Apoptotic Potential of Glucomoringin Isothiocyanate (GMG-ITC) Isolated from Moringa oleifera Lam Seeds on Human Prostate Cancer Cells (PC-3)
Abstract
:1. Introduction
2. Results
2.1. Crude Extraction and Phytochemical Analysis of M. oleifera Lam Seed
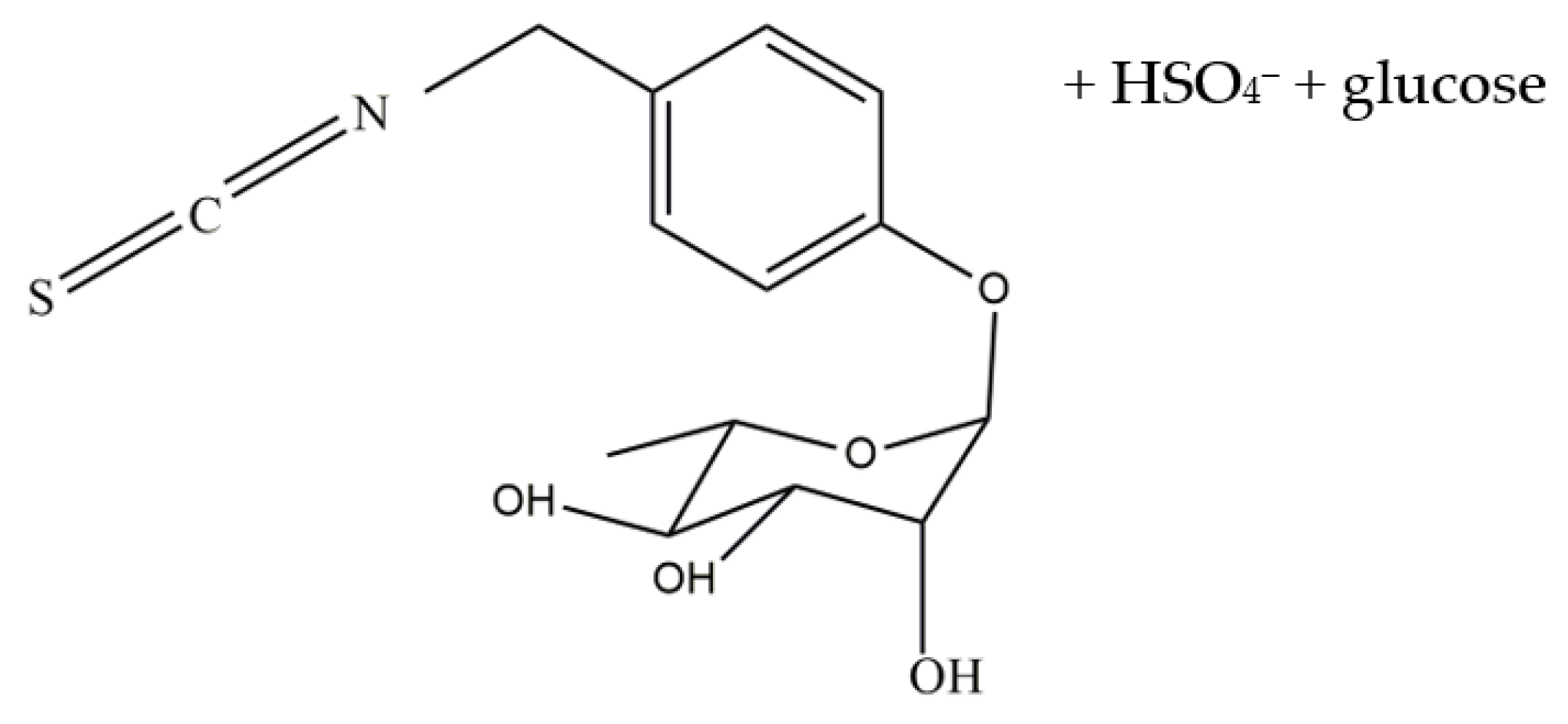
2.2. Characterization of the Isolated Glucomoringin
2.3. Cytotoxic Effect of GMG-ITC on PC-3 Human Prostate Cancer Cells
2.4. Assessment of Apoptosis in PC-3 Cells Induced by GMG-ITC
2.4.1. Morphological Assessment
2.4.2. Apoptosis Assessment Using Flow Cytometry
2.4.3. Apoptotic Gene and Protein Expression Modulated by GMG-ITC
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Crude Extractions
4.3. Phytochemical Analysis
4.4. Extraction and Purification of Glucomoringin
4.5. HPLC Analysis of Glucomoringin
4.6. NMR Analysis of Glucomoringin
4.7. Bioactivation of Glucomoringin
4.8. Cytotoxicity Study of GMG-ITC
4.9. Morphological Assessment of Apoptotic Cell Induction
4.9.1. Phase-Contrast Inverted Microscope Evaluation
4.9.2. Acridine Orange (AO) and Propidium Iodide (PI) Double Staining
4.9.3. Dead End Calorimetric Tunel Assay
4.10. Cell Cycle Analysis by Flow Cytometry
4.10.1. Annexin V-FITC by Flow Cytometry
4.10.2. Cell Cycle Arrest Analysis by Flow Cytometry
4.11. Quantitative Polymerase Chain Reaction (qPCR) for Gene Expression Analysis
4.12. Apoptotic Protein Detection by Cell Signaling Immunoassay
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Int. Agency Res. Cancer 2022, 3, 778–789. [Google Scholar] [CrossRef]
- Zagouri, F.; Peroukidis, S.; Tzannis, K.; Kouloulias, V.; Bamias, A. Hellenic Genito-Urinary Cancer Group (HCUCG) Current Clinical Practice Guidelines on Chemotherapy and Radiotherapy for the Treatment of Non-Metastatic Muscle-Invasive Urothelial Cancer: A Systematic Review and Critical Evaluation by the Hellenic Genito-Urinary Cancer Group (HGUCG). Crit. Rev. Oncol. Hematol. 2015, 93, 36–49. [Google Scholar] [CrossRef]
- Jeeshna, M.V.; Paulsam, S. Phytochemistry and Bioinformatics Approach for the Evaluation of Medicinal Properties of the Herb, Exacum Bicolor Roxb. IRJP 2011, 2, 163–168. [Google Scholar]
- Anwar, F.; Latif, S.; Ashraf, M.; Gilani, A.H. Moringa oleifera: A Food Plant with Multiple Medicinal Uses. Phytother. Res. PTR 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Clarke, J.D.; Dashwood, R.H.; Ho, E. Multi-Targeted Prevention of Cancer by Sulforaphane. Cancer Lett. 2008, 269, 291–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stearns, M.E.; Wang, M. Synergistic Effects of the Green Tea Extract Epigallocatechin-3-Gallate and Taxane in Eradication of Malignant Human Prostate Tumors. Transl. Oncol. 2011, 4, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Overbeek, A.; van den Berg, M.H.; van Leeuwen, F.E.; Kaspers, G.J.L.; Lambalk, C.B.; van Dulmen-den Broeder, E. Chemotherapy-Related Late Adverse Effects on Ovarian Function in Female Survivors of Childhood and Young Adult Cancer: A Systematic Review. Cancer Treat. Rev. 2017, 53, 10–24. [Google Scholar] [CrossRef]
