Diiron Aminocarbyne Complexes with NCE− Ligands (E = O, S, Se)
Abstract
:1. Introduction
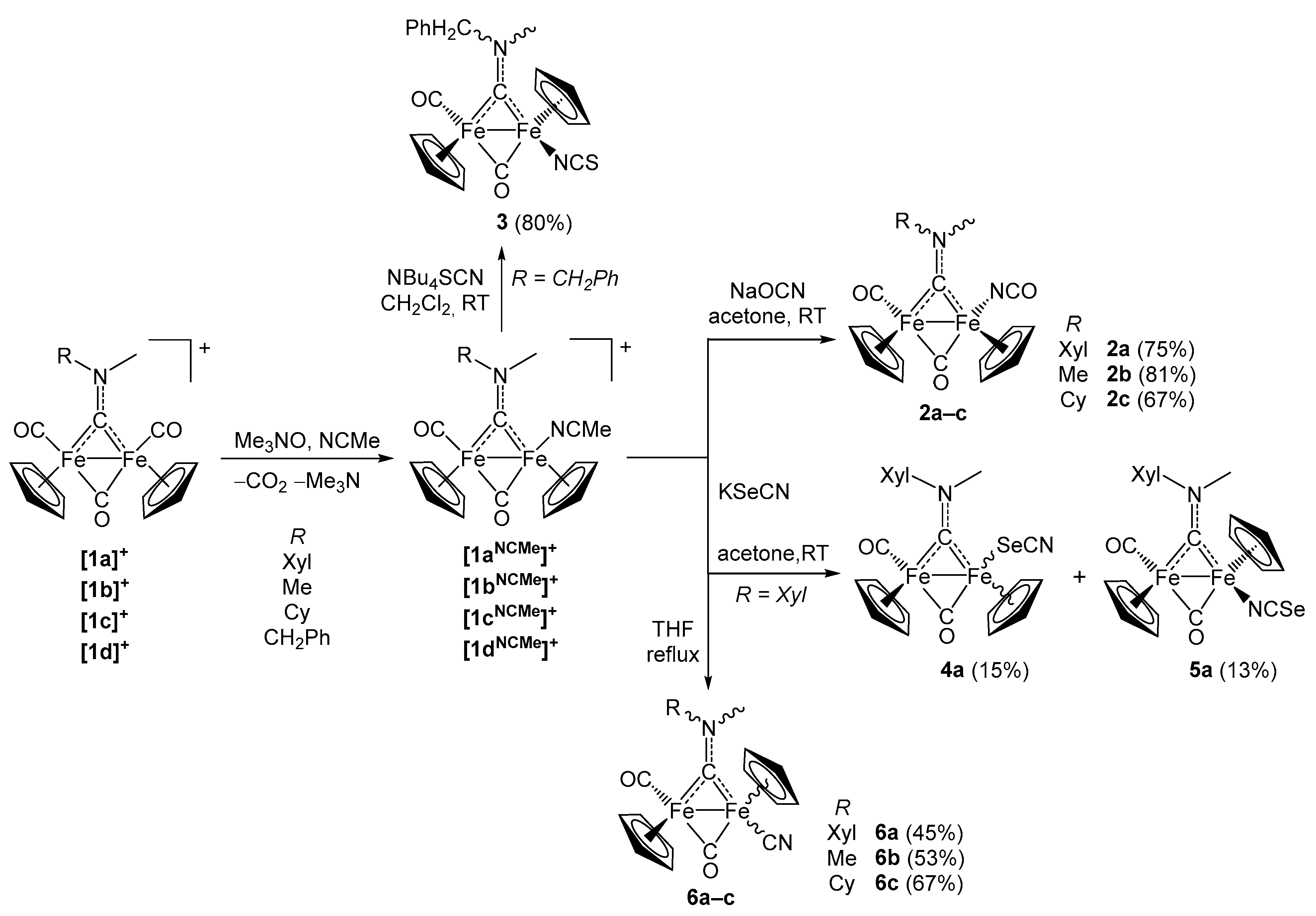
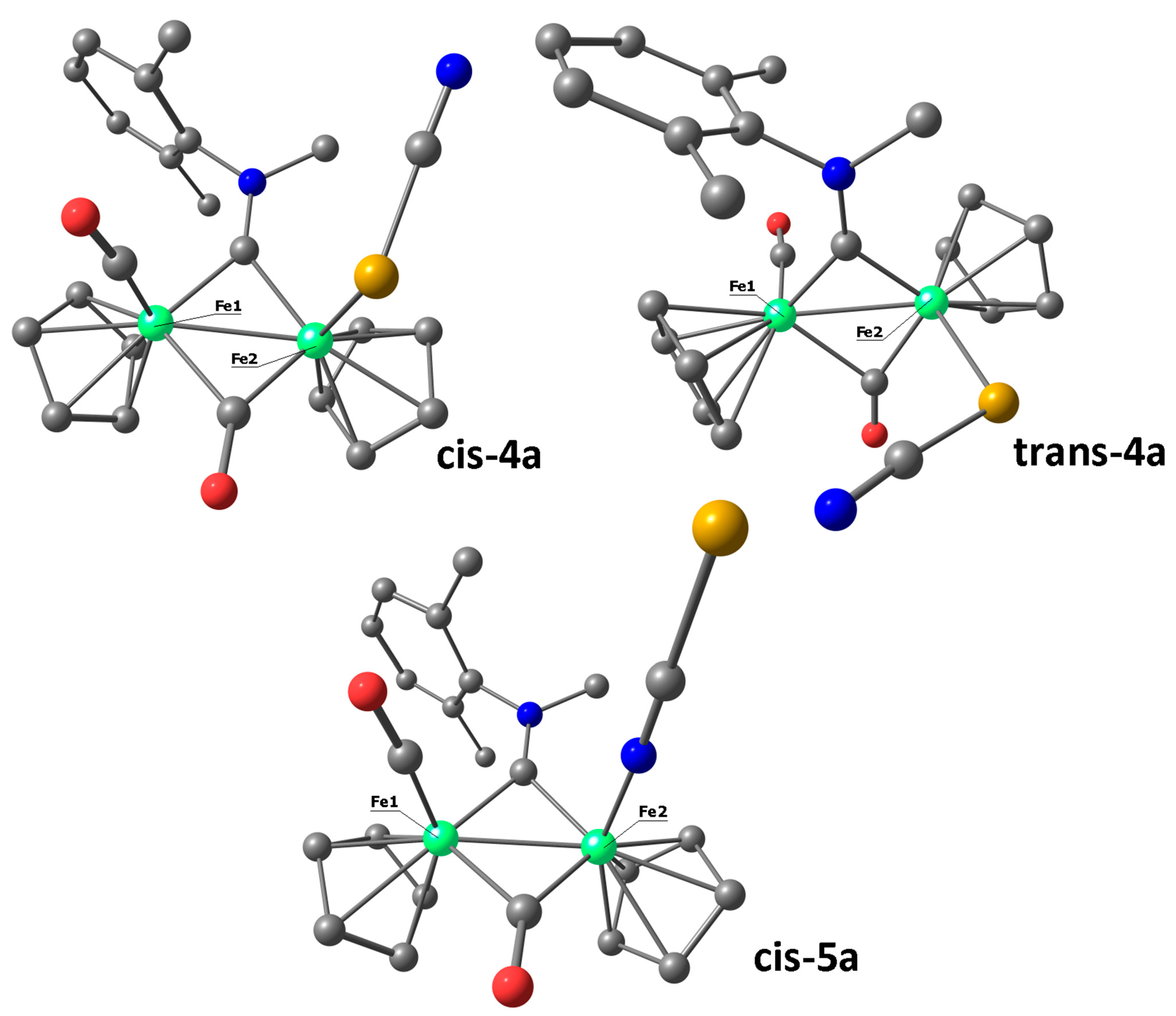
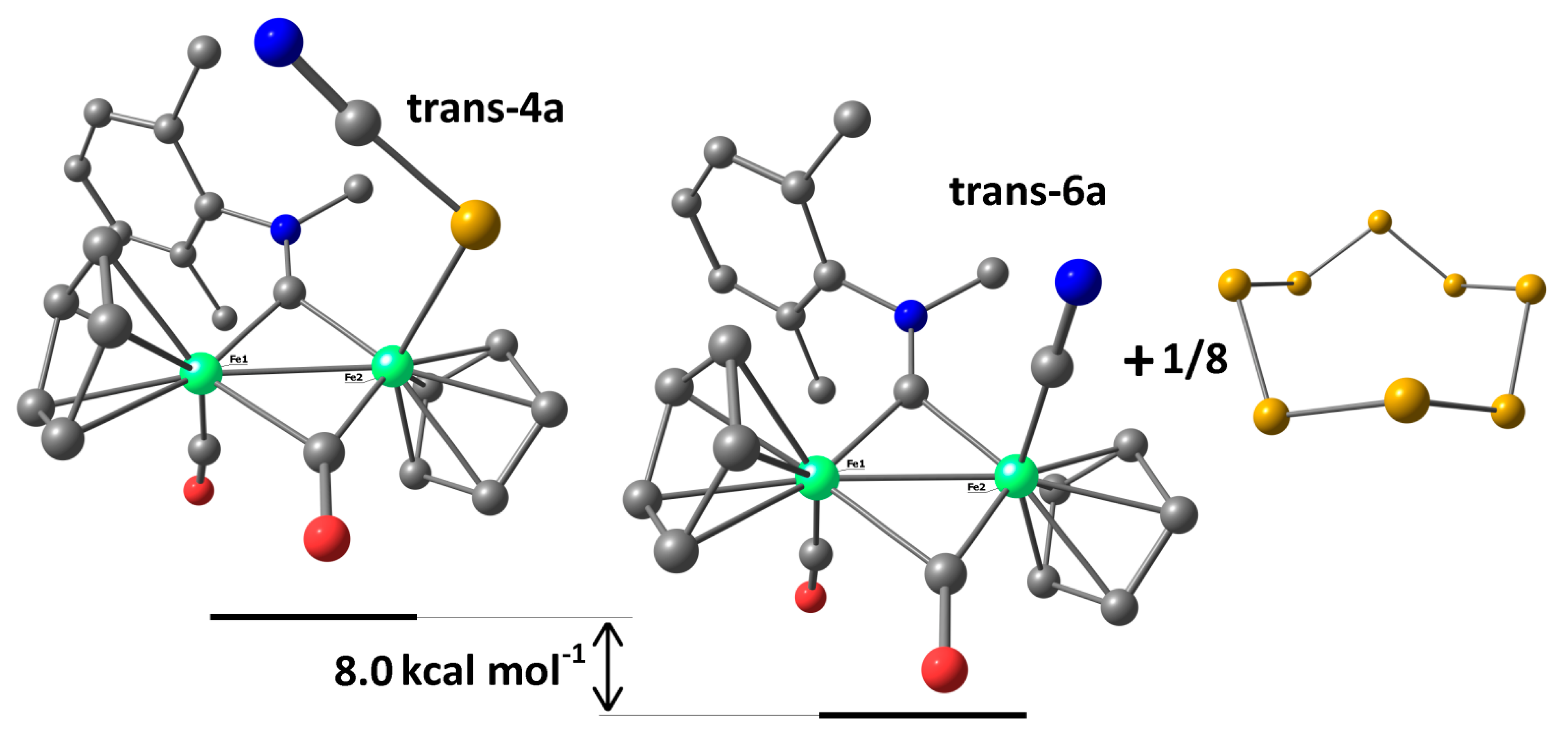
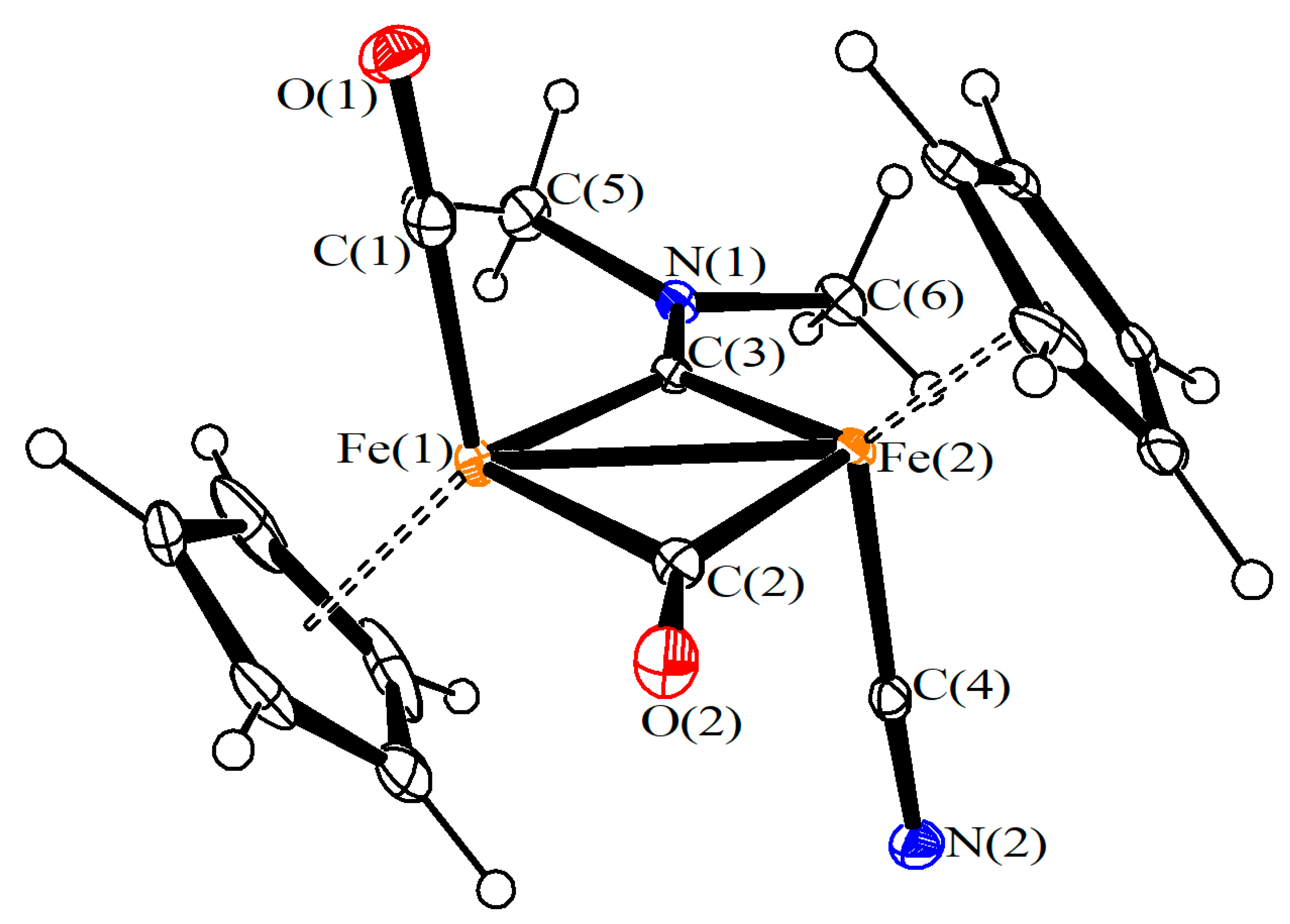
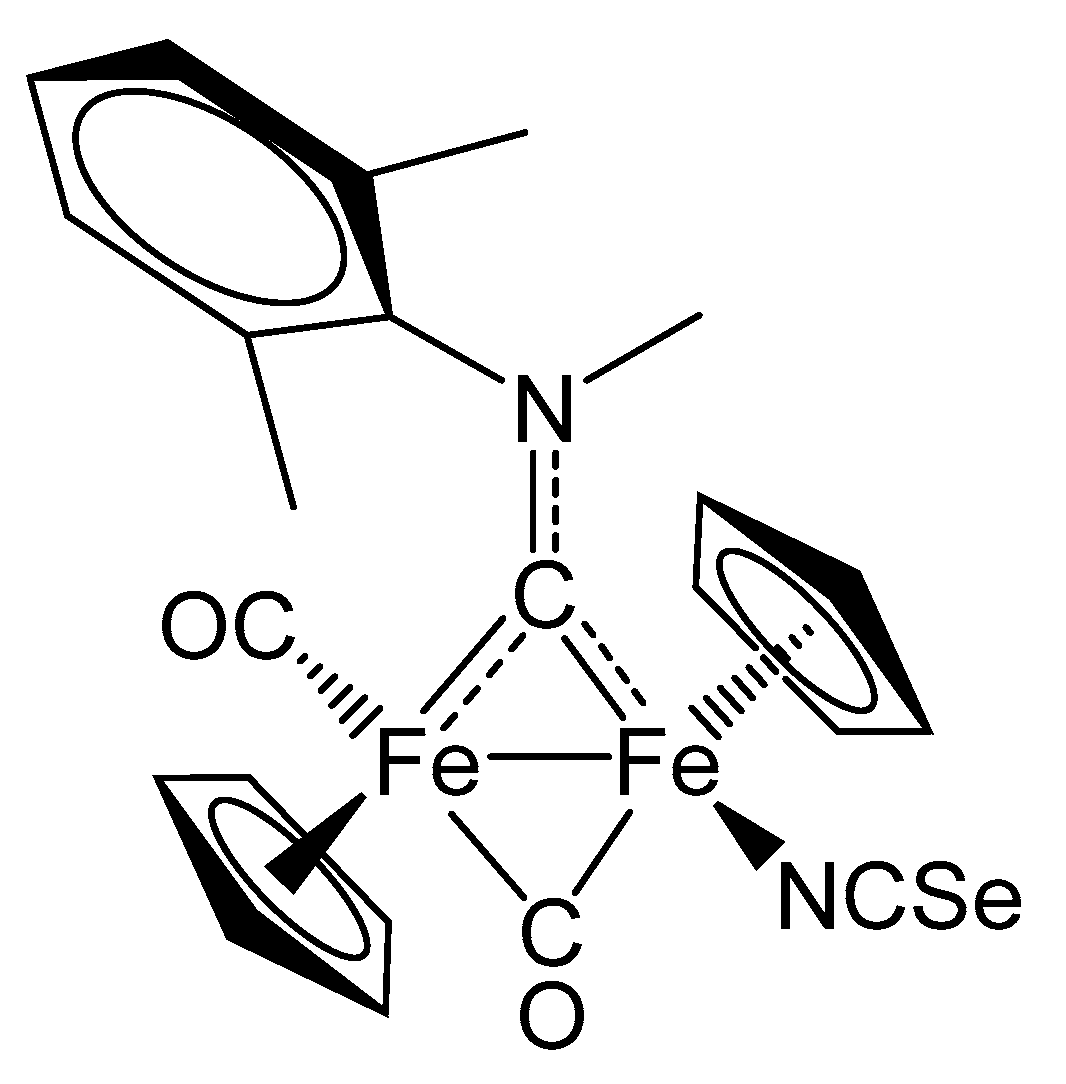
2. Results and Discussion
3. Experimental
3.1. Materials and Methods
3.2. General Procedure
3.3. General Procedure
4. X-ray Crystallography
5. Computational Details
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Burmeister, J.L. Recent Developments in the Coordination Chemistry of Ambidentate Ligands. Coord. Chem. Rev. 1966, 1, 205–221. [Google Scholar] [CrossRef]
- Malvolti, F.; Trujillo, A.; Cador, O.; Gendron, F.; Costuas, K.; Halet, J.-F.; Bondon, A.; Toupet, L.; Molard, Y.; Cordier, S.; et al. New Thiocyanato and Azido Adducts of the Redox-Active Fe(η5-C5Me5)(η2-Dppe) Center: Synthesis and Study of the Fe(II) and Fe(III) Complexes. Inorg. Chim. Acta 2011, 374, 288–301. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Brothers, S.M.; Reibenspies, J.H.; Hall, M.B.; Popescu, C.V.; Darensbourg, M.Y. Ambidentate Thiocyanate and Cyanate Ligands in Dinitrosyl Iron Complexes. Inorg. Chem. 2013, 52, 2119–2124. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.L.; Williams, L.E. Coordination Complexes of the Selenocyanate Ion. Inorg. Chem. 1966, 5, 1113–1117. [Google Scholar] [CrossRef]
- Wang, Y.-P.; Leu, H.-L.; Wang, Y.; Cheng, H.-Y.; Lin, T.-S. Cyclopentadienyl Chromium Complexes with Halide, Methyl, Isothiocyanate and Isoselenocyanate Ligands: Structures of [η5-(C5H4-COOCH3)]Cr(NO)2(Br) and [η5-(C5H4-COOCH3)]Cr(NO)2(NCS). J. Organomet. Chem. 2007, 692, 3340–3350. [Google Scholar] [CrossRef]
- Ribas, J.; Diaz, C.; Solans, X.; Font-Bardía, M. A New One-Dimensional System Starting from a Trinuclear Copper(II) Complex and Selenocyanate as Bridging Ligand. Comparison with the Thiocyanate Analogue. Comparison with the Thiocyanate Analogue. J. Chem. Soc. Dalton Trans. 1997, 35–38. [Google Scholar] [CrossRef]
- Bröring, M.; Prikhodovski, S.; Brandt, C.D.; Cónsul Tejero, E. Pillars, Layers, Pores and Networks from Nickeltripyrrins: A Porphyrin Fragment as a Versatile Building Block for the Construction of Supramolecular Assemblies. Chem. Eur. J. 2007, 13, 396–406. [Google Scholar] [CrossRef]
- Milenković, M.; Bacchi, A.; Cantoni, G.; Vilipić, J.; Sladić, D.; Vujčić, M.; Gligorijević, N.; Jovanović, K.; Radulović, S.; Anđelković, K. Synthesis, Characterization and Biological Activity of Three Square-Planar Complexes of Ni(II) with Ethyl (2E)-2-[2-(Diphenylphosphino)Benzylidene]Hydrazinecarboxylate and Monodentate Pseudohalides. Eur. J. Med. Chem. 2013, 68, 111–120. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, C.; Liu, J.; Zhang, M.; Liu, W.; Li, W.; Wu, C.; Cheng, G.; Yang, Q.; Wei, G.; et al. Phosphorescent [3 + 2 + 1] Coordinated Ir(III) Cyano Complexes for Achieving Efficient Phosphors and Their Application in OLED Devices. Chem. Sci. 2021, 12, 10165–10178. [Google Scholar] [CrossRef]
