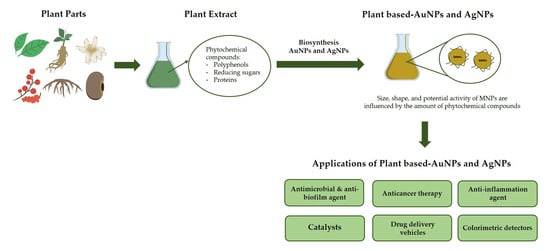
Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds
Abstract
:1. Introduction
2. Synthesis of Gold and Silver Nanoparticles Using Plant Extracts
2.1. Antimicrobial and Anti-Biofilm Agents
2.2. Anticancer Therapy
2.3. Anti-Inflammation Agents
2.4. Catalysts
2.5. Drug Delivery Vehicles
2.6. Colorimetric Detectors
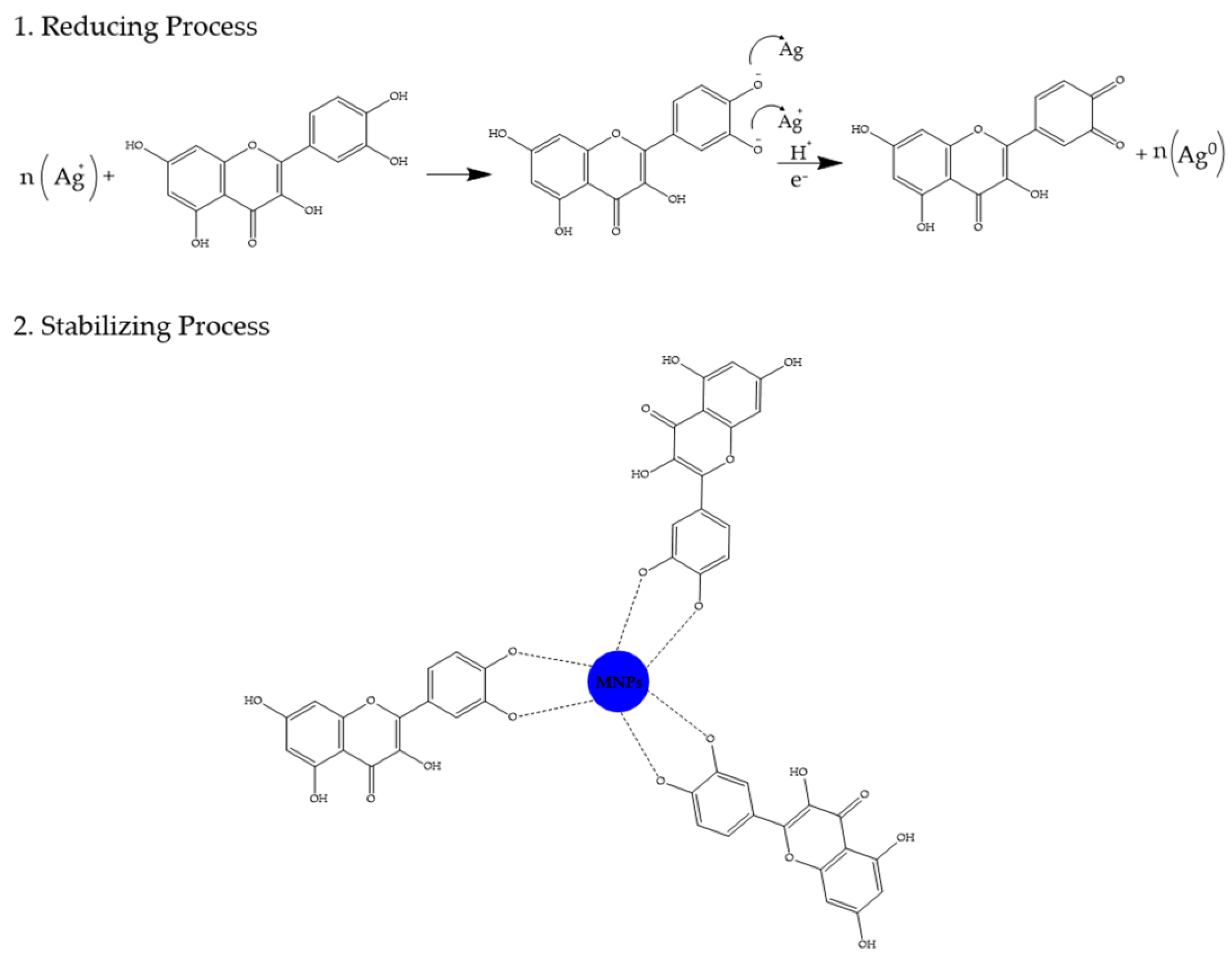
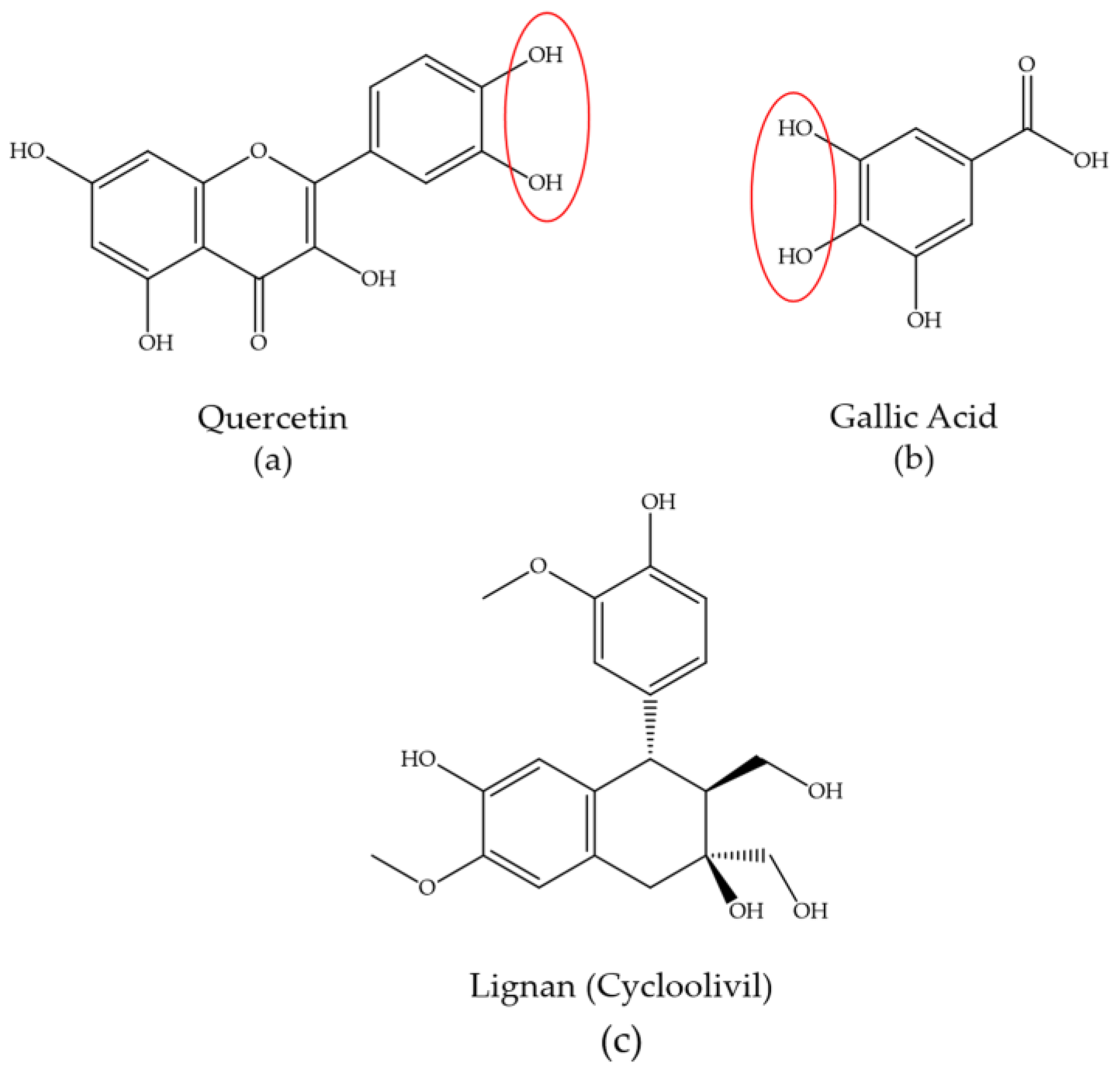
3. Phytochemical Content of Plants Used for Gold and Silver Nanoparticle Synthesis
4. Physical Properties of Nanoparticles Produced Using Plant Extracts
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Dewangan, R.; Sharma, A.K.; Kumar, N.; Maiti, S.K.; Singh, H.; Gangwar, A.K.; Shrivastava, S.; Kumar, A. In-Vitro Biocompatibility Determination of Bladder Acellular Matrix Graft. Trends Biomater. Artif. Organs 2012, 25, 161–171. [Google Scholar]
- Baig, N.; Kammakakam, I.; Falath, W.; Kammakakam, I. Nanomaterials: A Review of Synthesis Methods, Properties, Recent Progress, and Challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.Y. Synthesis, Bioactive Properties, and Biomedical Applications of Intrinsically Therapeutic Nanoparticles for Disease Treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Shukla, R.; Bansal, V.; Chaudhary, M.; Basu, A.; Bhonde, R.R.; Sastry, M. Biocompatibility of Gold Nanoparticles and Their Endocytotic Fate inside the Cellular Compartment: A Microscopic Overview. Langmuir 2005, 21, 10644–10654. [Google Scholar] [CrossRef]
- Burda, C.; Chen, X.; Narayanan, R.; El-Sayed, M.A. Chemistry and Properties of Nanocrystals of Different Shapes. ChemInform 2005, 36, 1025–1102. [Google Scholar] [CrossRef]
- Hammami, I.; Alabdallah, N.M. Gold Nanoparticles: Synthesis Properties and Applications. J. King Saud Univ. Sci. 2021, 33, 101560. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver Nanoparticle Applications and Human Health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef]
- Pulit-Prociak, J.; Grabowska, A.; Chwastowski, J.; Majka, T.M.; Banach, M. Safety of the Application of Nanosilver and Nanogold in Topical Cosmetic Preparations. Colloids Surf. B Biointerfaces 2019, 183, 110416. [Google Scholar] [CrossRef]
- Onitsuka, S.; Hamada, T.; Okamura, H. Preparation of Antimicrobial Gold and Silver Nanoparticles from Tea Leaf Extracts. Colloids Surf. B Biointerfaces 2019, 173, 242–248. [Google Scholar] [CrossRef]
- Chatterjee, S.; Lou, X.Y.; Liang, F.; Yang, Y.W. Surface-Functionalized Gold and Silver Nanoparticles for Colorimetric and Fluorescent Sensing of Metal Ions and Biomolecules. Coord. Chem. Rev. 2022, 459, 214461. [Google Scholar] [CrossRef]
- Francis, S.; Nair, K.M.; Paul, N.; Koshy, E.P.; Mathew, B. Catalytic Activities of Green Synthesized Silver and Gold Nanoparticles. Mater. Today Proc. 2019, 9, 97–104. [Google Scholar] [CrossRef]
- Yafout, M.; Ousaid, A.; Khayati, Y.; El Otmani, I.S. Gold Nanoparticles as a Drug Delivery System for Standard Chemotherapeutics: A New Lead for Targeted Pharmacological Cancer Treatments. Sci. Afr. 2021, 11, e00685. [Google Scholar] [CrossRef]
- Ningthoujam, R.; Singh, Y.D.; Babu, P.J.; Tirkey, A.; Pradhan, S.; Sarma, M. Nanocatalyst in Remediating Environmental Pollutants. Chem. Phys. Impact 2022, 4, 100064. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Zhang, X.F.; Liu, Z.G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Ghorbani, H.R. Green Synthesis of Gold Nanoparticles. Orient. J. Chem. 2015, 31, 303–305. [Google Scholar] [CrossRef] [Green Version]
- Alsammarraie, F.K.; Wang, W.; Zhou, P.; Mustapha, A.; Lin, M. Green Synthesis of Silver Nanoparticles Using Turmeric Extracts and Investigation of Their Antibacterial Activities. Colloids Surf. B Biointerfaces 2018, 171, 398–405. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Vishwanath, R.; Negi, B. Conventional and Green Methods of Synthesis of Silver Nanoparticles and Their Antimicrobial Properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100205. [Google Scholar] [CrossRef]
- Mukherji, S.; Bharti, S.; Shukla, G.; Mukherji, S. Synthesis and Characterization of Size- And Shape-Controlled Silver Nanoparticles. Phys. Sci. Rev. 2019, 4, 20170082. [Google Scholar] [CrossRef]
- Harish, V.; Ansari, M.M.; Tewari, D.; Gaur, M.; Yadav, A.B.; García-Betancourt, M.L.; Abdel-Haleem, F.M.; Bechelany, M.; Barhoum, A. Nanoparticle and Nanostructure Synthesis and Controlled Growth Methods. Nanomaterials 2022, 12, 3226. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Guan, Z.; Ofoegbu, P.C.; Clubb, P.; Rico, C.; He, F.; Hong, J. Green Synthesis of Nanoparticles: Current Developments and Limitations. Environ. Technol. Innov. 2022, 26, 102336. [Google Scholar] [CrossRef]
- Rahimzadeh, C.Y.; Barzinjy, A.A.; Mohammed, A.S.; Hamad, S.M. Green Synthesis of SiO2 Nanoparticles from Rhus coriaria L. Extract: Comparison with Chemically Synthesized SiO2 Nanoparticles. PLoS ONE 2022, 17, e0268184. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Green Synthesis of Biogenic Metal Nanoparticles by Terrestrial and Aquatic Phototrophic and Heterotrophic Eukaryotes and Biocompatible Agents. Adv. Colloid Interface Sci. 2011, 169, 59–79. [Google Scholar] [CrossRef]
- Teimouri, M.; Khosravi-Nejad, F.; Attar, F.; Saboury, A.A.; Kostova, I.; Benelli, G.; Falahati, M. Gold Nanoparticles Fabrication by Plant Extracts: Synthesis, Characterization, Degradation of 4-Nitrophenol from Industrial Wastewater, and Insecticidal Activity—A Review. J. Clean. Prod. 2018, 184, 740–753. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Rafa, N.; Chowdhury, A.T.; Chowdhury, S.; Nahrin, M.; Islam, A.B.M.S.; Ong, H.C. Green Approaches in Synthesising Nanomaterials for Environmental Nanobioremediation: Technological Advancements, Applications, Benefits and Challenges. Environ. Res. 2022, 204, 111967. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. “Green” Synthesis of Metals and Their Oxide Nanoparticles: Applications for Environmental Remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Bhardwaj, B.; Singh, P.; Kumar, A.; Kumar, S.; Budhwar, V. Eco-Friendly Greener Synthesis of Nanoparticles. Adv. Pharm. Bull. 2020, 10, 566–576. [Google Scholar] [CrossRef]
- Qiao, J.; Qi, L. Recent Progress in Plant-Gold Nanoparticles Fabrication Methods and Bio-Applications. Talanta 2021, 223, 121396. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.H. Green Nanobiotechnology: Factors Affecting Synthesis and Characterization Techniques. J. Nanomater. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Gour, A.; Jain, N.K. Advances in Green Synthesis of Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eljounaidi, K.; Lichman, B.R. Nature’s Chemists: The Discovery and Engineering of Phytochemical Biosynthesis. Front. Chem. 2020, 8, 596479. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Dumat, C.; Khalid, S.; Schreck, E.; Xiong, T.; Niazi, N.K. Foliar Heavy Metal Uptake, Toxicity and Detoxification in Plants: A Comparison of Foliar and Root Metal Uptake. J. Hazard. Mater. 2017, 325, 36–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peralta-Videa, J.R.; Huang, Y.; Parsons, J.G.; Zhao, L.; Lopez-Moreno, L.; Hernandez-Viezcas, J.A.; Gardea-Torresdey, J.L. Plant-Based Green Synthesis of Metallic Nanoparticles: Scientific Curiosity or a Realistic Alternative to Chemical Synthesis? Nanotechnol. Environ. Eng. 2016, 1, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Pal, G.; Rai, P.; Pandey, A. Green Synthesis of Nanoparticles: A Greener Approach for a Cleaner Future. In Green Synthesis, Characterization and Applications of Nanoparticles; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Dare, E.O.; Oseghale, C.O.; Labulo, A.H.; Adesuji, E.T.; Elemike, E.E.; Onwuka, J.C.; Bamgbose, J.T. Green Synthesis and Growth Kinetics of Nanosilver under Bio-Diversified Plant Extracts Influence. J. Nanostructure Chem. 2015, 5, 85–94. [Google Scholar] [CrossRef] [Green Version]
- Abbai, R.; Mathiyalagan, R.; Markus, J.; Kim, Y.J.; Wang, C.; Singh, P.; Ahn, S.; Farh, M.E.A.; Yang, D.C. Green Synthesis of Multifunctional Silver and Gold Nanoparticles from the Oriental Herbal Adaptogen: Siberian Ginseng. Int. J. Nanomed. 2016, 11, 3131–3143. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Al-Hamoud, K.; Liaqat, Z.; Shaik, M.R.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Mondeshki, M.; et al. Synthesis of Au, Ag, and Au–Ag Bimetallic Nanoparticles Using Pulicaria Undulata Extract and Their Catalytic Activity for the Reduction of 4-Nitrophenol. Nanomaterials 2020, 10, 1885. [Google Scholar] [CrossRef]
- Saleh, M.N.; Khoman Alwan, S. Bio-Synthesis of Silver Nanoparticles from Bacteria Klebsiella Pneumonia: Their Characterization and Antibacterial Studies. J. Phys. Conf. Ser. 2020, 1664, 012115. [Google Scholar] [CrossRef]
- Gupta, R.; Padmanabhan, P. Biogenic Synthesis and Characterization of Gold Nanoparticles by a Novel Marine Bacteria Marinobacter Algicola: Progression from Nanospheres to Various Geometrical Shapes. J. Microbiol. Biotechnol. Food Sci. 2018, 8, 732–737. [Google Scholar] [CrossRef] [Green Version]
