Ppb-Level Hydrogen Sulfide Gas Sensor Based on the Nanocomposite of MoS2 Octahedron/ZnO-Zn2SnO4 Nanoparticles
Abstract
1. Introduction
2. Results and Discussion
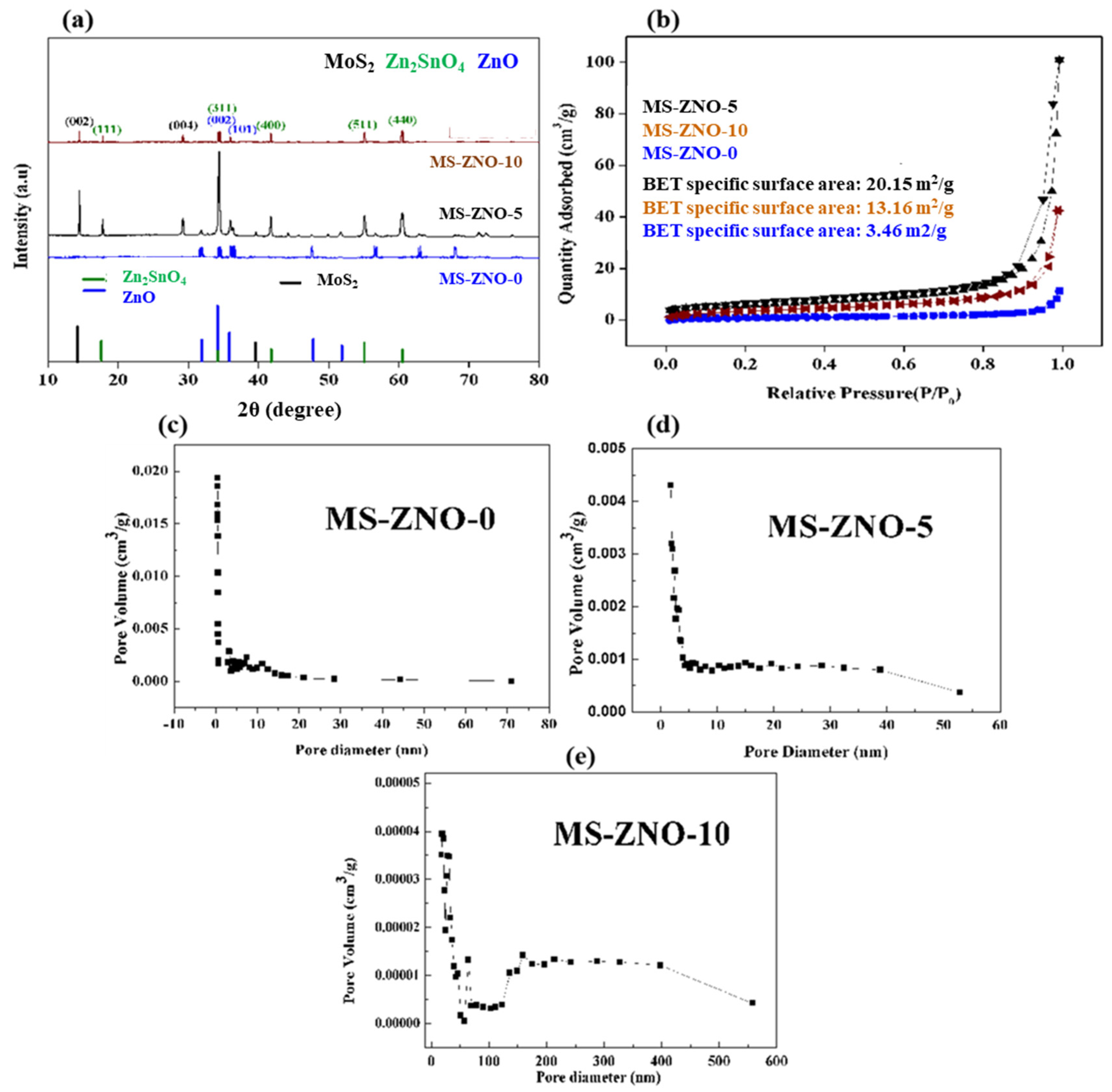
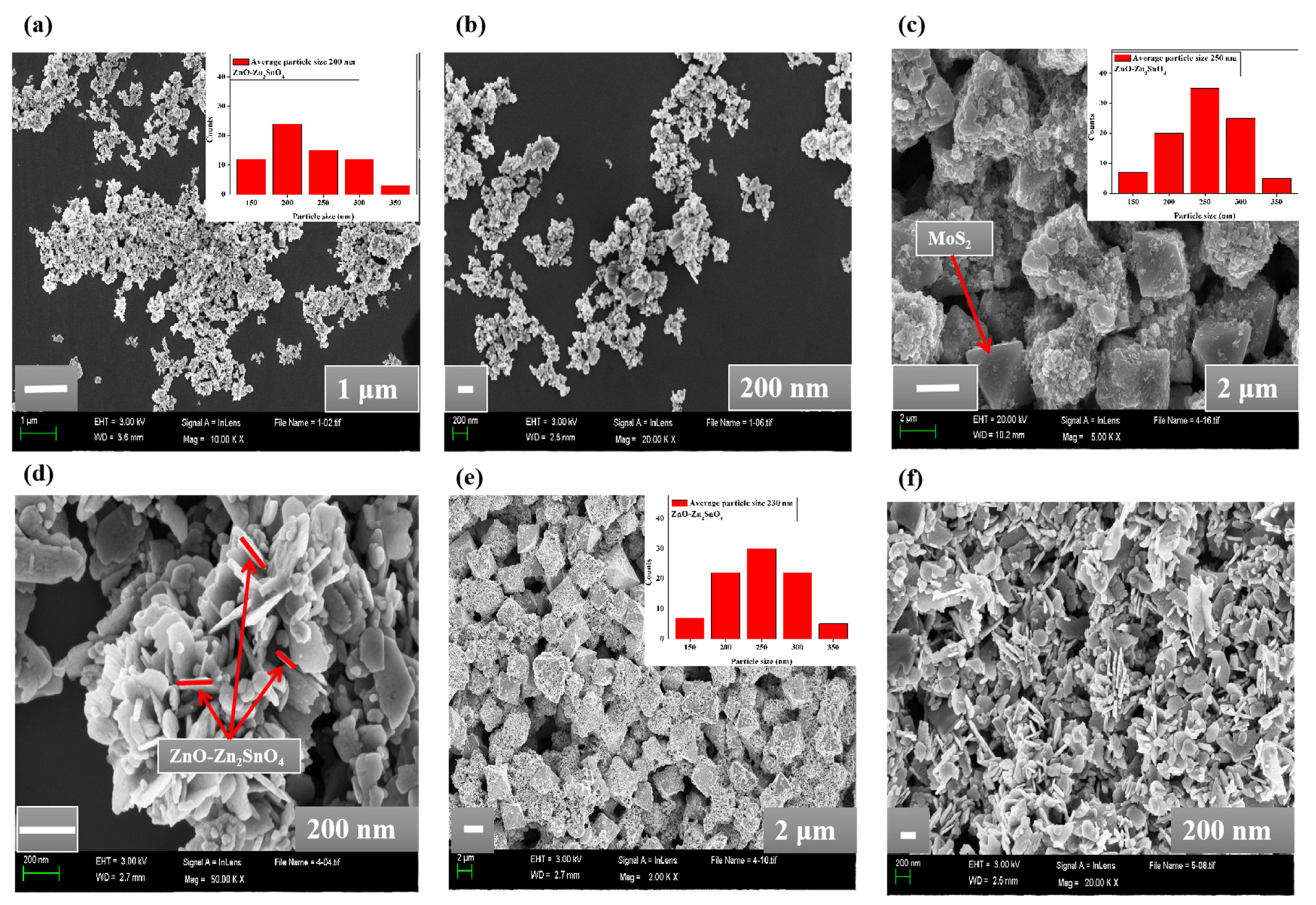
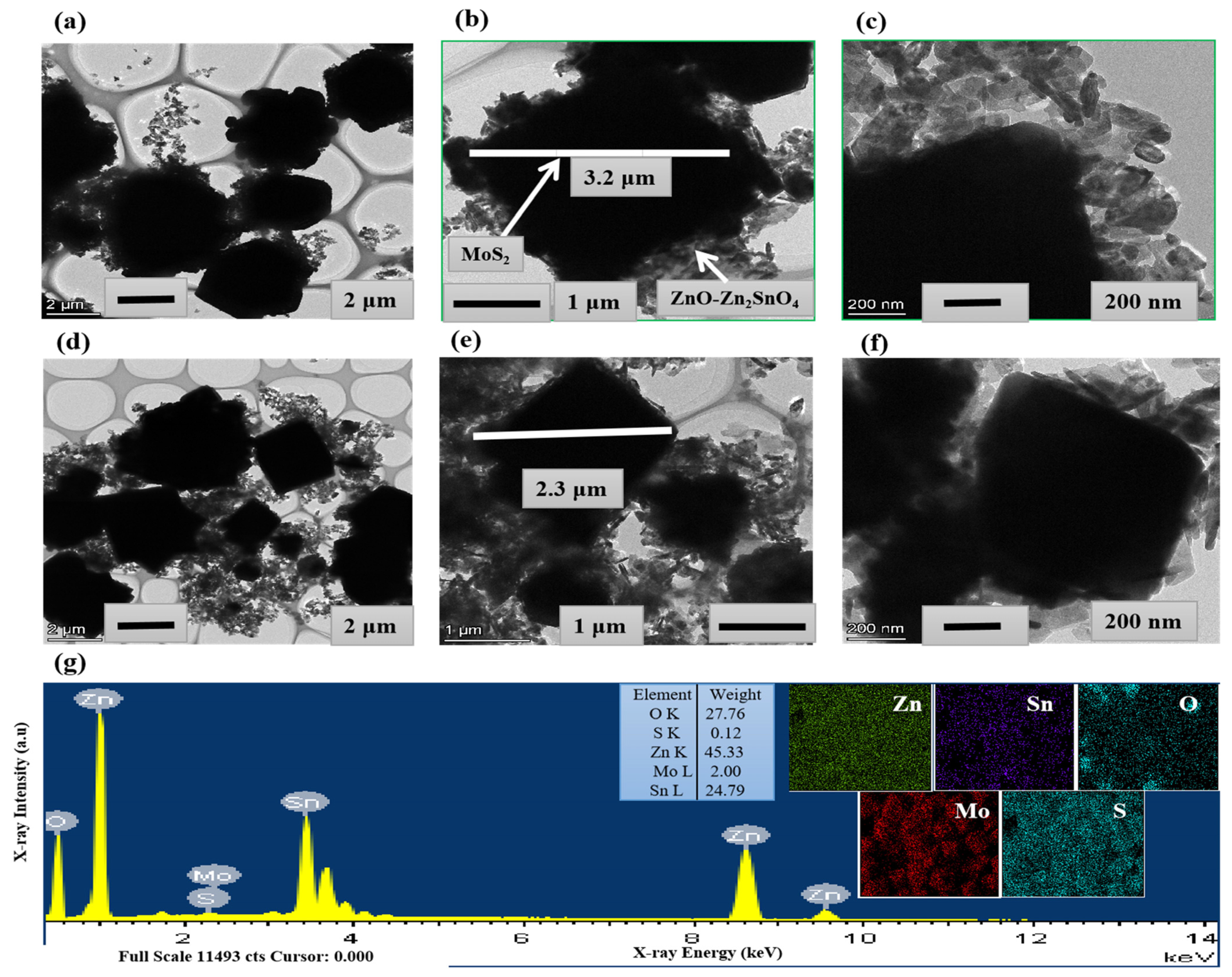
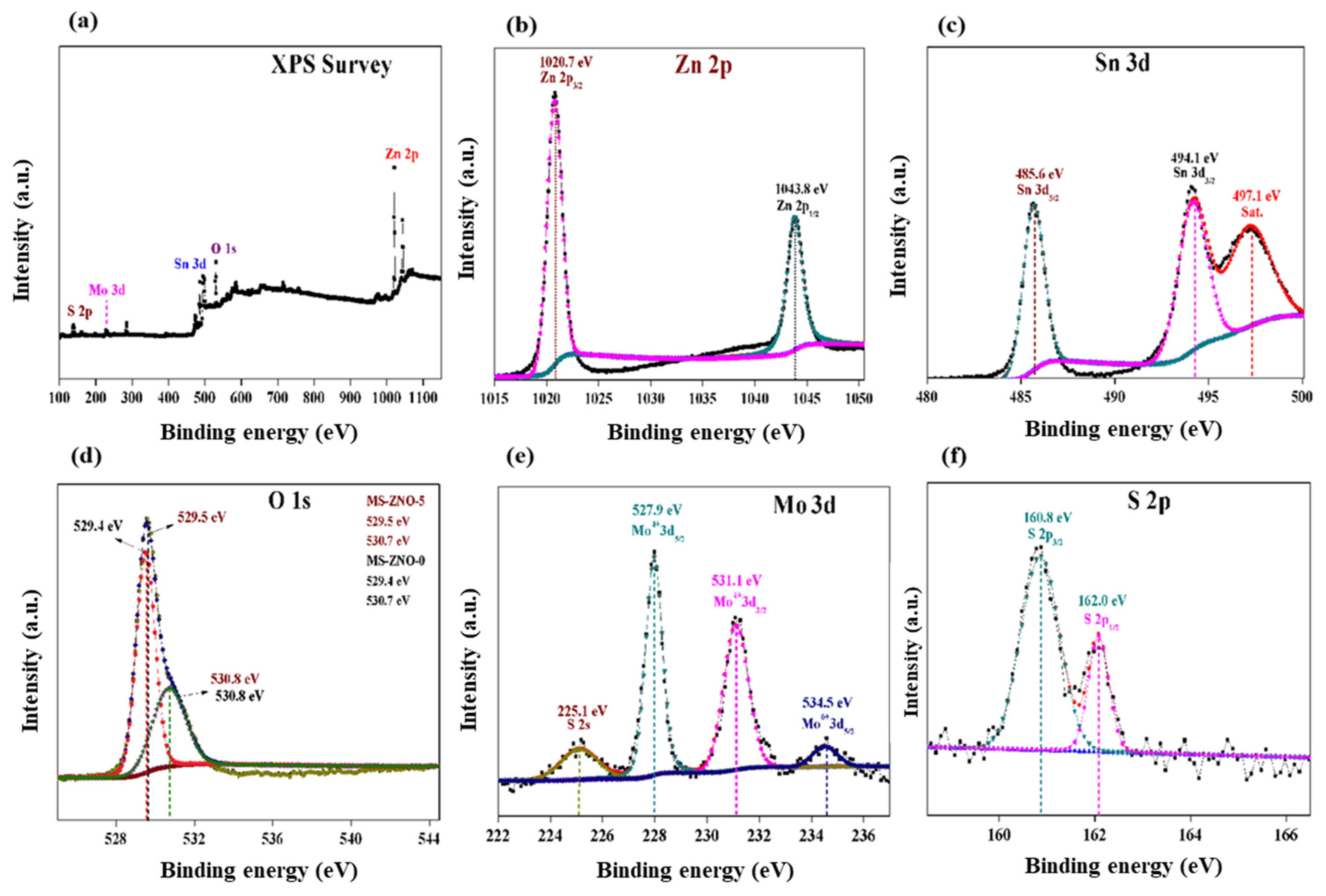
2.1. Characterizations of Materials
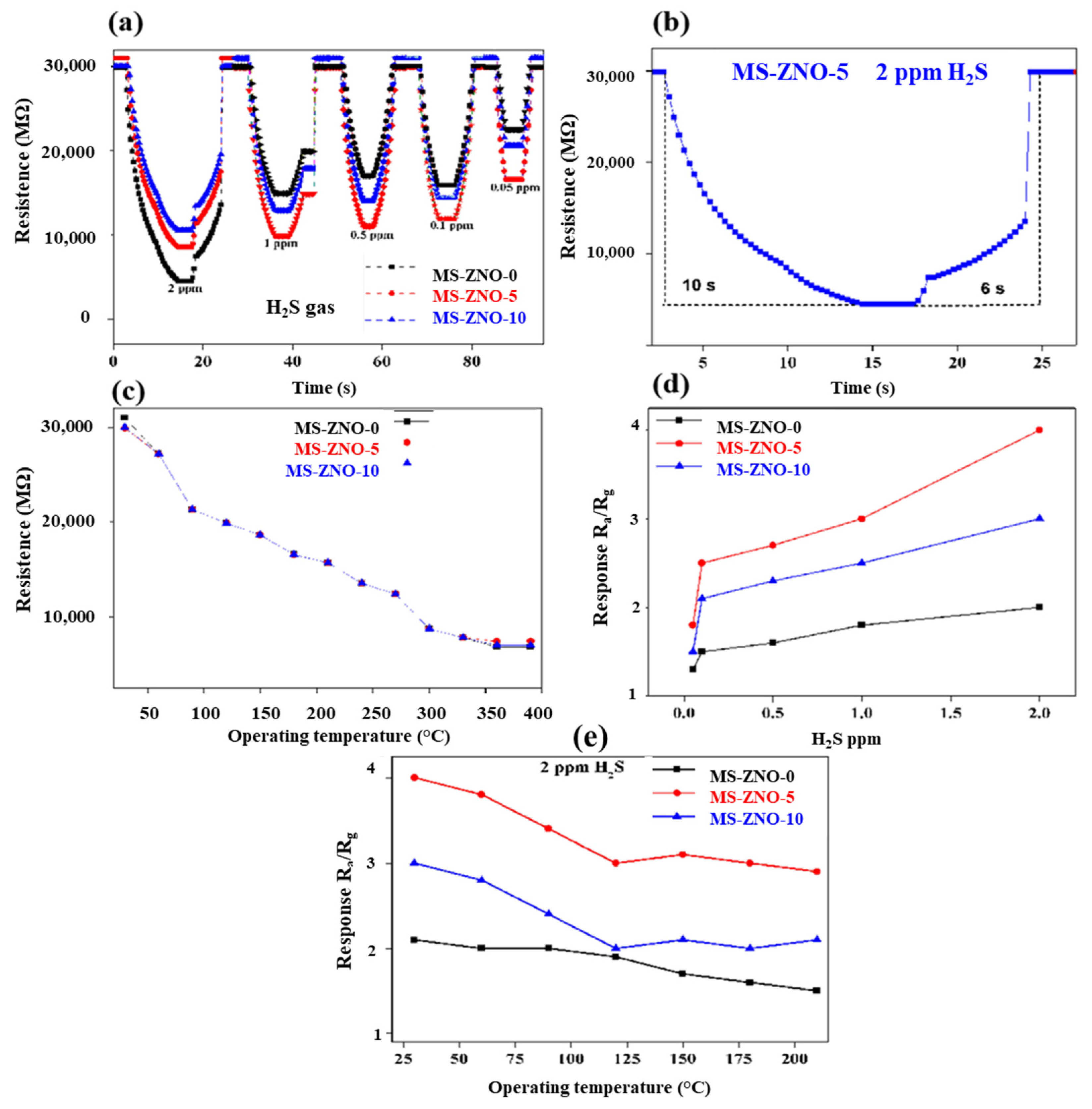
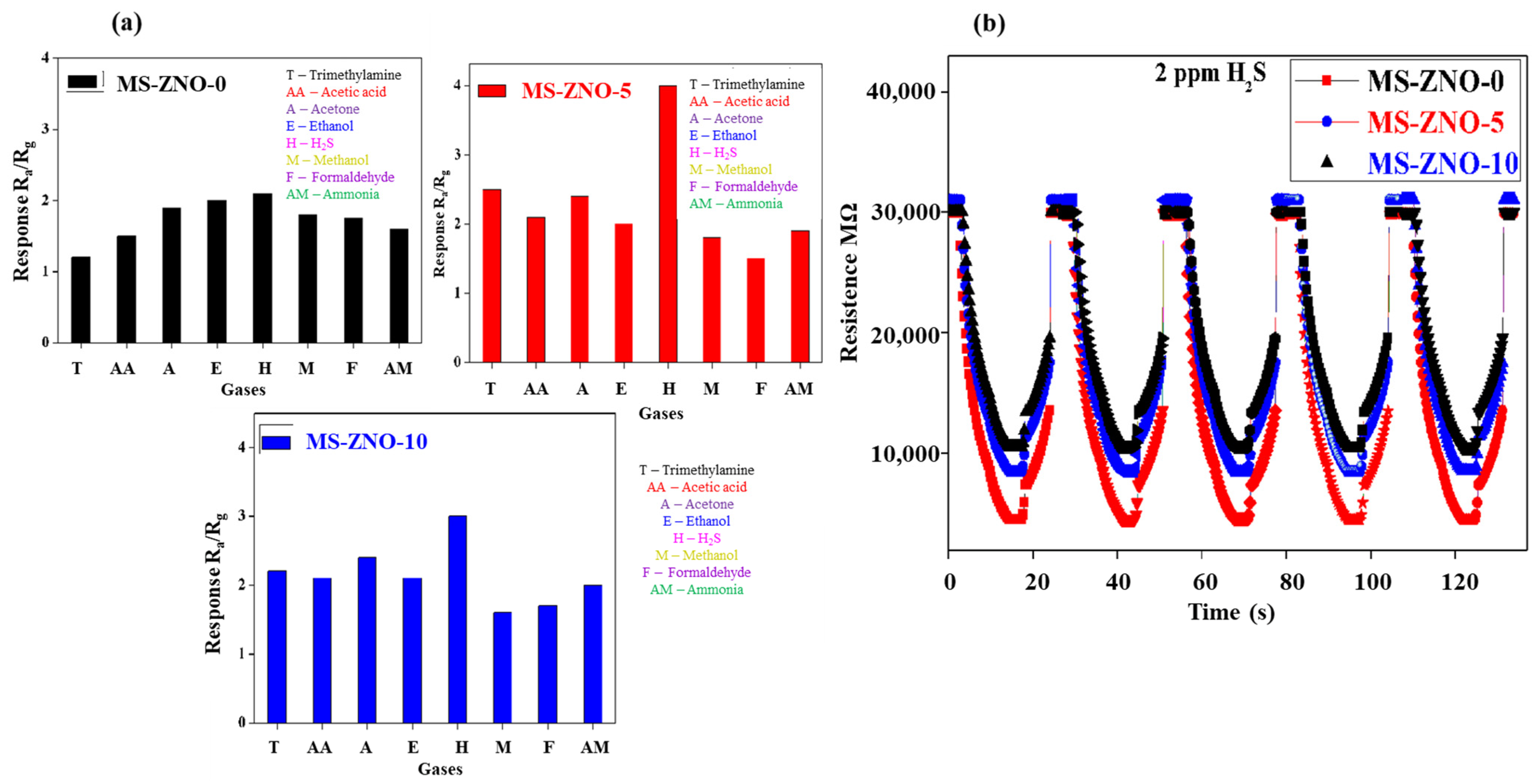
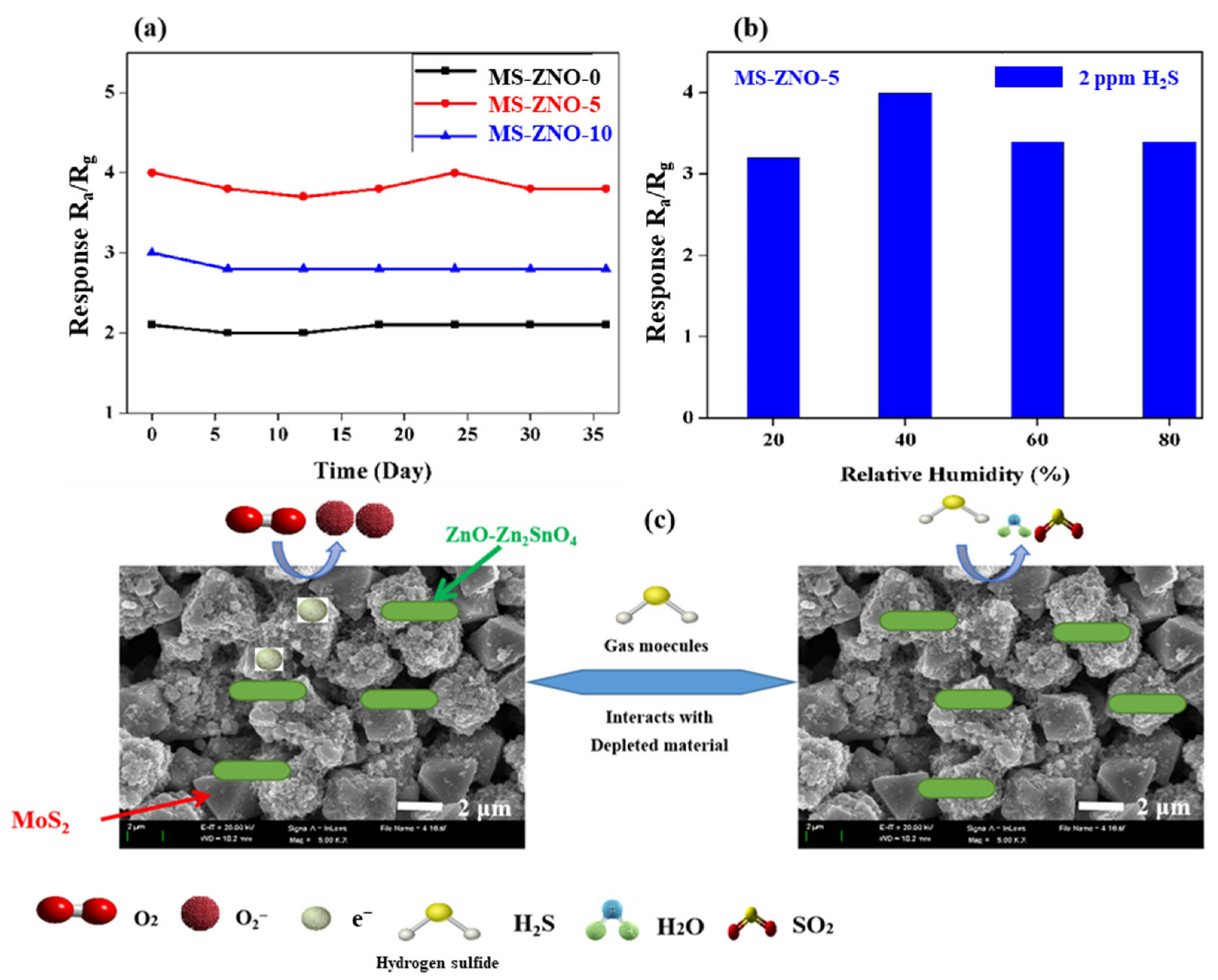
2.2. Gas Sensing Properties
2.3. Gas Sensing Mechanism
| Materials | Temp. (°C) | Gas/Conc. ppm | Response (Rg/Ra) | Selectivity | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|
| ZnSnO3 | 230 | ethanol/50 | 47 | 1.4 | 1 ppm | [46] |
| ZnO/Co3O4 | 250 | acetone/50 | 46 | - | 2 ppm | [47] |
| ZnO-ZnS | 150 | H2S/5 | 0.88 | - | 1 ppm | [48] |
| Pd/ZnO | 220 | CO/100 | 15 | - | 20 ppm | [49] |
| Zn2SnO4 | 133 | H2S/1 | - | - | 1 ppb | [50] |
| Nb2O5/SnO2 | 275 | H2S/20 | 4 | 3.8 | - | [51] |
| Ag-In2O3 | 30 | H2S/20 | 93719 | - | 0.005 ppm | [52] |
| MoS2-ZnO-Zn2SnO4 | 30 | H2S/2 | 4 | 1.6 | 0.05 ppm | This work |
3. Experimental Section
3.1. Chemicals
3.2. The Synthesis of ZnO-Zn2SnO4 Nanoparticles and MoS2-ZnO-Zn2SnO4 Nanocomposite
3.3. Characterizations of the Nanocomposites
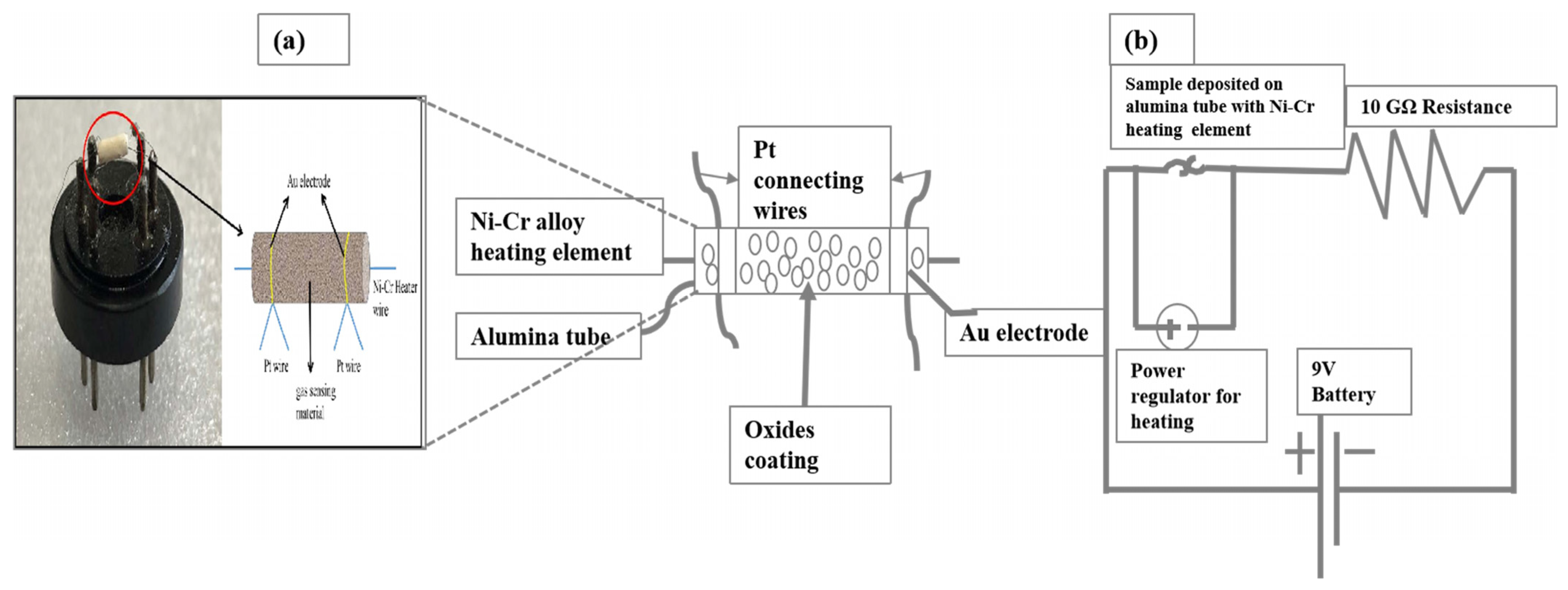
3.4. Fabrication of Gas Sensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tong, X.; Shen, W.; Chen, X.; Corriou, J.P. A fast response and recovery H2S gas sensor based on free-standing TiO2 nanotube array films prepared by one-step anodization method. Ceram. Int. 2017, 43, 14200–14209. [Google Scholar] [CrossRef]
- Han, C.; Li, X.; Shah, C.; Li, X.; Ma, J.; Zhang, X.; Liu, Y. Composition-controllable p-CuO/n-ZnO hollow nanofibers for high-performance H2S detection. Sens. Actuators B Chem. 2019, 285, 495–503. [Google Scholar] [CrossRef]
