Scutellarin Alleviates Ischemic Brain Injury in the Acute Phase by Affecting the Activity of Neurotransmitters in Neurons
Abstract
:1. Introduction
2. Results
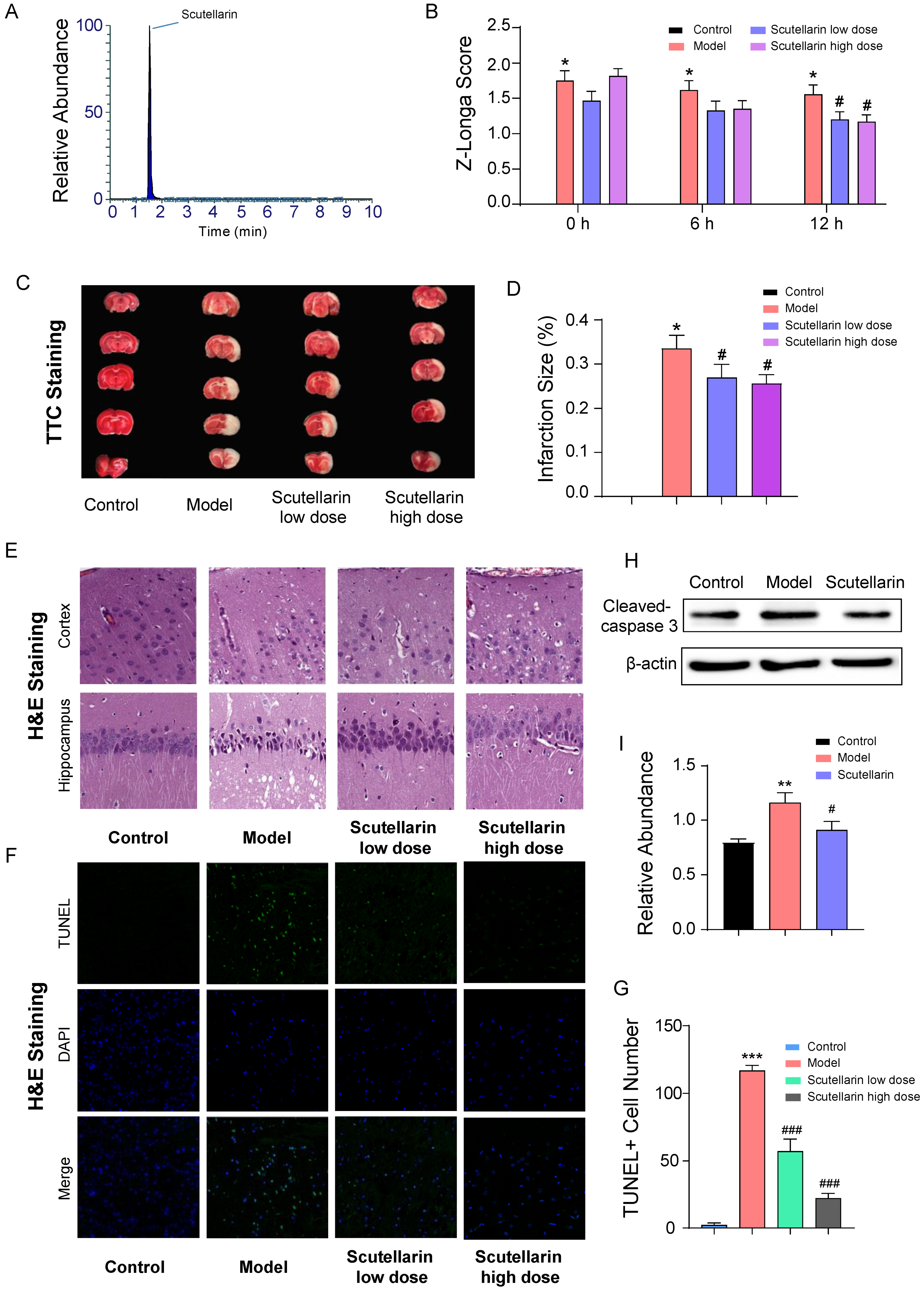
2.1. Scutellarin Protects Neurons against Ischemic Injury in Acute Phase
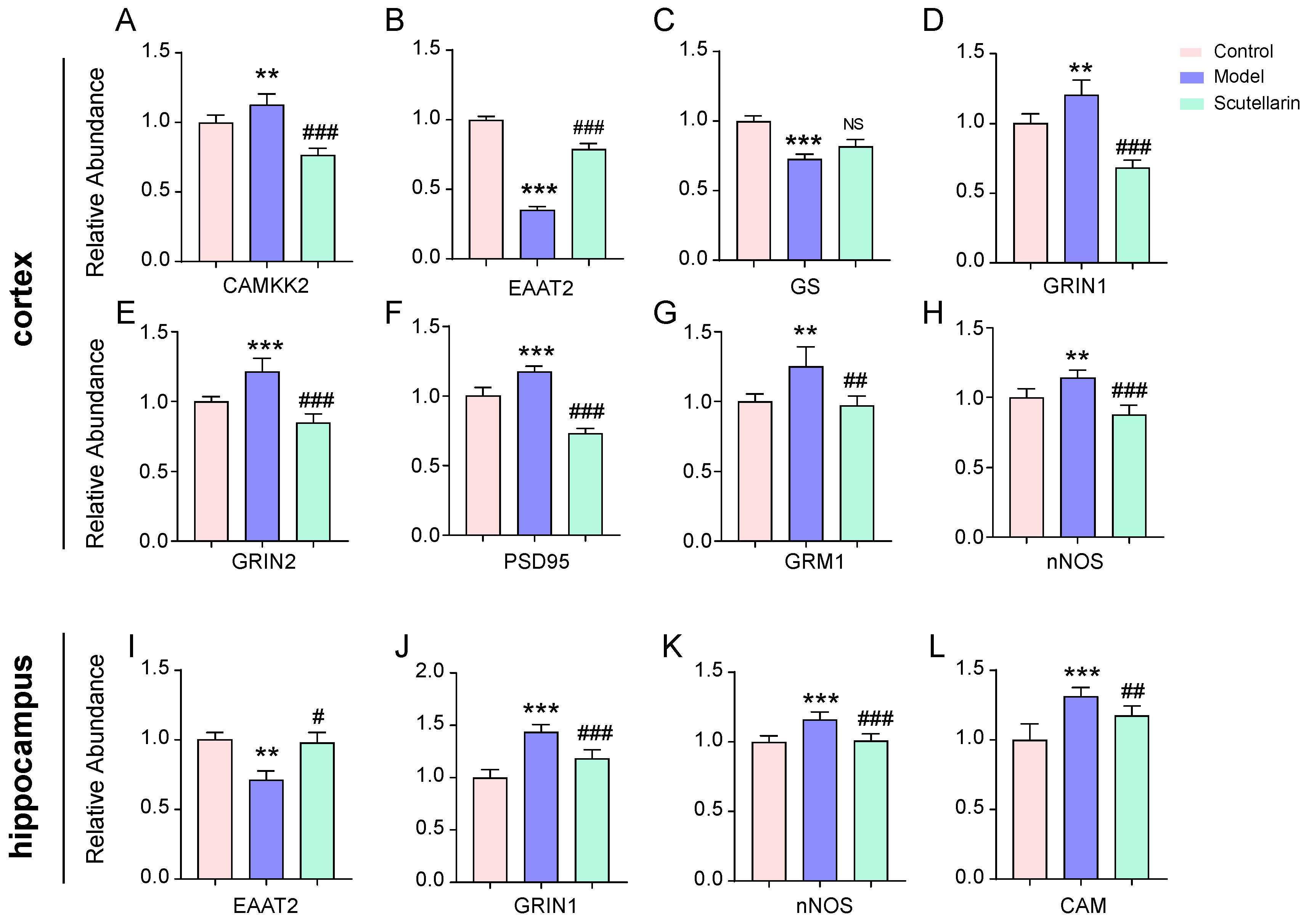
2.2. Scutellarin Reduces Ischemic Neuronal Apoptosis in the Acute Phase by Targeting Neurotransmitter Activities
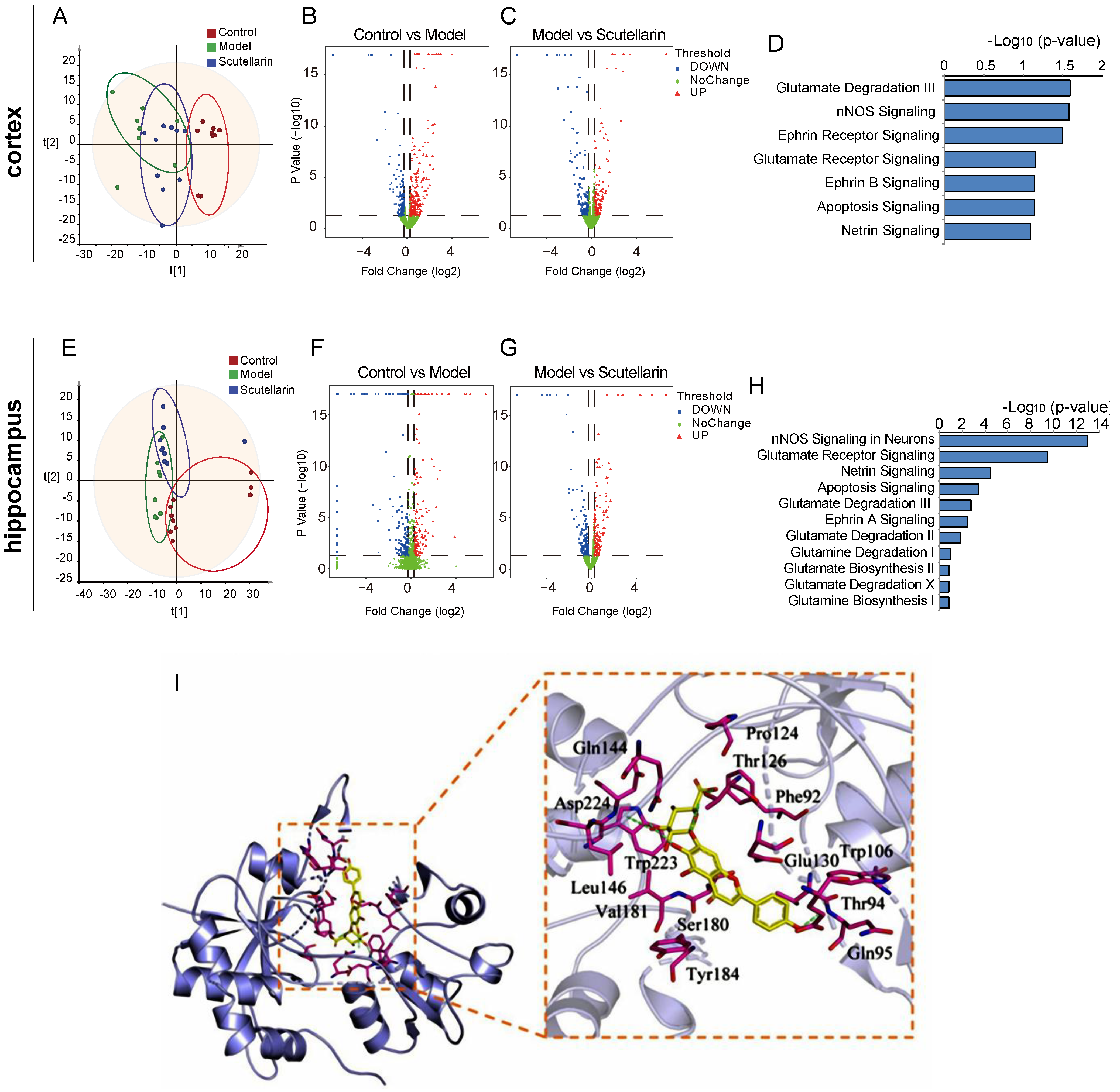
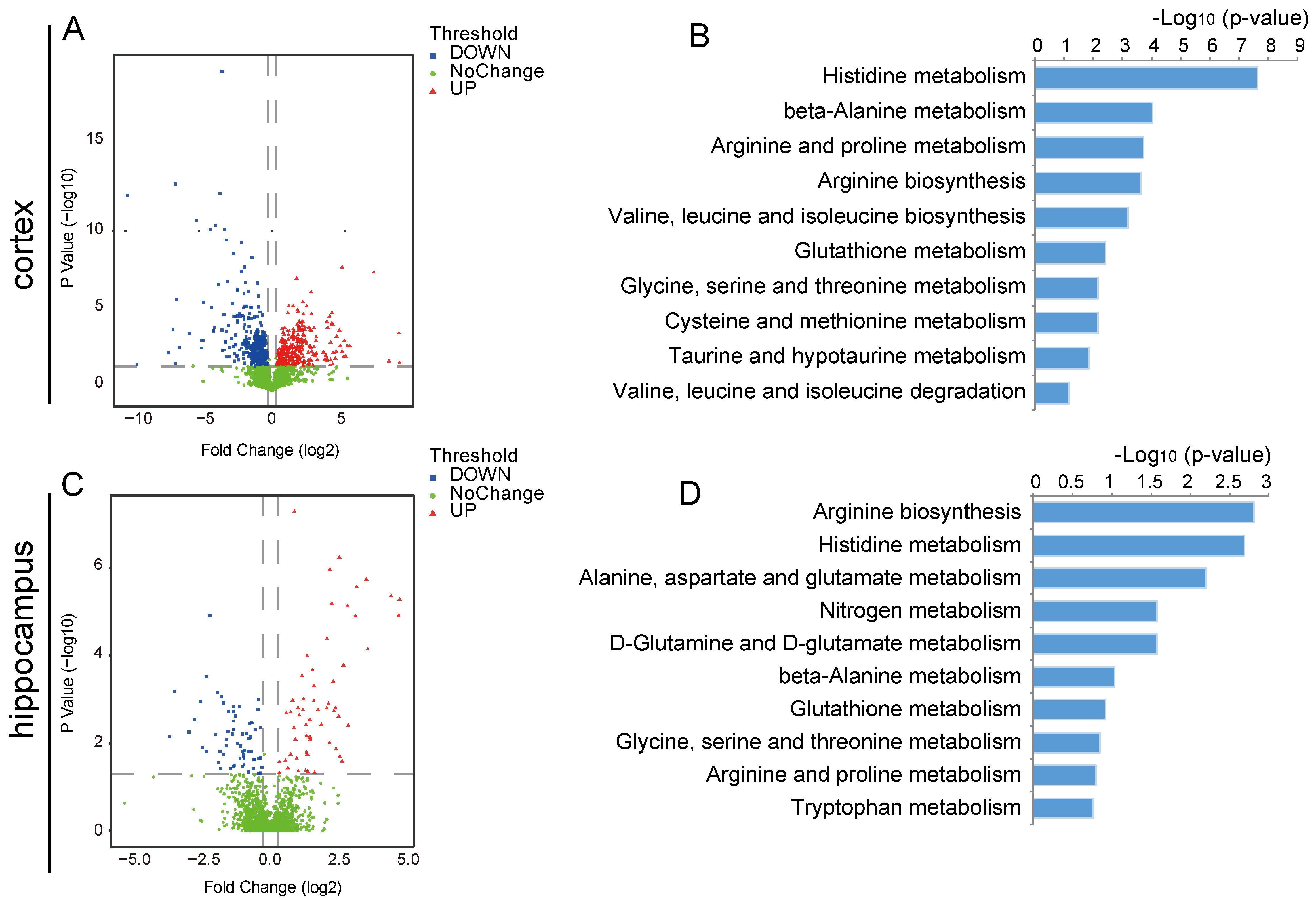
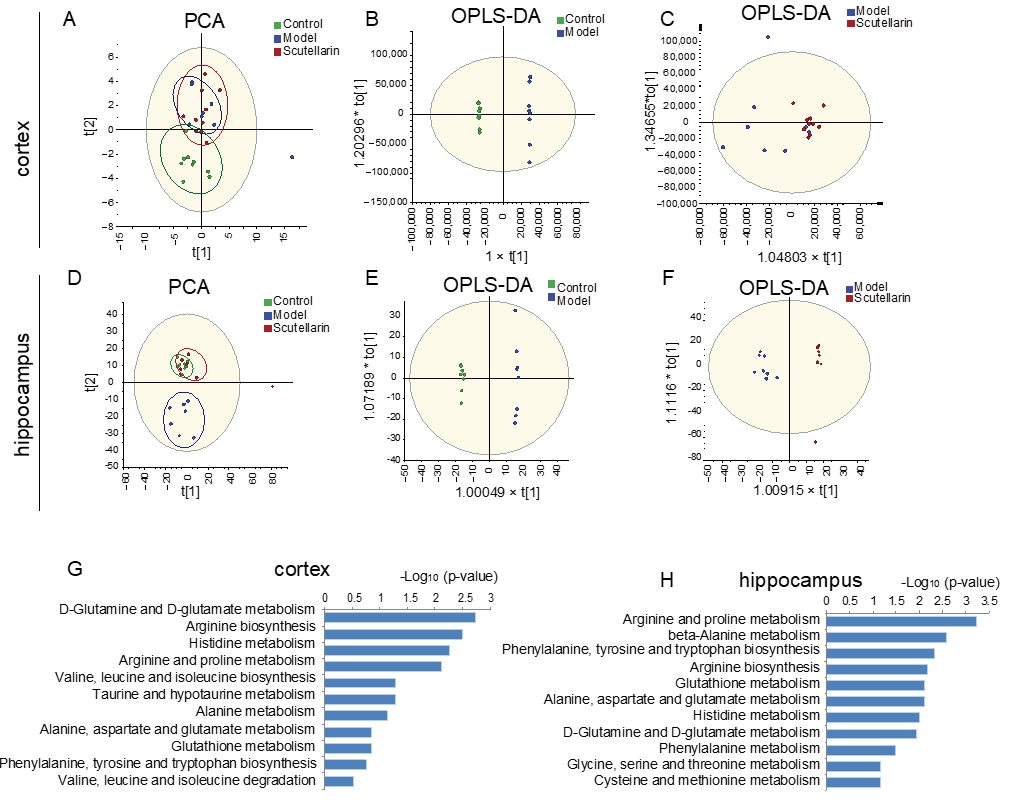
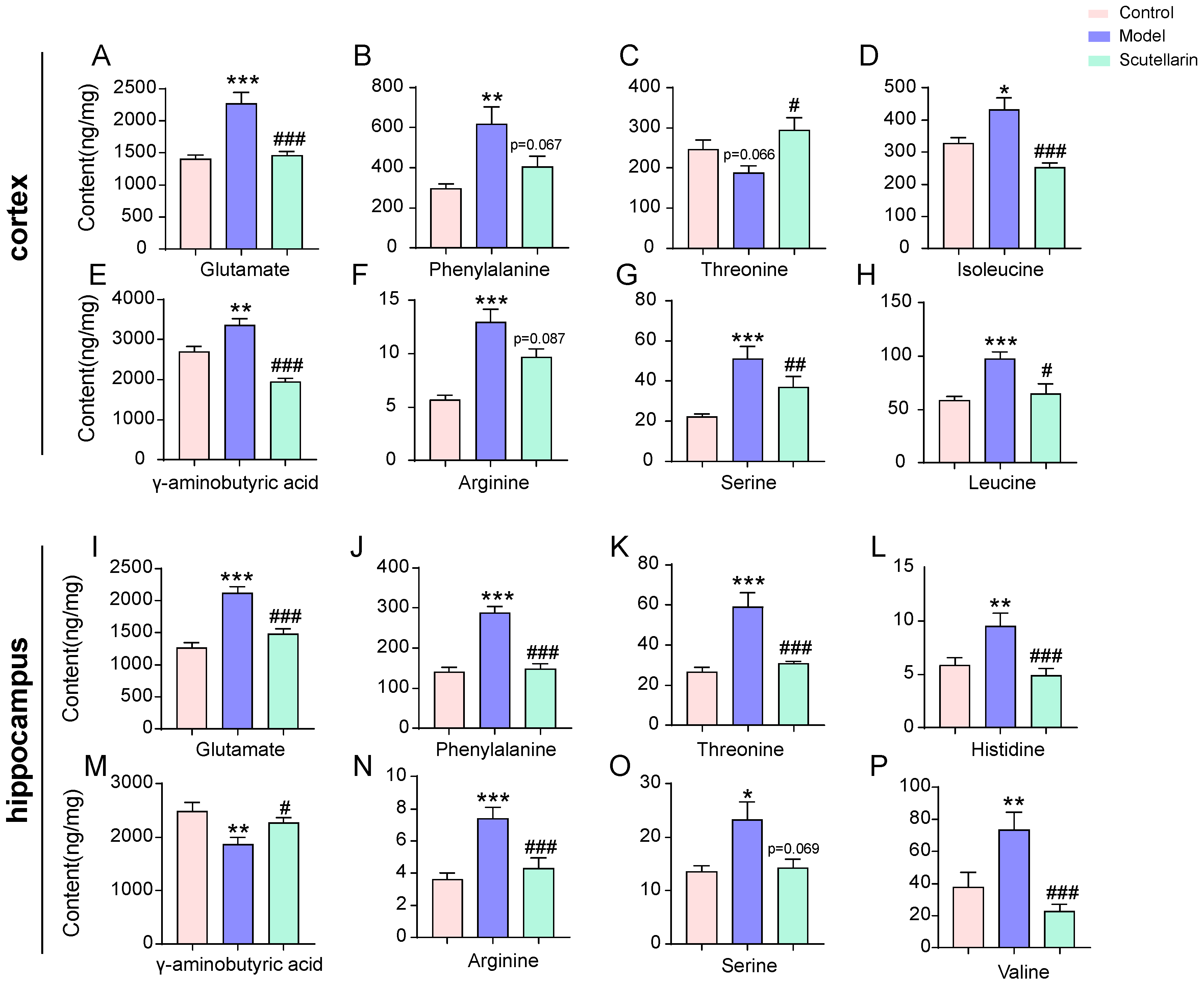
2.3. Changes in the Metabolic Profile of the Ischemic Brain in Acute Phase and Scutellarin Regulation
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Animals and Grouping
4.3. TTC and TUNEL Staining
4.4. Protein Sample Preparation and NanoLC-Orbitrap Fusion Lumos MS
4.5. Protein Identification and Quantification
4.6. Metabolite Sample Pretreatment and UHPLC-Q-Exactive Orbitrap MS Analysis
4.7. Bioinformatic Data Analysis and Molecular Docking
4.8. Parallel Reaction Monitoring-Mass Spectrometry (PRM-MS)
4.9. Western Blotting Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Weimar, C.; Mieck, T.; Buchthal, J.; Ehrenfeld, C.E.; Schmid, E.; Diener, H.C. Neurologic worsening during the acute phase of ischemic stroke. Arch. Neurol. 2005, 62, 393–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hacke, W.; Kaste, M.; Bluhmki, E.; Brozman, M.; Dávalos, A.; Guidetti, D.; Larrue, V.; Lees, K.R.; Medeghri, Z.; Machnig, T. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008, 359, 1317–1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansberg, M.G.; Bluhmki, E.; Thijs, V.N. Efficacy and safety of tissue plasminogen activator 3 to 4.5 hours after acute ischemic stroke: A metaanalysis. Stroke 2009, 40, 2438–2441. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.; Moro, M.A.; Nolte, C.H.; Dames, C.; Buckwalter, M.S.; Meisel, A. Immune pathways in etiology, acute phase, and chronic sequelae of ischemic stroke. Circ. Res. 2022, 130, 1167–1186. [Google Scholar] [CrossRef] [PubMed]
