Antifoaming Agent for Lubricating Oil: Preparation, Mechanism and Application
Abstract
:1. Introduction
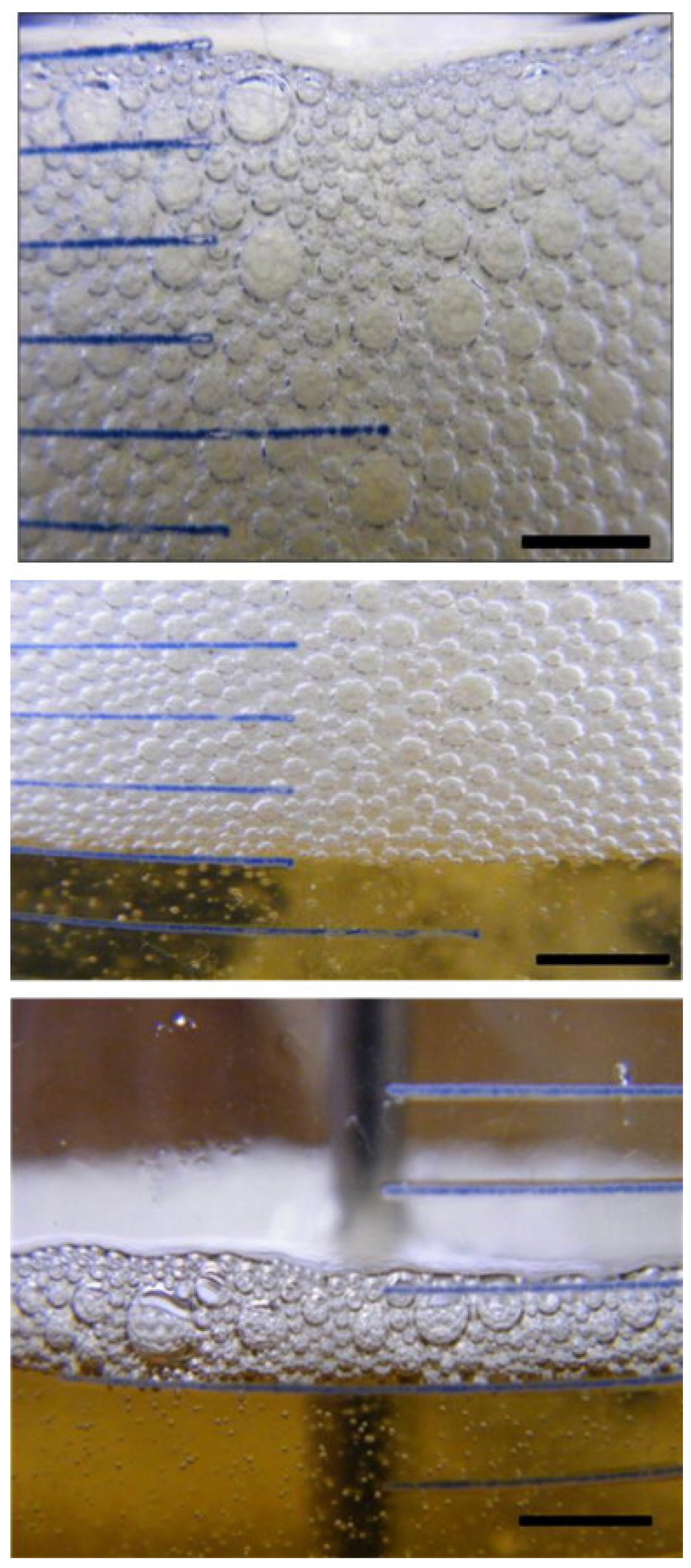
2. Formation and Harm of Foam
2.1. Formation of Foam
2.2. Harm of Foam
- (1)
- Degradation of lubrication and wear reduction performance:Foam destroys the continuity of the oil film at the friction pair where relative sliding occurs, reduces lubrication performance and causes the parts to lose sufficient lubrication protection, resulting in serious wear and even sintering [9].
- (2)
- Degradation of cooling and heat dissipation performance:Partial heat of mechanical equipment can be carried away and dissipated by the lubricating oil when it circulates. However, a large amount of air contained in lubricating oil affects the cooling effect and the heat dissipation effect of the lubricating oil on the machine [15].
- (3)
- Degradation of the cleaning and dispersing effect:The contact area between oil and air increases due to foam, and the oxidative metamorphism of lubricating oil at high temperatures intensifies, generating more carbides and sludge; at the same time, lubricating oil with insufficient fluidity cannot adequately flush away the dirty stuffs on the working surface of the parts [9].
- (4)
- Degradation of the anticorrosion and antirust effect:Lubricating oil is absorbed on the surface of the parts to form a layer of oil film to isolate oxygen, water, acidic substances and harmful gases in the air to prevent corrosion. Foam not only destroys the oil film but also releases bubbles at high temperatures, creating cavitation [9].
- (5)
- Phenomenon of air lock and flow interruption:Because of gas in the oil, on the one hand, the oil produces certain compressibility, which affects pressure transmission; on the other hand, steam resistance is generated, which blocks the oil circuit and affects the oil supply, thus affecting power transmission, making the system unable to work normally, or even interrupting flow and making the lubrication system unable to work normally [16].
- (6)
- Aggravating oxidation and deterioration of lubricating oil:When bubbles are generated on the surface or inside the tank, the contact area between the lubricating oil and air increases and, coupled with an increase in oil temperature, aggravates the oxidation and deterioration of the base oil, resulting in a large accumulation of sludge at the bottom of the tank [17].
