Persistent Lipid Accumulation Leads to Persistent Exacerbation of Endoplasmic Reticulum Stress and Inflammation in Progressive NASH via the IRE1α/TRAF2 Complex
Abstract
1. Introduction
2. Results
2.1. Continuous Lipid Accumulation Dysregulated the Concentration of Blood Lipids Related Indicators
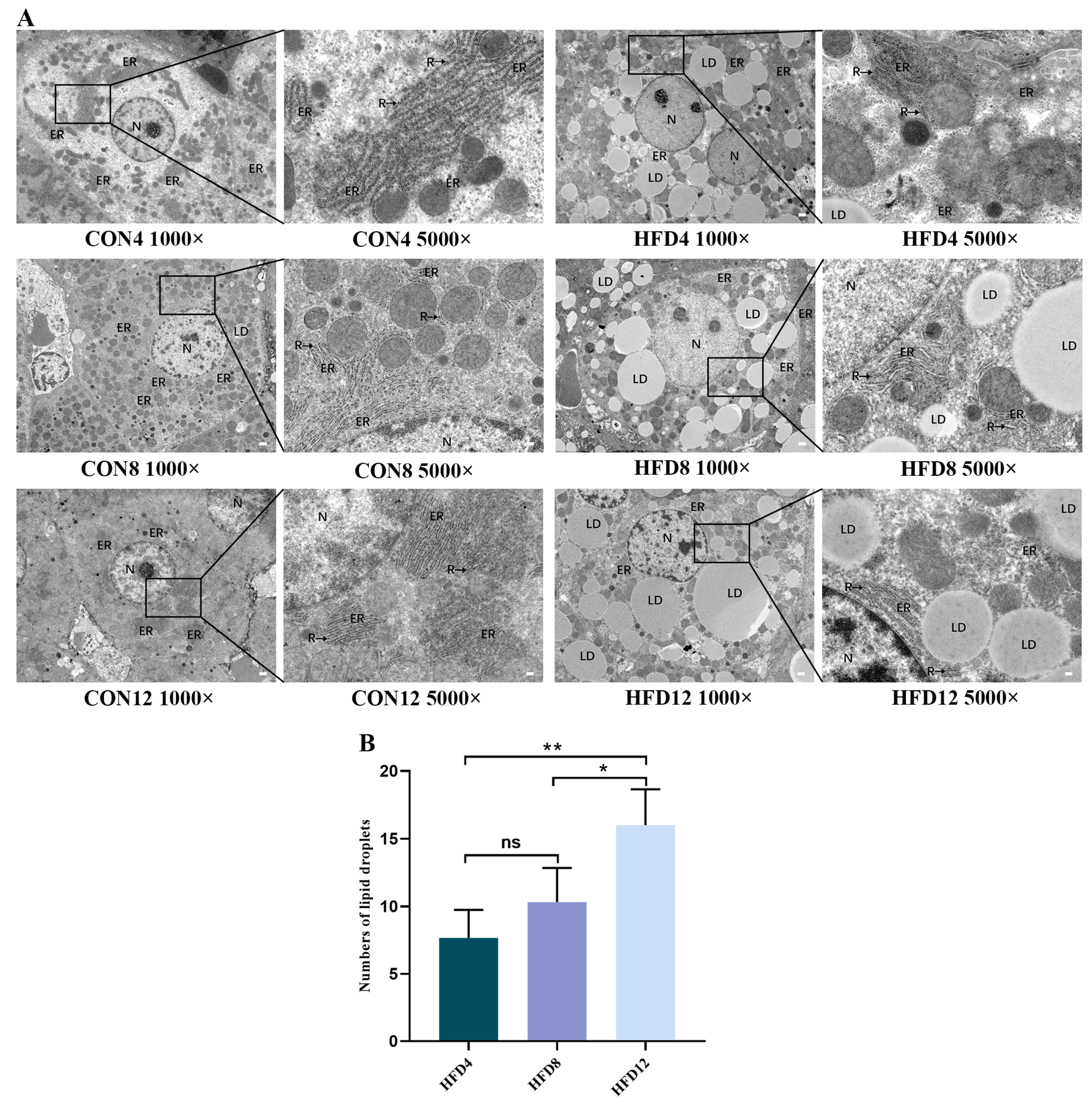
2.2. Persistent Lipid Accumulation Caused Hepatocyte Structure Impairment, Collagen Fibers, and Lipid Droplets Accumulation
2.3. Genes Expression Analysis
2.4. Differential Expressed Genes (DEGs) Analysis, Protein–Protein Interaction (PPI) Network Construction, and Core Genes Screening
2.5. Function Analysis of Differential Expressed Genes
2.6. Unremitting Lipid Accumulation Destroys the ER Structure and Increases Numbers of Lipid Droplets
2.7. Constant Lipid Accumulation Increased the Levels of TNF-α, IL-1β, and IL-6
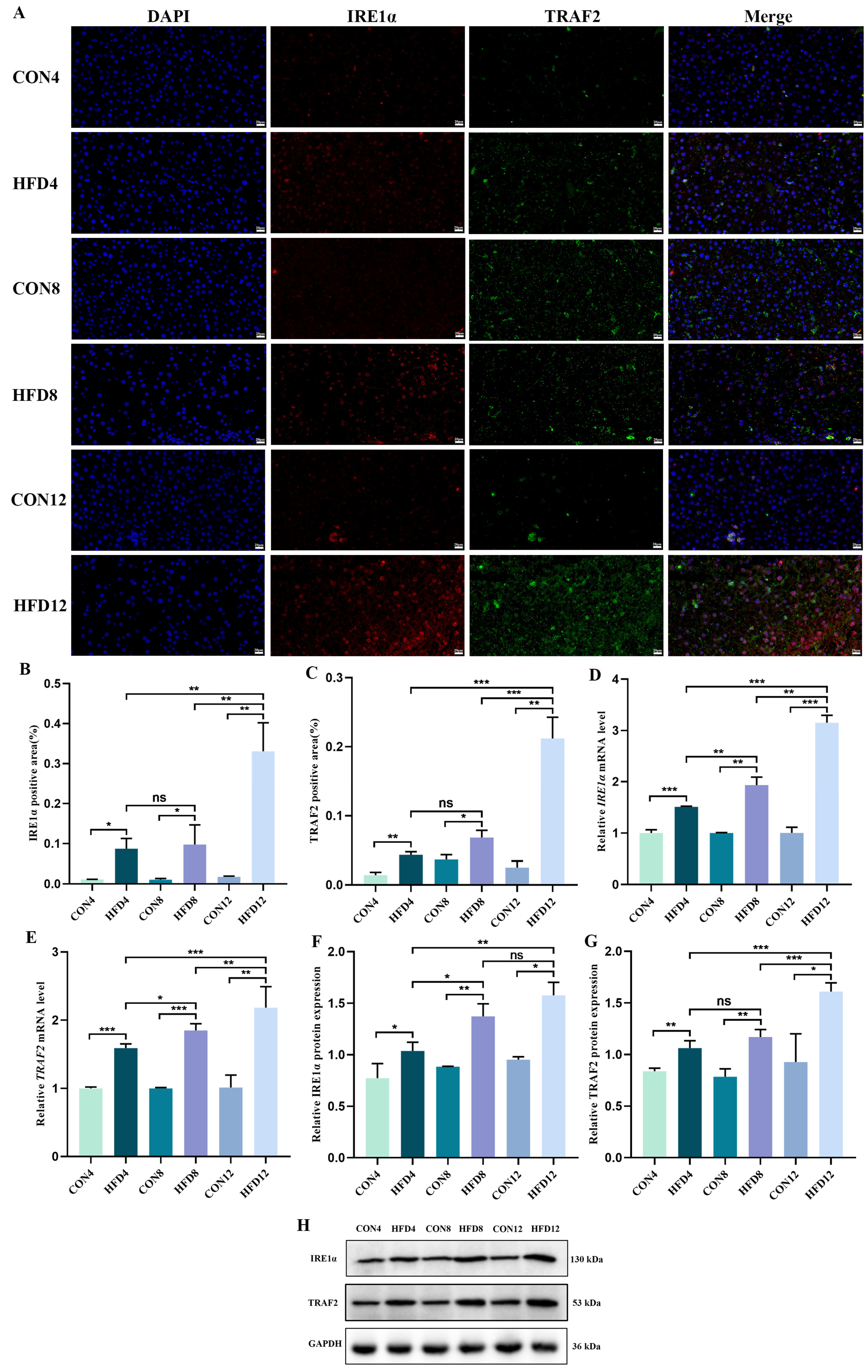
2.8. Sustaining Lipid Accumulation Activated the IRE1α/TRAF2 Complex
2.9. Continuous Lipid Accumulation Activated the IKK/IκB/NF-κB Signaling Pathway
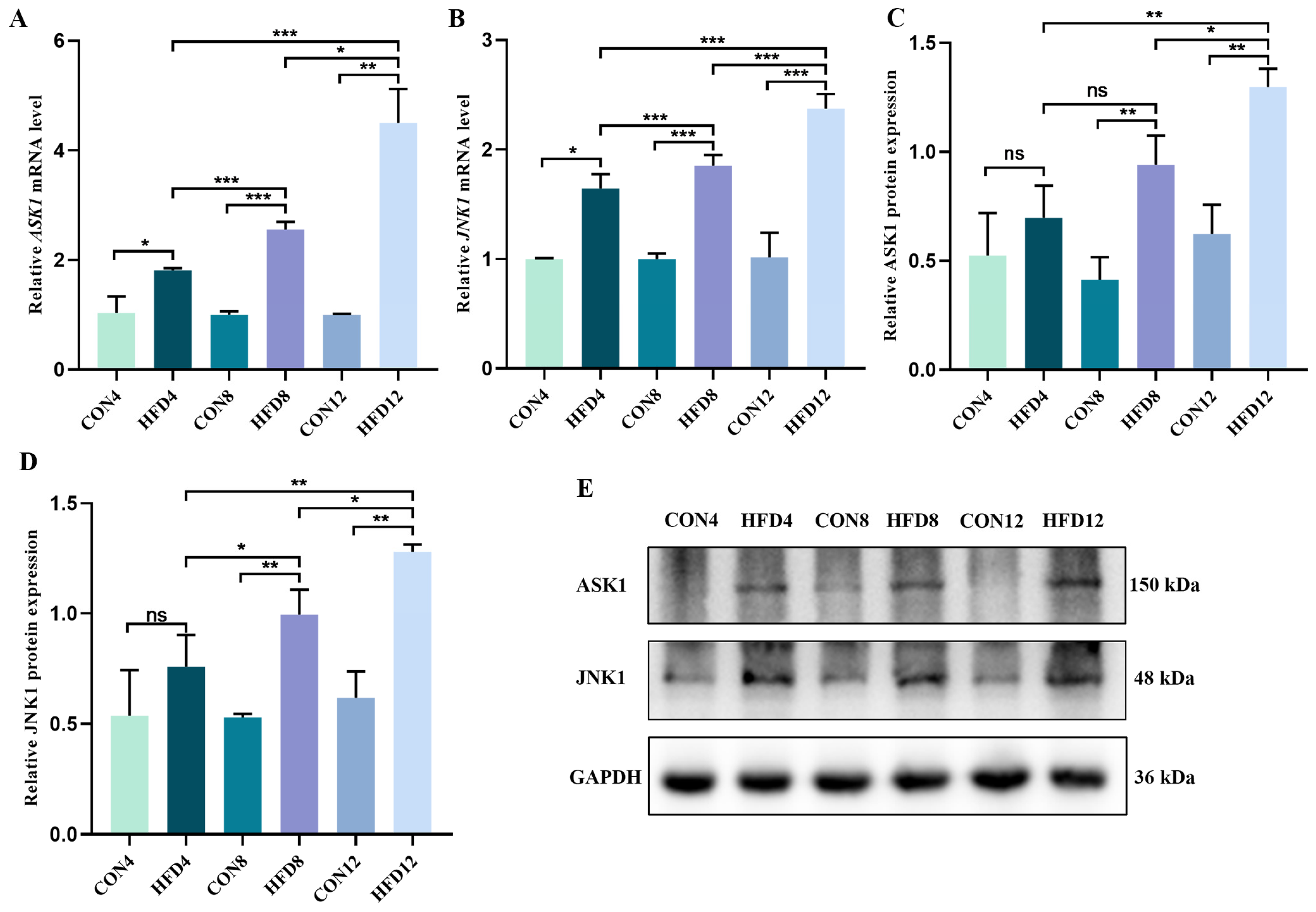
2.10. Persistent Lipid Accumulation Activated the ASK1/JNK1 Signaling Pathway
3. Discussion
4. Materials and Methods
4.1. Animals and High Fat Diet
4.2. NASH Model and Sample Collection
4.3. Blood Lipids Related Indicators Analysis
4.4. Histological Staining
4.5. MRNA Sequencing
4.6. PPI Network Construction and Gene Screening
4.7. Transmission Electron Microscopy
4.8. Enzyme-Linked Immunosorbent Assay
4.9. Immunofluorescence Staining
4.10. Quantitative Real-Time Polymerase Chain Reaction
4.11. Western Blotting
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Negro, F. Natural history of NASH and HCC. Liver Int. 2020, 40 (Suppl. 1), 72–76. [Google Scholar] [CrossRef] [PubMed]
- Parthasarathy, G.; Revelo, X.; Malhi, H. Pathogenesis of nonalcoholic steatohepatitis: An overview. Hepatol. Commun. 2020, 4, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Cabrera, D.; Arrese, M.; Feldstein, A.E. Triggering and resolution of inflammation in NASH. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Rotman, Y.; Sanyal, A.J. Current and upcoming pharmacotherapy for non-alcoholic fatty liver disease. Gut 2017, 66, 180–190. [Google Scholar] [CrossRef]
