Hydrogel-Film-Fabricated Fluorescent Biosensors with Aggregation-Induced Emission for Albumin Detection through the Real-Time Modulation of a Vortex Fluidic Device
Abstract
:1. Introduction
2. Results and Discussion
2.1. Kinetics of Fluorescent Response and VFD Modulation
2.2. Potential Mechanism
2.2.1. Diffusion Characterisation
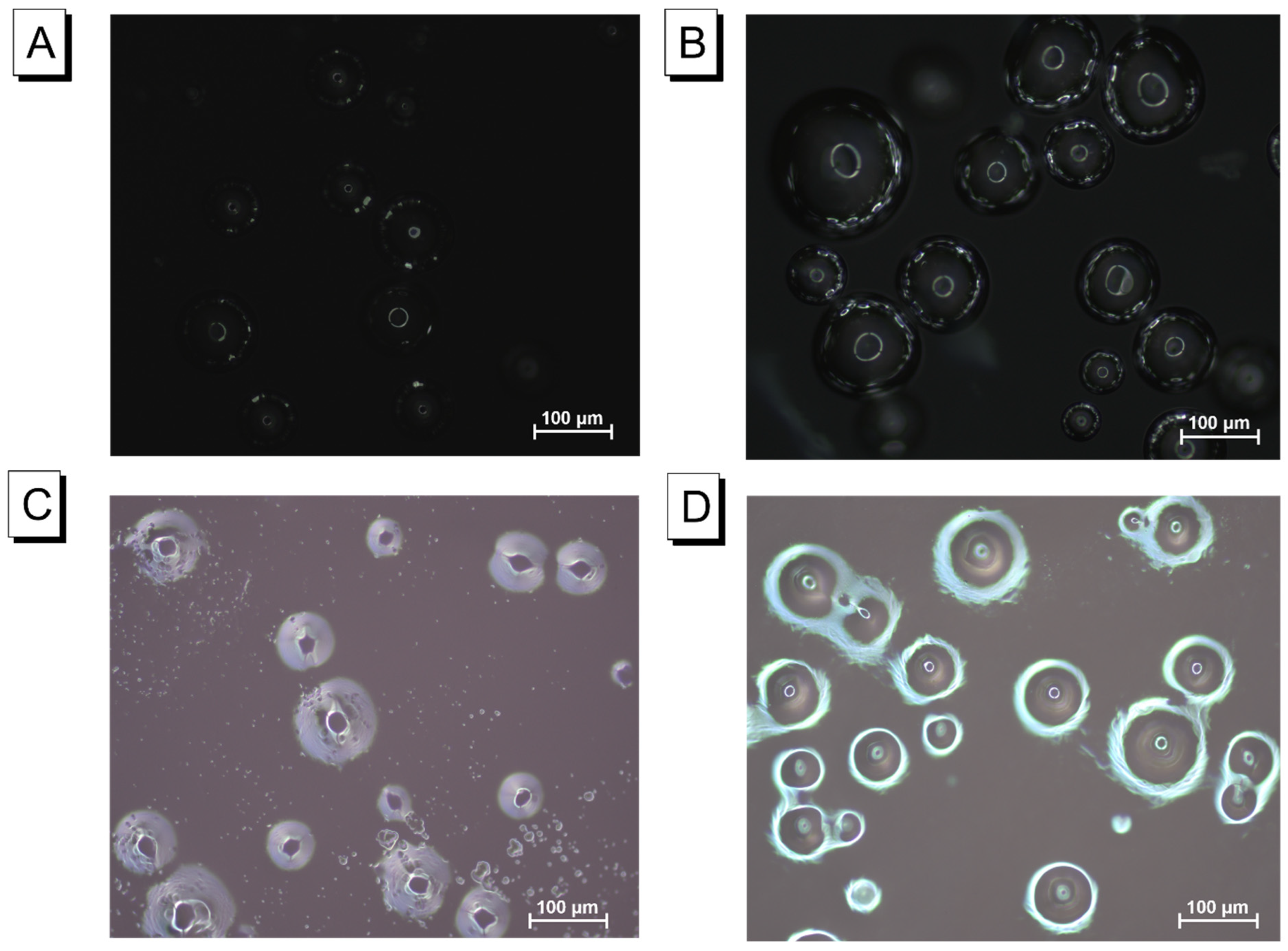
2.2.2. Microstructural Analysis
2.2.3. Motion Analysis
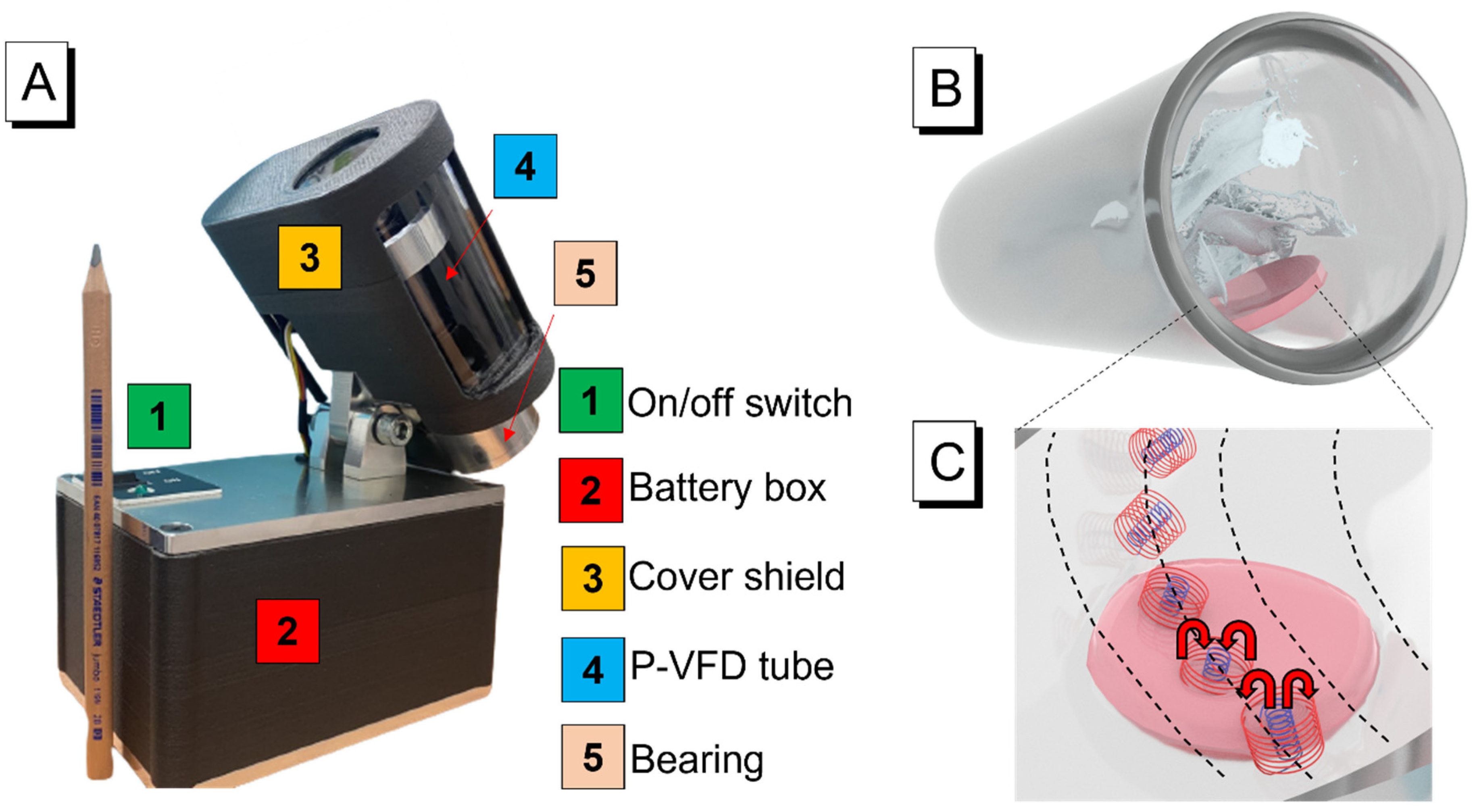
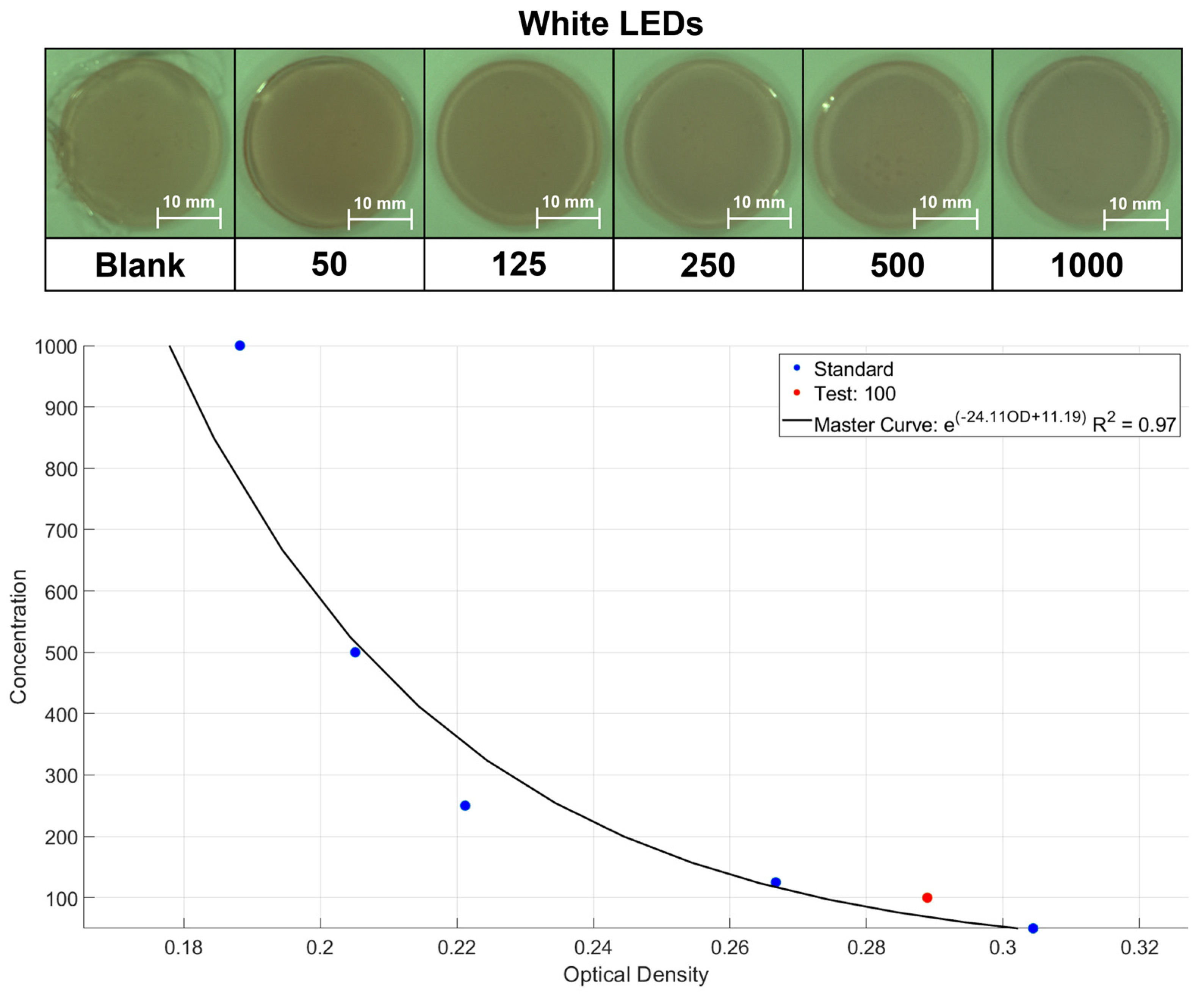
2.3. Application of Portable Device Based on Colorimetry
3. Experiments
3.1. Materials
3.1.1. HSA and TC426
3.1.2. AAm–Alg–Ca2+ Hydrogel Film
3.1.3. Carrageenan Hydrogel Film
3.2. Characterisations
3.3. Methods
3.3.1. Preparation of HSA and TC426 Solution
3.3.2. Hydrogel Fabrication of AAm–Alg–TC426
3.3.3. Hydrogel Fabrication of Carrageenan–TC426
3.3.4. VFD Operation
3.3.5. Protocols for TC426 + AAm–Alg/Carrageenan Hydrogels
3.3.6. Kinetics of Hydrogel Swelling
3.3.7. Fraction of Swelling Power
3.3.8. Transport Determination
3.3.9. Diffusion Coefficient
3.3.10. Dynamic Loss
3.3.11. Limit of Detection
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholson, J.; Wolmarans, M.; Park, G. The role of albumin in critical illness. Br. J. Anaesth. 2000, 85, 599–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Ding, D.; Tang, Y. Inorganic–organic hybrid materials to detect urinary biomarkers: Recent progress and future prospects. Mater. Chem. Front. 2022, 6, 2011–2033. [Google Scholar] [CrossRef]
- Fanali, G.; di Masi, A.; Trezza, V.; Marino, M.; Fasano, M.; Ascenzi, P. Human serum albumin: From bench to bedside. Mol. Asp. Med. 2012, 33, 209–290. [Google Scholar] [CrossRef] [PubMed]
- Prakash, S. Role of Human Serum Albumin and Oxidative Stress in Diabetes. J. Appl. Biotechnol. Bioeng. 2017, 3, 57. [Google Scholar] [CrossRef] [Green Version]
- Levitt, D.; Levitt, M. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, ume 9, 229–255. [Google Scholar] [CrossRef] [Green Version]