- (7)
- Potential safety hazard:Foam in the lubricating oil increases the volume of the lubricating oil, and lubricating oil may overflow from the oil tank, resulting in oil loss, fire and other unsafe factors [18].
3. Defoaming Mechanism
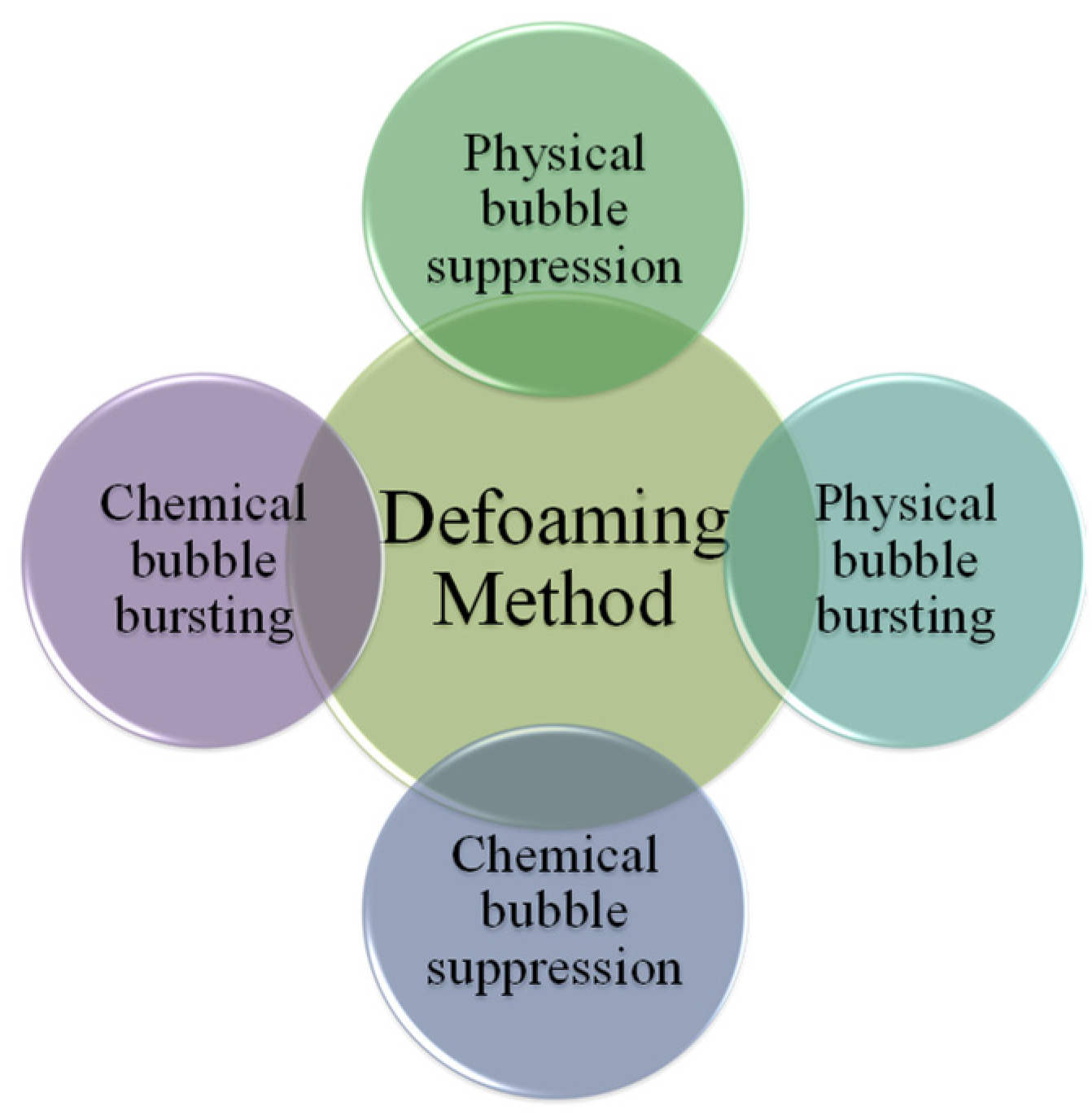
4. Defoaming Methods
4.1. Physical Defoaming
4.1.1. Physical Bubble Suppression
4.1.2. Physical Bubble Bursting
4.2. Chemical Defoaming
4.2.1. Chemical Bubble Suppression
4.2.2. Chemical Bubble Bursting
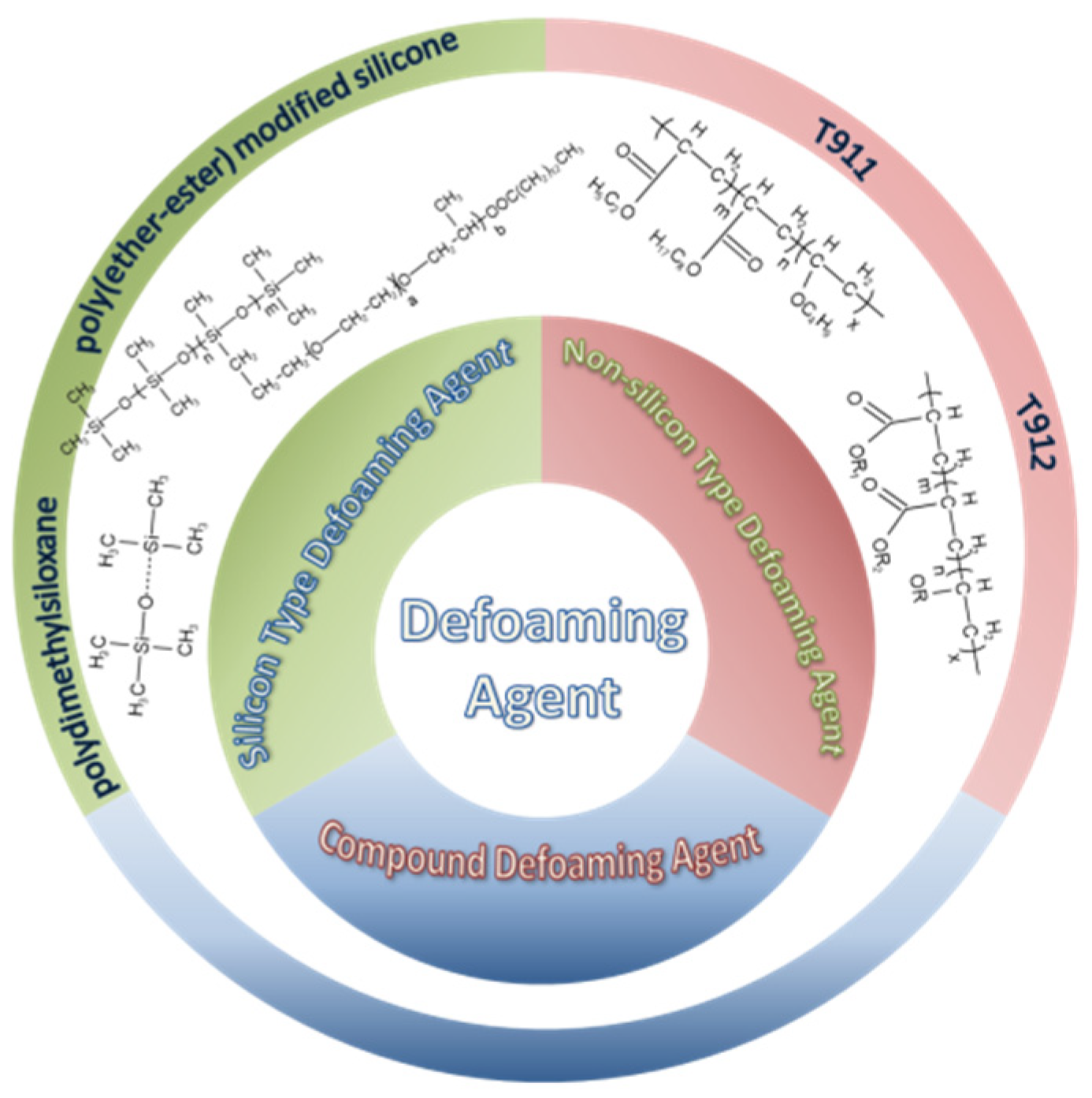
4.3. Defoaming Agent
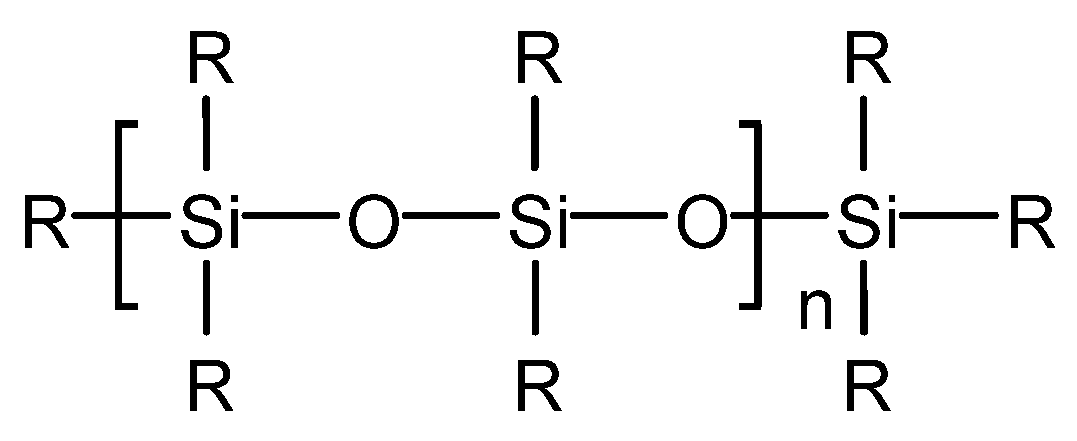
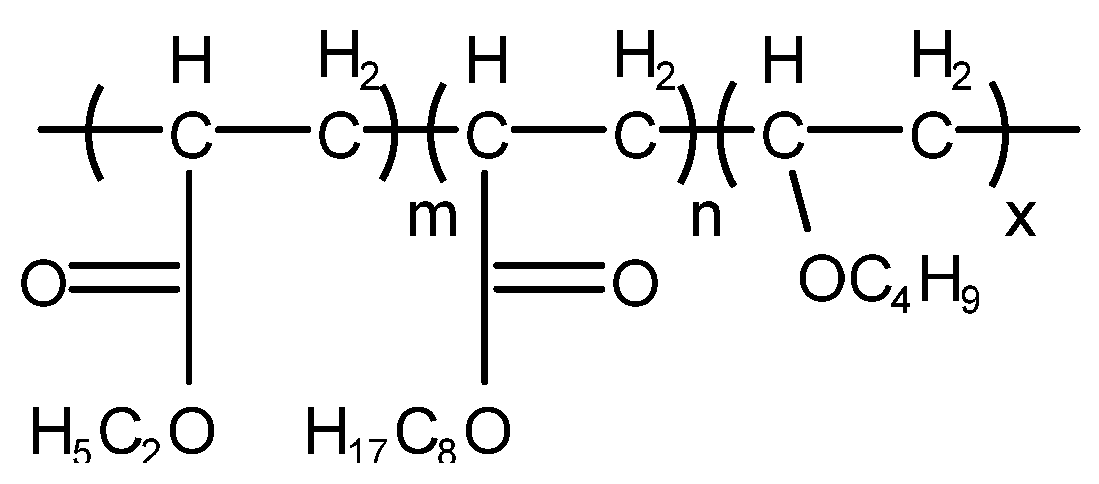
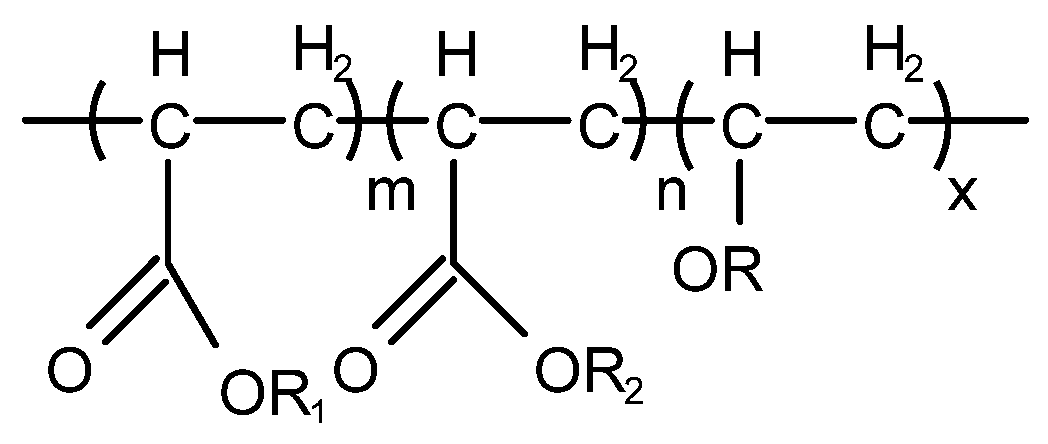
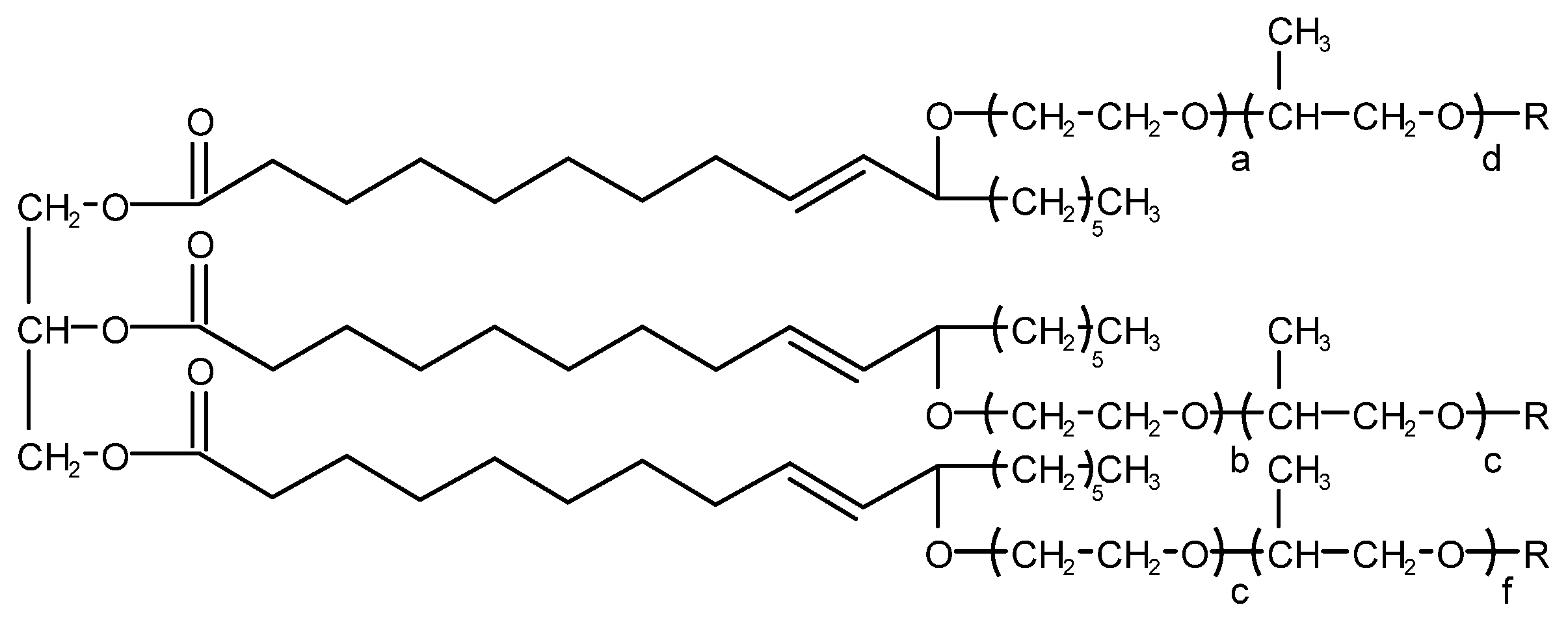
4.3.1. Silicone-Type Defoaming Agent
4.3.2. Non-Silicone-Type Defoaming Agent
4.3.3. Compound Defoaming Agent
| Defoaming Agents | Dosage | Foaming Characteristics (Foam Tendency/Foam Stability) (24 °C, mL/mL) | Oil for Test | Data Source | |
|---|---|---|---|---|---|
| silicone-type defoaming agent | Polydimethylsiloxane | 0 | 650/600 | TBN25 marine medium-speed oil | [12] [48] [49] [50] |
| (T901) | 0.03% | 570/470 | |||
| Non-silicone-type defoaming agent | Acrylate ether copolymer | 0 | 435/20 | Medium extreme-pressure gear oil | [51] |
| T911 | 0.03%~0.1% | 0/0 | [52] | ||
| Acrylate ether copolymer | 0 | 650/600 | TBN25 marine medium-speed oil | [51] | |
| T912 | 0.14% | 570/280 | [50] | ||
| 2-EHA/VAC copolymer high-efficiency defoaming agent | 0 | 600/520 | Cold heading gear oil | [43] | |
| 0.05% | 0/0 | ||||
| T921 | 0 | / | Advanced anti-wear hydraulic oil | [52] | |
| 0.005%~0.1% | 5/0 | ||||
| Compound defoaming agent | T922 | 0 | 620/560 | Shanghai 4040 medium-speed engine oil | [45] |
| 0.1% | 355/0 | ||||
| T923 | 0 | 620/560 | Shanghai 4040 medium-speed engine oil | [45] | |
| 0.05% | 10/0 | ||||
| 410 | 0 | 570/530 | Diesel engine three-generation oil | [46] | |
| 0.02% | 10/0 | ||||
| 412 | 0 | 650/600 | TBN25 marine medium-speed oil | [50] | |