- Gurunathan, S.; Kalishwaralal, K.; Vaidyanathan, R.; Venkataraman, D.; Pandian, S.R.K.; Muniyandi, J.; Hariharan, N.; Eom, S.H. Biosynthesis, Purification and Characterization of Silver Nanoparticles Using Escherichia coli . Colloids Surf. B Biointerfaces 2009, 74, 328–335. [Google Scholar] [CrossRef]
- Konishi, Y.; Ohno, K.; Saitoh, N.; Nomura, T.; Nagamine, S.; Hishida, H.; Takahashi, Y.; Uruga, T. Bioreductive Deposition of Platinum Nanoparticles on the Bacterium Shewanella algae . J. Biotechnol. 2007, 128, 648–653. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient Techniques for the Removal of Toxic Heavy Metals from Aquatic Environment: A Review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” Nanotechnologies: Synthesis of Metal Nanoparticles Using Plants. Acta Nat. 2014, 6. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakash, N.; Vijaya, J.J.; Kaviyarasu, K.; Kombaiah, K.; Kennedy, L.J.; Ramalingam, R.J.; Munusamy, M.A.; Al-Lohedan, H.A. Green Synthesis of Ag Nanoparticles Using Tamarind Fruit Extract for the Antibacterial Studies. J. Photochem. Photobiol. B Biol. 2017, 169, 178–185. [Google Scholar] [CrossRef]
- Akhtar, M.S.; Panwar, J.; Yun, Y.S. Biogenic Synthesis of Metallic Nanoparticles by Plant Extracts. ACS Sustain. Chem. Eng. 2013, 1, 591–602. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological Synthesis of Metallic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Tripathy, A.; Raichur, A.M.; Chandrasekaran, N.; Prathna, T.C.; Mukherjee, A. Process Variables in Biomimetic Synthesis of Silver Nanoparticles by Aqueous Extract of Azadirachta indica (Neem) Leaves. J. Nanoparticle Res. 2010, 12, 237–246. [Google Scholar] [CrossRef]
- Javed, R.; Zia, M.; Naz, S.; Aisida, S.O.; Ain, N.U.; Ao, Q. Role of Capping Agents in the Application of Nanoparticles in Biomedicine and Environmental Remediation: Recent Trends and Future Prospects. J. Nanobiotechnol. 2020, 18, 1–15. [Google Scholar] [CrossRef]
- Mikhailova, E.O. Gold Nanoparticles: Biosynthesis and Potential of Biomedical Application. J. Funct. Biomater. 2021, 12, 70. [Google Scholar] [CrossRef] [PubMed]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Yadi, M.; Mostafavi, E.; Saleh, B.; Davaran, S.; Aliyeva, I.; Khalilov, R.; Nikzamir, M.; Nikzamir, N.; Akbarzadeh, A.; Panahi, Y.; et al. Current Developments in Green Synthesis of Metallic Nanoparticles Using Plant Extracts: A Review. Artif. Cells Nanomed. Biotechnol. 2018, 46, 336–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Henríquez, L.; Alfaro-Aguilar, K.; Ugalde-álvarez, J.; Vega-Fernández, L.; de Oca-Vásquez, G.M.; Vega-Baudrit, J.R. Green Synthesis of Gold and Silver Nanoparticles from Plant Extracts and Their Possible Applications as Antimicrobial Agents in the Agricultural Area. Nanomater. 2020, 10, 1763. [Google Scholar] [CrossRef]
- Navya, P.N.; Daima, H.K. Rational Engineering of Physicochemical Properties of Nanomaterials for Biomedical Applications with Nanotoxicological Perspectives. Nano Converg. 2016, 3, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Abu Nayem, S.M.; Sultana, N.; Haque, M.A.; Miah, B.; Hasan, M.M.; Islam, T.; Hasan, M.M.; Awal, A.; Uddin, J.; Aziz, M.A.; et al. Green Synthesis of Gold and Silver Nanoparticles by Using Amorphophallus Paeoniifolius Tuber Extract and Evaluation of Their Antibacterial Activity. Molecules 2020, 25, 4773. [Google Scholar] [CrossRef]
- Gopinath, K.; Kumaraguru, S.; Bhakyaraj, K.; Mohan, S.; Venkatesh, K.S.; Esakkirajan, M.; Kaleeswarran, P.; Alharbi, N.S.; Kadaikunnan, S.; Govindarajan, M.; et al. Green Synthesis of Silver, Gold and Silver/Gold Bimetallic Nanoparticles Using the Gloriosa superba Leaf Extract and Their Antibacterial and Antibiofilm Activities. Microb. Pathog. 2016, 101, 1–11. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Beshay, M.; Mokkapati, V.R.S.S.; Garnaes, J.; Olsson, M.E.; Sultan, A.; Mackevica, A.; Mateiu, R.V.; Lütken, H.; et al. Anti-Biofilm Effects of Gold and Silver Nanoparticles Synthesized by the Rhodiola rosea Rhizome Extracts. Artif. Cells Nanomed. Biotechnol. 2018, 46, S886–S899. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.A.; Shahid, S.; Lee, C.S. Green Synthesis of Gold and Silver Nanoparticles Using Leaf Extract of Clerodendrum Inerme; Characterization, Antimicrobial, and Antioxidant Activities. Biomolecules 2020, 10, 835. [Google Scholar] [CrossRef]
- Jiménez Pérez, Z.E.; Mathiyalagan, R.; Markus, J.; Kim, Y.J.; Kang, H.M.; Abbai, R.; Seo, K.H.; Wang, D.; Soshnikova, V.; Yang, D.C. Ginseng-Berry-Mediated Gold and Silver Nanoparticle Synthesis and Evaluation of Their in Vitro Antioxidant, Antimicrobial, and Cytotoxicity Effects on Human Dermal Fibroblast and Murine Melanoma Skin Cell Lines. Int. J. Nanomed. 2017, 12, 709–723. [Google Scholar] [CrossRef] [Green Version]
- Naraginti, S.; Li, Y. Preliminary Investigation of Catalytic, Antioxidant, Anticancer and Bactericidal Activity of Green Synthesized Silver and Gold Nanoparticles Using Actinidia deliciosa . J. Photochem. Photobiol. B Biol. 2017, 170, 225–234. [Google Scholar] [CrossRef]
- Cardoso-Avila, P.E.; Patakfalvi, R.; Rodríguez-Pedroza, C.; Aparicio-Fernández, X.; Loza-Cornejo, S.; Villa-Cruz, V.; Martínez-Cano, E. One-Pot Green Synthesis of Gold and Silver Nanoparticles Using: Rosa canina L. Extract. RSC Adv. 2021, 11, 14624–14631. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Yi, T.H. Green and Rapid Synthesis of Silver Nanoparticles Using Borago Officinalis Leaf Extract: Anticancer and Antibacterial Activities. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1310–1316. [Google Scholar] [CrossRef] [Green Version]
- Amina, M.; Al Musayeib, N.M.; Alarfaj, N.A.; El-Tohamy, M.F.; Al-Hamoud, G.A. Antibacterial and Immunomodulatory Potentials of Biosynthesized Ag, Au, Ag-Au Bimetallic Alloy Nanoparticles Using the Asparagus racemosus Root Extract. Nanomaterials 2020, 10, 2453. [Google Scholar] [CrossRef]
- Dhayalan, M.; Denison, M.I.J.; Ayyar, M.; Gandhi, N.N.; Krishnan, K.; Abdulhadi, B. Biogenic Synthesis, Characterization of Gold and Silver Nanoparticles from Coleus forskohlii and Their Clinical Importance. J. Photochem. Photobiol. B Biol. 2018, 183, 251–257. [Google Scholar] [CrossRef]
- Naraginti, S.; Sivakumar, A. Eco-Friendly Synthesis of Silver and Gold Nanoparticles with Enhanced Bactericidal Activity and Study of Silver Catalyzed Reduction of 4-Nitrophenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 128, 357–362. [Google Scholar] [CrossRef]
- Francis, S.; Joseph, S.; Koshy, E.P.; Mathew, B. Green Synthesis and Characterization of Gold and Silver Nanoparticles Using Mussaenda Glabrata Leaf Extract and Their Environmental Applications to Dye Degradation. Environ. Sci. Pollut. Res. 2017, 24, 17347–17357. [Google Scholar] [CrossRef]
- Francis, S.; Koshy, E.P.; Mathew, B. Green Synthesis of Stereospermum Suaveolens Capped Silver and Gold Nanoparticles and Assessment of Their Innate Antioxidant, Antimicrobial and Antiproliferative Activities. Bioprocess Biosyst. Eng. 2018, 41, 939–951. [Google Scholar] [CrossRef]
- Godipurge, S.S.; Yallappa, S.; Biradar, N.J.; Biradar, J.S.; Dhananjaya, B.L.; Hegde, G.; Jagadish, K.; Hegde, G. A Facile and Green Strategy for the Synthesis of Au, Ag and Au–Ag Alloy Nanoparticles Using Aerial Parts of R. Hypocrateriformis Extract and Their Biological Evaluation. Enzym. Microb. Technol. 2016, 95, 174–184. [Google Scholar] [CrossRef]
- Huo, Y.; Singh, P.; Kim, Y.J.; Soshnikova, V.; Kang, J.; Markus, J.; Ahn, S.; Castro-Aceituno, V.; Mathiyalagan, R.; Chokkalingam, M.; et al. Biological Synthesis of Gold and Silver Chloride Nanoparticles by Glycyrrhiza uralensis and in Vitro Applications. Artif. Cells Nanomed. Biotechnol. 2018, 46, 303–312. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.; Bhuyan, B.; Purkayastha, D.D.; Dhar, S.S. Photocatalytic and Antibacterial Activities of Gold and Silver Nanoparticles Synthesized Using Biomass of Parkia Roxburghii Leaf. J. Photochem. Photobiol. B Biol. 2016, 154, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Ahmad, T.; Khan, A.; Maryam; Uddin, G.; Ahmad, B.; Mabkhot, Y.N.; Bawazeer, S.; Riaz, N.; Malikovna, B.K.; et al. Green Synthesis and Biomedicinal Applications of Silver and Gold Nanoparticles Functionalized with Methanolic Extract of Mentha longifolia . Artif. Cells Nanomed. Biotechnol. 2021, 49, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.J.; Cho, M.; Lee, S.M.; Park, J.H.; Oh, S.G.; Bang, K.S.; Oh, B.T. Green Synthesis of Silver and Gold Nanoparticles Using Zingiber officinale Root Extract and Antibacterial Activity of Silver Nanoparticles against Food Pathogens. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, R.; Joseph, S.; Mathew, B. Indigofera Tinctoria Leaf Extract Mediated Green Synthesis of Silver and Gold Nanoparticles and Assessment of Their Anticancer, Antimicrobial, Antioxidant and Catalytic Properties. Artif. Cells Nanomed. Biotechnol. 2017, 46, 861–871. [Google Scholar] [CrossRef] [Green Version]