- Lee, P.; Wu, X. Modifications of human serum albumin and their binding effect. Curr. Pharm. Des. 2015, 21, 1862–1865. [Google Scholar] [CrossRef] [Green Version]
- Tong, J.; Hu, T.; Qin, A.; Sun, J.Z.; Tang, B.Z. Deciphering the binding behaviours of BSA using ionic AIE-active fluorescent probes. Faraday Discuss. 2016, 196, 285–303. [Google Scholar] [CrossRef]
- Kragh-Hansen, U. Human Serum Albumin: A Multifunctional Protein. In Albumin in Medicine; Springer: Singapore, 2016; pp. 1–24. [Google Scholar]
- Xu, J.-F.; Yang, Y.-S.; Jiang, A.-Q.; Zhu, H.-L. Detection Methods and Research Progress of Human Serum Albumin. Crit. Rev. Anal. Chem. 2020, 52, 72–92. [Google Scholar] [CrossRef]
- Comper, W.D.; Osicka, T.M. Detection of urinary albumin. Adv. Chronic Kidney Dis. 2005, 12, 170–176. [Google Scholar] [CrossRef]
- Martin, H. Laboratory measurement of urine albumin and urine total protein in screening for proteinuria in chronic kidney disease. Clin. Biochem. Rev. 2011, 32, 97–102. [Google Scholar]
- Gaitonde, D.Y.; Cook, D.L.; Rivera, I.M. Chronic Kidney Disease: Detection and Evaluation. Am. Fam. Physician 2017, 96, 776–783. [Google Scholar] [PubMed]
- Hong, Y.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Phenomenon, mechanism and applications. Chem. Commun. 2009, 29, 4332–4353. [Google Scholar] [CrossRef]
- Li, H.; Kim, H.; Han, J.; Nguyen, V.N.; Peng, X.; Yoon, J. Activity-based smart AIEgens for detection, bioimaging, and therapeutics: Recent progress and outlook. Aggregate 2021, 2, e51. [Google Scholar] [CrossRef]
- He, X.; Xie, H.; Hu, L.; Liu, P.; Xu, C.; He, W.; Du, W.; Zhang, S.; Xing, H.; Liu, X.; et al. A versatile AIE fluorogen with selective reactivity to primary amines for monitoring amination, protein labeling, and mitochondrial staining. Aggregate 2023, 4, e239. [Google Scholar] [CrossRef]