- Samokhvalov, A.; Tatarchuk, B.J. Characterization of active sites, determination of mechanisms of H2S, COS and CS2 sorption and regeneration of ZnO low-temperature sorbents: Past, current and perspectives. Phys. Chem. Chem. Phys. 2011, 13, 3197–3209. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, T.J.; Ding, R.Y. A Room Temperature ZnO-NPs/MEMS Ammonia Gas Sensor. Nanomaterials 2022, 12, 3287. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Xu, Z.; Zhang, Z.; Tang, Z. Enhanced NO2 Sensing Performance of ZnO-SnO2 Heterojunction Derived from Metal-Organic Frameworks. Nanomaterials 2022, 12, 3726. [Google Scholar] [CrossRef]
- Horng, R.H.; Lin, S.H.; Hung, D.R.; Chao, P.H.; Fu, P.K.; Chen, C.H.; Chen, Y.C.; Shah, J.H.; Huang, C.Y.; Tarntair, F.G.; et al. Structure Effect on the Response of ZnGa2O4 Gas Sensor for Nitric Oxide Applications. Nanomaterials 2022, 12, 3759. [Google Scholar] [CrossRef]
- Yang, Y.; Maeng, B.; Jung, D.G.; Lee, J.; Kim, Y.; Kwon, J.; An, H.K.; Jung, D. Annealing Effects on SnO2 Thin Film for H2 Gas Sensing. Nanomaterials 2022, 12, 3227. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, A.; Sadaf, S.; Liu, J.; Wang, Y.; Wei, H.; Zhang, Q.; Fu, C.; Wang, J. Hydro-thermally synthesized spherical g-C3N4-NiCo2O4 nanocomposites for ppb level ethanol detection. J. Alloy. Compd. 2022, 911, 165048. [Google Scholar] [CrossRef]
- Akhtar, A.; Jaio, C.; Chu, X.F.; Liang, S.; Dong, Y.; He, L. Acetone sensing properties of the g–C3N4–CuO nanocomposites prepared by hydrothermal method. Mater. Chem. Phys. 2021, 265, 124375. [Google Scholar] [CrossRef]
- Akhtar, A.; Wen, H.; Chu, X.F.; Liang, S.; Dong, Y.; He, L.; Zhang, K. Synthesis of g-C3N4-Zn2SnO4 nanocomposites with enhanced sensing performance to ethanol vapor. Synth. Met. 2021, 278, 116829. [Google Scholar] [CrossRef]
