Fluorescent Molecular Rotors Based on Hinged Anthracene Carboxyimides
Abstract
:1. Introduction
2. Results and Discussion
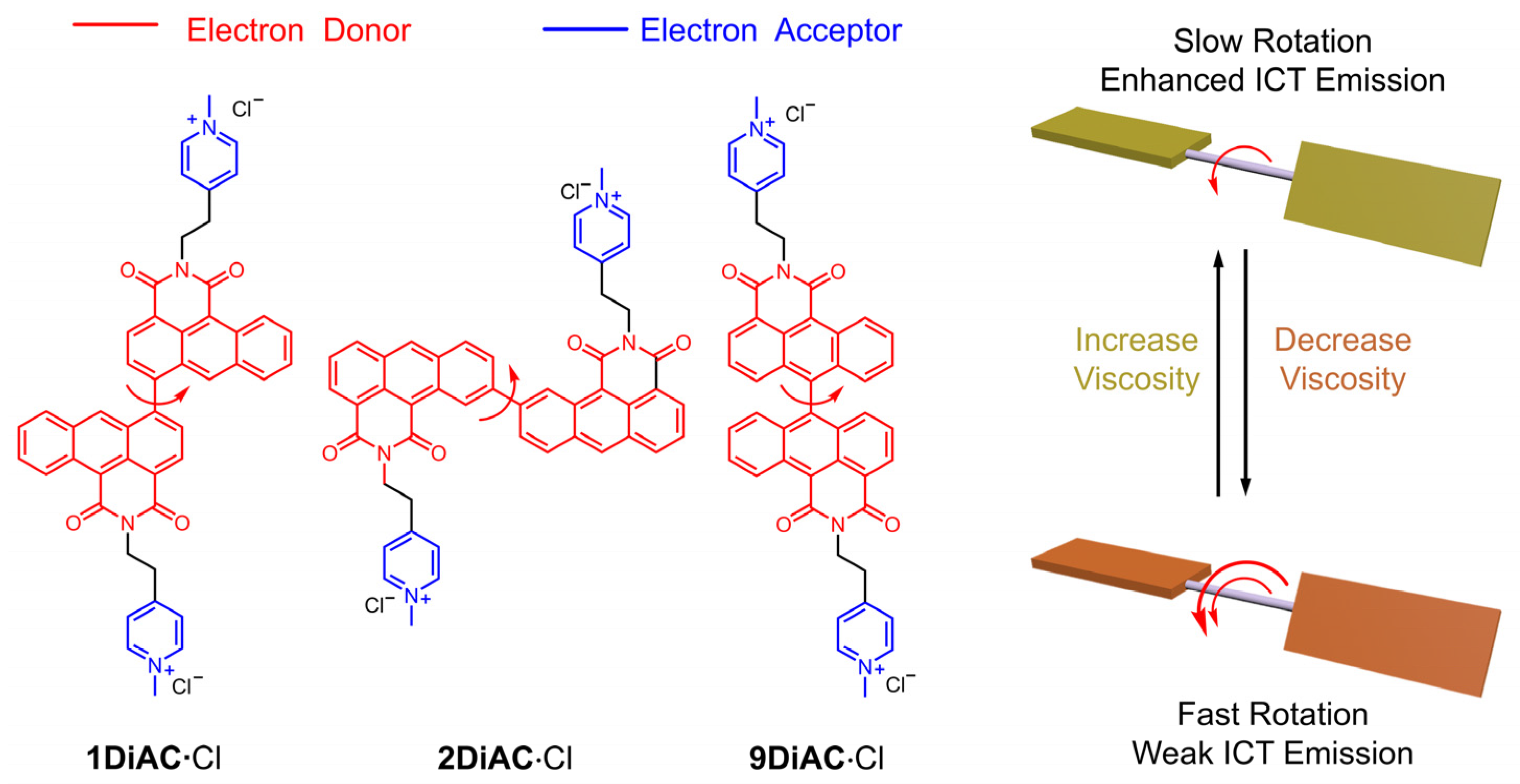
2.1. Design and Syntheses
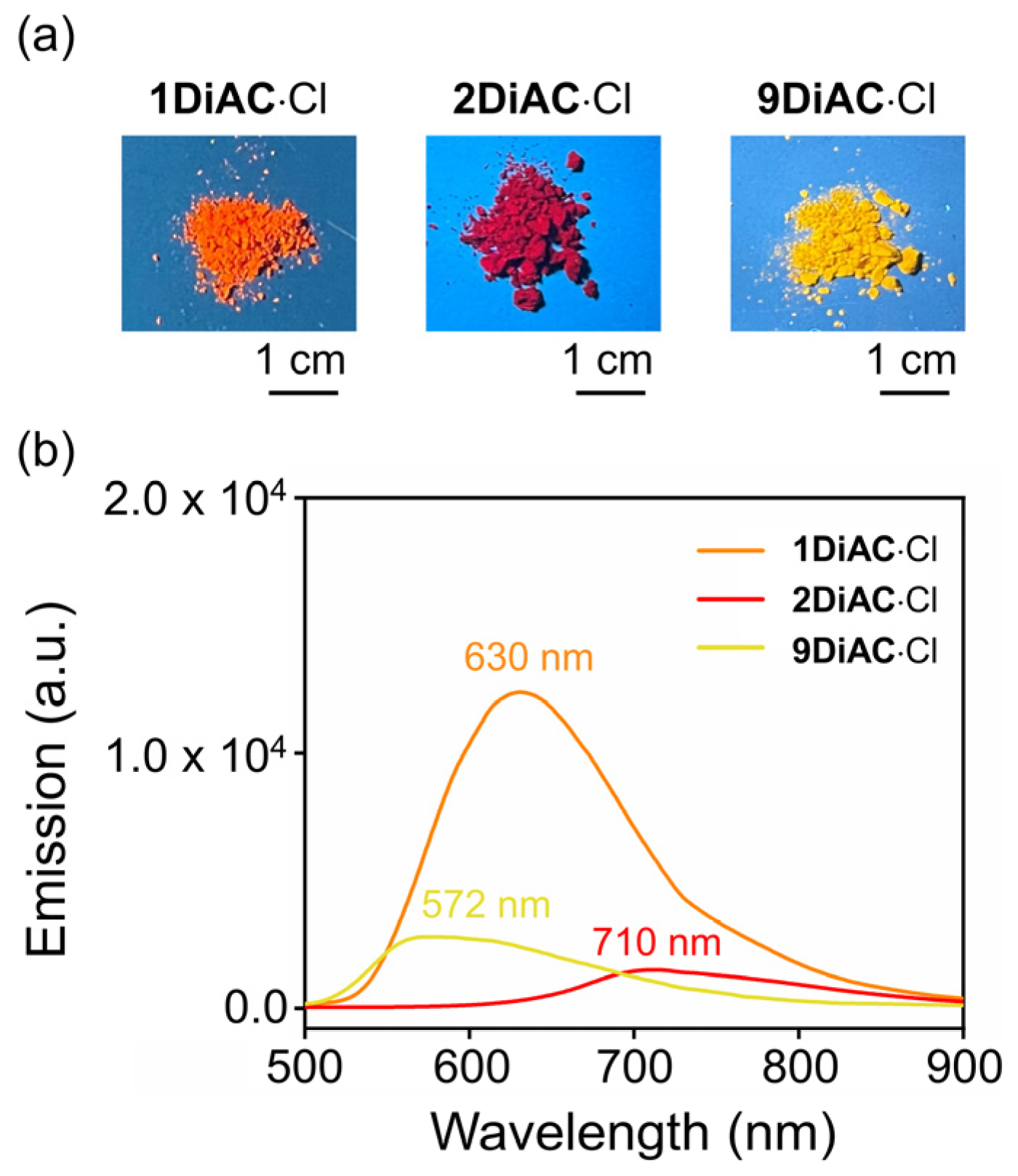
2.2. Photophysical Properties and Solvatochromism
2.3. Multilinear Analysis Using Kamlet–Taft and Catalán Parameters
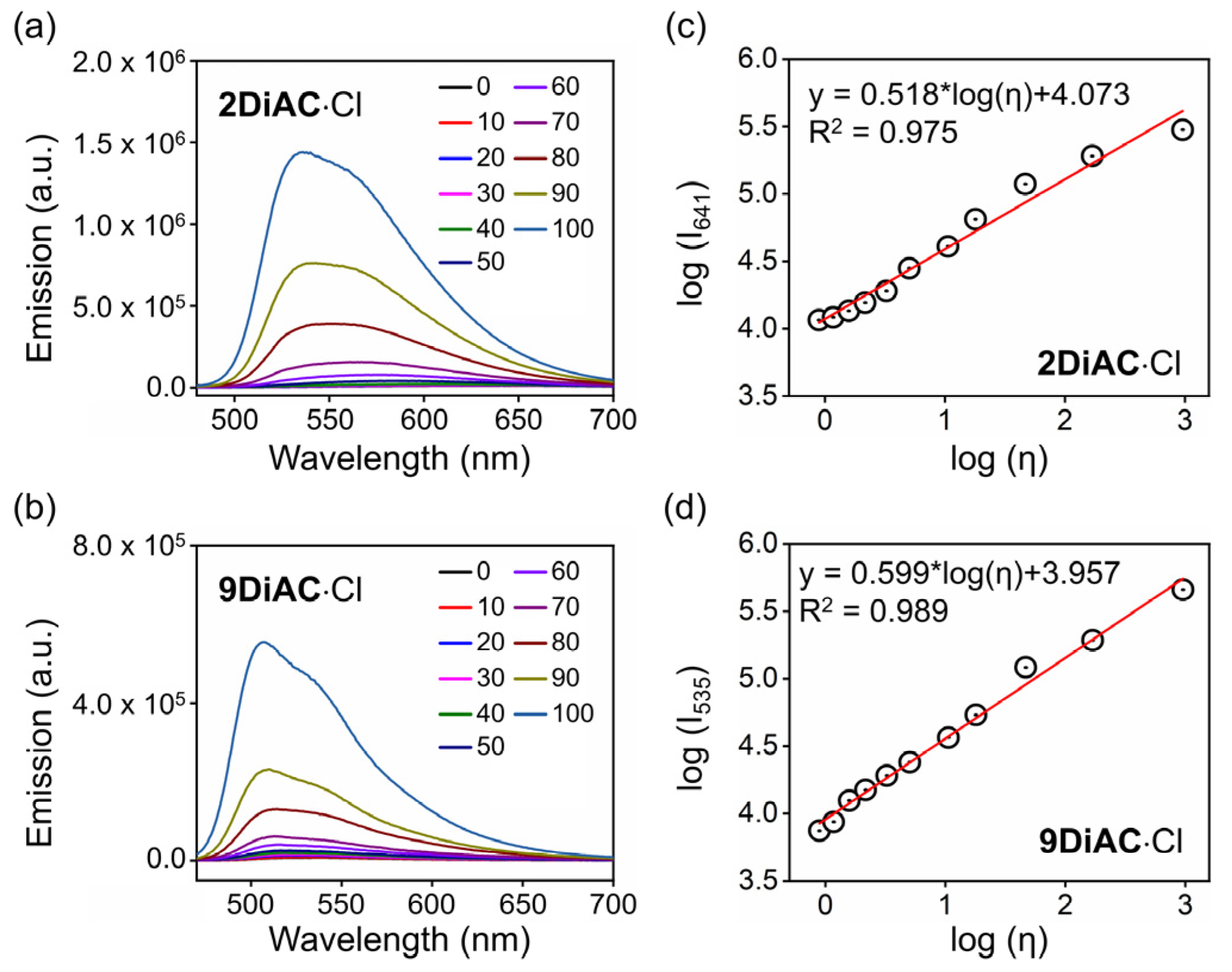
2.4. Viscosity Response of the Fluorescent Molecular Rotors
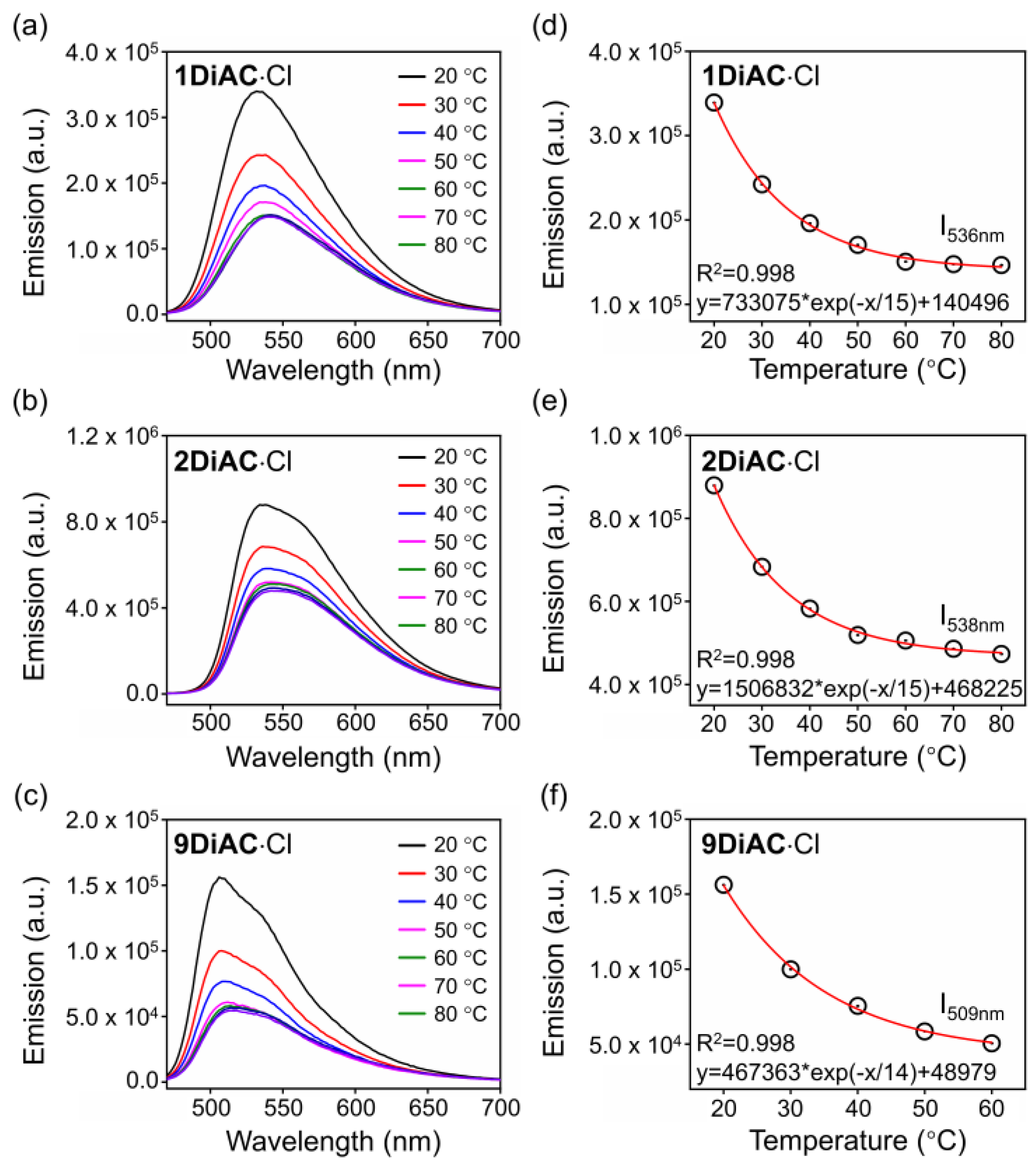
2.5. Viscosity-Related Temperature Response of the Fluorescent Molecular Rotors
3. Conclusions
4. Experimental Section
4.1. General
4.2. Syntheses
4.2.1. Synthesis of 1An Dione
4.2.2. Synthesis of 1An Anhydride
4.2.3. Synthesis of 1AC
4.2.4. Synthesis of 1DiAC
4.2.5. Synthesis of 1DiAC·Cl
4.2.6. Synthesis of 2An Dione
4.2.7. Synthesis of 2AC
4.2.8. Synthesis of 2DiAC·Cl
4.2.9. Synthesis of 9DiAn Dione
4.2.10. Synthesis of 9DiAn Anhydride
4.2.11. Synthesis of 9DiAC
4.2.12. Synthesis of 9DiAC·Cl
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. What is Solvatochromism? J. Phys. Chem. B 2010, 114, 17128–17135. [Google Scholar] [CrossRef] [PubMed]
- Kothavale, S.; Sekar, N. Methoxy supported, deep red emitting mono, bis and tris triphenylamine-isophorone based styryl colorants: Synthesis, photophysical properties, ICT, TICT emission and viscosity sensitivity. Dye. Pigment. 2017, 136, 116–130. [Google Scholar] [CrossRef]