- Tang, Y.; Tang, B.Z. Principles and Applications of Aggregation-Induced Emission; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Klymchenko, A.S. Solvatochromic and Fluorogenic Dyes as Environment-Sensitive Probes: Design and Biological Applications. Accounts Chem. Res. 2017, 50, 366–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hong, Y.; Feng, C.; Yu, Y.; Liu, J.; Lam, J.W.Y.; Luo, K.Q.; Tang, B.Z. Quantitation, Visualization, and Monitoring of Conformational Transitions of Human Serum Albumin by a Tetraphenylethene Derivative with Aggregation-Induced Emission Characteristics. Anal. Chem. 2010, 82, 7035–7043. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wei, G.; Zhang, S.; Zheng, B.; Xu, J.; Chen, G.; Li, M.; Song, S.; Fu, W.; Xiao, Z. Albumin tailoring fluorescence and photothermal conversion effect of near-infrared-II fluorophore with aggregation-induced emission characteristics. Nat. Commun. 2019, 10, 2206. [Google Scholar] [CrossRef] [Green Version]
- Liang, R.; Das, D.; Bakhtiiari, A. Protein confinement fine-tunes aggregation-induced emission in human serum albumin. Phys. Chem. Chem. Phys. 2021, 23, 26263–26272. [Google Scholar] [CrossRef]
- Tu, Y.; Yu, Y.; Zhou, Z.; Xie, S.; Yao, B.; Guan, S.; Situ, B.; Liu, Y.; Kwok, R.T.K.; Lam, J.W.Y.; et al. Specific and Quantitative Detection of Albumin in Biological Fluids by Tetrazolate-Functionalized Water-Soluble AIEgens. ACS Appl. Mater. Interfaces 2019, 11, 29619–29629. [Google Scholar] [CrossRef]
- Hu, Q.; Yao, B.; Owyong, T.C.; Prashanth, S.; Wang, C.; Zhang, X.; Wong, W.W.; Tang, Y.; Hong, Y. Detection of Urinary Albumin Using a “Turn-on” Fluorescent Probe with Aggregation-Induced Emission Characteristics. Chem. Asian J. 2021, 16, 1245–1252. [Google Scholar] [CrossRef]
- HofHoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Aswathy, S.; Narendrakumar, U.; Manjubala, I. Commercial hydrogels for biomedical applications. Heliyon 2020, 6, e03719. [Google Scholar] [CrossRef] [PubMed]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Ji, C.; Lü, B.; Rodin, M.; Paradies, J.H.H.; Yin, M.; Kuckling, D. Dually Crosslinked Supramolecular Hydrogel for Cancer Biomarker Sensing. ACS Appl. Mater. Interfaces 2020, 12, 36873–36881. [Google Scholar] [CrossRef] [PubMed]