- Yan, T.; Liu, H.; Sun, M.; Wang, X.; Li, M.; Yan, Q.; Xu, W.; Du, B. Efficient photocatalytic degradation of bisphenol A and dye pollutants over BiOI/Zn2SnO4 hetero-junction photocatalyst. RSC Adv. 2015, 5, 10688–10696. [Google Scholar] [CrossRef]
- Wang, K.; Shi, Y.; Guo, W.; Yu, X.; Ma, T. Zn2SnO4-Based Dye-Sensitized Solar Cells: Insight into Dye-Selectivity and Photoelectric Behaviors. Electrochim. Acta 2014, 135, 242–248. [Google Scholar] [CrossRef]
- Shu, S.; Wang, M.; Yang, W.; Liu, S. Synthesis of surface layered hierarchical octahedron-like structured Zn2SnO4/SnO2 with excellent sensing properties toward HCHO. Sens. Actuators B Chem. 2017, 243, 1171–1180. [Google Scholar] [CrossRef]
- An, D.; Mao, N.; Deng, G.; Zou, Y.; Li, Y.; Wei, T.; Lian, X. Ethanol gas-sensing characteristic of the Zn2SnO4 nanospheres. Ceram. Int. 2016, 42, 3535–3541. [Google Scholar] [CrossRef]
- Cao, F.F.; Li, C.P.; Li, M.J.; Li, H.J.; Huang, X.; Yang, B.H. Direct growth of Al-doped ZnO ultrathin nanosheets on electrode for ethanol gas sensor application. Appl. Surf. Sci. 2018, 447, 173–181. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, X.; Sun, H.; Liu, Q.; Zhang, J.; Li, G.; Gao, J.; Lu, H.; Wang, C. Porous ZnO–SnO2–Zn2SnO4 heterojunction nanofibers fabricated by electrospinning for enhanced ethanol sensing properties under UV irradiation. J. Alloy. Compd. 2021, 854, 157311. [Google Scholar] [CrossRef]
- Hanha, N.H.; Duy, L.V.; Hung, C.M.; Xuan, C.T.; Duy, N.V.; Hoa, N.D. High-performance acetone gas sensor based on Pt–Zn2SnO4 hollow octahedra for diabetic diagnosis. J Alloy. Compd. 2021, 886, 161284. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, Z.Q.; Zhu, L.F.; Yang, Y.H.; Wu, H.Y. Self-assembled and Pd decorated Zn2SnO4/ZnO wire-sheet shape nano-heterostructures networks hydrogen gas sensors. Sens. Actuators B Chem. 2014, 195, 549–561. [Google Scholar] [CrossRef]