- Wen, P.; Gao, Z.; Zhang, R.; Li, A.; Zhang, F.; Li, J.; Xie, J.; Wu, Y.; Wu, M.; Guo, K. A–π–D–π–A carbazole derivatives with remarkable solvatochromism and mechanoresponsive luminescence turn-on. J. Mater. Chem. C 2017, 5, 6136–6143. [Google Scholar] [CrossRef]
- Acree, W.E., Jr.; Wilkins, D.C.; Tucker, S.A.; Griffin, J.M.; Powell, J.R. Spectrochemical Investigations of Preferential Solvation. 2. Compatibility of Thermodynamic Models versus Spectrofluorometric Probe Methods for Tautomeric Solutes Dissolved in Binary Mixtures. J. Phys. Chem. 1994, 98, 2537–2544. [Google Scholar] [CrossRef]
- Królicki, R.; Jarzȩba, W.; Mostafavi, M.; Lampre, I. Preferential Solvation of Coumarin 153—The Role of Hydrogen Bonding. J. Phys. Chem. A 2002, 106, 1708–1713. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The solvatochromic comparison method. I. The .beta.-scale of solvent hydrogen-bond acceptor (HBA) basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The solvatochromic comparison method. 2. The .alpha.-scale of solvent hydrogen-bond donor (HBD) acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.; Taft, R.W. The solvatochromic comparison method. 6. The .pi.* scale of solvent polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, .pi.*, .alpha., and .beta., and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Catalán, J.; López, V.; Pérez, P.; Martin-Villamil, R.; Rodríguez, J.-G. Progress towards a generalized solvent polarity scale: The solvatochromism of 2-(dimethylamino)-7-nitrofluorene and its homomorph 2-fluoro-7-nitrofluorene. Liebigs Ann. 1995, 1995, 241–252. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C.; López, V.; Pérez, P.; De Paz, J.-L.G.; Rodríguez, J.G. A Generalized Solvent Basicity Scale: The Solvatochromism of 5-Nitroindoline and Its Homomorph 1-Methyl-5-nitroindoline. Liebigs Ann. 1996, 1996, 1785–1794. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C. A Generalized Solvent Acidity Scale: The Solvatochromism of o-tert-Butylstilbazolium Betaine Dye and Its Homomorph o,o′-Di-tert-butylstilbazolium Betaine Dye. Liebigs Ann. 1997, 1997, 1941–1949. [Google Scholar] [CrossRef]
- Catalán, J. Toward a Generalized Treatment of the Solvent Effect Based on Four Empirical Scales: Dipolarity (SdP, a New Scale), Polarizability (SP), Acidity (SA), and Basicity (SB) of the Medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Koenig, M.; Storti, B.; Bizzarri, R.; Guldi, D.M.; Brancato, G.; Bottari, G. A fluorescent molecular rotor showing vapochromism, aggregation-induced emission, and environmental sensing in living cells. J. Mater. Chem. C 2016, 4, 3018–3027. [Google Scholar] [CrossRef] [Green Version]
- Kuimova, M.K. Mapping viscosity in cells using molecular rotors. Phys. Chem. Chem. Phys. 2012, 14, 12671–12686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Rakhimbekova, A.; Duan, X.; Yin, Q.; Foss, C.A.; Fan, Y.; Xu, Y.; Li, X.; Cai, X.; Kutil, Z.; et al. A prostate-specific membrane antigen activated molecular rotor for real-time fluorescence imaging. Nat. Commun. 2021, 12, 5460. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Börner, R.; Mata, G.; Luedtke, N.W. A Highly Fluorescent Nucleobase Molecular Rotor. J. Am. Chem. Soc. 2020, 142, 14422–14426. [Google Scholar] [CrossRef] [PubMed]
