The Facet Dependence of CO2 Electroreduction Selectivity on a Pd3Au Bimetallic Catalyst: A DFT Study
Abstract
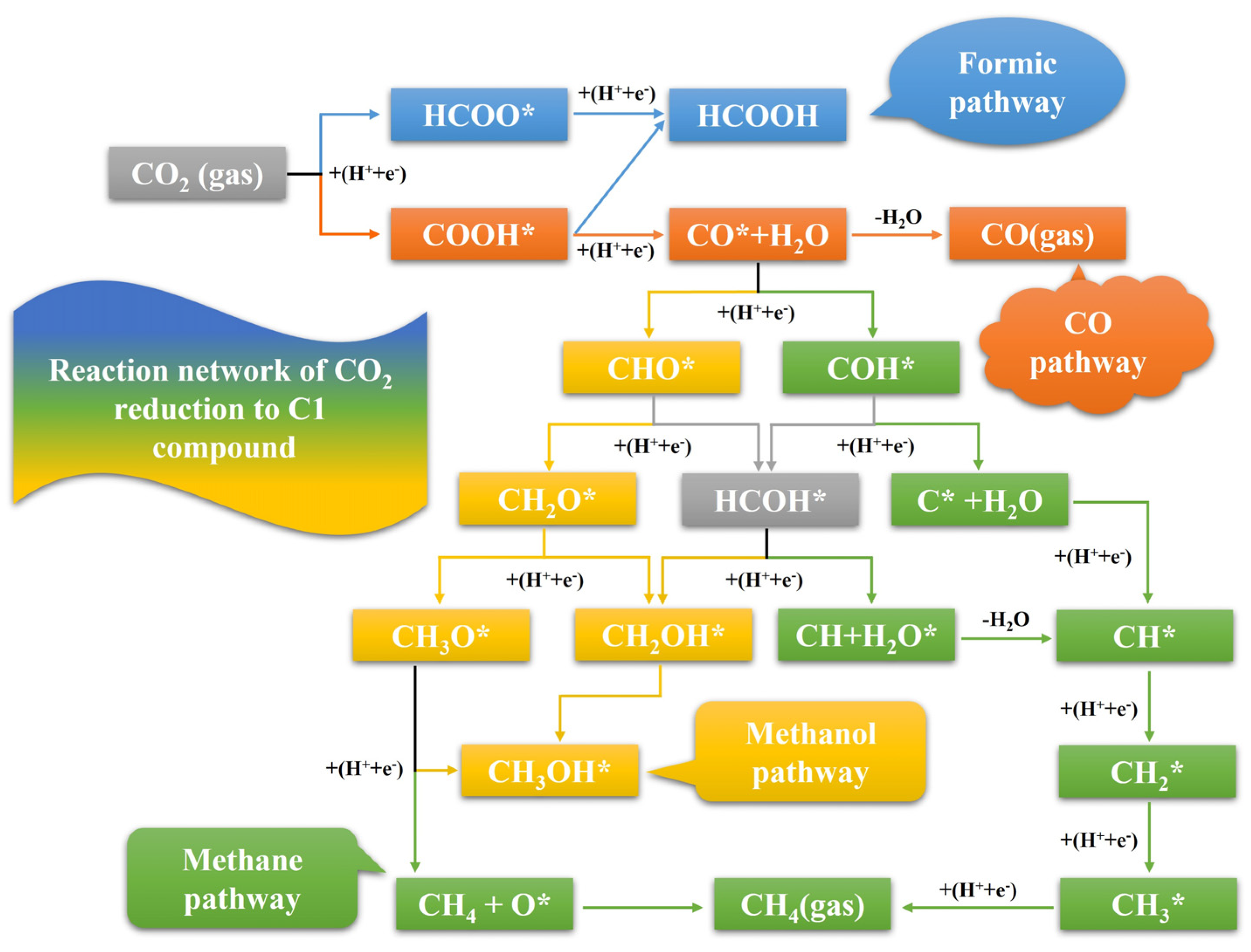
1. Introduction
2. Results and Discussions
2.1. The Properties of Different Pd3Au Surface
2.2. Competitive HER Reaction and Formic Acid Formation
2.3. Formation and Competition of Methane and Formic Acid
3. Computational Method
3.1. Models
3.2. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, D.; Xu, X.; Qin, Y.; Jiang, S.P.; Shao, Z. Rational Design of Ag-Based Catalysts for the Electrochemical CO2 Reduction to CO: A Review. Chemsuschem 2020, 13, 39–58. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; He, X.; Wang, W.; Xie, S.; Zhang, Q.; Wang, Y. Electrocatalytic reduction of CO2 and CO to multi-carbon compounds over Cu-based catalysts. Chem. Soc. Rev. 2021, 50, 12897–12914. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.L.; Wang, J.; Ma, Y.B.; Zhou, J.W.; Wang, Y.H.; Lu, P.Y.; Yin, J.W.; Ye, R.Q.; Zhu, Z.L.; Fan, Z.X. Recent Progresses in Electrochemical Carbon Dioxide Reduction on Copper-Based Catalysts toward Multicarbon Products. Adv. Funct. Mater. 2021, 31, 2102151. [Google Scholar] [CrossRef]
- Loiudice, A.; Lobaccaro, P.; Kamali, E.A.; Thao, T.; Huang, B.H.; Ager, J.W.; Buonsanti, R. Tailoring Copper Nanocrystals towards C2 Products in Electrochemical CO2 Reduction. Angew. Chem. Int. Ed. 2016, 55, 5789–5792. [Google Scholar] [CrossRef] [PubMed]
- Hori, Y.; Takahashi, I.; Koga, O.; Hoshi, N. Electrochemical reduction of carbon dioxide at various series of copper single crystal electrodes. J. Mol. Catal. A-Chem. 2003, 199, 39–47. [Google Scholar] [CrossRef]
- De Gregorio, G.L.; Burdyny, T.; Loiudice, A.; Iyengar, P.; Smith, W.A.; Buonsanti, R. Facet-Dependent Selectivity of Cu Catalysts in Electrochemical CO2 Reduction at Commercially Viable Current Densities. ACS Catal. 2020, 10, 4854–4862. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, G.; Zhang, Z.; Jin, M.; Yin, Y. Selectivity on Etching: Creation of High-Energy Facets on Copper Nanocrystals for CO2 Electrochemical Reduction. ACS Nano 2016, 10, 4559–4564. [Google Scholar] [CrossRef]
- Hoshi, N.; Kato, M.; Hori, Y. Electrochemical reduction of CO2 on single crystal electrodes of silver Ag(111), Ag(100) and Ag(110). J. Electroanal. Chem. 1997, 440, 283–286. [Google Scholar] [CrossRef]
- Hoshi, N.; Noma, M.; Suzuki, T.; Hori, Y. Structural effect on the rate of CO2 reduction on single crystal electrodes of palladium. J. Electroanal. Chem. 1997, 421, 15–18. [Google Scholar] [CrossRef]
- Klinkova, A.; De Luna, P.; Dinh, C.-T.; Voznyy, O.; Larin, E.M.; Kumacheva, E.; Sargent, E.H. Rational Design of Efficient Palladium Catalysts for Electroreduction of Carbon Dioxide to Formate. ACS Catal. 2016, 6, 8115–8120. [Google Scholar] [CrossRef]
- Singh, S.; Gautam, R.; Malik, K.; Verma, A. Ag-Co bimetallic catalyst for electrochemical reduction of CO2 to value added products. J. CO2 Util. 2017, 18, 139–146. [Google Scholar] [CrossRef]