- Serena, J.; Leira, R.; Castillo, J.; Pumar, J.M.; Castellanos, M.; Dávalos, A. Neurological deterioration in acute lacunar infarctions: The role of excitatory and inhibitory neurotransmitters. Stroke 2001, 32, 1154–1161. [Google Scholar] [CrossRef] [Green Version]
- Dirnagl, U.; Endres, M. Found in translation: Preclinical stroke research predicts human pathophysiology, clinical phenotypes, and therapeutic outcomes. Stroke 2014, 45, 1510–1518. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, M.D. Neuroprotection for ischemic stroke: Past, present and future. Neuropharmacology 2008, 55, 363–389. [Google Scholar] [CrossRef] [Green Version]
- O’Collins, V.E.; Macleod, M.R.; Donnan, G.A.; Horky, L.L.; Van Der Worp, B.H.; Howells, D.W. 1,026 experimental treatments in acute stroke. Ann. Neurol. 2006, 59, 467–477. [Google Scholar] [CrossRef]
- Minnerup, J.; Sutherland, B.A.; Buchan, A.M.; Kleinschnitz, C. Neuroprotection for stroke: Current status and future perspectives. Int. J. Mol. Sci. 2012, 13, 11753–11772. [Google Scholar] [CrossRef]
- Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Mechanisms, challenges and opportunities in stroke. Nat. Rev. Neurosci. 2003, 4, 399–414. [Google Scholar] [CrossRef]
- Mo, T.; Liu, X.; Liu, Y.; Wang, X.; Zhang, L.; Wang, J.; Zhang, Z.; Shi, S.; Tu, P. Expanded investigations of the aglycon promiscuity and catalysis characteristic of flavonol 3-O-rhamnosyltransferase AtUGT78D1 from Arabidopsis thaliana. RSC Adv. 2016, 6, 84616–84626. [Google Scholar] [CrossRef]
- Aritomi, M.; Kumori, T.; Kawasaki, T. Cyanogenic glycosides in leaves of Perilla frutescens var. acuta. Phytochemistry 1985, 24, 2438–2439. [Google Scholar] [CrossRef]