- Chatterjee, S.; Krause, J.A.; Madduma-Liyanage, K.; Connick, W.B. Platinum(II) Diimine Complexes with Halide/Pseudohalide Ligands and Dangling Trialkylamine or Ammonium Groups. Inorg. Chem. 2012, 51, 4572–4587. [Google Scholar] [CrossRef]
- Murakami, K.; Kitabayashi, A.; Yamauchi, S.; Nishi, K.; Fujinami, T.; Matsumoto, N.; Iijima, S.; Kojima, M. Iron(II) Complexes with a Linear Pentadentate Ligand H2L1=bis(N,N′-2-Methylimidazol-4-Yl-Methylideneaminopropyl)Methylamine and a Monodentate Ligand X (X=N3−, NCS−, NCSe−). Inorg. Chim. Acta 2013, 400, 244–249. [Google Scholar] [CrossRef]
- Barral, M.C.; Herrero, S.; Jiménez-Aparicio, R.; Priego, J.L.; Torres, M.R.; Urbanos, F.A. Activation of Isocyanate Ligands in Ru25+ Complexes. J. Mol. Struct. 2008, 890, 221–226. [Google Scholar] [CrossRef]
- Semproni, S.P.; Chirik, P.J. Activation of Dinitrogen-Derived Hafnium Nitrides for Nucleophilic N-C Bond Formation with a Terminal Isocyanate. Angew. Chem. Int. Ed. 2013, 52, 12965–12969. [Google Scholar] [CrossRef]
- Berndt, A.F.; Barnett, K.W. The Crystal and Molecular Structure of Cyclopentadienyliron Dicarbonyl Isothiocyanate. J. Organomet. Chem. 1980, 184, 211–219. [Google Scholar] [CrossRef]
- Poh, H.T.; Ho, P.C.; Fan, W.Y. Cyclopentadienyl Iron Dicarbonyl (CpFe(CO)2) Derivatives as Apoptosis-Inducing Agents. RSC Adv. 2016, 6, 18814–18823. [Google Scholar] [CrossRef]
- Johnson, K.A.; Lim, J.C.; Burmeister, J.L. Rates and Mechanisms of Substitution Reactions of Palladium(II) Thiocyanate and Selenocyanate Linkage Isomers. Inorg. Chem. 1973, 12, 124–128. [Google Scholar] [CrossRef]
- Chakravarty, B.; Adhikari, S. Formation of Linkage Isomers via the Substitution of Halides by Selenocyanate in Ruthenium(III) Complexes. Transit. Met. Chem. 1991, 16, 583–585. [Google Scholar] [CrossRef]
- Mochida, T.; Maekawa, S.; Sumitani, R. Photoinduced and Thermal Linkage Isomerizations of an Organometallic Ionic Liquid Containing a Half-Sandwich Ruthenium Thiocyanate Complex. Inorg. Chem. 2021, 60, 12386–12391. [Google Scholar] [CrossRef]
- Mahendrasinh, Z.; Suresh, E.; Kumar, S.B. Isothiocyanate and Selenocyanate Complexes of Cu(II), Ni(II), and Co(II) with a Pyridylpyrazole-Based Ligand: Synthesis, Characterization, and Structure. J. Coord. Chem. 2011, 64, 483–490. [Google Scholar] [CrossRef]
- Boeckmann, J.; Wriedt, M.; Näther, C. Metamagnetism and Single-Chain Magnetic Behavior in a Homospin One-Dimensional Iron(II) Coordination Polymer. Chem. Eur. J. 2012, 18, 5284–5289. [Google Scholar] [CrossRef]
- Mizoguchi, T.J.; Lippard, S.J. (μ-Oxo)Bis(μ-Carboxylato)Bis(2,2′-Bipyridyl)Bis(X)Diiron(III) Complexes, X = NCS−, NCSe−, and N3−: Synthetic Models of Pseudohalide Derivatives of Carboxylate-Bridged Diiron Proteins. Inorg. Chem. 1997, 36, 4526–4533. [Google Scholar] [CrossRef] [PubMed]