- Razis, A.F.A.; Arumugam, A.; Konsue, N. Glucosinolates and isothiocyanates: Cancer preventive effects. In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 199–210. [Google Scholar] [CrossRef]
- Ciapetti, G.; Granchi, D.; Savarino, L.; Cenni, E.; Magrini, E.; Baldini, N.; Giunti, A. In Vitro Testing of the Potential for Orthopedic Bone Cements to Cause Apoptosis of Osteoblast-like Cells. Biomaterials 2002, 23, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Jaafaru, M.S.; Nordin, N.; Shaari, K.; Rosli, R.; Abdull Razis, A.F. Isothiocyanate from Moringa oleifera seeds mitigates hydrogen peroxide-induced cytotoxicity and preserved morphological features of human neuronal cells. PLoS ONE 2018, 13, e0196403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaafaru, M.S.; Nordin, N.; Rosli, R.; Shaari, K.; Noor, N.M.; Razis, A.F.A. Prospective role of mitochondrial apoptotic pathway in mediating GMG-ITC to reduce cytotoxicity in H2O2-induced oxidative stress in differentiated SH-SY5Y cells. Biomed. Pharmacother. 2019, 119, 109445. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.G.; Qazi, G.N.; Ganju, R.K.; El-Tamer, M.; Singh, J.; Saxena, A.K.; Bedi, Y.S.; Taneja, S.C.; Bhat, H.K. Medicinal Plants and Cancer Chemoprevention. Curr. Drug Metab. 2008, 9, 581–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenwell, M.; Rahman, P.K.S.M. Medicinal Plants: Their Use in Anticancer Treatment. Int. J. Pharm. Sci. Res. 2015, 6, 4103–4112. [Google Scholar] [CrossRef] [PubMed]
- Meireles, D.; Gomes, J.; Lopes, L.; Hinzmann, M.; Machado, J. A Review of Properties, Nutritional and Pharmaceutical Applications of Moringa oleifera: Integrative Approach on Conventional and Traditional Asian Medicine. Adv. Tradit. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Chuang, P.-H.; Lee, C.-W.; Chou, J.-Y.; Murugan, M.; Shieh, B.-J.; Chen, H.-M. Anti-Fungal Activity of Crude Extracts and Essential Oil of Moringa oleifera Lam. Bioresour. Technol. 2007, 98, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.P.; Carvalho, A.F.U.; Farias, D.F.; Cariolano, N.G.; Melo, V.M.M.; Queiroz, M.G.R.; Martins, A.M.C.; Machado-Neto, J.G. Larvicidal Activity of the Water Extract of Moringa oleifera Seeds against Aedes Aegypti and Its Toxicity upon Laboratory Animals. Anais da Academia Brasileira de Ciências 2009, 81, 207–216. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; AL-Raqmi, K.A.S.; AL-Mijizy, Z.H.; Weli, A.M.; Al-Riyami, Q. Study of Total Phenol, Flavonoids Contents and Phytochemical Screening of Various Leaves Crude Extracts of Locally Grown Thymus Vulgaris. Asian Pac. J. Trop. Biomed. 2013, 3, 705–710. [Google Scholar] [CrossRef] [Green Version]