- Lee, S.; Park, G.; Lee, J. Importance of Ag-Cu Biphasic Boundaries for Selective Electrochemical Reduction of CO2 to Ethanol. Acs Catal. 2017, 7, 8594–8604. [Google Scholar] [CrossRef]
- Jedidi, A.; Rasul, S.; Masih, D.; Cavallo, L.; Takanabe, K. Generation of Cu–In alloy surfaces from CuInO2 as selective catalytic sites for CO2 electroreduction. J. Mater. Chem. A 2015, 3, 19085–19092. [Google Scholar] [CrossRef]
- Hahn, C.; Hatsukade, T.; Kim, Y.-G.; Vailionis, A.; Baricuatro, J.H.; Higgins, D.C.; Nitopi, S.A.; Soriaga, M.P.; Jaramillo, T.F. Engineering Cu surfaces for the electrocatalytic conversion of CO2: Controlling selectivity toward oxygenates and hydrocarbons. Proc. Natl. Acad. Sci. USA 2017, 114, 5918–5923. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Cheng, K.; Quan, X.; Fan, X.; Su, Y.; Chen, S.; Zhao, H.; Zhang, Y.; Yu, H.; et al. Selective Electrochemical Reduction of Carbon Dioxide to Ethanol on a Boron- and Nitrogen-Co-doped Nanodiamond. Angew. Chem. Int. Ed. 2017, 56, 15607–15611. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Choi, J.; Kim, H.; Hwang, E.; Ha, D.-H.; Ahn, S.H.; Kim, S.-K. AgIn dendrite catalysts for electrochemical reduction of CO2 to CO. Appl. Catal. B-Environ. 2017, 219, 123–131. [Google Scholar] [CrossRef]
- Kortlever, R.; Peters, I.; Balemans, C.; Kas, R.; Kwon, Y.; Mul, G.; Koper, M.T. Palladium-gold catalyst for the electrochemical reduction of CO2 to C1-C5 hydrocarbons. Chem. Commun. 2016, 52, 10229–10232. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, M.; Wang, X.; Zhou, X. Electrocatalytic Reduction of CO2 to C1 Compounds by Zn-Based Monatomic Alloys: A DFT Calculation. Catalysts 2022, 12, 1617. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, X.; Wang, Y.; Chen, G.; Li, M. Theoretical study on the reduction mechanism of CO2 to HCOOH on Pd3Au: An explicit solvent model is essential. J. Mater. Chem. A 2023, 11, 6591–6602. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.B.; Norskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Peterson, A.A.; Norskov, J.K. Activity Descriptors for CO2 Electroreduction to Methane on Transition-Metal Catalysts. J. Phys. Chem. Lett. 2012, 3, 251–258. [Google Scholar] [CrossRef]
- Clark, E.L.; Ringe, S.; Tang, M.; Walton, A.; Hahn, C.; Jaramillo, T.F.; Chan, K.; Bell, A.T. Influence of Atomic Surface Structure on the Activity of Ag for the Electrochemical Reduction of CO2 to CO. ACS Catal. 2019, 9, 4006–4014. [Google Scholar] [CrossRef]
- Srivastava, A.K. Single- and double-electron reductions of CO2 by using superalkalis: An ab initio study. Int. J. Quantum Chem. 2018, 118, e25598. [Google Scholar] [CrossRef]
- Czapla, M.; Skurski, P. Oxidizing CO2 with superhalogens. Phys. Chem. Chem. Phys. 2017, 19, 5435–5440. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Esopi, M.R.; Janik, M.J.; Asthagiri, A. Selectivity of CO2 Reduction on Copper Electrodes: The Role of the Kinetics of Elementary Steps. Angew. Chem. Int. Ed. 2013, 52, 2459–2462. [Google Scholar] [CrossRef]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 Electrochemical Reduction to Methane and Methanol on Copper-Based Alloys: Theoretical Insight. J. Phys. Chem. C 2015, 119, 8238–8249. [Google Scholar] [CrossRef]