- Abibat Salaudeen, A.; Kilner, C.A.; Halcrow, M.A. Mononuclear and Dinuclear Iron Thiocyanate and Selenocyanate Complexes of Tris-Pyrazolylmethane Ligands. Polyhedron 2008, 27, 2569–2576. [Google Scholar] [CrossRef]
- Manna, S.C.; Mistri, S.; Zangrando, E. Synthesis, Crystal Structure, Solid State Electronic Spectra and Thermal Study of Three Cobalt(II)–Selenocyanate Complexes: In Situ Room Temperature Transformation of 4,4′-Dipyridyldisulfide to 4,4′-Dipyridylsulfide. Inorg. Chim. Acta 2014, 413, 166–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Zhang, Y.; Wang, J.; Hu, L. KSeCN as an Efficient Cyanide Source for the One-Step Synthesis of Imino-1-Oxoisoindolines via Copper-Promoted C–H Activation. Tetrahedron Lett. 2021, 72, 153062. [Google Scholar] [CrossRef]
- Kabešová, M.; Pirskij, J.; Dunaj-Jurčo, M. Thermal Properties of Thio-and Selenocyanatocopper(II) Complexes with Bipyridine and Phenanthroline. J. Therm. Anal. 1988, 34, 1349–1358. [Google Scholar] [CrossRef]
- Mochizuki, R.; Higashi, K.; Okamoto, Y.; Abe, H.; Iwase, H.; Toida, T. Detection of Selenocyanate in Biological Samples by HPLC with Fluorescence Detection Using König Reaction. Chem. Pharm. Bull. 2019, 67, 884–887. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Huang, X.-B.; Zhou, Y.-B.; Liu, M.-C.; Wu, H.-Y. Metal-Free Synthesis of Aryl Selenocyanates and Selenaheterocycles with Elemental Selenium. Chem. Eur. J. 2021, 27, 944–948. [Google Scholar] [CrossRef]
- Lu, L.-G.; Bi, K.; Huang, X.-B.; Liu, M.-C.; Zhou, Y.-B.; Wu, H.-Y. Catalyst and Additive-Free Selective Ring-Opening Selenocyanation of Heterocycles with Elemental Selenium and TMSCN. Adv. Synth. Catal. 2021, 363, 1346–1351. [Google Scholar] [CrossRef]
- Mazzoni, R.; Salmi, M.; Zanotti, V. C-C Bond Formation in Diiron Complexes. Chem. Eur. J. 2012, 18, 10174–10194. [Google Scholar] [CrossRef]
- Van Beek, C.B.; Van Leest, N.P.; Lutz, M.; De Vos, S.D.; Gebbink, R.J.K.; De Bruin, B.; Broere, D.L. Combining Metal–Metal Cooperativity, Metal–Ligand Cooperativity and Chemical Non-Innocence in Diiron Carbonyl Complexes. Chem. Sci. 2022, 13, 2094–2104. [Google Scholar] [CrossRef]
- Arnett, C.H.; Agapie, T. Activation of an Open Shell, Carbyne-Bridged Diiron Complex Toward Binding of Dinitrogen. J. Am. Chem. Soc. 2020, 142, 10059–10068. [Google Scholar] [CrossRef]
- Fischer, S.; Rösel, A.; Kammer, A.; Barsch, E.; Schoch, R.; Junge, H.; Bauer, M.; Beller, M.; Ludwig, R. Diferrate [Fe2(CO)6(μ-CO){μ-P(Aryl)2}]− as Self-Assembling Iron/Phosphor-Based Catalyst for the Hydrogen Evolution Reaction in Photocatalytic Proton Reduction-Spectroscopic Insights. Chem. Eur. J. 2018, 24, 16052–16065. [Google Scholar] [CrossRef]
- Wenger, O.S. Is Iron the New Ruthenium? Chem. Eur. J. 2019, 25, 6043–6052. [Google Scholar] [CrossRef] [Green Version]