- Kuimova, M.K.; Botchway, S.W.; Parker, A.W.; Balaz, M.; Collins, H.A.; Anderson, H.L.; Suhling, K.; Ogilby, P.R. Imaging intracellular viscosity of a single cell during photoinduced cell death. Nat. Chem. 2009, 1, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Jin, Y.-J.; Dogra, R.; Cheong, I.W.; Kwak, G. Fluorescent Molecular Rotor-in-Paraffin Waxes for Thermometry and Biometric Identification. ACS Appl. Mater. Interfaces 2015, 7, 14485–14492. [Google Scholar] [CrossRef]
- Liu, Z.-F.; Chen, X.; Mou, Z.F.; Jin, W.J. Stimuli-responsive luminescent bithiophene-dicarbaldehyde molecular rotors by hydrogen bonding. J. Mater. Chem. C 2020, 8, 16100–16106. [Google Scholar] [CrossRef]
- Feng, J.; Tian, K.; Hu, D.; Wang, S.; Li, S.; Zeng, Y.; Li, Y.; Yang, G. A Triarylboron-Based Fluorescent Thermometer: Sensitive Over a Wide Temperature Range. Angew. Chem. Int. Ed. 2011, 50, 8072–8076. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; Cornell, B.; Sherin, P.S.; Maleckaitė, K.; Kubánková, M.; Izquierdo, M.A.; Vu, T.T.; Volkova, Y.A.; Budynina, E.M.; Molteni, C.; et al. Cyclopropyl Substituents Transform the Viscosity-Sensitive BODIPY Molecular Rotor into a Temperature Sensor. ACS Sens. 2021, 6, 2158–2167. [Google Scholar] [CrossRef]
- Vyšniauskas, A.; Qurashi, M.; Gallop, N.; Balaz, M.; Anderson, H.L.; Kuimova, M.K. Unravelling the effect of temperature on viscosity-sensitive fluorescent molecular rotors. Chem. Sci. 2015, 6, 5773–5778. [Google Scholar] [CrossRef] [Green Version]