- Dreiss, C.A. Hydrogel design strategies for drug delivery. Curr. Opin. Colloid Interface Sci. 2020, 48, 1–17. [Google Scholar] [CrossRef]
- Shang, J.; Le, X.; Zhang, J.; Chen, T.; Theato, P. Trends in polymeric shape memory hydrogels and hydrogel actuators. Polym. Chem. 2019, 10, 1036–1055. [Google Scholar] [CrossRef] [Green Version]
- Sun, J.-Y.; Zhao, X.; Illeperuma, W.R.K.; Chaudhuri, O.; Oh, K.H.; Mooney, D.J.; Vlassak, J.J.; Suo, Z. Highly stretchable and tough hydrogels. Nature 2012, 489, 133–136. [Google Scholar] [CrossRef] [Green Version]
- Kirsch, P. Carrageenan: A safe additive. Environ. Health Perspect. 2002, 110, A288. [Google Scholar] [CrossRef] [Green Version]
- Mihaila, S.M.; Gaharwar, A.K.; Reis, R.L.; Marques, A.P.; Gomes, M.E.; Khademhosseini, A. Photocrosslinkable Kappa-Carrageenan Hydrogels for Tissue Engineering Applications. Adv. Heal. Mater. 2013, 2, 895–907. [Google Scholar] [CrossRef]
- Li, J.; Wang, J.; Li, H.; Song, N.; Wang, D.; Tang, B.Z. Supramolecular materials based on AIE luminogens (AIEgens): Construction and applications. Chem. Soc. Rev. 2020, 49, 1144–1172. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Xie, S.; Tang, Y.; Zeng, Z.; Tang, B.Z. Hydrogel-derived luminescent scaffolds for biomedical applications. Mater. Chem. Front. 2021, 5, 3524–3548. [Google Scholar] [CrossRef]
- Lu, W.; Wei, S.; Shi, H.; Le, X.; Yin, G.; Chen, T. Progress in aggregation-induced emission-active fluorescent polymeric hydrogels. Aggregate 2021, 2, e37. [Google Scholar]
- Liow, S.S.; Dou, Q.; Kai, D.; Li, Z.; Sugiarto, S.; Yu, C.Y.Y.; Kwok, R.T.K.; Chen, X.; Wu, Y.-L.; Ong, S.T.; et al. Long-Term Real-Time In Vivo Drug Release Monitoring with AIE Thermogelling Polymer. Small 2017, 13, 1603404. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli, J.; Tang, Y. Hydrogel Based Sensors for Biomedical Applications: An Updated Review. Polymers 2017, 9, 364. [Google Scholar] [CrossRef] [Green Version]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar]