| 0.1% | 10/0 | ||||
| Compound defoaming agent 1 | 0 | 240/30 (150 °C) | Internal combustion engine oil | [47] | |
| 0.005% | 70/0 (150 °C) | SL5W-30 | |||
| Compound defoaming agent 2 | 0 | 210/0 (150 °C) | Internal combustion engine oil | [47] | |
| 0.01% | 70/0 (150 °C) | SM5W-30 | |||
| Compound defoaming agent 3 | 0 | 210/0 (150 °C) | Internal combustion engine oil | [47] | |
| 0.005% | 40/0 (150 °C) | SM5W-30 | |||
| Compound defoaming agent 4 | 0 | 210/0 (150 °C) | Internal combustion engine oil | [47] | |
| 0.005% | 30/0 (150 °C) | SM5W-30 | |||
| Compound defoaming agent 5 | 0 | 190/10 (150 °C) | Continuously variable transmission oil | [47] | |
| 0.005% | 50/0 (150 °C) | CVTF | |||
5. Foam Resistance Parameters of Defoaming Agent
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Xia, L.; Long, J.; Zhao, Y.; Wu, Z.; Dai, Z.; Wang, L. Molecular Dynamics Simulation on the Aggregation of Lubricant Oxidation Products. Tribol. Lett. 2018, 66, 104. [Google Scholar] [CrossRef]
- Dyson, C.J.; Priest, M.; Lee, P.M. Simulating the Misting of Lubricant in the Piston Assembly of an Automotive Gasoline Engine: The Effect of Viscosity Modifiers and Other Key Lubricant Components. Tribol. Lett. 2022, 70, 49. [Google Scholar] [CrossRef]
- Tuszynski, W.; Michalczewski, R.; Piekoszewski, W.; Szczerek, M. Effect of ageing automotive gear oils on scuffing and pitting. Tribol. Int. 2008, 41, 875–888. [Google Scholar] [CrossRef]
- Mohamed, A.; Ali, S.; Osman, T.A.; Kamel, B.M. Development and manufacturing an automated lubrication machine test for nano grease. J. Mater. Res. Technol. 2020, 9, 2054–2062. [Google Scholar] [CrossRef]
- Li, Z.T. Discussion on the mechanism and characteristics of antifoaming agent for lubricating oils. Synth. Lubr. 2020, 47, 38–41. [Google Scholar]
- Liu, Y.F.; Ge, X.Y.; Li, J.J. Graphene lubrication. Appl. Mater. Today 2020, 20, 100662. [Google Scholar] [CrossRef]