- Meng, L.; Lozano, Y.; Bombarda, I.; Gaydou, E.M.; Li, B. Polyphenol extraction from eight Perilla frutescens cultivars. Comptes Rendus Chim. 2009, 12, 602–611. [Google Scholar] [CrossRef]
- Horvath, C.R.; Martos, P.A.; Saxena, P.K. Identification and quantification of eight flavones in root and shoot tissues of the medicinal plant Huang-qin (Scutellaria baicalensis Georgi) using high-performance liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A 2005, 1062, 199–207. [Google Scholar] [CrossRef]
- Harborne, J.B.; Baxter, H. The Handbook of Natural Flavonoids. Volume 1 and Volume 2; John Wiley and Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Wang, L.; Ma, Q. Clinical benefits and pharmacology of scutellarin: A comprehensive review. Pharmacol. Ther. 2018, 190, 105–127. [Google Scholar] [CrossRef]
- Janardhan, V.; Qureshi, A.I. Mechanisms of ischemic brain injury. Curr. Cardiol. Rep. 2004, 6, 117–123. [Google Scholar] [CrossRef]
- Phillis, J.W.; O’Regan, M.H.; Estevez, A.Y.; Song, D.; VanderHeide, S.J. Cerebral energy metabolism during severe ischemia of varying duration and following reperfusion. J. Neurochem. 1996, 67, 1525–1531. [Google Scholar] [CrossRef]
- Tsang, A.; Stobbe, R.W.; Asdaghi, N.; Hussain, M.S.; Bhagat, Y.A.; Beaulieu, C.; Emery, D.; Butcher, K.S. Relationship between sodium intensity and perfusion deficits in acute ischemic stroke. J. Magn. Reson. Imaging 2011, 33, 41–47. [Google Scholar] [CrossRef]
- Benveniste, H.; Drejer, J.; Schousboe, A.; Diemer, N.H. Elevation of the extracellular concentrations of glutamate and aspartate in rat hippocampus during transient cerebral ischemia monitored by intracerebral microdialysis. J. Neurochem. 1984, 43, 1369–1374. [Google Scholar] [CrossRef]