- Vijayan, R.; Joseph, S.; Mathew, B. Anticancer, Antimicrobial, Antioxidant, and Catalytic Activities of Green-Synthesized Silver and Gold Nanoparticles Using Bauhinia purpurea Leaf Extract. Bioprocess Biosyst. Eng. 2019, 42, 305–319. [Google Scholar] [CrossRef]
- Wang, D.; Markus, J.; Wang, C.; Kim, Y.J.; Mathiyalagan, R.; Aceituno, V.C.; Ahn, S.; Yang, D.C. Green Synthesis of Gold and Silver Nanoparticles Using Aqueous Extract of Cibotium barometz Root. Artif. Cells Nanomed. Biotechnol. 2016, 45, 1548–1555. [Google Scholar] [CrossRef] [Green Version]
- Yallappa, S.; Manjanna, J.; Dhananjaya, B.L. Phytosynthesis of Stable Au, Ag and Au-Ag Alloy Nanoparticles Using J. Sambac Leaves Extract, and Their Enhanced Antimicrobial Activity in Presence of Organic Antimicrobials. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 236–243. [Google Scholar] [CrossRef]
- Rizzello, L.; Pompa, P.P. Nanosilver-Based Antibacterial Drugs and Devices: Mechanisms, Methodological Drawbacks, and Guidelines. Chem. Soc. Rev. 2014, 43, 1501–1518. [Google Scholar] [CrossRef]
- Ba Vinh, L.; Thi Minh Nguyet, N.; Young Yang, S.; Hoon Kim, J.; Thi Vien, L.; Thi Thanh Huong, P.; Van Thanh, N.; Xuan Cuong, N.; Hoai Nam, N.; Van Minh, C.; et al. A New Rearranged Abietane Diterpene from Clerodendrum inerme with Antioxidant and Cytotoxic Activities. Nat. Prod. Res. 2018, 32, 2001–2007. [Google Scholar] [CrossRef]
- Luo, L.J.; Lin, T.Y.; Yao, C.H.; Kuo, P.Y.; Matsusaki, M.; Harroun, S.G.; Huang, C.C.; Lai, J.Y. Dual-Functional Gelatin-Capped Silver Nanoparticles for Antibacterial and Antiangiogenic Treatment of Bacterial Keratitis. J. Colloid Interface Sci. 2019, 536, 112–126. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory; Cancer Today, International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- van den Boogaard, W.M.C.; Komninos, D.S.J.; Vermeij, W.P. Chemotherapy Side-Effects: Not All DNA Damage Is Equal. Cancers 2022, 14, 627. [Google Scholar] [CrossRef]
- Siddiqui, M.; Rajkumar, S.V. The High Cost of Cancer Drugs and What We Can Do about It. Mayo Clin. Proc. 2012, 87, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for Cancer Therapy: Current Progress and Perspectives. J. Hematol. Oncol. 2021, 14, 1–27. [Google Scholar] [CrossRef]
- Akinfenwa, A.O.; Abdul, N.S.; Docrat, F.T.; Marnewick, J.L.; Luckay, R.C.; Hussein, A.A. Cytotoxic Effects of Phytomediated Silver and Gold Nanoparticles Synthesised from Rooibos (Aspalathus linearis), and Aspalathin. Plants 2021, 10, 2460. [Google Scholar] [CrossRef]
- Ahmad, N.; Sharma, A.K.; Sharma, S.; Khan, I.; Sharma, D.K.; Shamsi, A.; Santhosh Kumar, T.R.; Seervi, M. Biosynthesized Composites of Au-Ag Nanoparticles Using Trapa Peel Extract Induced ROS-Mediated P53 Independent Apoptosis in Cancer Cells. Drug Chem. Toxicol. 2019, 42, 43–53. [Google Scholar] [CrossRef]
- Park, J.S.; Ahn, E.Y.; Park, Y. Asymmetric Dumbbell-Shaped Silver Nanoparticles and Spherical Gold Nanoparticles Green-Synthesized by Mangosteen (Garcinia mangostana) Pericarp Waste Extracts. Int. J. Nanomed. 2017, 12, 6895–6908. [Google Scholar] [CrossRef] [Green Version]
- Devi, G.K.; Sathishkumar, K. Synthesis of Gold and Silver Nanoparticles Using Mukia Maderaspatna Plant Extract and Its Anticancer Activity. IET Nanobiotechnol. 2017, 11, 143–151. [Google Scholar] [CrossRef]
- Wang, C.; Mathiyalagan, R.; Kim, Y.J.; Castro-Aceituno, V.; Singh, P.; Ahn, S.; Wang, D.; Yang, D.C. Rapid Green Synthesis of Silver and Gold Nanoparticles Using Dendropanax morbifera Leaf Extract and Their Anticancer Activities. Int. J. Nanomed. 2016, 11, 3691–3701. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.J.P.; Finub, J.S.; Narayanan, A. Synthesis of Silver Nanoparticles Using Piper Longum Leaf Extracts and Its Cytotoxic Activity against Hep-2 Cell Line. Colloids Surf. B Biointerfaces 2012, 91, 212–214. [Google Scholar] [CrossRef]
- Rajan, A.; Vilas, V.; Philip, D. Studies on Catalytic, Antioxidant, Antibacterial and Anticancer Activities of Biogenic Gold Nanoparticles. J. Mol. Liq. 2015, 212, 331–339. [Google Scholar] [CrossRef]
- Suman, T.Y.; Radhika Rajasree, S.R.; Kanchana, A.; Elizabeth, S.B. Biosynthesis, Characterization and Cytotoxic Effect of Plant Mediated Silver Nanoparticles Using Morinda citrifolia Root Extract. Colloids Surf. B Biointerfaces 2013, 106, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, T.; Sapi, A.; Das, G.; Debnath, T.; Ansari, A.Z.; Patra, J.K. Biosynthesis of Silver Nanoparticle Using Extract of Zea mays (Corn Flour) and Investigation of Its Cytotoxicity Effect and Radical Scavenging Potential. J. Photochem. Photobiol. B Biol. 2019, 193, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sammar, M.; Abu-Farich, B.; Rayan, I.; Falah, M.; Rayan, A. Correlation between Cytotoxicity in Cancer Cells and Free Radical-Scavenging Activity: In Vitro Evaluation of 57 Medicinal and Edible Plant Extracts. Oncol. Lett. 2019, 18, 6563–6571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.; Mahmood, M.; Xu, Y.; Watanabe, F.; Biris, A.S.; Hansen, D.K.; Inselman, A.; Casciano, D.; Patterson, T.A.; Paule, M.G.; et al. Effects of Silver Nanoparticles on Human and Rat Embryonic Neural Stem Cells. Front. Neurosci. 2015, 9, 115. [Google Scholar] [CrossRef] [Green Version]
- Guo, D.; Zhu, L.; Huang, Z.; Zhou, H.; Ge, Y.; Ma, W.; Wu, J.; Zhang, X.; Zhou, X.; Zhang, Y.; et al. Anti-Leukemia Activity of PVP-Coated Silver Nanoparticles via Generation of Reactive Oxygen Species and Release of Silver Ions. Biomaterials 2013, 34, 7884–7894. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shen, W.; Gurunathan, S. Silver Nanoparticle-Mediated Cellular Responses in Various Cell Lines: An in Vitro Model. Int. J. Mol. Sci. 2016, 17, 1603. [Google Scholar] [CrossRef] [Green Version]
- Roh, Y.J.; Rho, C.R.; Cho, W.K.; Kang, S. The Anti-Angiogenic Effects of Gold Nanoparticles on Experimental Choroidal Neovascularization in Mice. Acta Ophthalmol. 2016, 57, 6561–6567. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef] [Green Version]
- Tiloke, C.; Phulukdaree, A.; Anand, K.; Gengan, R.M.; Chuturgoon, A.A. Moringa Oleifera Gold Nanoparticles Modulate Oncogenes, Tumor Suppressor Genes, and Caspase-9 Splice Variants in A549 Cells. J. Cell. Biochem. 2016, 117, 2302–2314. [Google Scholar] [CrossRef]
- Kang, B.; Mackey, M.A.; El-Sayed, M.A. Nuclear Targeting of Gold Nanoparticles in Cancer Cells Induces DNA Damage, Causing Cytokinesis Arrest and Apoptosis. J. Am. Chem. Soc. 2010, 132, 1517–1519. [Google Scholar] [CrossRef]
- Isailovic, N.; Daigo, K.; Mantovani, A.; Selmi, C. Interleukin-17 and Innate Immunity in Infections and Chronic Inflammation. J. Autoimmun. 2015, 60, 1–11. [Google Scholar] [CrossRef]
- Liu, S.X.; Jin, H.Z.; Shan, L.; Zeng, H.W.; Chen, B.Y.; Sun, Q.Y.; Zhang, W.D. Inhibitory Effect of 4,4′-Dihydroxy-α-Truxillic Acid Derivatives on NO Production in Lipopolysaccharide-Induced RAW 264.7 Macrophages and Exploration of Structure-Activity Relationships. Bioorganic Med. Chem. Lett. 2013, 23, 2207–2211. [Google Scholar] [CrossRef]
- Yi, Z.J.; Gong, J.P.; Zhang, W. Transcriptional Co-Regulator RIP140: An Important Mediator of the Inflammatory Response and Its Associated Diseases (Review). Mol. Med. Rep. 2017, 16, 994–1000. [Google Scholar] [CrossRef] [Green Version]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic Inflammation in the Etiology of Disease across the Life Span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Deepa, S.; Sujatha, K.; Velmurugan, D. The Identification of Bioactive Compounds from Turbinaria ornata (Turner) J. Agaradh and Computational Studies. Pharmacogn. J. 2019, 11, 873–883. [Google Scholar] [CrossRef] [Green Version]
- Nunes, C.D.R.; Arantes, M.B.; de Faria Pereira, S.M.; da Cruz, L.L.; de Souza Passos, M.; de Moraes, L.P.; Vieira, I.J.C.; de Oliveira, D.B. Plants as Sources of Anti-Inflammatory Agents. Molecules 2020, 25, 3726. [Google Scholar] [CrossRef]
- Agarwal, H.; Nakara, A.; Shanmugam, V.K. Anti-Inflammatory Mechanism of Various Metal and Metal Oxide Nanoparticles Synthesized Using Plant Extracts: A Review. Biomed. Pharmacother. 2019, 109, 2561–2572. [Google Scholar] [CrossRef]
- Viscido, A.; Capannolo, A.; Latella, G.; Caprilli, R.; Frieri, G. Nanotechnology in the Treatment of Inflammatory Bowel Diseases. J. Crohn’s Colitis 2014, 8, 903–918. [Google Scholar] [CrossRef]
- Filip, G.A.; Moldovan, B.; Baldea, I.; Olteanu, D.; Suharoschi, R.; Decea, N.; Cismaru, C.M.; Gal, E.; Cenariu, M.; Clichici, S.; et al. UV-Light Mediated Green Synthesis of Silver and Gold Nanoparticles Using Cornelian cherry Fruit Extract and Their Comparative Effects in Experimental Inflammation. J. Photochem. Photobiol. B Biol. 2019, 191, 26–37. [Google Scholar] [CrossRef]