- Munusami, V.; Arutselvan, K.; Vadivel, S.; Govindasamy, S. High sensitivity LPG and H2 gas sensing behavior of MoS2/graphene hybrid sensors prepared by facile hydrothermal method. Ceram. Int. 2022, 48, 29322–29331. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-Based Charge Transfer in Two-Dimensional SnS2 for Selective and Reversible NO2 Gas Sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Zhou, H.; Xu, K.; Ha, N.; Cheng, Y.; Ou, R.; Ma, Q.; Hu, Y.; Trinh, V.; Ren, G.; Li, Z.; et al. Reversible Room Temperature H2 Gas Sensing Based on Self-Assembled Cobalt Oxysulfide. Sensors 2022, 22, 303. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Ren, B.; Xu, K.; Jeerapan, I.; Chen, H.; Li, Z.; Ou, J.Z. Recent progress in intrinsic and stimulated room-temperature gas sensors enabled by low-dimensional materials. J. Mater. Chem. C 2021, 9, 3026–3051. [Google Scholar] [CrossRef]
- Xu, K.; Ha, N.; Hu, Y.; Ma, Q.; Chen, W.; Wen, X.; Ou, R.; Trinh, V.; McConville, C.F.; Zhang, B.Y.; et al. A room temperature all-optical sensor based on two-dimensional SnS2 for highly sensitive and reversible NO2 sensing. J. Hazard. Mater. 2022, 426, 127813. [Google Scholar] [CrossRef]
- Alkathiri, T.; Xu, K.; Zhang, B.Y.; Khan, M.Y.; Jannat, A.; Syed, N.; Almutairi, A.F.M.; Ha, N.; Alsaif, M.M.Y.A.; Pillai, N.; et al. 2D Palladium Sulphate for Visible-Light-Driven Optoelectronic Reversible Gas Sensing at Room Temperature. Small Sci. 2022, 2, 2100097. [Google Scholar] [CrossRef]
- Li, K.; Du, C.; Gao, H.; Yin, T.; Yu, Y.; Wang, W. Ultra-fast and linear polarization-sensitive photodetectors based on ReSe2/MoS2 van der Waals heterostructures. J. Mater. 2022, 8, 1158–1164. [Google Scholar] [CrossRef]