- Globus, M.Y.T.; Busto, R.; Dietrich, W.D.; Martinez, E.; Valdes, I.; Ginsberg, M.D. Effect of ischemia on the in vivo release of striatal dopamine, glutamate, and γ-aminobutyric acid studied by intracerebral microdialysis. J. Neurochem. 1988, 51, 1455–1464. [Google Scholar] [CrossRef]
- Hillered, L.; Hallström, A.; Segersvärd, S.; Persson, L.; Ungerstedt, U. Dynamics of extracellular metabolites in the striatum after middle cerebral artery occlusion in the rat monitored by intracerebral microdialysis. J. Cereb. Blood Flow Metab. 1989, 9, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Rothman, S.M.; Olney, J.W. Glutamate and the pathophysiology of hypoxic–ischemic brain damage. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 1986, 19, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Lai, T.W.; Zhang, S.; Wang, Y.T. Excitotoxicity and stroke: Identifying novel targets for neuroprotection. Prog. Neurobiol. 2014, 115, 157–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yu, J.; Wu, J.; Qi, F.; Wang, H.; Wang, Z.; Xu, Z. Scutellarin protects cardiomyocyte ischemia–reperfusion injury by reducing apoptosis and oxidative stress. Life Sci. 2016, 157, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-L.; Liu, A.-J.; Liu, J.-G.; Yu, X.-H.; Qin, L.-P.; Su, D.-F. Protective effects of scutellarin and breviscapine on brain and heart ischemia in rats. J. Cardiovasc. Pharmacol. 2007, 50, 327–332. [Google Scholar] [CrossRef]
- Dai, J.; Li, C.; Zhao, L.; Guan, C.; Yang, C.; Zhang, N.; Zhou, B.; Zhang, Y.; Wang, L.; Jiang, W. Scutellarin protects the kidney from ischemia/reperfusion injury by targeting Nrf2. Nephrology 2022, 27, 690–700. [Google Scholar] [CrossRef]
- Hill, M.D.; Martin, R.H.; Mikulis, D.; Wong, J.H.; Silver, F.L.; Milot, G.; Clark, W.M.; MacDonald, R.L.; Kelly, M.E.; Boulton, M. Safety and efficacy of NA-1 in patients with iatrogenic stroke after endovascular aneurysm repair (ENACT): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2012, 11, 942–950. [Google Scholar] [CrossRef]