- Singh, P.; Ahn, S.; Kang, J.P.; Veronika, S.; Huo, Y.; Singh, H.; Chokkaligam, M.; El-Agamy Farh, M.; Aceituno, V.C.; Kim, Y.J.; et al. In Vitro Anti-Inflammatory Activity of Spherical Silver Nanoparticles and Monodisperse Hexagonal Gold Nanoparticles by Fruit Extract of Prunus serrulata: A Green Synthetic Approach. Artif. Cells Nanomed. Biotechnol. 2018, 46, 2022–2032. [Google Scholar] [CrossRef] [Green Version]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Mater. 2019, 12, 3602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonigala, B.; Kasukurthi, B.; Konduri, V.V.; Mangamuri, U.K.; Gorrepati, R.; Poda, S. Green Synthesis of Silver and Gold Nanoparticles Using Stemona Tuberosa Lour and Screening for Their Catalytic Activity in the Degradation of Toxic Chemicals. Environ. Sci. Pollut. Res. 2018, 25, 32540–32548. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Park, H.H.; Sakthivel, N. Extracellular Synthesis of Mycogenic Silver Nanoparticles by Cylindrocladium Floridanum and Its Homogeneous Catalytic Degradation of 4-Nitrophenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 116, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Vidhu, V.K.; Philip, D. Spectroscopic, Microscopic and Catalytic Properties of Silver Nanoparticles Synthesized Using Saraca Indica Flower. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 117, 102–108. [Google Scholar] [CrossRef]
- Joseph, S.; Mathew, B. Microwave Assisted Facile Green Synthesis of Silver and Gold Nanocatalysts Using the Leaf Extract of Aerva Lanata; Elsevier: Amsterdam, The Netherlands, 2015; Volume 136, ISBN 9148127310. [Google Scholar]
- Otari, S.V.; Patil, R.M.; Nadaf, N.H.; Ghosh, S.J.; Pawar, S.H. Green Synthesis of Silver Nanoparticles by Microorganism Using Organic Pollutant: Its Antimicrobial and Catalytic Application. Environ. Sci. Pollut. Res. 2014, 21, 1503–1513. [Google Scholar] [CrossRef]
- Gangula, A.; Podila, R.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic Reduction of 4-Nitrophenol Using Biogenic Gold and Silver Nanoparticles Derived from Breynia Rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef]
- Vilas, V.; Philip, D.; Mathew, J. Essential Oil Mediated Synthesis of Silver Nanocrystals for Environmental, Anti-Microbial and Antioxidant Applications. Mater. Sci. Eng. C 2016, 61, 429–436. [Google Scholar] [CrossRef]
- Kluenker, M.; Connolly, B.M.; Marolf, D.M.; Nawaz Tahir, M.; Korschelt, K.; Simon, P.; Köhler, U.; Plana-Ruiz, S.; Barton, B.; Panthöfer, M.; et al. Controlling the Morphology of Au-Pd Heterodimer Nanoparticles by Surface Ligands. Inorg. Chem. 2018, 57, 13640–13652. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic Analysis of Catalytic Reduction of 4-Nitrophenol by Metallic Nanoparticles Immobilized in Spherical Polyelectrolyte Brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, L.; Chen, B.; Ji, N.; Chen, F.; Zhang, Y.; Zhang, Z. Nanocomposites of Size-Controlled Gold Nanoparticles and Graphene Oxide: Formation and Applications in SERS and Catalysis. Nanoscale 2010, 2, 2733–2738. [Google Scholar] [CrossRef]
- Choi, Y.; Kang, S.; Cha, S.H.; Kim, H.S.; Song, K.; Lee, Y.J.; Kim, K.; Kim, Y.S.; Cho, S.; Park, Y. Platycodon Saponins from Platycodi Radix (Platycodon grandiflorum) for the Green Synthesis of Gold and Silver Nanoparticles. Nanoscale Res. Lett. 2018, 13, 23. [Google Scholar] [CrossRef]
- Wei, D.; Ye, Y.; Jia, X.; Yuan, C.; Qian, W. Chitosan as an Active Support for Assembly of Metal Nanoparticles and Application of the Resultant Bioconjugates in Catalysis. Carbohydr. Res. 2010, 345, 74–81. [Google Scholar] [CrossRef]
- Takale, B.S.; Bao, M.; Yamamoto, Y. Gold Nanoparticle (AuNPs) and Gold Nanopore (AuNPore) Catalysts in Organic Synthesis. Org. Biomol. Chem. 2014, 12, 2005–2027. [Google Scholar] [CrossRef]
- Shaik, M.R.; Ali, Z.J.Q.; Khan, M.; Kuniyil, M.; Assal, M.E.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H.; Khan, M.; Adil, S.F. Green Synthesis and Characterization of Palladium Nanoparticles Using Origanum vulgare L. Extract and Their Catalytic Activity. Molecules 2017, 22, 165. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Peng, Y.; Song, G. Silver Nitrate-Catalyzed Selective Air Oxidation of Benzylic and Allylic Alcohols to Corresponding Aldehydes or Ketones. J. Chin. Chem. Soc. 2014, 61, 517–520. [Google Scholar] [CrossRef]
- Musere, P.S.F.; Rahman, A.; Uahengo, V.; Naimhwaka, J.; Daniel, L.; Bhaskurani, S.V.H.S.; Jonnalagadda, S.B. Biosynthesis of Silver Nanoparticles Using Pearl Millet (Pennisetum glaucum) Husk to Remove Algae in the Water and Catalytic Oxidation of Benzyl Alcohol. J. Clean. Prod. 2021, 312. [Google Scholar] [CrossRef]
- Thanki, K.; Gangwal, R.P.; Sangamwar, A.T.; Jain, S. Oral Delivery of Anticancer Drugs: Challenges and Opportunities. J. Control. Release 2013, 170, 15–40. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Mundekkad, D.; Cho, W.C. Nanoparticles in Clinical Translation for Cancer Therapy. Int. J. Mol. Sci. 2022, 23, 1685. [Google Scholar] [CrossRef]
- Jain, K.K. Nanomedicine: Application of Nanobiotechnology in Medical Practice. Med. Princ. Pract. 2008, 17, 89–101. [Google Scholar] [CrossRef]
- Mishra, A.; Tripathy, S.K.; Yun, S. Il Fungus Mediated Synthesis of Gold Nanoparticles and Their Conjugation with Genomic DNA Isolated from Escherichia Coli and Staphylococcus Aureus. Process Biochem. 2012, 47, 701–711. [Google Scholar] [CrossRef]
- Naahidi, S.; Jafari, M.; Edalat, F.; Raymond, K.; Khademhosseini, A.; Chen, P. Biocompatibility of Engineered Nanoparticles for Drug Delivery. J. Control. Release 2013, 166, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in Medicine: Therapeutic Applications and Developments. Clin. Pharmacol. Ther. 2008, 83. [Google Scholar] [CrossRef] [PubMed]
- Bailly, A.L.; Correard, F.; Popov, A.; Tselikov, G.; Chaspoul, F.; Appay, R.; Al-Kattan, A.; Kabashin, A.V.; Braguer, D.; Esteve, M.A. In Vivo Evaluation of Safety, Biodistribution and Pharmacokinetics of Laser-Synthesized Gold Nanoparticles. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Alaqad, K.; Saleh, T.A. Gold and Silver Nanoparticles: Synthesis Methods, Characterization Routes and Applications towards Drugs. J. Environ. Anal. Toxicol. 2016, 6, 1–10. [Google Scholar] [CrossRef]
- Kang, M.S.; Lee, S.Y.; Kim, K.S.; Han, D.W. State of the Art Biocompatible Gold Nanoparticles for Cancer Theragnosis. Pharmaceutics 2020, 12, 701. [Google Scholar] [CrossRef]
- Markus, J.; Wang, D.; Kim, Y.J.; Ahn, S.; Mathiyalagan, R.; Wang, C.; Yang, D.C. Biosynthesis, Characterization, and Bioactivities Evaluation of Silver and Gold Nanoparticles Mediated by the Roots of Chinese Herbal Angelica Pubescens Maxim. Nanoscale Res. Lett. 2017, 12, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.K.; Mishra, S.; Jena, S.; Panigrahi, B.; Das, B.; Jayabalan, R.; Parhi, P.K.; Mandal, D. Rapid Colorimetric Sensing of Gadolinium by EGCG-Derived AgNPs: The Development of a Nanohybrid Bioimaging Probe. Chem. Commun. 2018, 54, 3981–3984. [Google Scholar] [CrossRef]
- Alberti, G.; Zanoni, C.; Magnaghi, L.R.; Biesuz, R. Gold and Silver Nanoparticle-Based Colorimetric Sensors: New Trends and Applications. Chemosensors 2021, 9, 305. [Google Scholar] [CrossRef]
- Qin, L.; Zeng, G.; Lai, C.; Huang, D.; Xu, P.; Zhang, C.; Cheng, M.; Liu, X.; Liu, S.; Li, B.; et al. “Gold Rush” in Modern Science: Fabrication Strategies and Typical Advanced Applications of Gold Nanoparticles in Sensing. Coord. Chem. Rev. 2018, 54, 3981–3984. [Google Scholar] [CrossRef]
- Liu, G.; Lu, M.; Huang, X.; Li, T.; Xu, D. Application of Gold-Nanoparticle Colorimetric Sensing to Rapid Food Safety Screening. Sensors 2018, 18, 4166. [Google Scholar] [CrossRef] [Green Version]
- Piriya, V.S.; Joseph, P.; Daniel, S.C.G.K.; Lakshmanan, S.; Kinoshita, T.; Muthusamy, S. Colorimetric Sensors for Rapid Detection of Various Analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Zayed, M.F.; Eisa, W.H.; El-kousy, S.M.; Mleha, W.K.; Kamal, N. Ficus Retusa-Stabilized Gold and Silver Nanoparticles: Controlled Synthesis, Spectroscopic Characterization, and Sensing Properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 214, 496–512. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M.; Esmail, G.A.; Al-Dhabi, N.A.; Arasu, M.V. Green Synthesis and Characterization of Silver Nanoparticles from Moringa Oleifera Flower and Assessment of Antimicrobial and Sensing Properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111836. [Google Scholar] [CrossRef]
- Kumar, V.; Singh, D.K.; Mohan, S.; Bano, D.; Gundampati, R.K.; Hasan, S.H. Green Synthesis of Silver Nanoparticle for the Selective and Sensitive Colorimetric Detection of Mercury (II) Ion. J. Photochem. Photobiol. B Biol. 2017, 168, 67–77. [Google Scholar] [CrossRef]
- Puente, C.; Gómez, I.; Kharisov, B.; López, I. Selective Colorimetric Sensing of Zn(II) Ions Using Green-Synthesized Silver Nanoparticles: Ficus Benjamina Extract as Reducing and Stabilizing Agent. Mater. Res. Bull. 2019, 112, 1–8. [Google Scholar] [CrossRef]
- Zohora, N.; Kumar, D.; Yazdani, M.; Rotello, V.M.; Ramanathan, R.; Bansal, V. Rapid Colorimetric Detection of Mercury Using Biosynthesized Gold Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 451–457. [Google Scholar] [CrossRef]