- Bisz, E.; Szostak, M. Iron-Catalyzed C−O Bond Activation: Opportunity for Sustainable Catalysis. ChemSusChem 2017, 10, 3964–3981. [Google Scholar] [CrossRef]
- Enthaler, S.; Junge, K.; Beller, M. Sustainable Metal Catalysis with Iron: From Rust to a Rising Star? Angew. Chem. Int. Ed. 2008, 47, 3317–3321. [Google Scholar] [CrossRef]
- Biancalana, L.; Marchetti, F. Aminocarbyne Ligands in Organometallic Chemistry. Coord. Chem. Rev. 2021, 449, 214203. [Google Scholar] [CrossRef]
- Marchetti, F. Constructing Organometallic Architectures from Aminoalkylidyne Diiron Complexes. Eur. J. Inorg. Chem. 2018, 2018, 3987–4003. [Google Scholar] [CrossRef] [Green Version]
- Bresciani, G.; Schoch, S.; Biancalana, L.; Zacchini, S.; Bortoluzzi, M.; Pampaloni, G.; Marchetti, F. Cyanide–Alkene Competition in a Diiron Complex and Isolation of a Multisite (Cyano)Alkylidene–Alkene Species. Dalton Trans. 2022, 51, 1936–1945. [Google Scholar] [CrossRef]
- Busetto, L.; Marchetti, F.; Zacchini, S.; Zanotti, V. Diiron and Diruthenium Aminocarbyne Complexes Containing Pseudohalides: Stereochemistry and Reactivity. Inorg. Chim. Acta 2005, 358, 1204–1216. [Google Scholar] [CrossRef]
- Bresciani, G.; Biancalana, L.; Pampaloni, G.; Zacchini, S.; Ciancaleoni, G.; Marchetti, F. A Comprehensive Analysis of the Metal–Nitrile Bonding in an Organo-Diiron System. Molecules 2021, 26, 7088. [Google Scholar] [CrossRef]
- Luh, T.-Y. Trimethylamine N-Oxide a Versatile Reagent for Organometallic Chemistry. Coord. Chem. Rev. 1984, 60, 255–276. [Google Scholar] [CrossRef]
- Bresciani, G.; Boni, S.; Zacchini, S.; Pampaloni, G.; Bortoluzzi, M.; Marchetti, F. Alkyne–Alkenyl Coupling at a Diruthenium Complex. Dalton Trans. 2022, 51, 15703–15715. [Google Scholar] [CrossRef] [PubMed]
- Bresciani, G.; Zacchini, S.; Pampaloni, G.; Marchetti, F. Carbon–Carbon Bond Coupling of Vinyl Molecules with an Allenyl Ligand at a Diruthenium Complex. Organometallics 2022, 41, 1006–1014. [Google Scholar] [CrossRef]
- Bresciani, G.; Zacchini, S.; Pampaloni, G.; Bortoluzzi, M.; Marchetti, F. η6-Coordinated Ruthenabenzenes from Three-Component Assembly on a Diruthenium μ-Allenyl Scaffold. Dalton Trans. 2022, 51, 8390–8400. [Google Scholar] [CrossRef]
- Scepaniak, J.J.; Bontchev, R.P.; Johnson, D.L.; Smith, J.M. Snapshots of Complete Nitrogen Atom Transfer from an Iron(IV) Nitrido Complex. Angew. Chem. 2011, 123, 6760–6763. [Google Scholar] [CrossRef]
- Lichtenberg, C.; Prokopchuk, D.E.; Adelhardt, M.; Viciu, L.; Meyer, K.; Grützmacher, H. Reactivity of an All-Ferrous Iron–Nitrogen Heterocubane under Reductive and Oxidative Conditions. Chem. Eur. J. 2015, 21, 15797–15805. [Google Scholar] [CrossRef]
- Reiners, M.; Maekawa, M.; Daniliuc, C.G.; Freytag, M.; Jones, P.G.; White, P.S.; Hohenberger, J.; Sutter, J.; Meyer, K.; Maron, L.; et al. Reactivity Studies on [Cp′Fe(μ-I)]2: Nitrido-, Sulfido- and Diselenide Iron Complexes Derived from Pseudohalide Activation. Chem. Sci. 2017, 8, 4108–4122. [Google Scholar] [CrossRef] [Green Version]