- Akinsulire, O.R.; Aibinu, I.E.; Adenipekun, T.; Adelowotan, T.; Odugbemi, T. In Vitro Antimicrobial Activity of Crude Extracts from Plants Bryophyllum Pinnatum and Kalanchoe Crenata. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Blažević, I.; Mastelić, J. Glucosinolate Degradation Products and Other Bound and Free Volatiles in the Leaves and Roots of Radish (Raphanus sativus L.). Food Chem. 2009, 113, 96–102. [Google Scholar] [CrossRef]
- Sofowra, A. Medicinal Plants and Traditional Medicine in Africa; Spectrum Books Ltd.: Ibadan, Nigeria, 1993. [Google Scholar]
- Matsuura, H.N.; Fett-Neto, A.G. Plant Alkaloids: Main Features, Toxicity, and Mechanisms of Action. In Plant Toxins; Gopalakrishnakone, P., Carlini, C.R., Ligabue-Braun, R., Eds.; Springer: Dordecht, The Netherlands, 2015; pp. 1–15. ISBN 978-94-007-6728-7. [Google Scholar]
- Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging Cytotoxic Alkaloids in the Battle against Cancer: Overview of Molecular Mechanisms. Molecules 2017, 22, 250. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Xu, Y.; Wang, B. Liriodenine Induces the Apoptosis of Human Laryngocarcinoma Cells via the Upregulation of P53 Expression. Oncol. Lett. 2015, 9, 1121–1127. [Google Scholar] [CrossRef] [Green Version]
- Koutová, D.; Havelek, R.; Peterová, E.; Muthná, D.; Královec, K.; Breiterová, K.; Cahlíková, L.; Řezáčová, M. Pancracine, a Montanine-Type Amaryllidaceae Alkaloid, Inhibits Proliferation of A549 Lung Adenocarcinoma Cells and Induces Apoptotic Cell Death in MOLT-4 Leukemic Cells. Int. J. Mol. Sci. 2021, 22, 7014. [Google Scholar] [CrossRef] [PubMed]
- Rattanawong, A.; Payon, V.; Limpanasittikul, W.; Boonkrai, C.; Mutirangura, A.; Wonganan, P. Cepharanthine Exhibits a Potent Anticancer Activity in P53-Mutated Colorectal Cancer Cells through Upregulation of P21Waf1/Cip1. Oncol. Rep. 2017, 39, 227–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flora, S.J.S.; Pachauri, V. Moringa (Moringa oleifera) Seed Extract and the Prevention of Oxidative Stress. In Nuts and Seeds in Health and Disease Prevention; Academic Press: Amsterdam, The Netherlands, 2011; pp. 775–785. ISBN 978-0-12-375688-6. [Google Scholar]
- Brunelli, D.; Tavecchio, M.; Falcioni, C.; Frapolli, R.; Erba, E.; Iori, R.; Rollin, P.; Barillari, J.; Manzotti, C.; Morazzoni, P.; et al. The Isothiocyanate Produced from Glucomoringin Inhibits NF-KB and Reduces Myeloma Growth in Nude Mice in Vivo. Biochem. Pharmacol. 2010, 79, 1141–1148. [Google Scholar] [CrossRef] [Green Version]