- Sarin, S.K.; Kumar, M.; Eslam, M.; George, J.; Al Mahtab, M.; Akbar, S.M.F.; Jia, J.; Tian, Q.; Aggarwal, R.; Muljono, D.H.; et al. Liver diseases in the Asia-Pacific region: A lancet gastroenterology & hepatology commission. Lancet Gastroenterol. Hepatol. 2020, 5, 167–228. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Reibe, S.; Febbraio, M.A. Relieving ER stress to target NASH-driven hepatocellular carcinoma. Nat. Rev. Endocrinol. 2019, 15, 73–74. [Google Scholar] [CrossRef]
- Rennert, C.; Heil, T.; Schicht, G.; Stilkerich, A.; Seidemann, L.; Kegel-Hubner, V.; Seehofer, D.; Damm, G. Prolonged lipid accumulation in cultured primary human hepatocytes rather leads to ER stress than oxidative stress. Int. J. Mol. Sci. 2020, 21, 7097. [Google Scholar] [CrossRef]
- Suzuki, M. Regulation of lipid metabolism via a connection between the endoplasmic reticulum and lipid droplets. Anat. Sci. Int. 2017, 92, 50–54. [Google Scholar] [CrossRef]
- Riaz, T.A.; Junjappa, R.P.; Handigund, M.; Ferdous, J.; Kim, H.R.; Chae, H.J. Role of endoplasmic reticulum stress sensor IRE1alpha in cellular physiology, calcium, ROS signaling, and metaflammation. Cells 2020, 9, 1160. [Google Scholar] [CrossRef]
- Di Conza, G.; Ho, P.C. ER stress responses: An emerging modulator for innate immunity. Cells 2020, 9, 695. [Google Scholar] [CrossRef]
- Yap, K.N.; Yamada, K.; Zikeli, S.; Kiaris, H.; Hood, W.R. Evaluating endoplasmic reticulum stress and unfolded protein response through the lens of ecology and evolution. Biol. Rev. 2021, 96, 541–556. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef]
- Park, S.H.; Kang, M.K.; Choi, Y.J.; Kim, Y.H.; Antika, L.D.; Lim, S.S.; Kang, Y.H. Dietary compound alpha-asarone alleviates ER stress-mediated apoptosis in 7beta-hydroxycholesterol-challenged macrophages. Mol. Nutr. Food Res. 2016, 60, 1033–1047. [Google Scholar] [CrossRef]