- Clough, C.R.; Müller, P.; Cummins, C.C. 6-Coordinate Tungsten(VI) Tris-n-Isopropylanilide Complexes: Products of Terminal Oxo and Nitrido Transformations Effected by Main Group Electrophiles. Dalton Trans. 2008, 4458–4463. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Armentano, D.; De Munno, G.; Lloret, F.; Julve, M.; Faus, J. Rhenium(IV) Cyanate Complexes: Synthesis, Crystal Structures and Magnetic Properties of NBu4[ReBr4(OCN)(DMF)] and (NBu4)2[ReBr(OCN)2(NCO)3]. Inorg. Chim. Acta 2006, 359, 4343–4349. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Bresciani, G.; Marchetti, F.; Pampaloni, G.; Zacchini, S. MoCl5 as an Effective Chlorinating Agent towards α-Amino Acids: Synthesis of α-Ammonium-Acylchloride Salts and α-Amino-Acylchloride Complexes. Dalton Trans. 2015, 44, 10030–10037. [Google Scholar] [CrossRef]
- Renili, F.; Marchetti, F.; Zacchini, S.; Zanotti, V. Assembly and Incorporation of a CO2Me Group into a Bridging Vinyliminium Ligand in a Diiron Complex. J. Organomet. Chem. 2011, 696, 1483–1486. [Google Scholar] [CrossRef]
- Busetto, L.; Marchetti, F.; Zacchini, S.; Zanotti, V. Acetylide Addition to Bridging Vinyliminium Ligands in Dinuclear Complexes. Eur. J. Inorg. Chem. 2007, 2007, 1799–1807. [Google Scholar] [CrossRef]
- Zhou, X.; Barton, B.E.; Chambers, G.M.; Rauchfuss, T.B.; Arrigoni, F.; Zampella, G. Preparation and Protonation of Fe2(Pdt)(CNR)6, Electron-Rich Analogues of Fe2(Pdt)(CO)6. Inorg. Chem. 2016, 55, 3401–3412. [Google Scholar] [CrossRef] [Green Version]
- Bresciani, G.; Antico, E.; Ciancaleoni, G.; Zacchini, S.; Pampaloni, G.; Marchetti, F. Bypassing the Inertness of Aziridine/CO2 Systems to Access 5-Aryl-2-Oxazolidinones: Catalyst-Free Synthesis Under Ambient Conditions. ChemSusChem 2020, 13, 5586–5594. [Google Scholar] [CrossRef]
- Bresciani, G.; Bortoluzzi, M.; Ghelarducci, C.; Marchetti, F.; Pampaloni, G. Synthesis of α-Alkylidene Cyclic Carbonates via CO2 Fixation under Ambient Conditions Promoted by an Easily Available Silver Carbamate. New J. Chem. 2021, 45, 4340–4346. [Google Scholar] [CrossRef]
- Bresciani, G.; Busto, N.; Ceccherini, V.; Bortoluzzi, M.; Pampaloni, G.; Garcia, B.; Marchetti, F. Screening the Biological Properties of Transition Metal Carbamates Reveals Gold(I) and Silver(I) Complexes as Potent Cytotoxic and Antimicrobial Agents. J. Inorg. Biochem. 2022, 227, 111667. [Google Scholar] [CrossRef]
- Gibson, V.C.; Redshaw, C.; Clegg, W.; Elsegood, M.R.J. Isocyanate versus Isothiocyanate Insertion into Alkoxo and Imido Ligands. J. Chem. Soc. Chem. Commun. 1994, 2635–2636. [Google Scholar] [CrossRef]
- Bruffaerts, J.; von Wolff, N.; Diskin-Posner, Y.; Ben-David, Y.; Milstein, D. Formamides as Isocyanate Surrogates: A Mechanistically Driven Approach to the Development of Atom-Efficient, Selective Catalytic Syntheses of Ureas, Carbamates, and Heterocycles. J. Am. Chem. Soc. 2019, 141, 16486–16493. [Google Scholar] [CrossRef]