- Vest, R.S.; O’Leary, H.; Coultrap, S.J.; Kindy, M.S.; Bayer, K.U. Effective post-insult neuroprotection by a novel Ca2+/calmodulin-dependent protein kinase II (CaMKII) inhibitor. J. Biol. Chem. 2010, 285, 20675–20682. [Google Scholar] [CrossRef] [Green Version]
- Fernstrom, J.D. Branched-chain amino acids and brain function. J. Nutr. 2005, 135, 1539S–1546S. [Google Scholar] [CrossRef] [Green Version]
- Fernstrom, J.D. Aromatic amino acids and monoamine synthesis in the central nervous system: Influence of the diet. J. Nutr. Biochem. 1990, 1, 508–517. [Google Scholar] [CrossRef]
- Hawkins, R.A.; O’Kane, R.L.; Simpson, I.A.; Vina, J.R. Structure of the blood–brain barrier and its role in the transport of amino acids. J. Nutr. 2006, 136, 218S–226S. [Google Scholar] [CrossRef] [Green Version]
- Kandasamy, P.; Gyimesi, G.; Kanai, Y.; Hediger, M.A. Amino acid transporters revisited: New views in health and disease. Trends Biochem. Sci. 2018, 43, 752–789. [Google Scholar] [CrossRef]
- Pardridge, W.M. Brain metabolism: A perspective from the blood-brain barrier. Physiol. Rev. 1983, 63, 1481–1535. [Google Scholar] [CrossRef]
- Yudkoff, M. Interactions in the metabolism of glutamate and the branched-chain amino acids and ketoacids in the CNS. Neurochem. Res. 2017, 42, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Contrusciere, V.; Paradisi, S.; Matteucci, A.; Malchiodi-Albedi, F. Branched-chain amino acids induce neurotoxicity in rat cortical cultures. Neurotox. Res. 2010, 17, 392–398. [Google Scholar] [CrossRef]
- Caioli, S.; Candelotti, E.; Pedersen, J.Z.; Saba, L.; Antonini, A.; Incerpi, S.; Zona, C. Baicalein reverts L-valine-induced persistent sodium current up-modulation in primary cortical neurons. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2016, 1862, 566–575. [Google Scholar] [CrossRef]