- Abdelazeez, A.A.A.; Trabelsi, A.B.G.; Alkallas, F.H.; Rabia, M. Successful 2D MoS2 nanosheets synthesis with SnSe grid-like nanoparticles: Photoelectrochemical hydrogen generation and solar cell applications. Sol. Energy 2022, 248, 251–259. [Google Scholar] [CrossRef]
- Zhao, W.; Yan, R.; Li, H.; Ding, K.; Chen, Y.; Xu, D. Highly sensitive NO2 gas sensor with a low detection limit based on Pt-modified MoS2 flakes. Mater. Lett. 2023, 330, 133386. [Google Scholar] [CrossRef]
- Li, W.; Li, H.; Qian, R.; Zhuo, S.; Ju, P.; Chen, Q. CTAB Enhanced Room-Temperature Detection of NO2 Based on MoS2-Reduced Graphene Oxide Nanohybrid. Nanomaterials 2022, 12, 1300. [Google Scholar] [CrossRef]
- Li, W.; Shahbazi, M.; Xing, K.; Tesfamichael, T.; Motta, N.; Qi, D.C. Highly Sensitive NO2 Gas Sensors Based on MoS2@MoO3 Magnetic Heterostructure. Nanomaterials 2022, 12, 1303. [Google Scholar] [CrossRef]
- Xia, Y.; Wu, Z.; Qin, Z.; Chen, F.; Lv, C.; Zhang, M.; Shaymurat, T.; Duan, H. Wool-Based Carbon Fiber/MoS2 Composite Prepared by Low-Temperature Catalytic Hydrothermal Method and Its Application in the Field of Gas Sensors. Nanomaterials 2022, 12, 1105. [Google Scholar] [CrossRef]
- Wang, S.; Chen, W.; Li, J.; Song, Z.; Zhang, H.; Zeng, W. Low Working Temperature of ZnO-MoS2 Nanocomposites for Delaying Aging with Good Acetylene Gas-Sensing Properties. Nanomaterials 2020, 10, 1902. [Google Scholar] [CrossRef]
- Parthibavarman, M.; Vallalperuman, K.; Sathishkumar, S.; Durairaj, M.; Thavamani, K. A novel microwave synthesis of nanocrystalline SnO2 and its structural optical and dielectric properties. J. Mater. Sci. Mater. Electron. 2014, 25, 730–735. [Google Scholar] [CrossRef]
- Zhang, C.; Shao, X.; Li, C.; Wang, M.; Zhang, M.; Liu, Y. Electrospun nanofibers of p type NiO/n-type ZnO heterojunctions with enhanced photocatalytic activity. ACS Appl. Mater. Interfaces 2010, 2, 2915–2923. [Google Scholar] [CrossRef]