- Toma, A.; Raţ, C.I.; Silvestru, A.; Rüffer, T.; Lang, H.; Mehring, M. Organoantimony(III) and -Bismuth(III) Hypervalent Pseudohalides. An Experimental and Theoretical Study. J. Organomet. Chem. 2013, 745–746, 71–79. [Google Scholar] [CrossRef]
- Baker, M.V.; Barnard, P.J.; Brayshaw, S.K.; Hickey, J.L.; Skelton, B.W.; White, A.H. Synthetic, Structural and Spectroscopic Studies of (Pseudo)Halo(1,3-Di-Tert-Butylimidazol-2-Ylidine)Gold Complexes. Dalton Trans. 2005, 37–43. [Google Scholar] [CrossRef]
- Schneider, D.; Nogai, S.; Schier, A.; Schmidbaur, H. Mono- and Dinuclear Gold(I) Thio- and Selenocyanate Complexes. Inorg. Chim. Acta 2003, 352, 179–187. [Google Scholar] [CrossRef]
- Parr, J.; Smith, M.B.; Slawin, A.M.Z. The Synthesis and Crystal Structures of the First Examples of Six-Membered Inorganic Iridacycles Containing the [(Ph2PE)2N]− Ligand (E = S or Se). J. Organomet. Chem. 1999, 588, 99–106. [Google Scholar] [CrossRef]
- Fettouhi, M.; Al-Maythalony, B.A.; Nasiruzzaman Shaikh, M.; Wazeer, M.I.M.; Isab, A.A. Alkyldiamine Bis(Selenocyanato)Cadmium(II) Complexes: Synthesis, 113Cd, 77Se, 15N and 13C NMR Spectroscopy and X-Ray Structure of a 2D Metal–Organic Framework. Polyhedron 2011, 30, 1262–1266. [Google Scholar] [CrossRef]
- Rohde, J.-U.; Preetz, W. Synthese und spektroskopische Charakterisierung von [Rh(SeCN)6]3− und trans-[Rh(CN)2(SeCN)4]3−, Kristallstruktur von (Me4N)3[Rh(SeCN)6]. Z. Anorg. Allg. Chem. 2000, 626, 1550–1556. [Google Scholar] [CrossRef]
- Pan, W.-H.; Fackler, J.P.; Kargol, J.A.; Burmeister, J.L. Selenium-77 Nuclear Magnetic Resonance Studies. 3. Chemical Shifts of Ionic, N- and Se-Coordinated Selenocyanate. Inorg. Chim. Acta 1980, 44, L95–L97. [Google Scholar] [CrossRef]
- Nuzzo, S.; Browne, M.P.; Twamley, B.; Lyons, M.E.G.; Baker, R.J. A Structural and Spectroscopic Study of the First Uranyl Selenocyanate, [Et4N]3[UO2(NCSe)5]. Inorganics 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Kargol, J.A.; Crecely, R.W.; Burmeister, J.L. Carbon-13 Nuclear Magnetic Resonance Study of Coordinated Thiocyanate, Selenocyanate, and Cyanate. Inorg. Chem. 1979, 18, 2532–2535. [Google Scholar] [CrossRef]
- Adams, R.D.; Cotton, F.A. Pathway of Bridge-Terminal Ligand Exchange in Some Binuclear Metal Carbonyls. Bis(Pentahapto-Cyclopentadienyldicarbonyliron) and Its Di(Methyl Isocyanide) Derivative and Bis(Pentahapto-Cyclopentadienylcarbonylnitrosylmanganese). J. Am. Chem. Soc. 1973, 95, 6589–6594. [Google Scholar] [CrossRef]
- Farrugia, L.J. Dynamics and Fluxionality in Metal Carbonyl Clusters: Some Old and New Problems. J. Chem. Soc. Dalton Trans. 1997, 1783–1792. [Google Scholar] [CrossRef]