- Parafati, M.; Kirby, R.J.; Khorasanizadeh, S.; Rastinejad, F.; Malany, S. A nonalcoholic fatty liver disease model in human induced pluripotent stem cell-derived hepatocytes, created by endoplasmic reticulum stress-induced steatosis. Dis. Model. Mech. 2018, 11, dmm033530. [Google Scholar] [CrossRef]
- Liu, Z.G. Molecular mechanism of TNF signaling and beyond. Cell Res. 2005, 15, 24–27. [Google Scholar] [CrossRef]
- Sovolyova, N.; Healy, S.; Samali, A.; Logue, S.E. Stressed to death—Mechanisms of ER stress-induced cell death. Biol. Chem. 2014, 395, 1–13. [Google Scholar] [CrossRef]
- Gardner, B.M.; Pincus, D.; Gotthardt, K.; Gallagher, C.M.; Walter, P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb. Perspect. Biol. 2013, 5, a013169. [Google Scholar] [CrossRef]
- Hollien, J. Evolution of the unfolded protein response. Biochim. Biophys. Acta 2013, 1833, 2458–2463. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, D.; Li, Y.; Qiao, H.; Shan, Z. Ketamine enhances autophagy and endoplasmic reticulum stress in rats and SV-HUC-1 cells via activating IRE1-TRAF2-ASK1-JNK pathway. Cell Cycle 2021, 20, 1907–1922. [Google Scholar] [CrossRef]
- Madhavan, A.; Kok, B.P.; Rius, B.; Grandjean, J.M.D.; Alabi, A.; Albert, V.; Sukiasyan, A.; Powers, E.T.; Galmozzi, A.; Saez, E.; et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. Nat. Commun. 2022, 13, 608. [Google Scholar] [CrossRef]
- Zilberman-Rudenko, J.; Shawver, L.M.; Wessel, A.W.; Luo, Y.; Pelletier, M.; Tsai, W.L.; Lee, Y.; Vonortas, S.; Cheng, L.; Ashwell, J.D.; et al. Recruitment of A20 by the C-terminal domain of NEMO suppresses NF-kappaB activation and autoinflammatory disease. Proc. Natl. Acad. Sci. USA 2016, 113, 1612–1617. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Yang, H.; Tang, C.; Yao, G.; Kong, L.; He, H.; Zhou, Y. Kaempferol alleviates insulin resistance via hepatic IKK/NF-kappaB signal in type 2 diabetic rats. Int. Immunopharmacol. 2015, 28, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Diehl, A.M.; Li, Z.P.; Lin, H.Z.; Yang, S.Q. Cytokines and the pathogenesis of non-alcoholic steatohepatitis. Gut 2005, 54, 303–306. [Google Scholar] [CrossRef]