- Britton, J.; Dalziel, S.B.; Raston, C.L. The synthesis of di-carboxylate esters using continuous flow vortex fluidics. Green Chem. 2015, 18, 2193–2200. [Google Scholar] [CrossRef] [Green Version]
- Britton, J.; Meneghini, L.M.; Raston, C.L.; Weiss, G.A. Accelerating Enzymatic Catalysis Using Vortex Fluidics. Angew. Chem. Int. Ed. 2016, 55, 11387–11391. [Google Scholar] [CrossRef] [Green Version]
- Yasmin, L.; Chen, X.; Stubbs, K.A.; Raston, C.L. Optimising a vortex fluidic device for controlling chemical reactivity and selectivity. Sci. Rep. 2013, 3, srep02282. [Google Scholar] [CrossRef] [Green Version]
- He, S.; Vimalanathan, K.; Su, P.; Jellicoe, M.; Luo, X.; Xing, W.; Cai, W.; Gibson, C.T.; Chen, Y.; Wong, J.W.C.; et al. Upsized Vortex Fluidic Device Enhancement of Mechanical Properties and the Microstructure of Biomass-Based Biodegradable Films. ACS Sustain. Chem. Eng. 2021, 9, 14588–14595. [Google Scholar] [CrossRef]
- Grzybowski, B.A.; Sobolev, Y.I.; Cybulski, O.; Mikulak-Klucznik, B. Materials, assemblies and reaction systems under rotation. Nat. Rev. Mater. 2022, 7, 338–354. [Google Scholar] [CrossRef]
- Britton, J.; Raston, C.L. Continuous flow vortex fluidic production of biodiesel. RSC Adv. 2014, 4, 49850–49854. [Google Scholar] [CrossRef]
- Sanders, E.C.; Sen, S.R.; Gelston, A.A.; Santos, A.M.; Luo, X.; Bhuvan, K.; Tang, D.Y.; Raston, C.L.; Weiss, G.A. Under-5-Minute Immunoblot Assays by Vortex Fluidic Device Acceleration. Angew. Chem. 2022, 134, e202202021. [Google Scholar]
- Britton, J.; Stubbs, K.A.; Weiss, G.A.; Raston, C.L. Vortex fluidic chemical transformations. Chem. A Eur. J. 2017, 23, 13270–13278. [Google Scholar] [CrossRef]
- Tavakoli, J.; Raston, C.L.; Tang, Y. Tuning Surface Morphology of Fluorescent Hydrogels Using a Vortex Fluidic Device. Molecules 2020, 25, 3445. [Google Scholar] [CrossRef]
