SPE–UPLC–MS/MS for Determination of 36 Monomers of Alkylphenol Ethoxylates in Tea
Abstract
:1. Introduction
2. Results and Discussion
2.1. UPLC–MS/MS Conditions Optimization
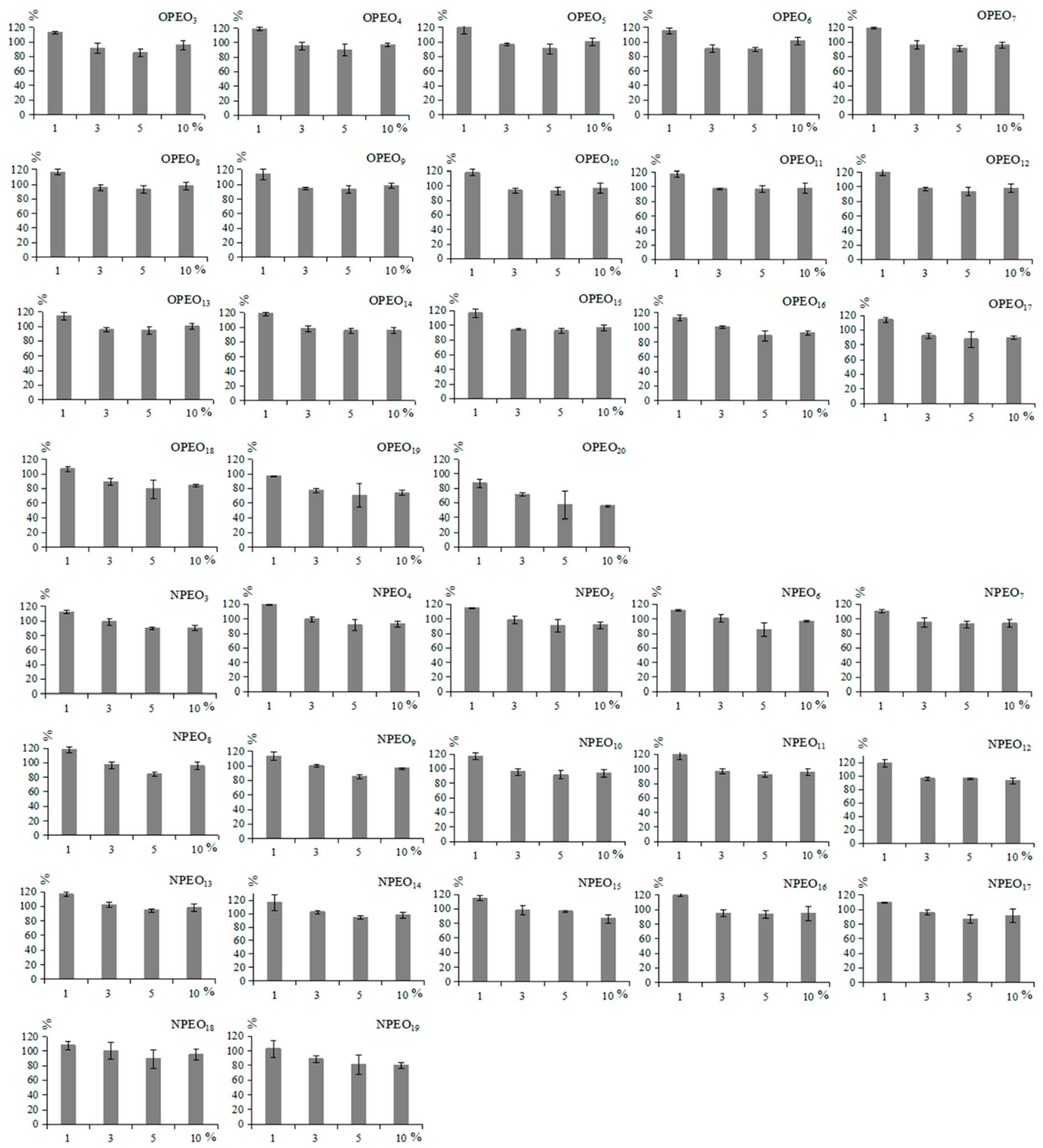
2.2. Optimization of Extraction and Clean-Up
2.3. Method Validation
2.4. Residues in Marketed Tea Samples
3. Materials and Methods
3.1. Reagents, Chemicals, and Materials
3.2. Blank Tea Samples
3.3. Sample Collection and Preparation
3.4. Instrumental Analysis
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Wu, Y.; An, Q.; Li, D.; Kang, L.; Zhou, C.; Zhang, J.; Pan, C. Multi-residue analytical method development and risk assessment of 56 pesticides and their metabolites in tea by chromatography tandem mass spectroscopy. Food Chem. 2021, 375, 131819. [Google Scholar] [CrossRef] [PubMed]
- Al-Zalabani, A.H.; Wesselius, A.; Yu, E.Y.-W.; Brandt, P.V.D.; Grant, E.J.; White, E.; Skeie, G.; Liedberg, F.; Weiderpass, E.; Zeegers, M.P. Tea consumption and risk of bladder cancer in the Bladder Cancer Epidemiology and Nutritional Determinants (BLEND) Study: Pooled analysis of 12 international cohort studies. Clin. Nutr. 2022, 41, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, K.; Majeed, I.; Bilal, M. Phytochemistry and diverse pharmacology of genus mimosa: A review. Biomolecules 2022, 12, 83. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Cheng, Z.; Chen, X.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Pan, X.; An, X.; Zheng, Y. Occurrences of eight common-used pesticide adjuvants in ten vegetable species and implications for dietary intake in North China. Food Chem. 2021, 347, 128984. [Google Scholar] [CrossRef] [PubMed]
- Kovarova, J.; Blahova, J.; Divisova, L.; Svobodova, Z. Alkylphenol ethoxylates and alkylphenols--update information on occurrence, fate and toxicity in aquatic environment. Pol. J. Vet. Sci. 2013, 16, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Shiu, R.F.; Jiang, J.J.; Kao, H.Y.; Fang, M.D.; Liang, Y.J.; Tang, C.C.; Lee, C.L. Alkylphenol ethoxylate metabolites in coastal sediments off southwestern Taiwan: Spatiotemporal variations, possible sources, and ecological risk. Chemosphere 2019, 225, 9–18. [Google Scholar] [CrossRef]
- Traverso-Soto, J.M.; Lara-Martín, P.A.; León, V.M.; González-Mazo, E. Analysis of alcohol polyethoxylates and polyethylene glycols in marine sediments. Talanta 2013, 110, 171–179. [Google Scholar] [CrossRef]
- McLaren, D.E.; Rawlins, A.J. Occurrence of alkylphenols and alkylphenol ethoxylates in North Sea sediment samples collected across oil and gas fields. Mar. Pollut. Bull. 2022, 178, 113655. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. National inventory of alkylphenol ethoxylate compounds in U.S. Sewage sludges and chemical fate in outdoor soil mesocosms. Environ. Pollut. 2013, 174, 189–193. [Google Scholar] [CrossRef] [Green Version]