- Ge, X.Y.; Chai, Z.Y.; Shi, Q.Y.; Liu, Y.F.; Wang, W.Z. Graphene superlubricity: A review. Friction 2023. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, S.; Li, J.; Ge, X.; Zhao, Z.; Wang, W. Quantum dots of graphene oxide as nano-additives trigger macroscale superlubricity with an extremely short running-in period. Mater. Today Nano 2022, 18, 100219. [Google Scholar] [CrossRef]
- Cen, Z.Y.; Chen, B.Y.; Yang, S.J. Talking about antifoaming agents in lubricating oils. J. Shandong Ind. Technol. 2019, 6, 38. [Google Scholar] [CrossRef]
- Mao, J.X.; Hu, J.Q.; Xu, X.; Guo, L. Application and development of defoaming method in lubricating oil. Chem. Ind. Times 2019, 33, 34–36. [Google Scholar]
- Zhan, C.; Saint-Jalmes, A.; Receveur, M.; El Bahi, H.; Rondelez, F.; Leroy, V. Detailed characterization of aeration in lubricating oils by an ultrasonic approach. Tribol. Int. 2022, 175, 107782. [Google Scholar] [CrossRef]
- Binks, B.P.; Davies, C.A.; Fletcher, P.D.I.; Sharp, E.L. Non-aqueous foams in lubricating oil systems. Colloids Surf. A 2010, 360, 198–204. [Google Scholar] [CrossRef]
- Kichkin, G.I. Foam formation in lubricating oils. Chem. Technol. Fuels Oils 1966, 2, 272–275. [Google Scholar] [CrossRef]
- Faujdar, E.; Negi, H.; Singh, R.K.; Varshney, V.K. Study on Biodegradable Poly(α-Olefins–co–α-Pinene) Architectures as Pour Point Depressant and Viscosity Index Improver Additive for Lubricating Oils. J. Polym. Environ. 2020, 28, 3019–3027. [Google Scholar] [CrossRef]
- Zhao, R.P. Causes, hazards and treatment measures of lubricating oil foam in gear box of wind turbine. Constr. Mach. Equip. 2020, 51, 108–112. [Google Scholar]
- Zou, X.R. The cause, harm and treatment of foaming of lubricating oil. Mech. Eng. Autom. 2001, S1, 164–165. [Google Scholar]
- Wu, Y. Study on the Foaming Problems of the Cold Heading Process Lubricating Oil; East China University of Science and Technology: Shanghai, China, 2015. [Google Scholar]
- Chang, J.H. Study on the Problems of Gear Oil Bubble Properties; East China University of Science and Technology: Shanghai, China, 2015. [Google Scholar]