- Bridi, R.; Araldi, J.N.; Sgarbi, M.B.; Testa, C.G.; Durigon, K.; Wajner, M.; Dutra-Filho, C.S. Induction of oxidative stress in rat brain by the metabolites accumulating in maple syrup urine disease. Int. J. Dev. Neurosci. 2003, 21, 327–332. [Google Scholar] [CrossRef]
- Buratta, S.; Hamberger, A.; Ryberg, H.; Nyström, B.; Sandberg, M.; Mozzi, R. Effect of serine and ethanolamine administration on phospholipid-related compounds and neurotransmitter amino acids in the rabbit hippocampus. J. Neurochem. 1998, 71, 2145–2150. [Google Scholar] [CrossRef]
- Horio, M.; Kohno, M.; Fujita, Y.; Ishima, T.; Inoue, R.; Mori, H.; Hashimoto, K. Levels of D-serine in the brain and peripheral organs of serine racemase (Srr) knock-out mice. Neurochem. Int. 2011, 59, 853–859. [Google Scholar] [CrossRef]
- Oja, S.S.; Lajtha, A.; Schousboe, A.; Saransaari, P. Handbook of Neurochemistry and Molecular Neurobiology: Amino Acids and Peptides in the Nervous System; Springer Nature: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- De Miranda, J.; Santoro, A.; Engelender, S.; Wolosker, H. Human serine racemase: Moleular cloning, genomic organization and functional analysis. Gene 2000, 256, 183–188. [Google Scholar] [CrossRef]
- Wolosker, H.; Blackshaw, S.; Snyder, S.H. Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. USA 1999, 96, 13409–13414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bird, M.I.; Nunn, P.B.; Lord, L.A. Formation of glycine and aminoacetone from L-threonine by rat liver mitochondria. Biochim. Biophys. Acta (BBA)-Gen. Subj. 1984, 802, 229–236. [Google Scholar] [CrossRef]