- Han, L.; Liu, J.; Wang, Z.; Zhang, K.; Luo, H.; Xu, B.; Zou, X.; Zheng, X.; Ye, B.; Yu, X. Shape-controlled synthesis of ZnSn(OH)6 crystallites and their HCHO-sensing properties. CrystEngComm 2012, 14, 3380–3386. [Google Scholar] [CrossRef]
- Chang, X.; Qiao, X.; Li, K.; Wang, P.; Xiong, Y.; Li, X.; Xia, F.; Xue, Q. UV assisted ppb level acetone detection based on hollow ZnO/MoS2 nanosheets core/shell heterostructures at low temperature. Sens. Actuators B Chem. 2020, 317, 128208. [Google Scholar] [CrossRef]
- Chakraborty, A.; Kebede, M. Preparation and characterization of WO3/Bi3O4Cl nanocomposite and its photocatalytic behavior under visible light irradiation. React. Kinet. Mech. Catal. 2012, 106, 83–98. [Google Scholar] [CrossRef]
- Lei, Y.; Guo, P.; Jia, M.; Wang, W.; Liu, J.; Zhai, J. One-step photo-deposition synthesis of TiO2 nanobelts/MoS2 quantum dots/rGO ternary composite with remarkably enhanced photocatalytic activity. J. Mater. Sci. 2020, 55, 14773–14786. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, B.; Jifan, H.; Qin, H.; Jiang, M. Preparation, structure, resistance and methane-gas sensing properties of nominal La1−xMgxFeO3. Sens. Actuators B Chem. 2008, 133, 340–344. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Bhattacharya, A.; Chu, X.; Liang, S.; Zeng, D. The formaldehyde sensing properties of CdGa2O4 prepared by co-precipitation method. Sens. Actuators B Chem. 2021, 343, 129834. [Google Scholar] [CrossRef]
- Liu, L.; Li, S.C.; Zhuang, J.; Wang, L.Y.; Zhang, J.B.; Li, H.Y.; Liu, Z.; Han, Y.; Jiang, X.X.; Zhang, P. Improved selective acetone sensing properties of Co-doped ZnO nanofibers by electrospinning. Sens. Actuator B Chem. 2011, 155, 782–788. [Google Scholar] [CrossRef]