- Galuppo, M.; Giacoppo, S.; De Nicola, G.R.; Iori, R.; Navarra, M.; Lombardo, G.E.; Bramanti, P.; Mazzon, E. Antiinflammatory Activity of Glucomoringin Isothiocyanate in a Mouse Model of Experimental Autoimmune Encephalomyelitis. Fitoterapia 2014, 95, 160–174. [Google Scholar] [CrossRef] [PubMed]
- de Graaf, R.M.; Krosse, S.; Swolfs, A.E.M.; te Brinke, E.; Prill, N.; Leimu, R.; van Galen, P.M.; Wang, Y.; Aarts, M.G.M.; van Dam, N.M. Isolation and Identification of 4-α-Rhamnosyloxy Benzyl Glucosinolate in Noccaea Caerulescens Showing Intraspecific Variation. Phytochemistry 2015, 110, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boik, J. Natural Compounds in Cancer Therapy: Promising Nontoxic Antitumor Agents from Plants and Other Natural Sources; Oregon Medical Press: Princeton, NJ, USA, 2001; ISBN 978-0-9648280-1-8. [Google Scholar]
- Doonan, F.; Cotter, T.G. Morphological Assessment of Apoptosis. Methods 2008, 44, 200–204. [Google Scholar] [CrossRef]
- Jaafaru, M.S.; Abd Karim, N.A.; Mohamed Eliaser, E.; Maitalata Waziri, P.; Ahmed, H.; Mustapha Barau, M.; Kong, L.; Abdull Razis, A.F. Nontoxic Glucomoringin-Isothiocyanate (GMG-ITC) Rich Soluble Extract Induces Apoptosis and Inhibits Proliferation of Human Prostate Adenocarcinoma Cells (PC-3). Nutrients 2018, 10, 1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgess, R.C.; Burman, B.; Kruhlak, M.J.; Misteli, T. Activation of DNA Damage Response Signaling by Condensed Chromatin. Cell Rep. 2014, 9, 1703–1717. [Google Scholar] [CrossRef] [Green Version]
- Whitefield, D.B.; Spagnol, S.T.; Armiger, T.J.; Lan, L.; Dahl, K.N. Quantifying Site-Specific Chromatin Mechanics and DNA Damage Response. Sci. Rep. 2018, 8, 18084. [Google Scholar] [CrossRef] [Green Version]
- Hantz, H.L.; Young, L.F.; Martin, K.R. Physiologically Attainable Concentrations of Lycopene Induce Mitochondrial Apoptosis in LNCaP Human Prostate Cancer Cells. Exp. Biol. Med. 2005, 230, 171–179. [Google Scholar] [CrossRef]
- Kundu, J.; Chun, K.-S.; Aruoma, O.I.; Kundu, J.K. Mechanistic Perspectives on Cancer Chemoprevention/Chemotherapeutic Effects of Thymoquinone. Mutat. Res. Mol. Mech. Mutagen. 2014, 768, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Su, J.; Li, L.; Chen, J.; Hu, S.; Zhang, X.; Chen, T. Mechanistic Elucidation of Apoptosis and Cell Cycle Arrest Induced by 5-Hydroxymethylfurfural, the Important Role of ROS-Mediated Signaling Pathways. Food Res. Int. 2014, 66, 186–196. [Google Scholar] [CrossRef]
- Pereira Soares, N.d.C.; Teodoro, A.J.; Oliveira, F.L.; Takiya, C.M.; Junior, A.P.; Nasciutti, L.E.; Lotsch, P.F.; Granjeiro, J.M.; Ferreira, L.B.; Pereira Gimba, E.R.; et al. Lycopene Induce Apoptosis in Human Prostate Cells and Alters the Expression of Bax and Bcl-2 Genes. LWT-Food Sci. Technol. 2014, 59, 1290–1297. [Google Scholar] [CrossRef]