- Singh, K.; Kukkar, D.; Singh, R.; Kukkar, P.; Kim, K.H. Exceptionally Stable Green-Synthesized Gold Nanoparticles for Highly Sensitive and Selective Colorimetric Detection of Trace Metal Ions and Volatile Aromatic Compounds. J. Ind. Eng. Chem. 2018, 68, 33–41. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal Plant Leaf Extract and Pure Flavonoid Mediated Green Synthesis of Silver Nanoparticles and Their Enhanced Antibacterial Property. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Asghar, H.M.A.; Bhattacharjee, S. Mechanistic Investigation of Phytochemicals Involved in Green Synthesis of Gold Nanoparticles Using Aqueous Elaeis Guineensis Leaves Extract: Role of Phenolic Compounds and Flavonoids. Biotechnol. Appl. Biochem. 2019, 66, 698–708. [Google Scholar] [CrossRef]
- Durmazel, S.; Üzer, A.; Erbil, B.; Sayln, B.; Apak, R. Silver Nanoparticle Formation-Based Colorimetric Determination of Reducing Sugars in Food Extracts via Tollens’ Reagent. ACS Omega 2019, 4, 7596–7604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheny, D.S.; Mathew, J.; Philip, D. Phytosynthesis of Au, Ag and Au-Ag Bimetallic Nanoparticles Using Aqueous Extract and Dried Leaf of Anacardium Occidentale. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 79, 254–262. [Google Scholar] [CrossRef]
- Gole, A.; Dash, C.; Ramakrishnan, V.; Sainkar, S.R.; Mandale, A.B.; Rao, M.; Sastry, M. Pepsin-Gold Colloid Conjugates: Preparation, Characterization, and Enzymatic Activity. Langmuir 2001, 17, 1674–1679. [Google Scholar] [CrossRef]
- Arunachalam, K.D.; Annamalai, S.K. Chrysopogon Zizanioides Aqueous Extract Mediated Synthesis, Characterization of Crystalline Silver and Gold Nanoparticles for Biomedical Applications. Int. J. Nanomed. 2013, 8, 2375–2384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunachalam, K.D.; Annamalai, S.K.; Hari, S. One-Step Green Synthesis and Characterization of Leaf Extract-Mediated Biocompatible Silver and Gold Nanoparticles from Memecylon Umbellatum. Int. J. Nanomed. 2013, 8, 1307–1315. [Google Scholar] [CrossRef] [Green Version]
- Elavazhagan, T.; Arunachalam, K.D. Memecylon Edule Leaf Extract Mediated Green Synthesis of Silver and Gold Nanoparticles. Int. J. Nanomed. 2011, 6, 1265–1278. [Google Scholar] [CrossRef] [Green Version]
- Philip, D.; Unni, C.; Aromal, S.A.; Vidhu, V.K. Murraya Koenigii Leaf-Assisted Rapid Green Synthesis of Silver and Gold Nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 899–904. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Noble Metal Nanoparticles: Plant-Mediated Synthesis, Mechanistic Aspects of Synthesis, and Applications. Ind. Eng. Chem. Res. 2016, 55, 9557–9577. [Google Scholar] [CrossRef]
- Ahmad Kuthi, N.; Chandren, S.; Basar, N.; Jamil, M.S.S. Biosynthesis of Gold Nanoisotrops Using Carallia Brachiata Leaf Extract and Their Catalytic Application in the Reduction of 4-Nitrophenol. Front. Chem. 2022, 9. [Google Scholar] [CrossRef]
- Sunita, P.; Palaniswamy, M. Effect of Extract Concentration and Ageing on Optical Properties of Biological Silver Nanoparticles. Int. J. Pharma Bio Sci. 2017, 8, 686–690. [Google Scholar] [CrossRef]
- Khan, M.; Khan, M.; Adil, S.F.; Tahir, M.N.; Tremel, W.; Alkhathlan, H.Z.; Al-Warthan, A.; Siddiqui, M.R.H. Green Synthesis of Silver Nanoparticles Mediated by Pulicaria Glutinosa Extract. Int. J. Nanomed. 2013, 8, 1507–1516. [Google Scholar] [CrossRef] [Green Version]
- Aromal, S.A.; Philip, D. Green Synthesis of Gold Nanoparticles Using Trigonella Foenum-Graecum and Its Size-Dependent Catalytic Activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef]
- Krutyakov, Y.A.; Kudrinskiy, A.A.; Olenin, A.Y.; Lisichkin, G.V. Synthesis and Properties of Silver Nanoparticles: Advances and Prospects. Russ. Chem. Rev. 2008, 77, 233. [Google Scholar] [CrossRef]
- Aditya, T.; Jana, J.; Singh, N.K.; Pal, A.; Pal, T. Remarkable Facet Selective Reduction of 4-Nitrophenol by Morphologically Tailored (111) Faceted Cu2O Nanocatalyst. ACS Omega 2017, 2, 1968–1984. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-León, E.; Rodríguez-Vázquez, B.E.; Martínez-Higuera, A.; Rodríguez-Beas, C.; Larios-Rodríguez, E.; Navarro, R.E.; López-Esparza, R.; Iñiguez-Palomares, R.A. Synthesis of Gold Nanoparticles Using Mimosa Tenuiflora Extract, Assessments of Cytotoxicity, Cellular Uptake, and Catalysis. Nanoscale Res. Lett. 2019, 14, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kuppusamy, P.; Yusoff, M.M.; Maniam, G.P.; Govindan, N. Biosynthesis of Metallic Nanoparticles Using Plant Derivatives and Their New Avenues in Pharmacological Applications—An Updated Report. Saudi Pharm. J. 2016, 24, 473–484. [Google Scholar] [CrossRef]
- Watzky, M.A.; Finke, R.G. Gold Nanoparticle Formation Kinetics and Mechanism: A Critical Analysis of the “Redox Crystallization” Mechanism. ACS Omega 2018, 3, 1555–1563. [Google Scholar] [CrossRef] [Green Version]
- Gomah, A.M.; Davies, R.I. Identification of the Active Ligands Chelating Zn in Some Plant Water Extracts. Plant Soil 1974, 40, 1–19. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Berta, L.; Coman, N.-A.; Rusu, A.; Tanase, C. A Review on Plant-Mediated Synthesis of Bimetallic Nanoparticles, Characterisation and Their Biological Applications. Materials 2021, 14, 7677. [Google Scholar] [CrossRef]
- Amin, M.; Anwar, F.; Janjua, M.R.S.A.; Iqbal, M.A.; Rashid, U. Green Synthesis of Silver Nanoparticles through Reduction with Solanum xanthocarpum L. Berry Extract: Characterization, Antimicrobial and Urease Inhibitory Activities against Helicobacter Pylori. Int. J. Mol. Sci. 2012, 13, 9923–9941. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Betts, J.W.; Kelly, S.M.; Schaller, J.; Heinze, T. Synthesis and Antibacterial Effects of Aqueous Colloidal Solutions of Silver Nanoparticles Using Aminocellulose as a Combined Reducing and Capping Reagent. Green Chem. 2013, 15, 989–998. [Google Scholar] [CrossRef]
- Zayed, M.F.; Eisa, W.H.; Abdel-Moneam, Y.K.; El-kousy, S.M.; Atia, A. Ziziphus Spina-Christi Based Bio-Synthesis of Ag Nanoparticles. J. Ind. Eng. Chem. 2015, 23, 50–56. [Google Scholar] [CrossRef]
- Sathishkumar, M.; Sneha, K.; Won, S.W.; Cho, C.W.; Kim, S.; Yun, Y.S. Cinnamon Zeylanicum Bark Extract and Powder Mediated Green Synthesis of Nano-Crystalline Silver Particles and Its Bactericidal Activity. Colloids Surf. B Biointerfaces 2009, 73, 332–338. [Google Scholar] [CrossRef]

| Method | Advantages | Disadvantages |
|---|---|---|
| Plant-based biosynthesis | High speed, eco-friendly, pollutant- and toxicity-free, more cost-effective (no cost for culture media and microorganism isolation), simple handling, stable and non-aggregated NPs, scalability | Cannot be genetically manipulated like microorganisms |
| Microorganism-based biosynthesis | Eco-friendly, non-toxic, clean, can be manipulated easily | Low speed, complicated process (sampling, isolation, culturing, storage of microorganisms, and downstream processing), difficult to control stability and aggregation, cost- and time-consuming process (need to culture microorganisms), probability of endotoxin presence |
| Plant | Part of the Plant | Target Pathogens | Activity | Concentration | Type of NP | Experimental Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Amorphophallus paeoniifolius | Tuber | Pseudomonas aeruginosa, Escherichia coli, Salmonella typhimurium, Citrobacter freundii, Bacillus subtilis, Staphylococcus aureus | Antibacterial | 25 µL/disk | Ag | The highest activity against P. aeruginosa with the ZOI was 20 nm at a concentration of 25 µL/disk | [57] |
| Au | Did not show activity (ZOI = 0.00 nm) | ||||||
| Gloriosa superba | Leaf | Bacillus subtilis, Escherichia coli | Antibacterial | 10–50 µL/disk | Ag | The highest activity against E. coli with the ZOI was 7.66 ± 0.33 mm at a concentration of 30 µL/disk | [58] |
| Au | Did not show activity (ZOI = 0.00 nm) | ||||||
| Rhodiola rosea | Rhizo-me | Pseudomonas aeruginosa, Escherichia coli | Antibacterial | 50–200 µg/mL | Ag | Significant activity against P. aeruginosa with an MIC and MBC at 50 µg/mL and 100 µg/mL; meanwhile, the MIC and MBC against E. coli were 100 µg/mL and 200 µg/mL | [59] |
| Au | Did not show activity | ||||||
| Antibiofilm | 1.6–200 µg/mL | Ag | Significant activity was at a concentration of ≥ 6.25 µg/mL | ||||
| Au | Significant activity was at a concentration of ≥ 12.5 µg/mL | ||||||
| Clerodendrum inerme | Leaf | Staphylococcus aureus, Bacillus subtilis, Klebsiella, Escherichia coli | Antibacterial | 250 µg/mL | Ag | The highest activity against Klebsiella with the ZOI was at 21 µg/mL | [60] |
| Au | The highest activity against E. coli with the ZOI was at 16 µg/mL | ||||||
| Aspergillus niger, Aspergillus flavus, Trichoderma harzianum | Antimycotic | 250 µg/mL | Ag | The highest activity against A. flavus with the ZOI was at 22 µg/mL | |||
| Au | the highest activity against A. flavus with the ZOI was at 20 µg/mL | ||||||
| Panax ginseng Meyer | Fruit | Escherichia coli, Staphylococcus aureus | Antibacterial | 1.15–3.45 µg/disk | Ag | The highest activity against S. aureus with the ZOI was 12.3 mm at a concentration of 3.45 µg/disk | [61] |