- Xu, J.Q.; Xue, Z.G.; Qin, N.; Cheng, Z.X.; Xiang, Q. The crystal facet-dependent gas sensing properties of ZnO nanosheets: Experimental and computational study. Sens. Actuator B-Chem. 2017, 242, 148–157. [Google Scholar] [CrossRef]
- Li, Q.; Chen, D.; Miao, J.; Lin, S.; Yu, Z.; Han, Y.; Yang, Z.; Zhi, X.; Cui, D.; An, Z. Agmodified 3D reduced graphene oxide aerogel-based sensor with an embedded microheater for a fast response and high-sensitive detection of NO2. ACS Appl. Mater. Interfaces 2020, 12, 25243–25252. [Google Scholar] [CrossRef]
- Chen, Y.J.; Yu, L.; Feng, D.D.; Zhuo, M.; Zhang, M.; Zhang, E.D.; Xu, Z.; Li, Q.H.; Wang, T.H. Superior ethanol-sensing properties based on Ni-doped SnO2 p–n hetero-junction hollow spheres. Sens. Actuators B Chem. 2012, 166–167, 61–67. [Google Scholar] [CrossRef]
- Wang, X.; Liu, F.; Xie, X.; Xu, G.; Tian, J.; Cui, H. Au modifified single crystalline and polycrystalline composite tin oxide for enhanced n-butanol sensing performance. Powder Technol. 2018, 331, 270–275. [Google Scholar] [CrossRef]
- Li, Y.; Song, Z.; Li, Y.; Chen, S.; Li, S.; Li, Y.; Wang, H.; Wang, Z. Hierarchical hollow MoS2 microspheres as materials for conductometric NO2 gas sensors. Sens. Actuators B Chem. 2019, 282, 259–267. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Y.; Fan, Y.; Luo, N.; Cheng, Z.; Xu, J. Micro-spherical ZnSnO3 material prepared by microwave-assisted method and its ethanol sensing properties. Chin. Chem. Lett. 2020, 31, 2087–2090. [Google Scholar] [CrossRef]
- Lei, M.; Zhou, X.; Zou, Y.; Ma, J.; Alharthic, F.A.; Alghamdi, A.; Yang, X.; Deng, Y. A facile construction of heterostructured ZnO/Co3O4 mesoporous spheres and superior acetone sensing performance. Chin. Chem. Lett. 2021, 32, 1998–2004. [Google Scholar] [CrossRef]
- Ding, P.; Xu, D.; Dong, N.; Chen, Y.; Xu, P.; Zheng, D.; Li, X. A high-sensitivity H2S gas sensor based on optimized ZnO-ZnS nano-heterojunction sensing material. Chin. Chem. Lett. 2020, 31, 2050–2054. [Google Scholar] [CrossRef]