- Wesolowski, S.R.; Kasmi, K.C.; Jonscher, K.R.; Friedman, J.E. Developmental origins of NAFLD: A womb with a clue. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 81–96. [Google Scholar] [CrossRef]
- Musso, G.; Cassader, M.; Gambino, R. Non-alcoholic steatohepatitis: Emerging molecular targets and therapeutic strategies. Nat. Rev. Drug Discov. 2016, 15, 249–274. [Google Scholar] [CrossRef]
- Michelotti, G.A.; Machado, M.V.; Diehl, A.M. NAFLD, NASH and liver cancer. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 656–665. [Google Scholar] [CrossRef]
- Khan, R.S.; Bril, F.; Cusi, K.; Newsome, P.N. Modulation of insulin resistance in nonalcoholic fatty liver disease. Hepatology 2019, 70, 711–724. [Google Scholar] [CrossRef]
- Roeb, E.; Geier, A. Nonalcoholic steatohepatitis (NASH)—Current treatment recommendations and future developments. Z. Gastroenterol. 2019, 57, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Besse-Patin, A.; Estall, J.L. An intimate relationship between ROS and insulin signalling: Implications for antioxidant treatment of fatty liver disease. Int. J. Cell Biol. 2014, 2014, 519153. [Google Scholar] [CrossRef]
- Lippman, S.M.; Klein, E.A.; Goodman, P.J.; Lucia, M.S.; Thompson, I.M.; Ford, L.G.; Parnes, H.L.; Minasian, L.M.; Gaziano, J.M.; Hartline, J.A.; et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: The Selenium and Vitamin E cancer prevention trial (SELECT). JAMA 2009, 301, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.A.; Alkhouri, N.; Davison, B.A.; Sanyal, A.; Edwards, C.; Colca, J.R.; Lee, B.H.; Loomba, R.; Cusi, K.; Kolterman, O.; et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. J. Hepatol. 2020, 72, 613–626. [Google Scholar] [CrossRef]
- Gawrieh, S.; Noureddin, M.; Loo, N.; Mohseni, R.; Awasty, V.; Cusi, K.; Kowdley, K.V.; Lai, M.; Schiff, E.; Parmar, D.; et al. Saroglitazar, a PPAR-alpha/gamma agonist, for treatment of NAFLD: A randomized controlled double-blind phase 2 trial. Hepatology 2021, 74, 1809–1824. [Google Scholar] [CrossRef]