- Xie, J.R. Talking about the effect of antifoaming agent on the performance of lubricating oil. N. Technol. New Prod. China 2013, 11, 20. [Google Scholar]
- Li, W.D.; Wang, D.; Liu, H.Y. Silicone oil’s dispersancy and anti-foaming characteristics in oil. Lubr. Oil 1996, 11, 33–35. [Google Scholar]
- Jiao, X.S. Preparation and Application of Antifoaming Agent; China Light Industry Press: Beijing, China, 2004; pp. 2–5. [Google Scholar]
- Wang, C.W.; Ni, H.J.; Wang, R.H.; Du, Y.K. Defoaming technology for foam drilling fluid. Drill. Prod. Technol. 2011, 34, 106–108. [Google Scholar]
- Blázquez, C.; Dalmazzone, C.; Emond, E.; Schneider, S. Crude Oil Foams: Testing and Ranking of Antifoams with the Depressurization Test. Energy Fuels 2017, 31, 1285–1294. [Google Scholar] [CrossRef]
- Kang, S.; Li, R.; Wu, Z.; Guo, S.; Gao, Y. Effective improvement of defoaming efficiency using foam breaker with synthetic sponge cylinders in foam fractionation. Chem. Eng. Process. 2016, 106, 26–32. [Google Scholar] [CrossRef]
- Garrett, P.R. Defoaming: Antifoams and mechanical methods. Curr. Opin. Colloid Interface Sci. 2015, 20, 81–91. [Google Scholar] [CrossRef]
- Chang, Q. Chapter 11—Emulsion, Foam, and Gel. In Colloid and Interface Chemistry for Water Quality Control; Chang, Q., Ed.; Academic Press: Beijing, China, 2016; pp. 227–245. [Google Scholar]
- Ge, C.C.; Wang, Y.S.; Yu, H.W.; Wei, Z. Study on foam and antifoaming agent. Dev. Appl. Mater. 2010, 25, 81–85. [Google Scholar]
- Zhu, M.Y. Study on Preparation and Performance of Multifunctional Composite Antifoam Agent; Zhengzhou University: Zhengzhou, China, 2020. [Google Scholar]
- Wei, Y.; Deng, C.L.; Xiao, Y.; Li, J.J.; Tang, X.D. Research on defoaming method of LuKeQin foam heavy oil. Yunnan Chem. Technol. 2019, 46, 16–18. [Google Scholar]