- Vazquez, A.; Bond, E.E.; Levine, A.J.; Bond, G.L. The Genetics of the P53 Pathway, Apoptosis and Cancer Therapy. Nat. Rev. Drug Discov. 2008, 7, 979–987. [Google Scholar] [CrossRef]
- Wei, M.C.; Zong, W.X.; Cheng, E.H.; Lindsten, T.; Panoutsakopoulou, V.; Ross, A.J.; Roth, K.A.; MacGregor, G.R.; Thompson, C.B.; Korsmeyer, S.J. Proapoptotic BAX and BAK: A Requisite Gateway to Mitochondrial Dysfunction and Death. Science 2001, 292, 727–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teijido, O.; Dejean, L. Upregulation of Bcl2 Inhibits Apoptosis-Driven BAX Insertion but Favors BAX Relocalization in Mitochondria. FEBS Lett. 2010, 584, 3305–3310. [Google Scholar] [CrossRef] [Green Version]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- McIlwain, D.R.; Berger, T.; Mak, T.W. Caspase Functions in Cell Death and Disease. Cold Spring Harb. Perspect. Biol. 2013, 5, a008656. [Google Scholar] [CrossRef] [Green Version]
- Abdull, R.A.R.; Noor, A.M. Cruciferous Vegetables: Dietary Phytochemicals for Cancer Prevention. Asian Pac. J. Cancer Prev. 2013, 14, 1565–1570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jed, W.F.; Kristina, L.W.; Katherine, K.S.; Yuzhu, S.; Hua, L.; Anita, A.P.; Collin, R.W.; Mark, E.O. Strategy to Deliver Precise Oral Doses of the Glucosinolates or Isothiocyanates from Moringa oleifera Leaves for Use in Clinical Studies. Nutrients 2019, 11, 1547. [Google Scholar]
- Birgit, H.; Gary, W. A critical review of the bioavailability of glucosinolates and related compounds. Nat. Prod. Rep. 2004, 21, 425–447. [Google Scholar]
- Jianghao, S.; Craig, S.C.; Janet, A.N.; Bing, P.; Liangli, Y.P.C. Profiling Glucosinolate Metabolites in Human Urine and Plasma After Broccoli Consumption Using Non-targeted and Targeted Metabolomic Analyses. Food Chem. 2020, 309, 125660. [Google Scholar]
- Tracey, L.L.; Shikha, S.; Federico, B.; George, M.S.; Perla, T.R.; Maria, H.T.; Robert, D.M.; Richard, Y.B.; Richard, F.M. Accumulation of Sulforaphane and Alliin in Human Prostate Tissue. Nutrients 2022, 14, 3263. [Google Scholar]
- Chu, Q.; Lee, D.T.; Tsao, S.W.; Wang, X.; Wong, Y.C. S-allylcysteine, a water-soluble garlic derivative, suppresses the growth of a human androgen-independent prostate cancer xenograft, CWR22R, under in vivo conditions. BJU Int. 2007, 99, 925–932. [Google Scholar] [CrossRef]
- Tiloke, C.; Phulukdaree, A.; Chuturgoon, A.A. The Antiproliferative Effect of Moringa oleifera Crude Aqueous Leaf Extract on Human Esophageal Cancer Cells. J. Med. Food 2016, 19, 398–403. [Google Scholar] [CrossRef]