- Chapman, R.W.; Lynch, K.D. Obeticholic acid-a new therapy in PBC and NASH. Br. Med. Bull. 2020, 133, 95–104. [Google Scholar] [CrossRef] [PubMed]
- An, P.; Wei, G.Y.; Huang, P.Z.; Li, W.D.; Qi, X.L.; Lin, Y.; Vaid, K.A.; Wang, J.; Zhang, S.C.; Li, Y.; et al. A novel non-bile acid FXR agonist EDP-305 potently suppresses liver injury and fibrosis without worsening of ductular reaction. Liver Int. 2020, 40, 1655–1669. [Google Scholar] [CrossRef] [PubMed]
- Ebersbach-Silva, P.; Poletto, A.C.; David-Silva, A.; Seraphim, P.M.; Anhe, G.F.; Passarelli, M.; Furuya, D.T.; Machado, U.F. Palmitate-induced Slc2a4/GLUT4 downregulation in L6 muscle cells: Evidence of inflammatory and endoplasmic reticulum stress involvement. Lipids Health Dis. 2018, 17, 64. [Google Scholar] [CrossRef]
- Bertolotti, A.; Zhang, Y.; Hendershot, L.M.; Harding, H.P.; Ron, D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat. Cell Biol. 2000, 2, 326–332. [Google Scholar] [CrossRef]
- Keestra-Gounder, A.M.; Byndloss, M.X.; Seyffert, N.; Young, B.M.; Chavez-Arroyo, A.; Tsai, A.Y.; Cevallos, S.A.; Winter, M.G.; Pham, O.H.; Tiffany, C.R.; et al. NOD1 and NOD2 signalling links ER stress with inflammation. Nature 2016, 532, 394–397. [Google Scholar] [CrossRef]
- Kaser, A.; Lee, A.H.; Franke, A.; Glickman, J.N.; Zeissig, S.; Tilg, H.; Nieuwenhuis, E.E.; Higgins, D.E.; Schreiber, S.; Glimcher, L.H.; et al. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell 2008, 134, 743–756. [Google Scholar] [CrossRef]
- Montane, J.; Cadavez, L.; Novials, A. Stress and the inflammatory process: A major cause of pancreatic cell death in type 2 diabetes. Diabetes Metab. Syndr. Obes. 2014, 7, 25–34. [Google Scholar] [CrossRef]
- Zhu, Z.; Gao, S.; Chen, C.; Xu, W.; Xiao, P.; Chen, Z.; Du, C.; Chen, B.; Gao, Y.; Wang, C.; et al. The natural product salicin alleviates osteoarthritis progression by binding to IRE1alpha and inhibiting endoplasmic reticulum stress through the IRE1alpha-IkappaBalpha-p65 signaling pathway. Exp. Mol. Med. 2022, 54, 1927–1939. [Google Scholar] [CrossRef] [PubMed]