- Feng, Z.F. Review of antifoaming agents for lubricating oils. In Proceedings of the 2001 Annual Meeting of China Petroleum Lubricant Science and Technology Information Station, Beijing, China, 1 September 2001; pp. 310–317. [Google Scholar]
- Zhang, L.; Wang, Y. Effect of antifoaming agent on lubricating oil. Lubr. Oil 2019, 34, 40–43. [Google Scholar]
- Zhang, F.J.; Wang, M. Research progress of silicone defoamer. J. Xuchang Univ. 2012, 31, 76–79. [Google Scholar]
- Cevada, E.; Hernández, E.; Flores, C.; Zavala, G.; Álvarez, F.; Vázquez, F. Novel silicon free defoaming agents, based on alkylacrylates, for petroleum: Effect of the molecular weight on their efficiency. Fuel 2020, 278, 118401. [Google Scholar] [CrossRef]
- Dou, Y.C.; Guo, R.; Qiao, Y.; Shi, D.N. Preparation of poly(ether-ester) modified silicone defoaming agent. Fine Chem. 2014, 31, 36–39. [Google Scholar]
- An, Q.F.; Guo, K.; Li, M.T.; Huang, L.X. Synthesis and characterization of polyether-b-polysiloxane and its applications in antifoaming agent. Silicone Mater. 2008, 22, 344–348. [Google Scholar]
- Hu, T.; Zhang, G.X.; Lu, Y.; Zhang, Y.; Cheng, Y.; Wei, Q. Synthesis of silicone defoamer and evaluation of its residue defoaming performance. Spec. Petrochem. 2022, 39, 28–30. [Google Scholar]
- Guo, L.; Hu, J.Q.; Yao, T. Antifoam Additives in Lubricants. Chem. Ind. Times 2015, 29, 27–29. [Google Scholar]
- Feng, H.C.; Ge, Q.W.; Wang, X.L.; Wang, Y.H.; Ji, C.Y. Effect of the defoaming agent on the properties of lubricating oil. Lubr. Oil 2010, 25, 24–27. [Google Scholar]
- Huang, W.X. Lecture 17: The action mechanism, main varieties and applications of antifoaming agents. Pet Prod. Appl. Res. 2018, 36, 83–94. [Google Scholar]
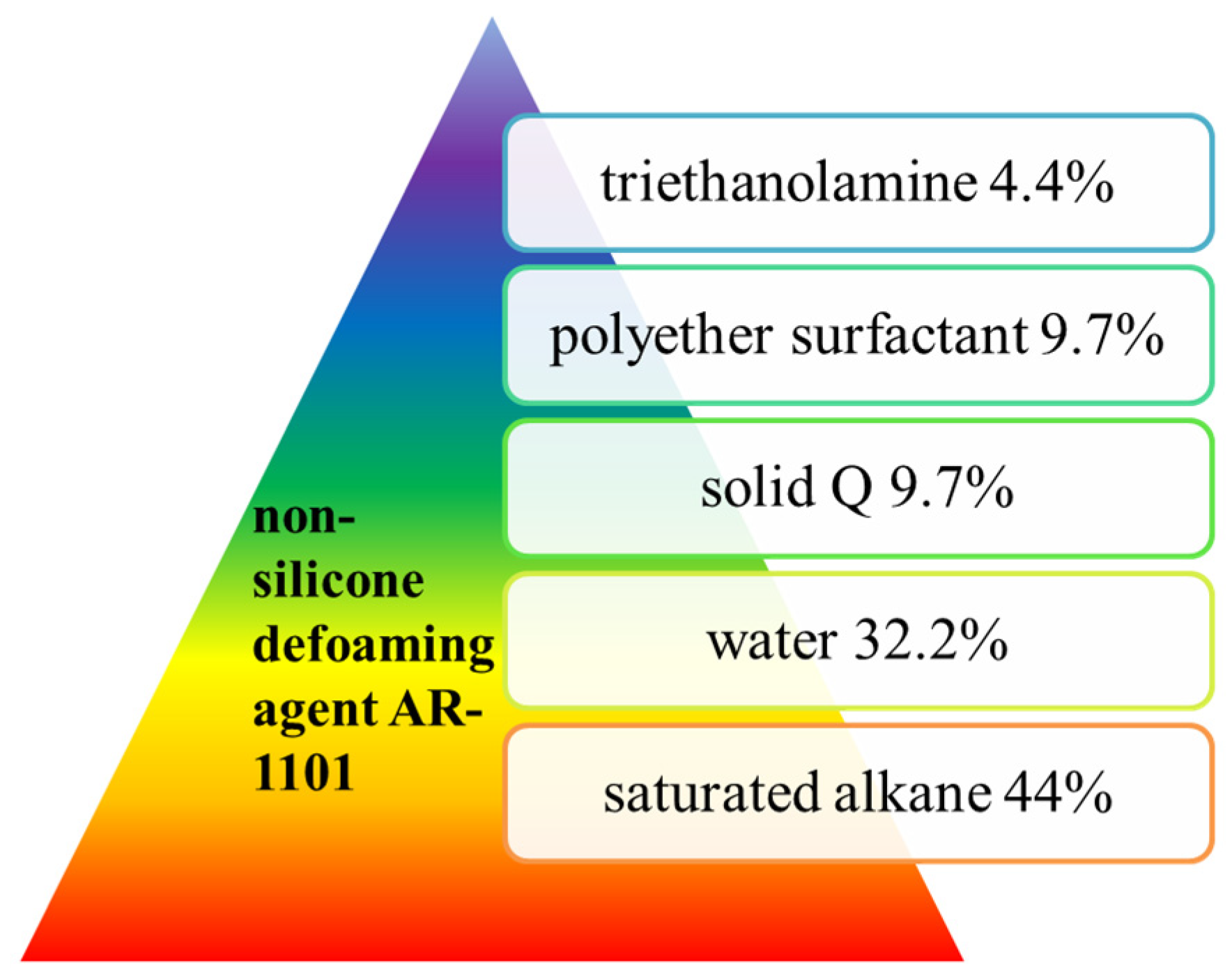
- Zhou, H.; Wang, Z.X.; Li, J.M.; Lin, C.M. Preparation and application of AR-1101 non-silicon defoaming agent. Text Aux 1987, 4, 25–29. [Google Scholar]
- Li, L.; Xiong, J. A Terpolymer Type Non-Silicone Anti-Foaming Agent and Its Preparation Method. China Patent CN108359513B, 11 May 2021. [Google Scholar]