- Abeywickrama, C.S.; Wijesinghe, K.J.; Plescia, C.B.; Fisher, L.S.; Goodson, T., III; Stahelin, R.V.; Pang, Y. A pyrene-based two-photon excitable fluorescent probe to visualize nuclei in live cells. Photochem. Photobiol. Sci. 2020, 19, 1152–1159. [Google Scholar] [CrossRef]
- Hirsch, T.; Port, H.; Wolf, H.C.; Miehlich, B.; Effenberger, F. Intramolecular Charge Separation and Transition State Dynamics in Anthracene/Pyridinium Supermolecules. J. Phys. Chem. B 1997, 101, 4525–4535. [Google Scholar] [CrossRef]
- Ikbal, M.; Banerjee, R.; Atta, S.; Dhara, D.; Anoop, A.; Singh, N.D.P. Synthesis, Photophysical and Photochemical Properties of Photoacid Generators Based on N-Hydroxyanthracene-1,9-dicarboxyimide and Their Application toward Modification of Silicon Surfaces. J. Org. Chem. 2012, 77, 10557–10567. [Google Scholar] [CrossRef] [PubMed]
- Sami, S.M.; Dorr, R.T.; Alberts, D.S.; Remers, W.A. 2-Substituted 1,2-dihydro-3H-dibenz[de,h]isoquinoline-1,3-diones. A new class of antitumor agent. J. Med. Chem. 1993, 36, 765–770. [Google Scholar] [CrossRef]
- Yao, J.H.; Chi, C.; Wu, J.; Loh, K.-P. Bisanthracene Bis(dicarboxylic imide)s as Soluble and Stable NIR Dyes. Chem. Eur. J. 2009, 15, 9299–9302. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Han, B.; Chen, K.; Sun, J.; Hou, X. A novel single-fluorophore-based ratiometric fluorescent probe for direct detection of isocyanates in air. Chem. Commun. 2017, 53, 6231–6234. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Niu, G.; Wei, X.; Lan, M.; Zeng, L.; Kinsella, J.M.; Sheng, R. A family of multi-color anthracene carboxyimides: Synthesis, spectroscopic properties, solvatochromic fluorescence and bio-imaging application. Dye. Pigment. 2017, 139, 166–173. [Google Scholar] [CrossRef]
- Liang, H.; Tang, L.; He, J.; Li, J.; Chen, Z.; Cai, S.; Pang, J.; Mahmood, Z.; Chen, W.; Li, M.-D.; et al. Modulating the intersystem crossing mechanism of anthracene carboxyimide-based photosensitizers via structural adjustments and application as a potent photodynamic therapeutic reagent. Phys. Chem. Chem. Phys. 2022, 24, 20901–20912. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.-P.; Hancock, G.; Toogood, M.J.; McKendrick, K.G. Quantitative Laser-Induced Fluorescence Spectroscopy of the CF A2Σ+−X2Π Transition: Electronic Transition Dipole Moment Function and Predissociation. J. Phys. Chem. 1996, 100, 47–53. [Google Scholar] [CrossRef]
- Catalán, J.; Díaz, C.; López, V.; Pérez, P.; Claramunt, R.M. The TICT Mechanism in 9,9′-Biaryl Compounds: Solvatochromism of 9,9′-Bianthryl, N-(9-Anthryl)carbazole, and N,N′-Bicarbazyl. J. Phys. Chem. 1996, 100, 18392–18398. [Google Scholar] [CrossRef]
- Rohman, M.A.; Phanrang, P.T.; Chamlagai, D.; Mitra, S. Deciphering Spectroscopic and Structural Insights into the Photophysical Behavior of 2,2′-Dipyridylamine: An Efficient Environment Sensitive Fluorescence Probe. J. Phys. Chem. A 2021, 125, 6964–6975. [Google Scholar] [CrossRef] [PubMed]
