Anthocyanins method development in botanical supplement raw materials | - | [68] |
| 4 | Elderberry juice (2004) | Cy-3,5-O-diglc; Cy-3-O-sam; | Commercial juice concentrate | - | Capcell Pak C18 UG120 (150 × 4.6 mm, 5 µm) | A: 0.1% TFA in water; B: 0.1% TFA in 50% Acetonitrile 0–60 min, 15–30% B; 0.5 mL/min; 40 °C CT | HPLC-PDA-ESI-MS 520 nm | Chemical profiling of anthocyanins | Antioxidant activity (DPPH) | [19] |
| 5 | S. nigra and S. canadensis berries | Cy-3-O-sam-5-O-glc; Cy-3,5-O-diglc; Cy-3-O-sam; Cy-3-O-glc; Cy-3-O-rut; Pg-3-O-glc; Cy based anthocyanin; Dp-3-O-rut; Cy-3-O-(Z/E)-p-coumaroyl-sam-5-O-glc; Cy-3-O-p-coumaroyl-glc; Pt-3-O-rut; Cy-3-O-p-coumaroyl-sam; | 5 g of powdered sample extracted using 10 mL of acidified methanol (0.1%FA) for 10 min | Anthocyanins S. nigra: 391–806 mg/100 g FW; S. canadensis: 207–1005 mg/100 g FW | Synergi Hydro RP (150 × 2 mm, 4 µm) | A: Water: Acetonitrile: AA: TFA—84.8: 5: 10:0.2 v/v; B: Acetonitrile; 0–30 min, 99–90% A; 30–40 min, 90–70% A; 40–45 min, 70–60% A; 0.2 mL/min; 25 °C; | LC-MS | Anthocyanins and other polyphenolics variation study | - | [37] |
| 6 | S. nigra berries (2008) | Anthocyanins, flavonoids, and HCA in berries | 80% MeOH extraction solvent | - | Synergi Polar RP (150 × 2 mm) | Fruit: A: Water: Acetonitrile: AA: TFA—50.4:48.5:1:0.1 v/v; B: 0.1%TFA in Water; 0–20 min, 20–60% A; 20–21 min, 60–20% A; 21–30 min, 20% A; 30 °C CT; | HPLC-PDA | Phenolic compounds | - | [43] |
| 7 | S. nigra berries (2013) | Cy-3-O-sam-5-O-glc; Cy-3,5-O-diglc; Cy-3-O-sam; Cy-3-O-glc; | 5 g of berries extracted using acidified methanol (0.3% HCl v/v) | Anthocyanins: 272.9 mg/100 g FW | Zorbax SB-C18 (100 × 3.0 mm, 3.5 µm) | A: MeOH; B: 0.1% acetic acid in Water; 0–35 min, 5–42% A; 1.0 mL/min; 48 °C CT; | LC-MS | Chemical characterization of polyphenols | Antioxidant activity (DPPH) | [71] |
| 8 | S. nigra berries (2014) | Anthocyanins: Cy-3-O-sam-5-glc; Cy-3-O-sam; Cy-3-O-glc; | Fruit extracted with water and left to macerate for an hour | - | Aquasil C18 (150 × 2.1 mm, 5 µm) | A: 0.3% FA in acetonitrile; B: 0.3% FA in Water; 0–50 min, 28% A; 50–60 min, 28–57% A; 60–65 min, 57% A; 0.2 mL/min; 30 °C CT; | LC-MS | Polyphenol patterns in various berry extracts | Antioxidant activity (DPPH) | [72] |
| 9 | Elderberry juices (2015) | Cyanidin derivatives; Peonidin derivatives; Pelargonidin derivatives | Commercial juices | Phenolics: 2.2–7.2 mg/mL; Anthocyanins: 0.1–5.3 mg/mL | BEH RP C18 (50 × 2.1 mm, 1.7 µm) | A: 4.5% FA in Water; B: 0.1%FA in acetonitrile; 0–4 min, 5–95%B; 0.4 mL/min; | UPLC-MS/MS | Determination of anthocyanins and total polyphenols | - | [66] |