| Au | - | ||||||
| Siberian ginseng (Eleutherococcus senticosus) | Stem | Escherichia coli, Vibrio parahaemolyticus, Staphylococcus aureus, Bacillus anthracis | Antibacterial | 10–30 µg/mL | Ag | The highest activity against S. aureus with the ZOI was 13.8 ± 0.2 mm at a concentration of 30 µg/mL | [39] |
| Au | Did not show activity (ZOI = 0.00 nm) | ||||||
| Actinidia deliciosa | Fruit | Pseudomonas aeruginosa | Antibacterial | 10–30 µg/mL | Ag, Au | AgNPs showed a higher ZOI than AuNPs at each concentration | [62] |
| Rosa canina L. | Rose-hip | Escherichia coli | Antibacterial | 0.5 µg/mL | Ag | MIC was 0.5 µg/mL | [63] |
| Au | - | ||||||
| Borago officinalis | Leaf | Pseudomonas aeruginosa, Vibrio parahaemolyticus, Staphylococcus aureus, Escherichia coli | Antibacterial | 15 µL/disk | Ag | The highest activity against P. aeruginosa with the ZOI was 13.7 ± 0.5 mm at a concentration of 15 µL/disk | [64] |
| Antibiofilm | 2–10 µg/mL | Ag | Significant activity was at a concentration of 10 µg/mL | ||||
| Asparagus racemosus | Root | Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli, Bacillus subtilis, Klebsiella pneumonia | Antibacterial | 20–80 µg/mL | Ag | The highest activity against P. aeruginosa with the ZOI was 28 mm at a concentration of 80 µg/mL | [65] |
| Au | The highest activity against P. aeruginosa with the ZOI was 26 mm at a concentration of 80 µg/mL | ||||||
| Coleus forskohlii | Root | Proteus vulgaris, Micrococcus luteus, Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli | Antibacterial | 15–35 µL/disk | Ag | The highest activity against E. coli with the ZOI was 26 mm at a concentration of 35 µL/disk | [66,67] |
| Au | The highest activity against E. coli with the ZOI was 21 mm at a concentration of 35 µL/disk | ||||||
| Mussaenda glabrata | Leaf | Pseudomonas aeruginosa, Bacillus pumilus, Escherichia coli, Staphylococcus aureus, | Antibacterial | - | Ag | The highest activity against S. aureus with the ZOI was 19 mm | [68] |
| Au | The highest activity against S. aureus with the ZOI was 14 mm | ||||||
| Aspergillus niger, Penicillium chrysogenum | Antimycotic | - | Ag | The highest activity against P. chrysogenum with the ZOI was 13 mm | |||
| Au | The highest activity against P. chrysogenum with ZOI was 12 mm | ||||||
| Stereospermum chelonoides | Root bark | Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli | Antibacterial | 1000 µg/mL | Ag | The highest activity against P. aeruginosa with the ZOI was 20 mm | [69] |
| Au | The highest activity against E. coli with the ZOI was 17 mm | ||||||
| Aspergillus flavus, Aspergillus nidulans | Antimycotic | 1000 µg/mL | Ag | The highest activity against A. flavus with the ZOI was 23 mm | |||
| Au | The highest activity against A. flavus with the ZOI was 21 mm | ||||||
| Rivea hypocrateriformis | Aerial part | Klebsiella pneumonia, Bacillus subtilis, Staphylococcus aureus, Pseudomonas aeruginosa, Escherichia coli | Antibacterial | 25–100 µg/mL | Ag | The highest activity against E. coli with the ZOI was 13 mm at a concentration of 100 µg/mL | [70] |
| Au | The highest activity against E. coli with the ZOI was 12 mm at a concentration of 100 µg/mL | ||||||
| Trichophyton rubrum, Candida albicans, Chrysosporium indicum | Antimycotic | 25–100 µg/mL | Ag | The highest activity against C. indicum with the ZOI was 7 mm at a concentration of100 µg/mL | |||
| Au | The highest activity against C. indicum with the ZOI was 6 mm at a concentration of 100 µg/mL | ||||||
| Glycyrrhiza uralensis | Root | Pseudomonas aeruginosa, Salmonella enterica, Staphylococcus aureus, Escherichia coli | Antibacterial | 15–45 µg/disk | Ag | The highest activity against S. aureus with the ZOI was 17.3 ± 0.57 mm at a concentration of 45 µg/disk | [71] |
| Au | Did not show activity | ||||||
| Parkia roxburghii | Leaf | Escherichia coli, Staphylococcus aureus | Antibacterial | 25 µL/disk | Ag, Au | AgNPs showed the higher ZOI than AuNPs at each strain | [72] |
| Mentha longifolia | Leaf | Staphylococcus aureus, Bacillus subtilis | Antibacterial | 3000 µg/mL | Ag | The highest activity against S. aureus with the ZOI was 12 ± 0.03 mm | [73] |
| Au | The highest activity against S. aureus with the ZOI was 10 ± 0.01 mm | ||||||
| Zingiber officinale | Root | Listeria spp., Staphylococcus spp. | Antibacterial | 25 µL/disk | Ag | The highest activity against Listeria spp. with the ZOI was 8.9 ± 0.6 mm | [74] |
| Indigofera tinctoria | Leaf | Staphylococcus aureus, Bacillus pumilus, Pseudomonas aeruginosa, Escherichia coli | Antibacterial | 100 µL/disk | Ag, Au | AgNPs showed the higher ZOI than AuNPs at each strain | [75] |
| Aspergillus fumigatus, Aspergillus niger | Antimycotic | 100 µL/disk | Ag, Au | AgNPs showed the higher ZOI than AuNPs at each strain | |||
| Bauhinia purpurea | Leaf | Staphylococcus aureus, Bacillus subtilis, Pseudomonas aeruginosa, Escherichia coli | Antibacterial | 50 µL/disk | Ag, Au | AgNPs showed the higher ZOI than AuNPs at each strain | [76] |
| Aspergillus nidulans, Aspergillus flavus | Antimycotic | 50 µL/disk | Ag, Au | AgNPs showed the higher ZOI than AuNPs at each strain | |||
| Cibotium barometz | Root | Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella enterica, Escherichia coli | Antibacterial | 15–45 µL/disk | Ag | The highest activity against S. aureus with the ZOI was ± 16 nm | [77] |
| Jasminum sambac | Leaf | Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli | Antibacterial | 10 µg/disk | Ag | The highest activity against E. coli with the ZOI was 7 nm | [78] |
| Au | The highest activity against E. coli with the ZOI was 4 nm |
| Plant | Part of the Plant | Cell Lines Targeted | Concentration | Type of NP | Experimental Outcomes | Ref. |
|---|---|---|---|---|---|---|
| Rivea hypocrateriformis | Aerial part | Breast cancer cell (MCF-7) | 25 µg/mL | Ag | 51.30% cytotoxic effect | [70] |
| Au | 52% cytotoxic effect | |||||
| Glycyrrhiza uralensis | Root | Breast cancer cell (MCF-7) | 10 µg/mL | Ag | 20% cytotoxic effect | [71] |
| - | Au | Did not show cytotoxic effect | ||||
| Murine macrophage (RAW264.7) | - | Ag | Did not show cytotoxic effect | |||
| 25 µg/mL | Au | 15% cytotoxic effect | ||||
| Borago officinalis | Leaf | Cervical cancer cell (HeLa) | 5 µg/mL | Ag | 74.90% cytotoxic effect | [64] |
| Lung cancer cell (A549) | 10 µg/mL | Ag | 64.30% cytotoxic effect | |||
| Murine macrophage (RAW264.7) | 2–10 µg/mL | Ag | 20–25% cytotoxic effect | |||
| Indigofera tinctoria | Leaf | Lung cancer cell (A549) | 56.62 ± 0.86 µg/mL (IC50) | Ag | AgNPs and AuNPs showed more cytotoxic effect than plant extract alone (IC50 = 71.92 ± 0.76 µg/mL) | [75] |
| 59.33 ± 0.57 µg/mL (IC50) | Au | |||||
| Bauhinia purpurea | Leaf | Lung cancer cell (A549) | 100 µg/mL | Ag | 68% cytotoxic effect | [76] |
| Au | 69% cytotoxic effect | |||||
| Rooibos (Aspalathus linearis) | Leaf and stem | Human neuroblastoma (SH-SY5Y) | 25–500 µg/mL | Ag | Anticancer activity with IC50 was 108.80 µg/mL | [86] |
| Au | Did not show cytotoxic effect | |||||
| Liver cancer cell (HepG2) | 25–500 µg/mL | Ag | Anticancer activity with IC50 was 183.40 µg/mL | |||
| Au | Did not show cytotoxic effect | |||||
| Coleus forskohlii | Root | Liver cancer cell (HepG2) | 10 µg/mL | Ag, Au | 20% cytotoxic effect | [66] |
| Siberian ginseng (Eleutherococcus senticosus) | Stem | Breast cancer cell (MCF-7) | 10 µg/mL | Ag | 40% cytotoxic effect | [39] |
| - | Au | Did not show cytotoxic effect | ||||
| Human keratinocyte cell (HaCaT) | 10 µg/mL | Ag | 17% cytotoxic effect | |||
| - | Au | Did not show cytotoxic effect | ||||
| Mangosteen (Garcinia mangostana) | Pericarp | Lung cancer cell (A549) | 18.75 µg/mL | Ag | 11.9% cytotoxic effect | [88] |
| 75 µg/mL | Au | 23.5% cytotoxic effect | ||||
| NIH3T3 cell | 37.5 µg/mL | Ag | 63% cytotoxic effect | |||
| 75 µg/mL | Au | 6.2% cytotoxic effect | ||||
| Actinidia deliciosa | Fruit | Colon cancer cell (HCT116) | 350 µg/mL | Ag | 22% cytotoxic effect | [62] |
| Au | 29% cytotoxic effect | |||||
| Mukia maderaspatana | Leaf | Breast cancer cell (MCF-7) | 1–100 µg/mL | Ag | Anticancer activity with IC50 was 51.30 µg/mL | [89] |
| Au | Anticancer activity with IC50 was 44.80 µg/mL | |||||
| Dendropanax morbifera | Leaf | Human keratinocyte cell (HaCaT) | 100 µg/mL | Ag | 40% cytotoxic effect | [90] |
| Au | Did not show cytotoxic effect | |||||
| Lung cancer cell (A549) | 100 µg/mL | Ag | 70% cytotoxic effect | |||
| Au | Did not show cytotoxic effect |
| Plant | Part of the Plant | Type of NP | Experimental Outcome | Ref. |
|---|---|---|---|---|
| Cornelian cherry (Cornus mas) | Fruit | Au, Ag | ↓ Inflammation and apoptosis in early stage | [111] |
| Prunus serrulata | Fruit | Au, Ag | ↓ Expression of inflammatory mediators in lipopolysaccharide-induced RAW 264.7 | [112] |
| Mentha longifolia | Leaf | Au, Ag | The activity of AuNPs and AgNPs (100 mg/kg) was comparable with the standard drug (10 mg/kg) | [73] |