- Junjappa, R.P.; Patil, P.; Bhattarai, K.R.; Kim, H.R.; Chae, H.J. IRE1alpha implications in endoplasmic reticulum stress-mediated development and pathogenesis of autoimmune diseases. Front. Immunol. 2018, 9, 1289. [Google Scholar] [CrossRef] [PubMed]
- Malli, R.; Graier, W.F. IRE1alpha modulates ER and mitochondria crosstalk. Nat. Cell Biol. 2019, 21, 667–668. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Ji, Z.; Fu, J.; Wang, X.F.; Zhang, L.S. Endosulfan induces endothelial inflammation and dysfunction via IRE1alpha/NF-kappaB signaling pathway. Environ. Sci. Pollut. Res. Int. 2020, 27, 26163–26171. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, M.; Zhu, M.; Gu, J.; Song, J.; Cui, L.; Liu, D.; Ning, Q.; Jia, X.; Feng, L. Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1alpha/NF-kappaB signaling pathway. Food Funct. 2018, 9, 2386–2397. [Google Scholar] [CrossRef]
- Hu, N.; Wang, C.; Dai, X.; Zhou, M.; Gong, L.; Yu, L.; Peng, C.; Li, Y. Phillygenin inhibits LPS-induced activation and inflammation of LX2 cells by TLR4/MyD88/NF-kappaB signaling pathway. J. Ethnopharmacol. 2020, 248, 112361. [Google Scholar] [CrossRef]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1beta, IL-6 and TNF-alpha production by AP-1 and NF-kappaB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Yuan, F.; Xu, X.; Wu, Y.; Duan, S.; Wu, H. Gastrodin ameliorates acute rejection via IRE1alpha/TRAF2/NF-kappaB in rats receiving liver allografts. Biomed. Res. Int. 2019, 2019, 9276831. [Google Scholar] [CrossRef]
- Ye, L.; Zeng, Q.; Dai, H.; Zhang, W.; Wang, X.; Ma, R.; Hong, X.; Zhao, C.; Pan, L. Endoplasmic reticulum stress is involved in ventilator-induced lung injury in mice via the IRE1alpha-TRAF2-NF-kappaB pathway. Int. Immunopharmacol. 2020, 78, 106069. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yuan, Y.; Wu, C.; Jiang, T.; Wang, B.; Xiong, J.; Zheng, P.; Li, Y.; Xu, J.; Xu, K.; et al. The reciprocal causation of the ASK1-JNK1/2 pathway and endoplasmic reticulum stress in diabetes-induced cognitive decline. Front. Cell Dev. Biol. 2020, 8, 602. [Google Scholar] [CrossRef]