- Hiroo, Y.; Shunsuke, K.; Toshitaka, H.; Masahito, S. Distribution and geochemical behaviour of anionic surfactants determined as ethyl violet active substances in Lake Biwa, Japan. Water Environ. J. 2015, 29, 221–227. [Google Scholar]
- Zhang, Z.; Ren, N.; Kannan, K.; Nan, J.; Liu, L.; Ma, W.; Qi, H.; Li, Y. Occurrence of endocrine-disrupting phenols and estrogens in water and sediment of the Songhua River, northeastern China. Arch. Environ. Contam. Toxicol. 2014, 66, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Chokwe, T.B.; Okonkwo, J.O.; Sibali, L.L.; Ncube, E.J. Alkylphenol ethoxylates and brominated flame retardants in water, fish (carp) and sediment samples from the Vaal river, South Africa. Environ. Sci. Pollut. Res. 2015, 22, 11922–11929. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, R.; Lacorte, S.; Ginebreda, A.; Barceló, D. Occurrence and fate of alkylphenols and alkylphenol ethoxylates in sewage treatment plants and impact on receiving waters along the Ter River (Catalonia, NE Spain). Environ. Pollut. 2008, 153, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.J.; Cruzeiro, C.; Reis, M.; Pardal, M.A.; Rocha, E. Pollution by endocrine disruptors in a southwest European temperate coastal lagoon (Ria de Aveiro, Portugal). Environ. Monit. Assess. 2016, 188, 101. [Google Scholar] [CrossRef]
- Ringbeck, B.; Weber, T.; Bury, D.; Kasper-Sonnenberg, M.; Pälmke, C.; Brüning, T.; Koch, H.M.; Kolossa-Gehring, M. Nonylphenol (np) exposure in germany between 1991 and 2021: Urinary biomarker analyses in the German environmental specimen bank (esb). Int. J. Hyg. Environ. Health 2022, 245, 114010. [Google Scholar] [CrossRef]
- Nishio, E.; Ichiki, Y.; Tamura, H.; Morita, S.; Watanabe, K.; Yoshikawa, H. Isolation of bacterial strains that produce the endocrine disruptor, octylphenol diethoxylates, in paddy fields. Biosc. Biotech. Bioch. 2002, 66, 1792–1798. [Google Scholar] [CrossRef] [Green Version]
- Mahalakshmi, R.; Pugazhendhi, A.; Brindhadevi, K.; Ramesh, N. Analysis of alkylphenol ethoxylates (APEOs) from tannery sediments using LC-MS and their environmental risks. Process Biochem. 2020, 97, 37–42. [Google Scholar] [CrossRef]
- Chiu, T.S.; Hsieh, C.Y.; Miaw, C.L.; Lin, C.N.; Chang, T.C.; Yen, C.H.; Chiou, M.T. Alkylphenol polyethoxylate derivatives in groundwater and blood samples collected from pig herds in Taiwan. J. Vet. Med. Sci. 2014, 76, 971–975. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Z.J.; Cao, X.L.; Li, H.; Zhang, C.; Abd El-Aty, A.M.; Jin, F.; Shao, H.; Jin, M.J.; Wang, S.S.; She, Y.X.; et al. Fast determination of alkylphenol ethoxylates in leafy vegetables using a modified quick, easy, cheap, effective, rugged, and safe method and ultra-high performance supercritical fluid chromatography-tandem mass spectrometry. J. Chromatogr. A 2017, 1525, 161–172. [Google Scholar] [CrossRef]