- Shi, C.; Hansen, H.A.; Lausche, A.C.; Nørskov, J.K. Trends in electrochemical CO2 reduction activity for open and close-packed metal surfaces. Phys. Chem. Chem. Phys. 2014, 16, 4720–4727. [Google Scholar] [CrossRef]
- Luo, W.; Nie, X.; Janik, M.J.; Asthagiri, A. Facet Dependence of CO2 Reduction Paths on Cu Electrodes. ACS Catal. 2015, 6, 219–229. [Google Scholar] [CrossRef]
- Durand, W.J.; Peterson, A.A.; Studt, F.; Abild-Pedersen, F.; Nørskov, J.K. Structure effects on the energetics of the electrochemical reduction of CO2 by copper surfaces. Surf. Sci. 2011, 605, 1354–1359. [Google Scholar] [CrossRef]
- Zhu, S.Q.; Wang, Q.; Qin, X.P.; Gu, M.; Tao, R.; Lee, B.P.; Zhang, L.L.; Yao, Y.Z.; Li, T.H.; Shao, M.H. Tuning Structural and Compositional Effects in Pd-Au Nanowires for Highly Selective and Active CO2 Electrochemical Reduction Reaction. Adv. Energy Mater. 2018, 8, 1802238. [Google Scholar] [CrossRef]
- Hahn, C.; Abram, D.N.; Hansen, H.A.; Hatsukade, T.; Jackson, A.; Johnson, N.C.; Hellstern, T.R.; Kuhl, K.P.; Cave, E.R.; Feaster, J.T.; et al. Synthesis of thin film AuPd alloys and their investigation for electrocatalytic CO2 reduction. J. Mater. Chem. A 2015, 3, 20185–20194. [Google Scholar] [CrossRef]
- Zheng, M.; Zhou, X.; Zhou, Y.; Li, M. Theoretical insights into mechanisms of electrochemical reduction of CO2 to ethylene catalyzed by Pd3Au. Appl. Surf. Sci. 2022, 572, 151474. [Google Scholar] [CrossRef]
- Zhang, X.-G.; Feng, S.; Zhan, C.; Wu, D.-Y.; Zhao, Y.; Tian, Z.-Q. Electroreduction Reaction Mechanism of Carbon Dioxide to C2 Products via Cu/Au Bimetallic Catalysis: A Theoretical Prediction. J. Phys. Chem. Lett. 2020, 11, 6593–6599. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Xie, S.; Zhang, X.-G.; Sun, F.; Kang, J.; Jiang, Z.; Zhang, Q.; Wu, D.-Y.; Wang, Y. Promoting electrocatalytic CO2 reduction to formate via sulfur-boosting water activation on indium surfaces. Nat. Commun. 2019, 10, 892. [Google Scholar] [CrossRef] [PubMed]
- Chiang, C.-L.; Lin, K.-S.; Chuang, H.-W. Direct synthesis of formic acid via CO2 hydrogenation over Cu/ZnO/Al2O3 catalyst. J. Clean. Prod. 2018, 172, 1957–1977. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, S.; Zhao, Y.; Ma, X. Hydrogenation of CO2 to formic acid catalyzed by heterogeneous Ru-PPh3/Al2O3 catalysts. Fuel Process. Technol. 2018, 178, 98–103. [Google Scholar] [CrossRef]
- Filonenko, G.A.; Vrijburg, W.L.; Hensen, E.J.M.; Pidko, E.A. On the activity of supported Au catalysts in the liquid phase hydrogenation of CO2 to formates. J. Catal. 2016, 343, 97–105. [Google Scholar] [CrossRef]
- Zhu, G.; Li, Y.; Zhu, H.; Su, H.; Chan, S.H.; Sun, Q. Curvature-Dependent Selectivity of CO2 Electrocatalytic Reduction on Cobalt Porphyrin Nanotubes. ACS Catal. 2016, 6, 6294–6301. [Google Scholar] [CrossRef]