- Maher, T.J.; Wurtman, R.J. L-threonine administration increases glycine concentrations in the rat central nervous system. Life Sci. 1980, 26, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Fernstrom, J.D.; Fernstrom, M.H. Tyrosine, phenylalanine, and catecholamine synthesis and function in the brain. J. Nutr. 2007, 137, 1539S–1547S. [Google Scholar] [CrossRef] [Green Version]
- Haas, H.L.; Sergeeva, O.A.; Selbach, O. Histamine in the nervous system. Physiol. Rev. 2008, 88, 1183–1241. [Google Scholar] [CrossRef]
- Wiesinger, H. Arginine metabolism and the synthesis of nitric oxide in the nervous system. Prog. Neurobiol. 2001, 64, 365–391. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Naz, S.; Vallejo, M.; García, A.; Barbas, C. Method validation strategies involved in non-targeted metabolomics. J. Chromatogr. A 2014, 1353, 99–105. [Google Scholar] [CrossRef]
| Protein | Grid Size | Docking Score (kcal/mol) | |
|---|---|---|---|
| Memantine | Scutellarin | ||
| GRIN1 GRIN2 GRM1 | 40 × 40 × 40 40 × 40 × 40 40 × 40 × 40 | −7.4 −6.0 −6.4 | −10.1 −8.4 −8.7 |
| Protein | Protein Accession | Unipeptide Sequence Applied |
|---|---|---|
| EAAT2 | Peptide 1 | KNDEVSSLDAFLDLIR |
| Peptide 2 | SELDTIDSQHR | |
| Peptide 3 | MHEDIEMTK | |
| Peptide 4 | SADC[+57]SVEEEPWKR | |
| GS | Peptide 1 | DIVEAHYR |
| Peptide 2 | RPSANC[+57]DPYAVTEAIVR | |
| Peptide 3 | TC[+57]LLNETGDEPFQYK | |
| GLS | Peptide 1 | VADYIPQLAK |
| Peptide 2 | FSPDLWGVSVC[+57]TVDGQR | |
| Peptide 3 | MAGNEYVGFSNATFQSER | |
| Grin1 | Peptide 1 | VEFNEDGDRK |
| Peptide 2 | IIWPGGETEKPR | |
| Peptide 3 | YADGVTGR | |
| Grin2b | Peptide 1 | IISENKTDEEPGYIK |
| Peptide 2 | NLTNVDWEDR | |
| Grm1 | Peptide 1 | GEVSC[+57]C[+57]WIC[+57]TAC[+57]K |
| Peptide 2 | KPGAGNANSNGKSVSWSEPGGR | |
| Grik5 | Peptide 1 | ETLSVRMLDDSRDPTPLLK |
| Peptide 2 | MVEYDGLTGR | |
| Gria2 | Peptide 1 | GADQEYSAFR |
| Peptide 2 | ADIAIAPLTITLVR | |
| Gria3 | Peptide 1 | INTILEQVVILGK |
| Peptide 2 | YTSALTHDAILVIAEAFR | |