- Chen, J.; Mullin, C.A. Determination of nonylphenol ethoxylate and octylphenol ethoxylate surfactants in beehive samples by high performance liquid chromatography coupled to mass spectrometry. Food Chem. 2014, 158, 473–479. [Google Scholar] [CrossRef]
- Ferrara, F.; Ademollo, N.; Delise, M.; Fabietti, F.; Funari, E. Alkylphenols and their ethoxylates in seafood from the Tyrrhenian Sea. Chemosphere 2008, 72, 1279–1285. [Google Scholar] [CrossRef] [PubMed]
- Viñas, P.; Pastor-Belda, M.; Torres, A.; Campillo, N.; Hernández-Córdoba, M. Use of oleic-acid functionalized nanoparticles for the magnetic solid-phase microextraction of alkylphenols in fruit juices using liquid chromatography-tandem mass spectrometry. Talanta 2016, 151, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lou, J.; Yuan, J.; Xu, J.; Fan, X. Laccase immobilization on core-shell magnetic metal-organic framework microspheres for alkylphenol ethoxylate compound removal. J. Environ. Chem. Eng. 2021, 9, 105000. [Google Scholar] [CrossRef]
- Sonnenschein, C.; Soto, A.M. An updated review of environmental estrogen and androgen mimics and antagonists. J. Steroid Biochem. 1998, 65, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, L.; Lara-Martín, P.A.; Giger, W.; Hollender, J.; Berg, M. Synthetic surfactants in Swiss sewage sludges: Analytical challenges, concentrations and per capita loads. Sci. Total Environ. 2022, 808, 151361. [Google Scholar] [CrossRef]
- Acir, I.H.; Guenther, K. Endocrine-disrupting metabolites of alkylphenol ethoxylates—a critical review of analytical methods, environmental occurrences, toxicity, and regulation. Sci. Total Environ. 2018, 635, 1530–1546. [Google Scholar] [CrossRef] [PubMed]
- Li, B.X.; Pang, X.Y.; Zhang, P.; Lin, J.; Li, X.X.; Liu, Y.; Li, H.; Liu, F.; Mu, W. Alcohol ethoxylates significantly synergize pesticides than alkylphenol ethoxylates considering bioactivity against three pests and joint toxicity to Daphnia magna. Sci. Total Environ. 2018, 644, 1452–1459. [Google Scholar] [CrossRef]
- Bokern, M.; Raid, P.; Harms, H. Toxicity, uptake and metabolism of 4-n-nonylphenol in root cultures and intact plants under septic and aseptic conditions. Environ. Sci. Pollut. Res. 1998, 5, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, F.; Ademollo, N.; Orrù, M.A.; Silvestroni, L.; Funari, E. Alkylphenols in adipose tissues of Italian population. Chemosphere 2011, 82, 1044–1049. [Google Scholar] [CrossRef]
- Ademollo, N.; Ferrara, F.; Delise, M.; Fabietti, F.; Funari, E. Nonylphenol and octylphenol in human breast milk. Environ. Int. 2008, 34, 984–987. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhu, P.; Xu, B.; Zhao, R.; Qiao, S.; Chen, X.; Tang, R.; Wu, D.; Song, L.; Wang, S.; et al. Determination of nine environmental phenols in urine by ultra-high-performance liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2012, 36, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Guo, J.; Zou, Y.; Wang, L.; Kaw, H.Y.; Quinto, M.; Meng, L.Y.; Dong, M. Fast removal of phenolic compounds from water using hierarchical porous carbon nanofibers membrane. J. Chromatogr. A 2022, 1685, 463624. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Iqbal, S. Design, synthesis, in silico testing, and in vitro evaluation of thiazolidinone-based benzothiazole derivatives as inhibitors of α-amylase and α-glucosidase. Pharmaceuticals 2022, 15, 1164. [Google Scholar] [CrossRef] [PubMed]