- Luo, X.; Mohammed Al-Antaki, A.H.; Igder, A.; Stubbs, K.A.; Su, P.; Zhang, W.; Weiss, G.A.; Raston, C.L. Vortex flu-idic-mediated fabrication of fast gelated silica hydrogels with embedded laccase nanoflowers for real-time biosensing under flow. ACS Appl. Mater. Interfaces 2020, 12, 51999–52007. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.T.T.; Tohl, D.; Wallace, A.; Hu, Q.; Li, J.; Reynolds, K.J.; Tang, Y. Developing a fluorescent sensing based portable medical open-platform—A case study for albuminuria measurement in chronic kidney disease screening and monitoring. Sens. Bio-Sens. Res. 2022, 37, 100504. [Google Scholar] [CrossRef]
- Pham, A.T.T.; Tohl, D.; Hu, Q.; Li, J.; Reynolds, K.J.; Tang, Y. Portable Colorimetric Device with Commercial Microplates for Quantitative Detection of Urine Biomarkers: Design, Development, and Clinical Evaluation. Biosensors 2022, 12, 723. [Google Scholar] [CrossRef] [PubMed]
- Tohl, D.; Teferra, M.N.; Wallace, A.; Pham, A.T.T.; Tang, Y. Re-Referencing and Calibration for Robust Ratiometric Light Intensity Measurement. IEEE Trans. Instrum. Meas. 2022, 71, 100504. [Google Scholar] [CrossRef]
- Yuk, H.; Zhang, T.; Lin, S.; Parada, G.A.; Zhao, X. Tough bonding of hydrogels to diverse non-porous surfaces. Nat. Mater. 2015, 15, 190–196. [Google Scholar] [CrossRef] [Green Version]
- Ganji, F.; Vasheghani, F.S.; Vasheghani, F.E. Theoretical description of hydrogel swelling: A review. Iran. Polym. J. 2010, 19, 375–398. [Google Scholar]
- Karadağ, E.; Saraydın, D. Swelling studies of super water retainer acrylamide/crotonic acid hydrogels crosslinked by trimethylolpropane triacrylate and 1,4-butanediol dimethacrylate. Polym. Bull. 2002, 48, 299–307. [Google Scholar] [CrossRef]
- Ganji, F.; Vasheghani, F.E. Hydrogels in controlled drug delivery systems. Iran. Polym. J. 2009, 18, 63–88. [Google Scholar]
- Elaloufi, R.; Carminati, R.; Greffet, J.-J. Definition of the diffusion coefficient in scattering and absorbing media. J. Opt. Soc. Am. A 2003, 20, 678–685. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Rad, M.; Mata, J.; Mahmodi, H.; Kabakova, I.; Raston, C.; Tang, Y.; Tipper, J.; Tavakoli, J. Hyperbranched polymers tune the physicochemical, mechanical, and biomedical properties of alginate hydrogels. Mater. Today Chem. 2021, 23, 100656. [Google Scholar] [CrossRef]