- Law, K.Y. Fluorescence probe for microenvironments: Anomalous viscosity dependence of the fluorescence quantum yield of p-N,N-dialkylaminobenzylidenemalononitrile in 1-alkanols. Chem. Phys. Lett. 1980, 75, 545–549. [Google Scholar] [CrossRef]
- Lee, S.-C.; Heo, J.; Woo, H.C.; Lee, J.-A.; Seo, Y.H.; Lee, C.-L.; Kim, S.; Kwon, O.P. Fluorescent Molecular Rotors for Viscosity Sensors. Chem. Eur. J. 2018, 24, 13706–13718. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, N.P.; Gonzalez-Rodriguez, E.; Hughes, A.; dos Passos Gomes, G.; White, F.D.; Kuriakose, F.; Alabugin, I.V. Radical Alkyne peri-Annulation Reactions for the Synthesis of Functionalized Phenalenes, Benzanthrenes, and Olympicene. Angew. Chem. Int. Ed. 2018, 57, 3651–3655. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar]
- Dennington, R.; Keith, T.A.; Millam, J.M. GaussView, Version 5; Semichem Inc.: Shawnee, KS, USA, 2016. [Google Scholar]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, Y.; Fang, W.; Olson, M.A. Fluorescent Molecular Rotors Based on Hinged Anthracene Carboxyimides. Molecules 2023, 28, 3217. https://doi.org/10.3390/molecules28073217
Ni Y, Fang W, Olson MA. Fluorescent Molecular Rotors Based on Hinged Anthracene Carboxyimides. Molecules. 2023; 28(7):3217. https://doi.org/10.3390/molecules28073217
Chicago/Turabian StyleNi, Yanhai, Wangjian Fang, and Mark A. Olson. 2023. "Fluorescent Molecular Rotors Based on Hinged Anthracene Carboxyimides" Molecules 28, no. 7: 3217. https://doi.org/10.3390/molecules28073217