- Chen, Y.J.; Zhang, Z.; Liu, Y.; Cao, T.; Huang, W.; D’Arcy, A.K.; Chen, J. A Non-Silicone Defoamer and Its Preparation Method. China Patent CN108786189B, 2 July 2019. [Google Scholar]
- Wu, Y.; Li, S.P.; Chang, J.H. Synthesis and performance of 2-EHA/VAC copolymer super-antifoaming agents for lubricating oils. Mod. Chem. Ind. 2014, 34, 92–95. [Google Scholar]
- Chen, P.H.; Jiang, H.L.; Shu, H.Y.; Zhao, T.T. Study and progress trend of organicsilicon antifoam. Jiangxi Chem. Ind. 2007, 3, 5–7. [Google Scholar]
- Wang, K.Y.; Xu, W. Preparation and application of No.3 compound anti-foaming agent. Lubr. Oil 2002, 17, 48–52. [Google Scholar]
- Cao, Y.F. Development and application of 410 antifoam package. Lubr. Oil 1997, 12, 43–45. [Google Scholar]
- Xu, W.; Shui, L.; Zhang, J.; Zhang, G.R.; Zhou, Y.; Song, Z.X.; Xue, Y.L.; An, A.F.; Chen, L.; Zhao, H.P. The Utility Model Relates to a Compound Antifoaming Agent for Lubricating Oil and Its Application. China Patent CN104232246B, 1 February 2017. [Google Scholar]
- Chen, J.; Huang, X.; He, L.; Luo, X. Foaming of Oils: Effect of Poly(dimethylsiloxanes) and Silica Nanoparticles. ACS Omega 2019, 4, 6502–6510. [Google Scholar] [CrossRef] [Green Version]
- Politova-Brinkova, N.; Hristova, M.; Georgiev, V.; Tcholakova, S.; Denkov, N.; Grandl, M.; Achenbach, F. Role of surfactant adsorption and surface properties for the efficiency of PDMS-silica antifoams. Colloids Surf. A 2021, 610, 125747. [Google Scholar] [CrossRef]
- Liu, H.C. Application of 412 compound anti-foam agent. Lubr. Oil 1998, 13, 45–46. [Google Scholar]
- Hu, N.; Hu, M.M.; Li, X.; Li, Z.X.; Pan, M.H.; Gao, L.F.; Yin, J.H. Study on Preparation and Performance of a Highly Efficient GPES Defoamer. J. Salt. Sci. Chem. Ind. 2021, 50, 9–13. [Google Scholar]
- Wang, K.Y. The properties and application of series products of non-silicon antifoamers. Lubr. Oil 1993, 36–38. [Google Scholar]