- Bresciani, G.; Biancalana, L.; Zacchini, S.; Pampaloni, G.; Ciancaleoni, G.; Marchetti, F. Diiron Bis-cyclopentadienyl Complexes as Transfer Hydrogenation Catalysts: The Key Role of the Bridging Aminocarbyne Ligand. Appl. Organomet. Chem. 2023, 37, e6990. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Adams, M.J.; Yarbrough, J.C.; Phelps, A.L. Synthesis and Structural Characterization of Potassium Salts of Phosphane-Substituted (Cyclopentadienyl)Iron Dicyanides, and Their Use as Bridging Ligands for Copper(I) Phosphane Derivatives. Eur. J. Inorg. Chem. 2003, 2003, 3639–3648. [Google Scholar] [CrossRef]
- Nakazawa, H.; Kawasaki, T.; Miyoshi, K.; Suresh, C.H.; Koga, N. C–C Bond Cleavage of Acetonitrile by a Carbonyl Iron Complex with a Silyl Ligand. Organometallics 2004, 23, 117–126. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lee, W.-Z.; Miller, M.L.; Reibenspies, J.H.; Darensbourg, D.J.; Darensbourg, M.Y. Responses of the Fe(CN)2(CO) Unit to Electronic Changes as Related to Its Role in [NiFe]Hydrogenase. J. Am. Chem. Soc. 1998, 120, 10103–10114. [Google Scholar] [CrossRef]
- Boss, K.; Dowling, C.; Manning, A.R. Preparation, Spectra and Structure of [Fe2(η-C5H5)2(L)(CN)(µ-CO){µ-CN(R’) R}], [Fe2(η-C5H5)2(CO)(CN){µ-CNMe2}2]+ and [Fe2(η-C5H5)2(CN)2(µ-CNMe2}2] Zwitterions (L = CO or Organoisocyanide) and Their Reactions with Alkyl and Protic Electrophiles. J. Organomet. Chem. 1996, 509, 197–207. [Google Scholar] [CrossRef]
- Albano, V.G.; Busetto, L.; Monari, M.; Zanotti, V. Reactions of Acetonitrile Di-Iron μ-Aminocarbyne Complexes; Synthesis and Structure of [Fe2(μ-CNMe2)(μ-H)(CO)2(Cp)2]. J. Organomet. Chem. 2000, 606, 163–168. [Google Scholar] [CrossRef]
- Arrigoni, F.; Bertini, L.; De Gioia, L.; Cingolani, A.; Mazzoni, R.; Zanotti, V.; Zampella, G. Mechanistic Insight into Electrocatalytic H2 Production by [Fe2(CN){μ-CN(Me)2}(μ-CO)(CO)(Cp)2]: Effects of Dithiolate Replacement in [FeFe] Hydrogenase Models. Inorg. Chem. 2017, 56, 13852–13864. [Google Scholar] [CrossRef]
- Boss, K.; Manning, A.R.; Müller-Bunz, H. The Structure of the Red-Brown Form of [Fe(η5-C5H4Me)2(CO)(CN)(μ-CO)(μ-CNMe2)] and Its Confirmation as a Cis Isomer. Z. Krist.-Cryst. Mater. 2006, 221, 266–269. [Google Scholar] [CrossRef]
- Biancalana, L.; De Franco, M.; Ciancaleoni, G.; Zacchini, S.; Pampaloni, G.; Gandin, V.; Marchetti, F. Easily Available, Amphiphilic Diiron Cyclopentadienyl Complexes Exhibit in Vitro Anticancer Activity in 2D and 3D Human Cancer Cells through Redox Modulation Triggered by CO Release. Chem. Eur. J. 2021, 27, 10169–10185. [Google Scholar] [CrossRef]
- Agonigi, G.; Bortoluzzi, M.; Marchetti, F.; Pampaloni, G.; Zacchini, S.; Zanotti, V. Regioselective Nucleophilic Additions to Diiron Carbonyl Complexes Containing a Bridging Aminocarbyne Ligand: A Synthetic, Crystallographic and DFT Study. Eur. J. Inorg. Chem. 2018, 2018, 960–971. [Google Scholar] [CrossRef]
- Menges, F. Spectragryph-Optical Spectroscopy Software 1.2.15.0; COMODO: Clifton, NJ, USA, 2022. [Google Scholar]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef] [Green Version]