- Lebeaupin, C.; Vallee, D.; Hazari, Y.; Hetz, C.; Chevet, E.; Bailly-Maitre, B. Endoplasmic reticulum stress signalling and the pathogenesis of non-alcoholic fatty liver disease. J. Hepatol. 2018, 69, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.S.; Harrison, D.J.; Kisielewski, D.; Cassidy, D.M.; McNeilly, A.D.; Gallagher, J.R.; Walsh, S.V.; Honda, T.; McCrimmon, R.J.; Dinkova-Kostova, A.T.; et al. Experimental nonalcoholic steatohepatitis and liver fibrosis are ameliorated by pharmacologic activation of Nrf2 (Nf-e2 p45-related factor 2). Cell Mol. Gastroenterol. Hepatol. 2018, 5, 367–398. [Google Scholar] [CrossRef] [PubMed]
- Kar, S.; Paglialunga, S.; Jaycox, S.H.; Islam, R.; Paredes, A.H. Assay validation and clinical performance of chronic inflammatory and chemokine biomarkers of NASH fibrosis. PLoS ONE 2019, 14, e0217263. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hu, S.; Sheng, X.; Liu, Y. Naringenin loaded multifunctional nanoparticles to enhance the chemotherapeutic efficacy in hepatic fibrosis. Biomed. Microdevices 2020, 22, 68. [Google Scholar] [CrossRef] [PubMed]
- Dennis, G., Jr.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, P3. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Tan, Q.; Kir, J.; Liu, D.; Bryant, D.; Guo, Y.; Stephens, R.; Baseler, M.W.; Lane, H.C.; et al. DAVID Bioinformatics Resources: Expanded annotation database and novel algorithms to better extract biology from large gene lists. Nucleic Acids Res. 2007, 35, W169–W175. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef]
- Deng, J.L.; Xu, Y.H.; Wang, G. Identification of potential crucial genes and key pathways in breast cancer using bioinformatic analysis. Front. Genet. 2019, 10, 695. [Google Scholar] [CrossRef]



| Gene Name | Forward/Reverse | Primer Sequences (5′-3′) |
|---|---|---|
| GAPDH | Forward | ACG GCA AGT TCA ACG GCA CAG |
| Reverse | GAA GAC GCC AGT AGA CTC CAC GAC | |