- Monika, G.; Shweta, T.; Anuradha, S.; Sudhakar, G. Qualitative and Quantitative Analysis of Phytochemicals and Pharmacological Value of Some Dye Yielding Medicinal Plants. Orient. J. Chem. 2013, 29, 475–481. [Google Scholar]
- Visentin, M.; Tava, A.; Iori, R.; Palmieri, S. Isolation and Identification for Trans-4-(Methylthio)-3-Butenyl Glucosinolate from Radish Roots (Raphanus sativus L.). J. Agric. Food Chem. 1992, 40, 1687–1691. [Google Scholar] [CrossRef]
- Barillari, J.; Gueyrard, D.; Rollin, P.; Iori, R. Barbarea Verna as a Source of 2-Phenylethyl Glucosinolate, Precursor of Cancer Chemopreventive Phenylethyl Isothiocyanate. Fitoterapia 2001, 72, 760–764. [Google Scholar] [CrossRef]
- Song, L.; Morrison, J.J.; Botting, N.P.; Thornalley, P.J. Analysis of Glucosinolates, Isothiocyanates, and Amine Degradation Products in Vegetable Extracts and Blood Plasma by LC-MS/MS. Anal. Biochem. 2005, 347, 234–243. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Abdul, A.B.; Zain, Z.N.M.; Hadi, A.H.A. Zerumbone inhibits interleukin-6 and induces apoptosis and cell cycle arrest in ovarian and cervical cancer cells. Int. Immunopharmacol. 2012, 12, 594–602. [Google Scholar] [CrossRef]
- Arbab, I.A.; Abdul, A.B.; Sukari, M.A.; Abdullah, R.; Syam, S.; Kamalidehghan, B.; Mohan, S. Dentatin isolated from Clausena excavata induces apoptosis in MCF-7 cells through the intrinsic pathway with involvement of NF-κB signalling and G0/G1 cell cycle arrest: A bioassay-guided approach. J. Ethnopharmacol. 2013, 145, 343–354. [Google Scholar] [CrossRef]








| Initial Weight of Sample (g) | Yield of Crude Water Extract Per 1 g of Sample | Yield of Compound (mg/g) of Crude | ||
|---|---|---|---|---|
| 10 | g | % | mg | % |
| 2.17 ± 0.13 | 21.78 | 94.33 ± 0.01 | 9.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Karim, N.A.; Adam, A.H.B.; Jaafaru, M.S.; Rukayadi, Y.; Abdull Razis, A.F. Apoptotic Potential of Glucomoringin Isothiocyanate (GMG-ITC) Isolated from Moringa oleifera Lam Seeds on Human Prostate Cancer Cells (PC-3). Molecules 2023, 28, 3214. https://doi.org/10.3390/molecules28073214
Abd Karim NA, Adam AHB, Jaafaru MS, Rukayadi Y, Abdull Razis AF. Apoptotic Potential of Glucomoringin Isothiocyanate (GMG-ITC) Isolated from Moringa oleifera Lam Seeds on Human Prostate Cancer Cells (PC-3). Molecules. 2023; 28(7):3214. https://doi.org/10.3390/molecules28073214
Chicago/Turabian StyleAbd Karim, Nurul Ashikin, Aziza Hussein Bakheit Adam, Mohammed Sani Jaafaru, Yaya Rukayadi, and Ahmad Faizal Abdull Razis. 2023. "Apoptotic Potential of Glucomoringin Isothiocyanate (GMG-ITC) Isolated from Moringa oleifera Lam Seeds on Human Prostate Cancer Cells (PC-3)" Molecules 28, no. 7: 3214. https://doi.org/10.3390/molecules28073214