- Willker, W.; Leibfritz, D.; Kerssebaum, R.; Bermel, W. Gradient Selection in Inverse Heteronuclear Correlation Spectroscopy. Magn. Reson. Chem. 1993, 31, 287–292. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimme, S.; Brandenburg, J.G.; Bannwarth, C.; Hansen, A. Consistent Structures and Interactions by Density Functional Theory with Small Atomic Orbital Basis Sets. J. Chem. Phys. 2015, 143, 054107. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057. [Google Scholar] [CrossRef]
- Kruse, H.; Grimme, S. A Geometrical Correction for the Inter- and Intra-Molecular Basis Set Superposition Error in Hartree-Fock and Density Functional Theory Calculations for Large Systems. J. Chem. Phys. 2012, 136, 154101. [Google Scholar] [CrossRef] [Green Version]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [Green Version]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, Structures, and Electronic Properties of Molecules in Solution with the C-PCM Solvation Model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cramer, C.J. Essentials of Computational Chemistry: Theories and Models, 2nd ed.; Wiley: Chichester, UK; Hoboken, NJ, USA, 2004; ISBN 978-0-470-09182-1. [Google Scholar]
- Neese, F. Software Update: The ORCA Program System. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]

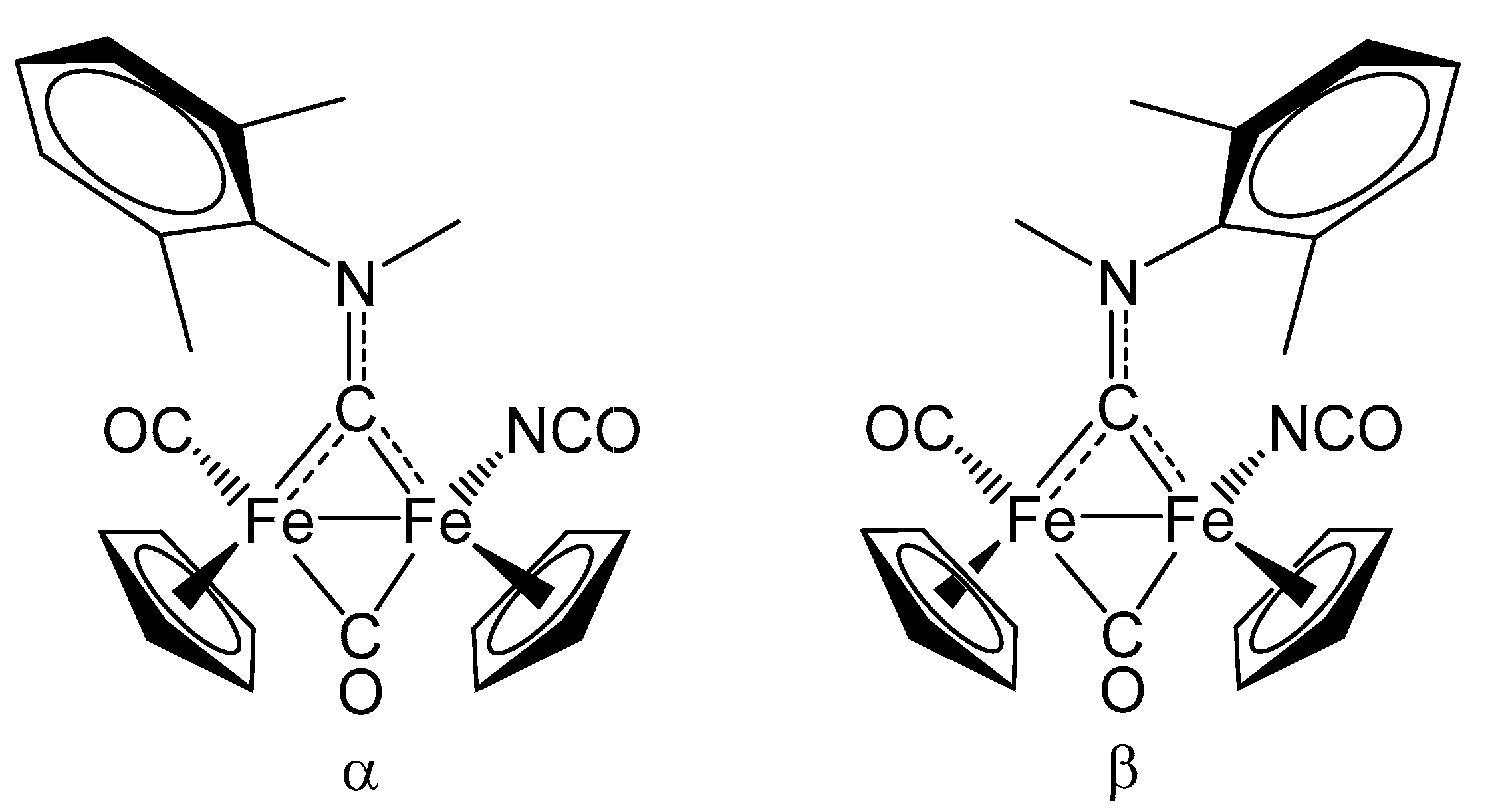
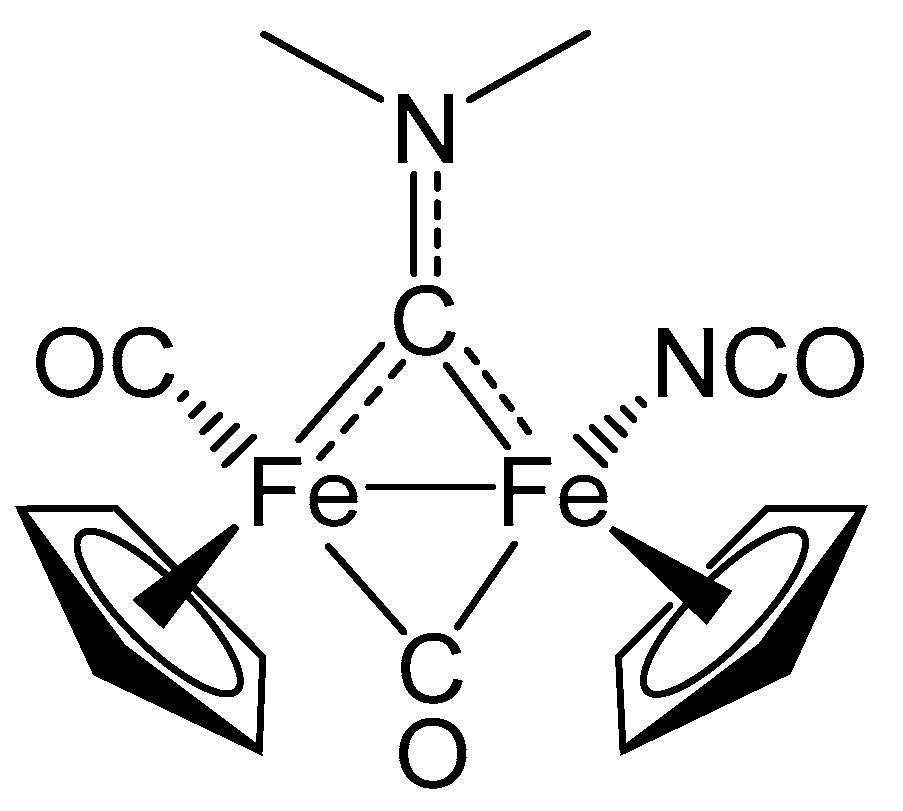
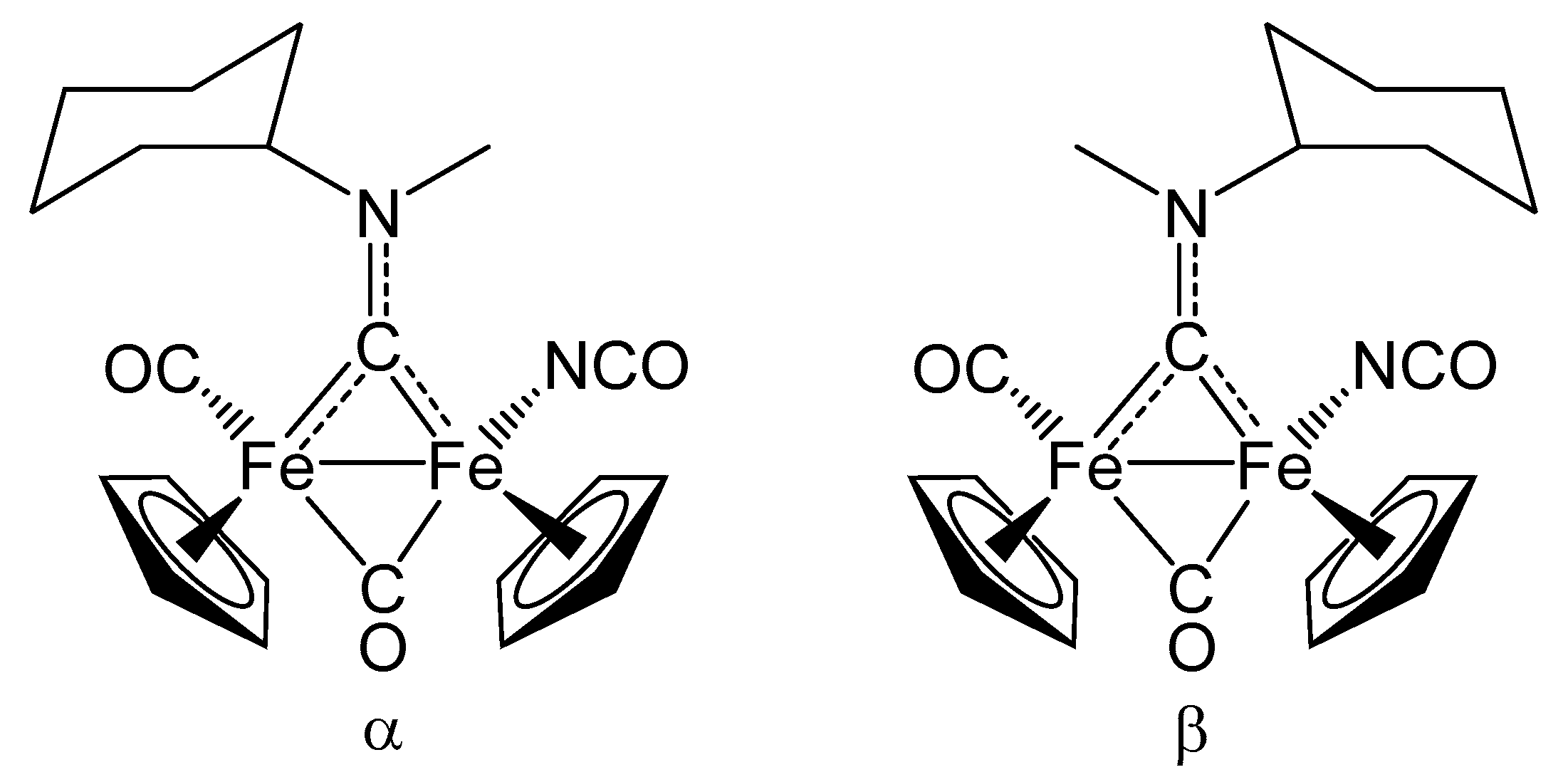

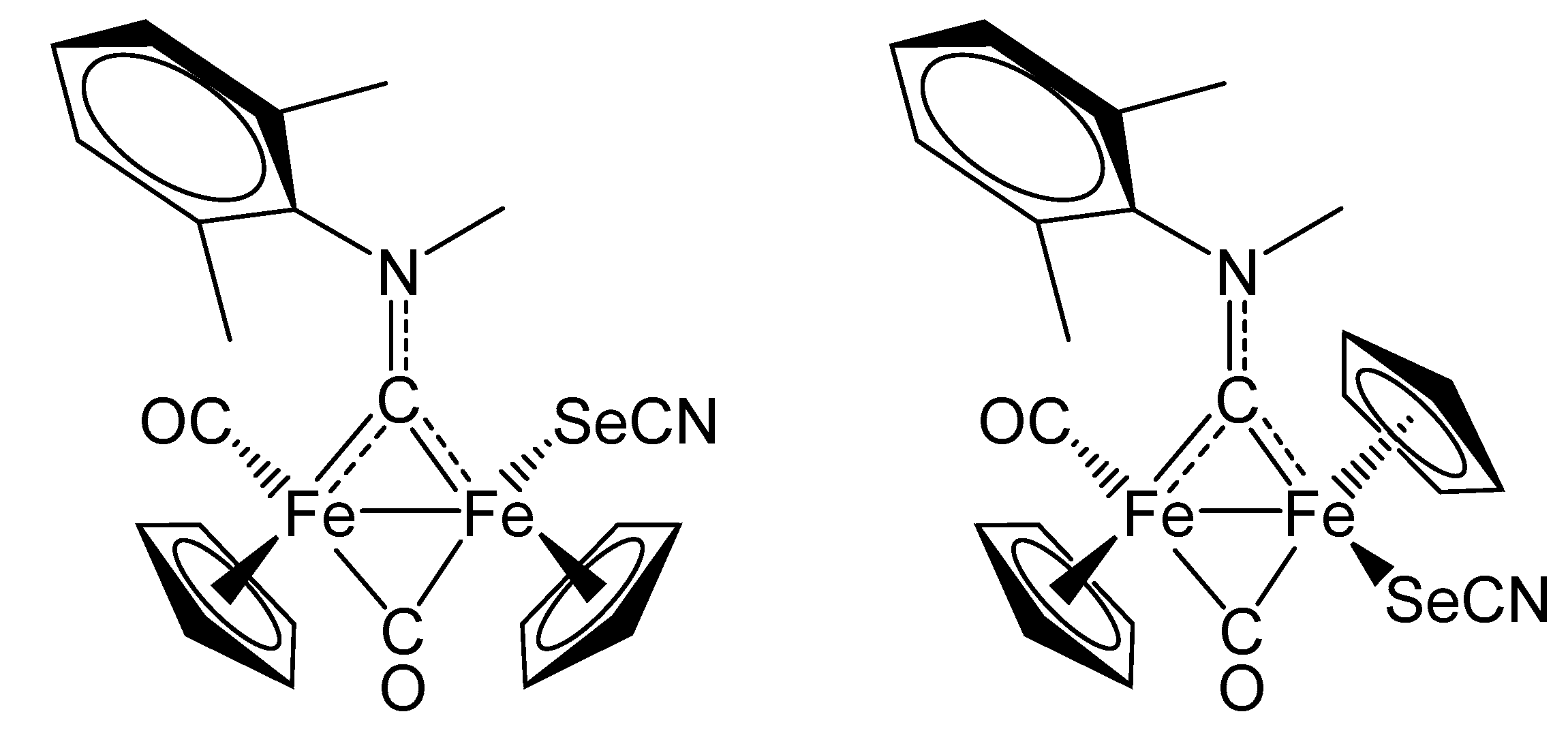



| trans-6b | |
|---|---|
| Formula | C16H16Fe2N2O2 |
| FW | 380.01 |
| T, K | 100(2) |
| λ, Å | 0.71073 |
| Crystal system | Monoclinic |
| Space group | P21/c |
| a, Å | 8.7226(2) |
| b, Å | 12.4479(3) |
| c, Å | 14.1827(4) |
| β, ° | 107.2940(10) |
| Cell Volume, Å3 | 1470.31(6) |
| Z | 4 |
| Dc, g∙cm−3 | 1.717 |
| μ, mm−1 | 1.980 |
| F(000) | 776 |
| Crystal size, mm | 0.16 × 0.15 × 0.13 |
| θ limits, ° | 2.222–26.996 |
| Reflections collected | 23,142 |
| Independent reflections | 3205 [Rin = 0.0474] |
| Data/restraints/parameters | 3205/0/201 |
| Goodness on fit on F2 | 1.074 |
| R1 (I > 2σ(I)) | 0.0283 |
| wR2 (all data) | 0.0677 |
| Largest diff. peak and hole, e Å−3 | 0.562/−0.444 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bresciani, G.; Zacchini, S.; Pampaloni, G.; Bortoluzzi, M.; Marchetti, F. Diiron Aminocarbyne Complexes with NCE− Ligands (E = O, S, Se). Molecules 2023, 28, 3251. https://doi.org/10.3390/molecules28073251
Bresciani G, Zacchini S, Pampaloni G, Bortoluzzi M, Marchetti F. Diiron Aminocarbyne Complexes with NCE− Ligands (E = O, S, Se). Molecules. 2023; 28(7):3251. https://doi.org/10.3390/molecules28073251
Chicago/Turabian StyleBresciani, Giulio, Stefano Zacchini, Guido Pampaloni, Marco Bortoluzzi, and Fabio Marchetti. 2023. "Diiron Aminocarbyne Complexes with NCE− Ligands (E = O, S, Se)" Molecules 28, no. 7: 3251. https://doi.org/10.3390/molecules28073251