| Plant | Part of the Plant | Reactions | Type of NP | Experimental Outcomes | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| ↓ Intensity λmax | New Band | Reaction Time | Discoloration | |||||
| Stemona tuberosa Lour | Aerial parts | Reduction of 4-nitrophenol | Au | ✓ | ✓ | NE | ✓ | [114] |
| Ag | ✓ | ✓ | NE | ✓ | ||||
| Reduction of methyl orange | Au | ✓ | ✓ | NE | ✓ | |||
| Ag | ✓ | ✓ | NE | ✓ | ||||
| Reduction of methyl red | Au | ✓ | ✓ | NE | ✓ | |||
| Ag | ✓ | ✓ | NE | ✓ | ||||
| Reduction of methylene blue | Au | ✓ | ✓ | NE | ✓ | |||
| Ag | ✓ | ✓ | NE | ✓ | ||||
| Mussaenda glabrata | Leaf | Reduction of 4-nitrophenol | Au | ✓ | ✓ | 7 min | NE | [68] |
| Ag | ✓ | ✓ | 9 min | NE | ||||
| Reduction of rhodamine B | Au | ✓ | ✓ | 5 min | NE | |||
| Ag | ✓ | ✓ | 9 min | NE | ||||
| Reduction of methyl orange | Au | ✓ | ✓ | 4 min | NE | |||
| Ag | ✓ | ✓ | 7 min | NE | ||||
| Indigofera tinctoria | Leaf | Reduction of o- and p-nitroaniline | Au | ✓ | ✓ | 18 min | NE | [75] |
| Ag | ✓ | ✓ | 10 min | NE | ||||
| Bauhinia purpurea | Leaf | Reduction of rhodamine B | Au | ✓ | ✓ | 4 min | ✓ | [76] |
| Ag | ✓ | ✓ | 6 min | ✓ | ||||
| Reduction of methylene blue | Au | ✓ | ✓ | 4 min | ✓ | |||
| Ag | ✓ | ✓ | 6 min | ✓ | ||||
| Aerva lanata | Leaf | Reduction of 4-nitrophenol | Au | ✓ | ✓ | 11 min | ✓ | [117] |
| Ag | ✓ | ✓ | 13 min | ✓ | ||||
| Platycodon grandiflorum | Radix | Reduction of 4-nitrophenol | Au | ✓ | ✓ | 720 s | ✓ | [124] |
| Pulicaria undulata | Aerial part | Reduction of 4-nitrophenol | Au | ✓ | ✓ | ~2 h | ✓ | [40] |
| Ag | ✓ | ✓ | > 10 h | ✓ | ||||
| Actinidia deliciosa | Fruit | Reduction of methylene blue | Au | ✓ | ✓ | 14 min | ✓ | [62] |
| Ag | ✓ | ✓ | 22 min | ✓ | ||||
| Rosa canina L. | Rosehip | Reduction of 4-nitrophenol | Au | ✓ | ✓ | NE | NE | [63] |
| Coleus forskohlii | Root | Reduction of 4-nitrophenol | Ag | ✓ | ✓ | 24 min | ✓ | [67] |
| Glycyrrhiza uralensis | Root | Reduction of methylene blue | Au, Ag | ✓ | ✓ | ~60 min | NE | [71] |
| Plant | Part of the Plant | Type of NP | Experimental Outcomes | Ref. |
|---|---|---|---|---|
| Mangosteen (Garcinia mangostana) | Pericarp | Au | Low cytotoxic effect at the highest concentration (6.2%) | [88] |
| Dendropanax morbifera | Leaf | Au | No cytotoxic effect | [90] |
| Cibotium barometz | Root | Au | No cytotoxic effect | [77] |
| Angelica pubescens Maxim | Root | Au | No cytotoxic effect | [140] |
| Plant | Part of the Plant | Type of NP | Ion Target | Ref. |
|---|---|---|---|---|
| Ficus retusa | Leaf | Ag | Fe3+ | [146] |
| Moringa oleifera | Flower | Ag | Cu4+ | [147] |
| Murraya koenigii | Leaf | Ag | Hg2+ | [148] |
| Ficus benjamina | Leaf | Ag | Zn2+ | [149] |
| Cinnamomum tamala | Leaf | Au | Hg2+ | [150] |
| Momordica charantia | Fruit | Au | Cd2+ | [151] |
| Plant | Part of the Plant | Phytochemical Contents | Concentration of Phytochemicals | Detection Method | Solvent | Synthesis Condition | Type of NP | Size (nm) | Shape | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Panax ginseng Meyer | Fruit | Ginsenosides | - | HPLC | Water | Add 1 mM HAuCl4·3H2O to 5% extract incubated at 80 °C for 45 min | Au | 5–10 | Spherical | [61] |
| Polyphenols | 0.403 ± 0.03 mg/g (measured as gallic acid) | UV-Vis | ||||||||
| Reducing sugars | 5.02 ± 1.70 mg/g (measured as glucose) | Add 1 mM AgNO3 to 5% extract incubated at 80 °C for 3.5 h | Ag | 10–20 | ||||||
| Acidic polysaccharides | 0.137 ± 5.70 mg/g | |||||||||
| Siberian ginseng (Eleutherococcus senticosus) | Stem | Phenolic compounds | 0.241 mg/g (measured as gallic acid) | LC-MS | Water | Add 1 mM HAuCl4·3H2O to 1:1 diluted extract incubated at room temperature for 9 min | Au | 189 | Face-centred cubical | [39] |
| Reducing sugars | 4.5 mg/g (measured as glucose) | Add 1 mM AgNO3 to 1:1 diluted extract incubated at 80 °C for 1.5 h | Ag | 126 | ||||||
| Proteins | - | |||||||||
| Cornelian cherry (Cornus mas) | Fruit | Polyphenolic compounds | - | UV-Vis | Water | Add 10 mL extract to 30 mL 1 mM HAuCl4 irradiated UV light at room temperature for 15 min | Au | 5–30 | Pseudo-spherical | [111] |
| Add 10 mL extract to 30 mL 1 mM AgNO3 irradiated UV light at room temperature for 2.5 h | Ag | 10–25 | Spherical | |||||||
| Pulicaria undulata | Aerial part | Phenolic compounds (quercetin, kaempferol, dihydrokaempferol, caffeic acid) | - | - | Water | Add 1 mL HAuCl4·3H2O 1 M to 0.2 mL extract 10 mg/mL stirred at room temperature for 2 h | Au | 5–12 | Regular shape (spherical); irregular shape (triangular, hexagonal, quasi-elongated plates) | [40] |
| Add 1 mL AgNO3 1 M to 0.2 mL extract 10 mg/mL stirred at room temperature for 60 min | Ag | |||||||||
| Rhodiola rosea | Rhizome | Flavonoids, polyphenols, terpenoids, polysaccharides, alkaloids, vitamins, amino acids, organic acids | - | FTIR | Water | Add 5 mM HAuCl4 to 2:8 diluted extract incubated at room temperature for 4 s | Au | 12–18 | Irregular shape | [59] |
| Add 5 mM AgNO3 to 2:8 diluted extract incubated at 90 °C for 10 min | Ag | 12–30 | Spherical | |||||||
| Mangosteen (Garcinia mangostana) | Pericarp | Flavonoids, phenolic compounds, carbohydrates, glycosides | - | Phytochemical screening, FTIR | Methanol | Mix 0.35 M HAuCl4·3H2O and 0.02% extract vortexed 5 s then incubated at room temperature for 5 h | Au | 15.37–44.20 | Spherical | [88] |
| Mix 0.35 M AgNO3 and 0.02% extract vortexed 5 s then incubated at room temperature for 5 h | Ag | 13.65–31.08 | Asymmetric nano-dumbbell | |||||||
| Stemona tuberosa Lour | Aerial parts | - | - | - | Water | Add 1 mL extract to 9 mL 1 mM HAuCl4 incubated at 80 °C for 20 min | Au | 20–30 | Irregular shape | [114] |
| Add 1 mL extract to 9 mL 1 mM AgNO3 incubated at 80 °C for 5 min | Ag | 10–12 | Spherical, irregular shape | |||||||
| Actinidia deliciosa | Fruit | Proteins | - | FTIR | - | Add 1 mL extract to 49 mL 1 mM HAuCl4 incubated for 2 h | Au | 7–20 | Spherical | [62] |
| Add 10 mL extract to 190 mL 1 mM AgNO3 incubated for 2 h | Ag | 25–40 | ||||||||
| Rosa canina L. | Rosehip | Phenolic compounds | - | FTIR | Water | Add 80% diluted extract to 1 mM HAuCl4 (1:1) incubated for 15 min | Au | 26 | Quasi-spherical | [63] |
| Add 80% diluted extract to 10 mM AgNO3 (1:1) incubated for 15 min | Ag | 34 | ||||||||
| Rooibos (Aspalathus linearis) | Leaf and stem | Polyphenols, aspalathin | - | FTIR | Water | Add 10 mL 5% extract to 90 mL 1 mM HAuCl4 (heated at 70 °C), stirred under reflux for 28 min | Au | 7.5 ± 0.34 | Hydra-like shape | [86] |
| Add 10 mL 5% extract to 90 mL 1 mM AgNO3 (heated at 70 °C), stirred under reflux for 30 min | Ag | 6.7 ± 0.39 | Quasi-spherical | |||||||
| Borago officinalis | Leaf | Reducing sugars, saccharides, proteins, flavonoids | - | FTIR | Water | Add AgNO3 to 25% extract with final concentration 1 mM, incubated at 65 °C for 65 s | Ag | 30–80 | Spherical, hexagonal, irregular shape | [64] |
| Ficus retusa | Leaf | Phenolic compounds | - | FTIR | Ethanol | Add 100 µL extract to 0.75 mM HAuCl4 at pH 6 for 75 min | Au | 10–25 | Spherical | [146] |
| Add 200 µL extract to 1.5 mM AgNO34 at pH 9 for 60 min | Ag | 15 | ||||||||
| Mukia maderaspatana | Leaf | Flavonol (quercetin, phloroglucinol) | - | Phytochemical screening | Water | Add 2 mM HAuCl4 to 10 mL extract incubated at 80 °C for 4 h | Au | 20–50 | Spherical, triangular, circular | [89] |
| Add 1 mM AgNO3 to 10 mL extract incubated at 70 °C for 30 min | Ag | 20–50 | Irregular shape | |||||||
| Clerodendrum inerme | Leaf | Phenolics, flavonoids, cardiac glycosides, anthraquinones, carbohydrates | - | - | Water | Add 1 mM HAuCl4·3H2O to 25 mL extract heated at 80 °C for 65 min with continuous stirring | Au | 5.82 | Spherical | [60] |
| Add 1 mM AgNO3 to 25 mL extract heated at 70 °C for 65 min with continuous stirring | Ag | 5.54 | ||||||||
| Trapa natans var. bispinosa Roxb. | Peel | Phenolic compounds (gallic acids, quinones) | - | - | Water | Add 0.025 M HAuCl4 to extract incubated at 40–60 °C | Au | 25 ± 2 | Spherical | [87] |
| Add 1 mM AgNO3 to 25 mL extract incubated at 40–60 °C | Ag | 15 ± 2 | ||||||||
| Asparagus racemosus | Root | Phenolics, flavonoids, spiroketal compounds, steroids, reducing sugar, amines, carboxylic acid | - | FTIR | Ethyl acetate | Add 10 mL extract to 50 mL 1 mM HAuCl4 irradiated at microwave 700 w and 2.45 GHz for 20 min, incubated 24 h | Au | 10–50 | Spherical | [65] |
| Add 10 mL extract to 50 mL 1 mM AgNO3 irradiated at microwave 700 w and 2.45 GHz for 20 min, incubated 24 h | Ag | |||||||||
| Chrysopogon zizanioides | Leaf | Alkaloids, phytosterols | - | Phytochemical screening, FTIR | Water | Add 10 mL extract to 10 mL 1 mM HAuCl4 incubated in 150 rpm rotary shaker in the dark for 2 h | Au | 123–138 | Cubic | [157] |
| Add 10 mL extract to 10 mL 1 mM AgNO3 incubated in 150 rpm rotary shaker in the dark for 30 min | Ag | 85–110 | ||||||||
| Memecylon umbellatum | Leaf | High saponins, phenolic compounds, protein, quinones | - | Phytochemical screening, FTIR | Water | Add 15 mL extract to 10 mL 1 mM HAuCl4 incubated in 150 rpm shaker in the dark for 1 h | Au | 15–25 | Spherical, triangular, hexagonal | [158] |