| Peptide 3 | ADIAVAPLTITLVR | |
| CaM | Peptide 1 | EAFSLFDKDGDGTITTK |
| Peptide 2 | ELGTVMR | |
| Peptide 3 | MKDTDSEEEIR | |
| Camkk1 | Peptide 1 | MERSPAVC[+57]C[+57]QDPRAELVER |
| Peptide 2 | SPAVC[+57]C[+57]QDPR | |
| Peptide 3 | HGEEPLPSEEEHC[+57]SVVEVTEEEVK | |
| Camkk2 | Peptide 1 | GPIEQVYQEIAILK |
| Peptide 2 | IFSGKALDVWAMGVTLYC[+57]FVFGQC[+57]PFM[+16]DER | |
| nNOS | Peptide 1 | MDLDM[+16]RK |
| Peptide 2 | LSEEDAGVFISR | |
| Peptide 3 | SQAYAKTLC[+57]EIFK | |
| Peptide 4 | FSVFGLGSR | |
| PSD95 | Peptide 1 | GNSGLGFSIAGGTDNPHIGDDPSIFITK |
| Peptide 2 | FGDVLHVIDAGDEEWWQAR | |
| Peptide 3 | ANDDLLSEFPDK | |
| Peptide 4 | FGSC[+57]VPHTTRPK | |
| PLA2 | Peptide 1 | DPRYGASPLHWAK |
| Peptide 2 | QPAELHLFRNYDAPEAVR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Liu, Y.; Liu, X.; Zhang, Y.; Yan, X.; Deng, X.; Shi, J. Scutellarin Alleviates Ischemic Brain Injury in the Acute Phase by Affecting the Activity of Neurotransmitters in Neurons. Molecules 2023, 28, 3181. https://doi.org/10.3390/molecules28073181
Wang C, Liu Y, Liu X, Zhang Y, Yan X, Deng X, Shi J. Scutellarin Alleviates Ischemic Brain Injury in the Acute Phase by Affecting the Activity of Neurotransmitters in Neurons. Molecules. 2023; 28(7):3181. https://doi.org/10.3390/molecules28073181
Chicago/Turabian StyleWang, Chunguo, Yaoyu Liu, Xi Liu, Yuting Zhang, Xingli Yan, Xinqi Deng, and Jinli Shi. 2023. "Scutellarin Alleviates Ischemic Brain Injury in the Acute Phase by Affecting the Activity of Neurotransmitters in Neurons" Molecules 28, no. 7: 3181. https://doi.org/10.3390/molecules28073181
APA StyleWang, C., Liu, Y., Liu, X., Zhang, Y., Yan, X., Deng, X., & Shi, J. (2023). Scutellarin Alleviates Ischemic Brain Injury in the Acute Phase by Affecting the Activity of Neurotransmitters in Neurons. Molecules, 28(7), 3181. https://doi.org/10.3390/molecules28073181