| Hydrogel Material | Testing Type | Diffusional Exponent (n) | Type of Transport | Behaviour | Diffusion Coefficient (D × 10−10/m2 s−1) |
|---|---|---|---|---|---|
| AAm–Alg | Normal soaking | 0.768 | Non-Fickian diffusion | Rwater penetration > Rpolymer chain relaxation | 3143.6 |
| VFD | 0.917 | 8950.1 | |||
| Carrageenan | Normal soaking | 0.247 | Less-Fickian diffusion | Rwater penetration Rpolymer chain relaxation | 7.187 |
| VFD | 0.403 | 131.632 |
| Transport | Diffusional Exponent (n) | Actual Behaviours |
|---|---|---|
| Less-Fickian | n < 0.45 | Rwater penetration ≪ Rpolymer chain relaxation |
| Fickian | 0.45 < n < 0.5 | Rwater penetration < Rpolymer chain relaxation |
| Non-Fickian | 0.50 < n < 1.0 | Rwater penetration > Rpolymer chain relaxation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Luo, X.; Tohl, D.; Pham, A.T.T.; Raston, C.; Tang, Y. Hydrogel-Film-Fabricated Fluorescent Biosensors with Aggregation-Induced Emission for Albumin Detection through the Real-Time Modulation of a Vortex Fluidic Device. Molecules 2023, 28, 3244. https://doi.org/10.3390/molecules28073244
Hu Q, Luo X, Tohl D, Pham ATT, Raston C, Tang Y. Hydrogel-Film-Fabricated Fluorescent Biosensors with Aggregation-Induced Emission for Albumin Detection through the Real-Time Modulation of a Vortex Fluidic Device. Molecules. 2023; 28(7):3244. https://doi.org/10.3390/molecules28073244
Chicago/Turabian StyleHu, Qi, Xuan Luo, Damian Tohl, Anh Tran Tam Pham, Colin Raston, and Youhong Tang. 2023. "Hydrogel-Film-Fabricated Fluorescent Biosensors with Aggregation-Induced Emission for Albumin Detection through the Real-Time Modulation of a Vortex Fluidic Device" Molecules 28, no. 7: 3244. https://doi.org/10.3390/molecules28073244
APA StyleHu, Q., Luo, X., Tohl, D., Pham, A. T. T., Raston, C., & Tang, Y. (2023). Hydrogel-Film-Fabricated Fluorescent Biosensors with Aggregation-Induced Emission for Albumin Detection through the Real-Time Modulation of a Vortex Fluidic Device. Molecules, 28(7), 3244. https://doi.org/10.3390/molecules28073244