| Add 15 mL extract to 10 mL 1 mM AgNO3 incubated in 150 rpm shaker in the dark for 3 h | Ag | 15–20 | Spherical | |||||||
| Platycodon >grandiflorum | Radix | Triterpenoidal platycodon saponin | - | FTIR | Water | Add 0.05% fraction to 0.2 mM HAuCl4 incubated at room temperature for 5 min | Au | 14–15 | Spherical (major), triangular (minor) | [124] |
| Add 0.01% fraction to 0.8 mM AgNO3 incubated at 80 °C for 3 h and then at room temperature for 21 h | Ag | 17–18 | Spherical | |||||||
| Coleus forskohlii | Root | Phenolic compounds | - | FTIR | Water | Add 1 mL extract to 0.4 mL 0.1 mM HAuCl4 at pH 7 | Au | 10–30 | Spherical | [66] |
| Add 1 mL extract to 0.4 mL 1 mM AgNO34 at pH 7 | Ag | 5–35 | Elliptical | |||||||
| Root | Forskolin, proteins | - | FTIR | Water | Add 5 mL extract to 10 mL 1 mM boiled HAuCl4 stirred at 80 °C for 15 min | Au | 15–35 | Hexagonal | [67] | |
| Add 5 mL extract to 10 mL 1 mM AgNO34 stirred at 80 °C for 15 min | Ag | 35–55 | Face-centred cubical | |||||||
| Memecylon edule | Leaf | Saponin | - | FTIR | Water | Add 15 mL extract to 10 mL 1 mM HAuCl4 incubated in 150 rpm shaker in the dark for 1 h | Au | 10–45 | Triangular, circular, hexagonal | [159] |
| Add 15 mL extract to 10 mL 1 mM AgNO3 incubated in 150 rpm shaker in the dark for 3 h | Ag | 50–90 | Square | |||||||
| Mussaenda glabrata | Leaf | Alkaloids, tannins, flavonoids, steroids | - | FTIR | Water | Mix 1 mM HAuCl4·3H2O with diluted extract (9:1) incubated at room temperature for 5 min | Au | 10.59 | Spherical | [68] |
| Add 1 mM AgNO3 with diluted extract (9:1) incubated at room temperature for 10 min | Ag | 51.32 | ||||||||
| Stereospermum chelonoides | Root bark | Polyphenolic compound (lignans) | - | FTIR | Water | Mix 90 mL 1 mM HAuCl4/AgNO3 to 10 mL extract irradiated at microwave for 1 min | Au | 27.19 ± 5.96 | Spherical | [69] |
| Ag | 49.77 ± 11.64 | |||||||||
| Rivea hypocrateriformis | Aerial part | Polyphenols | - | FTIR | Water | Add 20 mL extract to 50 mL 1 mM HAuCl4/ AgNO3 irradiated at microwave 700 w and 2.45 GHz for 7 min, incubated for 24 h | Au | 20–30 | Spherical | [70] |
| Ag | ||||||||||
| Gloriosa superba | Leaf | Glycosides, water soluble tannins | - | FTIR | Water | Add 5 mL extract to 100 mL 1 mM HAuCl4 at pH 2.26 for 10 min | Au | 20–50 | Triangular, spherical | [58] |
| Add 5 mL extract to 100 mL 1 mM AgNO34 at pH 4.35 for 10 min | Ag | |||||||||
| Glycyrrhiza uralensis | Root | Flavonoids, polyphenols, glycyrrhizin | - | FTIR | Water | Add 1 mM HAuCl4·3H2O to extract incubated at 80 °C for 4 min | Au | 10–15 | Spherical | [71] |
| Add 1 mM AgNO3 to extract incubated at 80 °C for 40 min | Ag | 5–15 | ||||||||
| Aerva lanata | Leaf | Polyphenols, flavonoids, alkaloids, proteins, sugars, tannins | - | FTIR | Water | Add 10 mL extract to 10 mL 10 mM HAuCl4 (final concentration 1 mM) irradiated at microwave 800 w and 2.45 GHz for 90 s | Au | 10–30 | Spherical, hexagonal, triangular plate | [117] |
| Add 10 mL extract to 90 mL 1 mM AgNO3 irradiated at microwave 800 w and 2.45 GHz for 1 min | Ag | 10–34 | Spherical | |||||||
| Dendropanax morbifera | Leaf | Polysaccharides | - | - | Water | Add 1 mM HAuCl4·3H2O to 1:9 diluted extract incubated at 80 °C for 3 min | Au | 10–20 | Polygonal, hexagonal | [90] |
| Add 1 mM AgNO3 to 1:9 diluted extract incubated at 80 °C for 1 h | Ag | 100–150 | Polygonal, hexagonal, triangle | |||||||
| Angelica pubescens Maxim | Root | Flavonoids, phenols, sesquiterpenes | - | FTIR | Water | Add 7 mM HAuCl4·3H2O to 70% diluted extract incubated at 80 °C for 10 min | Au | 10–30 | Spherical icosahedral | [140] |
| Add 5 mM AgNO3 to 50% diluted extract incubated at 80 °C for 50 min | Ag | 20–50 | Quasi-spherical | |||||||
| Amorphophallus paeoniifolius | Tuber | Phenolic compounds (flavonoid–quercetin) | - | FTIR | Water | Mix 0.8 mM HAuCl4·3H2O with extract (1:4) incubated at falcon tube for 1 h | Au | 13.3 | Spherical, polygonal | [57] |
| Mix 0.01 mM AgNO3 with extract (1:1) incubated at falcon tube and exposed to sunlight for 2–3 min | Ag | 22.48 | ||||||||
| Parkia roxburghii | Leaf | Proteins | - | FTIR | Water | Mix 1 g leaf powder with 100 mL 1 mM HAuCl4/AgNO3 aqueous solution stirred at room temperature for 12 h | Au | 5–25 | Spherical | [72] |
| Ag | 5–25 | Quasi-spherical | ||||||||
| Murraya koenigii | Leaf | Polyphenols, flavonoids, alkaloids | FTIR | Water | Mix 5 mL extract with 25 mL 1 mM HAuCl4 aqueous solution stirred vigorously at 300 K for 2 min | Au | 20 | Spherical, triangle | [160] | |
| Mix 15 mL extract with 100 mL 1 mM AgNO3 boiling aqueous solution, boiling continued for 1 min | Ag | 10 | Spherical | |||||||
| Mentha longifolia | Leaf | Phenolic flavonoids, tannins, saponins and monoterpenes, alkaloids | - | Phytochemical screening, FTIR | Methanol | Mix 1 mM HAuCl4/AgNO3 with extract (1:1) stirred at 70 °C | Au | 10.23 ± 2 | Oval | [73] |
| Ag | 13.45 ± 2 | |||||||||
| Anacardium occidentale | Leaf | Proteins | - | FTIR | Water | Mix 12 mg leaf powder with 30 mL 0.59 mM HAuCl4 stirred for 1 min and filtered | Au | 17 | Spherical | [155] |
| Polyols, gallic acid, water soluble tannins | - | Mix 5 mg leaf powder with 30 mL 0.59 mM AgNO3 stirred for 1 min and filtered | Ag | 15.5 | ||||||
| Prunus serrulata | Fruit | Phenolic compounds | - | FTIR | Water | Add HAuCl4·3H2O to 30 mL 1:9 diluted extract (final concentration 1 mM) incubated at 80 °C for 30 s | Au | 20–50 | Hexagonal | [112] |
| Proteins | - | Add AgNO3to 30 mL 1:9 diluted extract (final concentration 1 mM) incubated at 80 °C for 50 min | Ag | 20–100 | Spherical | |||||
| Zingiber officinale | Root | Ascorbic acid, oxalic acid | - | FTIR | Water | Mix 5 mL extract with 1 mM HAuCl4/AgNO3 and add 45 mL Ultrapure water at pH alkaline, incubated for 10 h | Au | 5–20 | Spherical, irregular shape (hexagonal, triangular, truncated triangular) | [74] |
| Ag | 10–20 | Spherical | ||||||||
| Indigofera tinctoria | Leaf | Phenolic compounds, tannins, alkaloids, saponins, flavonoids, amino acids, carbohydrates, glycosides, steroids | - | FTIR | Water | Add 10 mL extract to 90 mL 1 mM HAuCl4 irradiated at microwave 800 w and 2.45 GHz for 30 s | Au | 6–29 | Spherical, hexagonal, triangular | [75] |
| Add 10 mL extract to 90 mL 1 mM AgNO3 irradiated at microwave 800 w and 2.45 GHz for 60 s | Ag | 9–26 | Spherical | |||||||
| Bauhinia purpurea | Leaf | Polyphenols | - | FTIR | Water | Add extract to 1 mM HAuCl4 (1:10) irradiated at microwave 800 w and 2.45 GHz for 30 s | Au | 20–100 | Triangular, hexagonal, nanorods | [76] |
| Add extract to 1 mM AgNO3 (1:10) irradiated at microwave 800 w and 2.45 GHz for 60 s | Ag | 20–100 | Spherical | |||||||
| Cibotium barometz | Root | Flavonoids, phenolic acids, fatty acid | - | FTIR | Water | Add HAuCl4·3H2O to 5 mL extract diluted with 25 aquadest (final concentration 1 mM), incubated at 80 °C for 50 min | Au | 5–20 | Spherical | [77] |
| Add AgNO3 to to 5 mL extract diluted with 25 aquadest (final concentration 1 mM), incubated at 80 °C for 1 h | Ag | 5–40 | ||||||||
| Jasminum sambac | Leaf | Polyphenols, flavonoids, terpenoids | - | FTIR | Water | Add 10 mL extract to 50 mL 1 mM HAuCl4 irradiated at microwave 700 w and 2.45 GHz for 90 s | Au | 20–0 | Spherical | [78] |
| Add 10 mL extract to 50 mL 1 mM AgNO3 irradiated at microwave 700 w and 2.45 GHz for 3 min | Ag |
| Plant | Type of NP | Optimum Quantity (OQ) | Conditions | Ref. | |
|---|---|---|---|---|---|
| Before Reaching OQ | After Reaching OQ | ||||
| Anacardium occidentale leaf | Au | 12 mg | ↑ Quantity (5–12 mg) causing ↓ λmax = ↓ Particle size | Not measured | [155] |
| Ag | 5 mg | ↑ Quantity (2–5 mg) causing ↓ λmax = ↓ Particle size | ↑ Quantity (10 mg) causing ↑ λmax = ↑ Particle size | ||
| Panax ginseng fruit | Au, Ag | 5% | ↑ Quantity (1–5%) causing ↓ λmax = ↓ Particle size | ↑ Quantity (6–8%) causing ↑ λmax = ↑ Particle size | [61] |
| Siberian ginseng (Eleutherococcus senticosus) stem | Au, Ag | Proportion metal ions to extract 1:1 | Not measured | ↑ Quantity (1:2, 1:3) causing ↑ λmax = ↑ Particle size | [39] |
| Fenugreek (Trigonella foenum-graecum) seed | Au | 3 mL | ↑ Quantity (0.5–3 mL) causing ↓ λmax = ↓ Particle size | Not measured | [165] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuhrotun, A.; Oktaviani, D.J.; Hasanah, A.N. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules 2023, 28, 3240. https://doi.org/10.3390/molecules28073240
Zuhrotun A, Oktaviani DJ, Hasanah AN. Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules. 2023; 28(7):3240. https://doi.org/10.3390/molecules28073240
Chicago/Turabian StyleZuhrotun, Ade, Dede Jihan Oktaviani, and Aliya Nur Hasanah. 2023. "Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds" Molecules 28, no. 7: 3240. https://doi.org/10.3390/molecules28073240
APA StyleZuhrotun, A., Oktaviani, D. J., & Hasanah, A. N. (2023). Biosynthesis of Gold and Silver Nanoparticles Using Phytochemical Compounds. Molecules, 28(7), 3240. https://doi.org/10.3390/molecules28073240