- Kozuch, S.; Shaik, S. How to Conceptualize Catalytic Cycles? The Energetic Span Model. Acc. Chem. Res. 2011, 44, 101–110. [Google Scholar] [CrossRef]
- Hongzhiwei Technology, Device Studio, Version 2021A, China. 2021. Available online: https://iresearch.net.cn/cloud-software (accessed on 1 April 2023).
- Hafner, J. Ab-initio simulations of materials using VASP: Density-functional theory and beyond. J. Comput. Chem. 2008, 29, 2044–2078. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blochl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Mathew, K.; Sundararaman, R.; Letchworth-Weaver, K.; Arias, T.A.; Hennig, R.G. Implicit solvation model for density-functional study of nanocrystal surfaces and reaction pathways. J. Chem. Phys. 2014, 140, 084106. [Google Scholar] [CrossRef]
- Mathew, K.; Kolluru, V.S.C.; Mula, S.; Steinmann, S.N.; Hennig, R.G. Implicit self-consistent electrolyte model in plane-wave density-functional theory. J. Chem. Phys. 2019, 151, 234101. [Google Scholar] [CrossRef]
- Bader, R.F.W. A quantum theory of molecular structure and its applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Tang, W.; Sanville, E.; Henkelman, G. A grid-based Bader analysis algorithm without lattice bias. J. Phys. Condens. Matter 2009, 21, 084204. [Google Scholar] [CrossRef]
- Norskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jonsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Exner, K.S. Is Thermodynamics a Good Descriptor for the Activity? Re-Investigation of Sabatier’s Principle by the Free Energy Diagram in Electrocatalysis. ACS Catal. 2019, 9, 5320–5329. [Google Scholar] [CrossRef]
- van Santen, R.A.; Neurock, M.; Shetty, S.G. Reactivity Theory of Transition-Metal Surfaces: A Brønsted−Evans−Polanyi Linear Activation Energy−Free-Energy Analysis. Chem. Rev. 2010, 110, 2005–2048. [Google Scholar] [CrossRef] [PubMed]




| Ead(CO) [eV] | Charge Transfer [|e|] | Charge Density Difference | |
|---|---|---|---|
| Pd3Au (111) | −2.26 | 0.21 |  |
| Pd3Au (100) | −1.68 | 0.11 |  |
| Pd3Au (110) | −1.96 | 0.17 |  |
| Pd3Au (211) | −2.27 | 0.22 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, M.; Zhou, X.; Wang, Y.; Chen, G.; Li, M. The Facet Dependence of CO2 Electroreduction Selectivity on a Pd3Au Bimetallic Catalyst: A DFT Study. Molecules 2023, 28, 3169. https://doi.org/10.3390/molecules28073169
Zheng M, Zhou X, Wang Y, Chen G, Li M. The Facet Dependence of CO2 Electroreduction Selectivity on a Pd3Au Bimetallic Catalyst: A DFT Study. Molecules. 2023; 28(7):3169. https://doi.org/10.3390/molecules28073169
Chicago/Turabian StyleZheng, Ming, Xin Zhou, Yixin Wang, Gang Chen, and Mingxia Li. 2023. "The Facet Dependence of CO2 Electroreduction Selectivity on a Pd3Au Bimetallic Catalyst: A DFT Study" Molecules 28, no. 7: 3169. https://doi.org/10.3390/molecules28073169
APA StyleZheng, M., Zhou, X., Wang, Y., Chen, G., & Li, M. (2023). The Facet Dependence of CO2 Electroreduction Selectivity on a Pd3Au Bimetallic Catalyst: A DFT Study. Molecules, 28(7), 3169. https://doi.org/10.3390/molecules28073169