- Loyo-Rosales, J.E.; Rice, C.P.; Torrents, A. Octyl and nonylphenol ethoxylates and carboxylates in wastewater and sediments by liquid chromatography/tandem mass spectrometry. Chemosphere 2007, 68, 2118–2127. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C. Multiresidue method for the determination of pesticides in oolong tea using QuEChERS by gas chromatography-triple quadrupole tandem mass spectrometry. Food Chem. 2017, 229, 580–587. [Google Scholar] [CrossRef]
- Wiest, L.; Giroud, B.; Assoumani, A.; Lestremau, F.; Vulliet, E. A multi-family offline SPE LC-MS/MS analytical method for anionic, cationic and non-ionic surfactants quantification in surface water. Talanta 2021, 232, 122441. [Google Scholar] [CrossRef]
- Fontanals, N.; Galià, M.; Cormack, P.A.; Marcé, R.M.; Sherrington, D.C.; Borrull, F. Evaluation of a new hypercrosslinked polymer as a sorbent for solid-phase extraction of polar compounds. J. Chromatogr. A 2005, 1075, 51–56. [Google Scholar] [CrossRef]
- Kong, X.J.; Wei, X.; Wang, N.; Bu, Y.Q.; Shan, Z.J. A review on the environmental behavior of the polyoxyethylene type nonionic surfactants adjuvants in pesticides. J. Agric. Resour. Environ. 2017, 34, 197–206. [Google Scholar]
- Staples, C.A.; Klecka, G.M.; Naylor, C.G.; Losey, B.S. C8- and C9-alkylphenols and ethoxylates: I. Identity, physical characterization, and biodegradation pathways analysis. Hum. Ecol. Risk Assess. 2008, 14, 1007–1024. [Google Scholar] [CrossRef]
- DeArmond, P.D.; DiGoregorio, A.L. Rapid liquid chromatography-tandem mass spectrometry-based method for the analysis of alcohol ethoxylates and alkylphenol ethoxylates in environmental samples. J. Chromatogr. A 2013, 1305, 154–163. [Google Scholar] [CrossRef]
- Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Determination of alkylphenol polyethoxylates, bisphenol-a, 17α-ethynylestradiol and 17β-estradiol and its metabolites in sewage samples by SPE and LC/MS/MS. J. Hazard. Mater. 2010, 183, 701–711. [Google Scholar] [CrossRef]
- Andreu, V.; Ferrer, E.; Rubio, J.L.; Font, G.; Picó, Y. Quantitative determination of octylphenol, nonylphenol, alkylphenol ethoxylates and alcohol ethoxylates by pressurized liquid extraction and liquid chromatography-mass spectrometry in soils treated with sewage sludges. Sci. Total Environ. 2007, 378, 124–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega-Morales, T.