- Luo, N.; Zhang, B.; Zhang, D.; Xu, J. Enhanced CO sensing properties of Pd modified ZnO porous nanosheets. Chin. Chem. Lett. 2020, 31, 2033–2036. [Google Scholar] [CrossRef]
- Xu, T.T.; Zhang, X.F.; Dong, X.; Deng, Z.P.; Huo, L.H.; Gao, S. Enhanced H2S gas-sensing performance of Zn2SnO4 hierarchical quasi-microspheres constructed from nanosheets and octahedra. J. Hazard. Mater. 2019, 361, B49–B55. [Google Scholar] [CrossRef]
- Mao, L.W.; Zhu, L.Y.; Wu, T.T.; Xu, L.; Jin, X.H.; Lu, H.L. Excellent long-term stable H2S gas sensor based on Nb2O5/SnO2 core-shell heterostructure nanorods. Appl. Surf. Sci. 2022, 602, 154339. [Google Scholar] [CrossRef]
- Yan, S.; Li, Z.; Li, H.; Wu, Z.; Wang, J.; Shen, W.; Fu, Y.Q. Ultra-sensitive room-temperature H2S sensor using Ag–In2O3 nanorod composites. J. Mater. Sci. 2018, 53, 16331–16344. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, D.; Akhtar, A. Ppb-Level Hydrogen Sulfide Gas Sensor Based on the Nanocomposite of MoS2 Octahedron/ZnO-Zn2SnO4 Nanoparticles. Molecules 2023, 28, 3230. https://doi.org/10.3390/molecules28073230
Wu D, Akhtar A. Ppb-Level Hydrogen Sulfide Gas Sensor Based on the Nanocomposite of MoS2 Octahedron/ZnO-Zn2SnO4 Nanoparticles. Molecules. 2023; 28(7):3230. https://doi.org/10.3390/molecules28073230
Chicago/Turabian StyleWu, Di, and Ali Akhtar. 2023. "Ppb-Level Hydrogen Sulfide Gas Sensor Based on the Nanocomposite of MoS2 Octahedron/ZnO-Zn2SnO4 Nanoparticles" Molecules 28, no. 7: 3230. https://doi.org/10.3390/molecules28073230
APA StyleWu, D., & Akhtar, A. (2023). Ppb-Level Hydrogen Sulfide Gas Sensor Based on the Nanocomposite of MoS2 Octahedron/ZnO-Zn2SnO4 Nanoparticles. Molecules, 28(7), 3230. https://doi.org/10.3390/molecules28073230