| IRE1α | Forward | CCT GGC ACT GAA GGT TGG AT |
| Reverse | GAG TGG AAG CAG TCA AGG CT | |
| TRAF2 | Forward | AGC CTT CTT CAC AAG CAG ATA TG |
| Reverse | GGT CCA GCA ACA TCA AAG TCA | |
| IKK-β | Forward | TGA ACG AGG ATG AGA AGA CTG T |
| Reverse | TGG AAG GCT GGG ACA TTA GAT | |
| IκB-α | Forward | GTC TCG CTC CTG TTG AAG TG |
| Reverse | GTG TCA TAG CTC TCC TCA TCC T | |
| NF-κB | Forward | AGA GAA GCA CAG ATA CCA CTA AGA |
| Reverse | GTT CAG CCT CAT AGA AGC CAT C | |
| ASK1 | Forward | ACC TGA ACG CTC CTG GTA CA |
| Reverse | TCC TCA GCC AGA AAC CGA CT | |
| JNK1 | Forward | CTC TCC AGC ACC CGT ACA TC |
| Reverse | CGC CAT TCT TAG TTC GCT CC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lei, N.; Song, H.; Zeng, L.; Ji, S.; Meng, X.; Zhu, X.; Li, X.; Feng, Q.; Liu, J.; Mu, J. Persistent Lipid Accumulation Leads to Persistent Exacerbation of Endoplasmic Reticulum Stress and Inflammation in Progressive NASH via the IRE1α/TRAF2 Complex. Molecules 2023, 28, 3185. https://doi.org/10.3390/molecules28073185
Lei N, Song H, Zeng L, Ji S, Meng X, Zhu X, Li X, Feng Q, Liu J, Mu J. Persistent Lipid Accumulation Leads to Persistent Exacerbation of Endoplasmic Reticulum Stress and Inflammation in Progressive NASH via the IRE1α/TRAF2 Complex. Molecules. 2023; 28(7):3185. https://doi.org/10.3390/molecules28073185
Chicago/Turabian StyleLei, Na, Hongfei Song, Ling Zeng, Shaoxiu Ji, Xiangbo Meng, Xiuying Zhu, Xiuyan Li, Quansheng Feng, Jibin Liu, and Jie Mu. 2023. "Persistent Lipid Accumulation Leads to Persistent Exacerbation of Endoplasmic Reticulum Stress and Inflammation in Progressive NASH via the IRE1α/TRAF2 Complex" Molecules 28, no. 7: 3185. https://doi.org/10.3390/molecules28073185
APA StyleLei, N., Song, H., Zeng, L., Ji, S., Meng, X., Zhu, X., Li, X., Feng, Q., Liu, J., & Mu, J. (2023). Persistent Lipid Accumulation Leads to Persistent Exacerbation of Endoplasmic Reticulum Stress and Inflammation in Progressive NASH via the IRE1α/TRAF2 Complex. Molecules, 28(7), 3185. https://doi.org/10.3390/molecules28073185