| 10 | S. nigra berries (2015) | Cy-3,5-O-diglc; Cy-3-O-sam-5-glc; Cy-3-O-glc; Cy-3-O-sam; Cy-3-O-rut; Pg-3-O-glc; Pg-3-O-sam | 70% EtOH–Water solution acidified by acetic acid | Crude extract: 6.0 mg/100 g DW; Purified extract: 48.5 mg/100 g DW | Gemini C18 (150 × 3 mm, 5.0 µm) | A: 0.1% FA in 3% acetonitrile; B: 0.1% FA in 3% Water; 0.25 mL/min; 45 °C CT; | LC-MS/IT-TOF | Identification and quantification of anthocyanins | - | [69] |
| 11 | Elderberry juice (2019) | Cy-3-O-sam-5-O-glc; Cy-3-O-sam; Cy-3-O-glc | - | Anthocyanin content: 1.1 mg/mL | Aqua C18 (150 × 4.6 mm, 5 µm) | A: 0.1% TFA in Water; B: Acetonitrile; Gradient program; | LC-MSn | Anthocyanin profile of elderberry juice | Antioxidant activity (DPPH) | [67] |
| 12 | S. nigra fruit (2020) | Cy-hex; Pg-sambu; Cy-pent-hex; Cy-rha-hex; Cy-dihex; Cy-sambu-rha/glc; Dp-dirham-hex | 2 g of sample extracted using 10 mL of 1% formic acid in methanol | - | Anthocyanins: Pursuit C18 (150 × 2 mm, 3 µm) | Anthocyanins: A: Water; B: 0.1% FA in MeOH; 0–6 min, 10–15%B; 6–12 min, 15–25%B; 12–16 min, 25–30%B; 16–30 min, 30%B; 30–42 min, 30–100%B; 0.2 mL/min | HPLC-DAD-HRMS | Identification of polyphenolic compounds in berries | - | [73] |
| 13 | Sambucus spp berries and commercial products and adulterants | Anthocyanins: Cy-3-O-sam-5-O-glc; Cy-3-O-sam; Cy-3-O-glc; Cy-3-O-(6-O-E-p-cou-2-O-β-D-xylopyranosyl)-β-D-glcpyr-5-O-β-D-glcpyr | 0.5 g of powdered sample sonicated in 2 mL of acidified methanol (1% FA) for 15 min. Procedure repeated four more times | - | HSS C18 (150 × 2.1 mm, 1.8 µm) | A: 1%FA in Water; B: 1%FA in acetonitrile; 0–8 min, 11–23%B; 8–13 min, 23–35%B; 13–18 min, 35–100%B; 0.135 mL/min; 45 °C CT; | LC-QDa-MS and LC-QToF-MS | Chemical profiling of anthocyanins and flavonoids (qualitative and quantitative) | - | [16] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Osman, A.G.; Avula, B.; Katragunta, K.; Ali, Z.; Chittiboyina, A.G.; Khan, I.A. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules 2023, 28, 3148. https://doi.org/10.3390/molecules28073148
Osman AG, Avula B, Katragunta K, Ali Z, Chittiboyina AG, Khan IA. Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules. 2023; 28(7):3148. https://doi.org/10.3390/molecules28073148
Chicago/Turabian StyleOsman, Ahmed G., Bharathi Avula, Kumar Katragunta, Zulfiqar Ali, Amar G. Chittiboyina, and Ikhlas A. Khan. 2023. "Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders" Molecules 28, no. 7: 3148. https://doi.org/10.3390/molecules28073148
APA StyleOsman, A. G., Avula, B., Katragunta, K., Ali, Z., Chittiboyina, A. G., & Khan, I. A. (2023). Elderberry Extracts: Characterization of the Polyphenolic Chemical Composition, Quality Consistency, Safety, Adulteration, and Attenuation of Oxidative Stress- and Inflammation-Induced Health Disorders. Molecules, 28(7), 3148. https://doi.org/10.3390/molecules28073148