- Cevada, E.; Fuentes, J.V.; Zamora, E.B.; Hernandez, E.I.; Flores, C.A.; Zavala, G.; Alvarez-Ramirez, F.; Vazquez, F. Effect of the Chemical Structure of Alkyl Acrylates on Their Defoaming Activity in Crude Oil: Experimental and Theoretical Studies. Energy Fuels 2021, 35, 9047–9058. [Google Scholar] [CrossRef]
- Zhu, T.I.; Li, M.; Cheng, L.; Liu, X.L.; Zhu, J.; Ma, X.Y.; Sun, X.T. Types and characteristics introduction of antifoaming agent. Lubr. Oil 2017, 32, 23–25. [Google Scholar]
- Wang, K.Y. Study on application of complex antifoamers. Pet. Process. Petrochem. 1994, 25, 15–19. [Google Scholar]
- Pugh, R.J. Foaming, foam films, antifoaming and defoaming. Adv. Colloid Interface Sci. 1996, 64, 67–142. [Google Scholar] [CrossRef]
- McClure, D.D.; Lamy, M.; Black, L.; Kavanagh, J.M.; Barton, G.W. An experimental investigation into the behaviour of antifoaming agents. Chem. Eng. Sci. 2017, 160, 269–274. [Google Scholar] [CrossRef]
- Hu, N.; Hu, M.M.; Li, Z.X.; Li, X.; Gao, L.F.; Yin, J.H. Research Progress and Prospect of Defoamer. J. Salt. Sci. Chem. Ind. 2021, 50, 10–16. [Google Scholar]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, C.; Zhang, X.; Jia, M.; Ma, C.; Li, J.; Shi, M.; Niu, Y. Antifoaming Agent for Lubricating Oil: Preparation, Mechanism and Application. Molecules 2023, 28, 3152. https://doi.org/10.3390/molecules28073152
Ren C, Zhang X, Jia M, Ma C, Li J, Shi M, Niu Y. Antifoaming Agent for Lubricating Oil: Preparation, Mechanism and Application. Molecules. 2023; 28(7):3152. https://doi.org/10.3390/molecules28073152
Chicago/Turabian StyleRen, Chenfei, Xingxing Zhang, Ming Jia, Chenming Ma, Jiaxin Li, Miaomiao Shi, and Yunyin Niu. 2023. "Antifoaming Agent for Lubricating Oil: Preparation, Mechanism and Application" Molecules 28, no. 7: 3152. https://doi.org/10.3390/molecules28073152
APA StyleRen, C., Zhang, X., Jia, M., Ma, C., Li, J., Shi, M., & Niu, Y. (2023). Antifoaming Agent for Lubricating Oil: Preparation, Mechanism and Application. Molecules, 28(7), 3152. https://doi.org/10.3390/molecules28073152