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J. Development and optimisation of an on-line solid phase extraction coupled to ultra-high-performance liquid chromatography-tandem mass spectrometry methodology for the simultaneous determination of endocrine disrupting compounds in wastewater samples. J. Chromatogr. A 2012, 1230, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Ciofi, L.; Ancillotti, C.; Chiuminatto, U.; Fibbi, D.; Pasquini, B.; Bruzzoniti, M.C.; Rivoira, L.; Bubba, M.D. Fully automated on-line solid phase extraction coupled to liquid chromatography-tandem mass spectrometry for the simultaneous analysis of alkylphenol polyethoxylates and their carboxylic and phenolic metabolites in wastewater samples. Anal. Bioanal. Chem. 2016, 408, 3331–3347. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, D.; Li, Z.; Wang, Y.; Yang, Y.; Liu, M.; Li, D.; Sun, G.; Zeng, B. Concentrations, leachability, and health risks of mercury in green tea from major production areas in China. Ecotoxicol Environ Saf. 2022, 232, 113279. [Google Scholar] [CrossRef] [PubMed]
- State Administration for Market Regulation and Standardization Administration (China). Textiles–Determination of surfactant–Alkyphenols and Alkylphenol Ethoxylates. Available online: https://openstd.samr.gov.cn/bzgk/gb/newGbInfo?hcno=22CC306762497933A6EC5EEF88B511F0 (accessed on 25 February 2023).





| Analyte | Matrix | Enrichment | Optimisation of Extraction | Detection | Recovery (%) | Ref. |
|---|---|---|---|---|---|---|
| APEO3–13 | Beehive samples | QuEChERS | – | LC–MS | 74–111 | [20] |
| OPEO7–11 | Water | SPE | Cartridges | LC–MS/MS | 58.7–68.4 (absolute recovery) | [36] |
| APEO2–20 | Water | SPE | Cartridges Elution solvents | HPLC–MS/MS | 37–69 (absolute recovery) | [40] |
| APEO1–12 | Sewage | SPE | Cartridges pH and ionic strength Sample volume Wash step Elution solvents | LC–MS/MS | 60–108 | [41] |
| APEO1–15 | Soil sludge | PLE *–SPE | Solvents | LC–APCI–MS | 89–102 | [42] |
| APEO1–8 | Sewage | SPE | Sample volume pH Wash step Elution solvents | LC–MS/MS | 74–106 | [43] |
| APEO1–8 | Wastewater | On-line SPE | Sorbents Loading volume Elution solvents | LC–MS/MS | 50–120 | [44] |
| APEO3–20 | Tea | SPE | Elution solvents Wash step Elution solvents volume | LC–MS/MS | 70.3–110.7 (APEO20 61.8%–62.9%) | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Q.; Qin, Y.; Sun, H.; Wang, X.; Yang, M.; Zhang, X.; Zhou, L.; Luo, F. SPE–UPLC–MS/MS for Determination of 36 Monomers of Alkylphenol Ethoxylates in Tea. Molecules 2023, 28, 3216. https://doi.org/10.3390/molecules28073216
Lin Q, Qin Y, Sun H, Wang X, Yang M, Zhang X, Zhou L, Luo F. SPE–UPLC–MS/MS for Determination of 36 Monomers of Alkylphenol Ethoxylates in Tea. Molecules. 2023; 28(7):3216. https://doi.org/10.3390/molecules28073216
Chicago/Turabian StyleLin, Qin, Yujie Qin, Hezhi Sun, Xinru Wang, Mei Yang, Xinzhong Zhang, Li Zhou, and Fengjian Luo. 2023. "SPE–UPLC–MS/MS for Determination of 36 Monomers of Alkylphenol Ethoxylates in Tea" Molecules 28, no. 7: 3216. https://doi.org/10.3390/molecules28073216





