Recent Advances in Graphitic Carbon Nitride Based Electro-Catalysts for CO2 Reduction Reactions
Abstract
:1. Introduction
1.1. Fundamentals of Electrocatalytic Reduction of CO2
1.2. Unique Properties of g-C3N4 as an Electrocatalyst
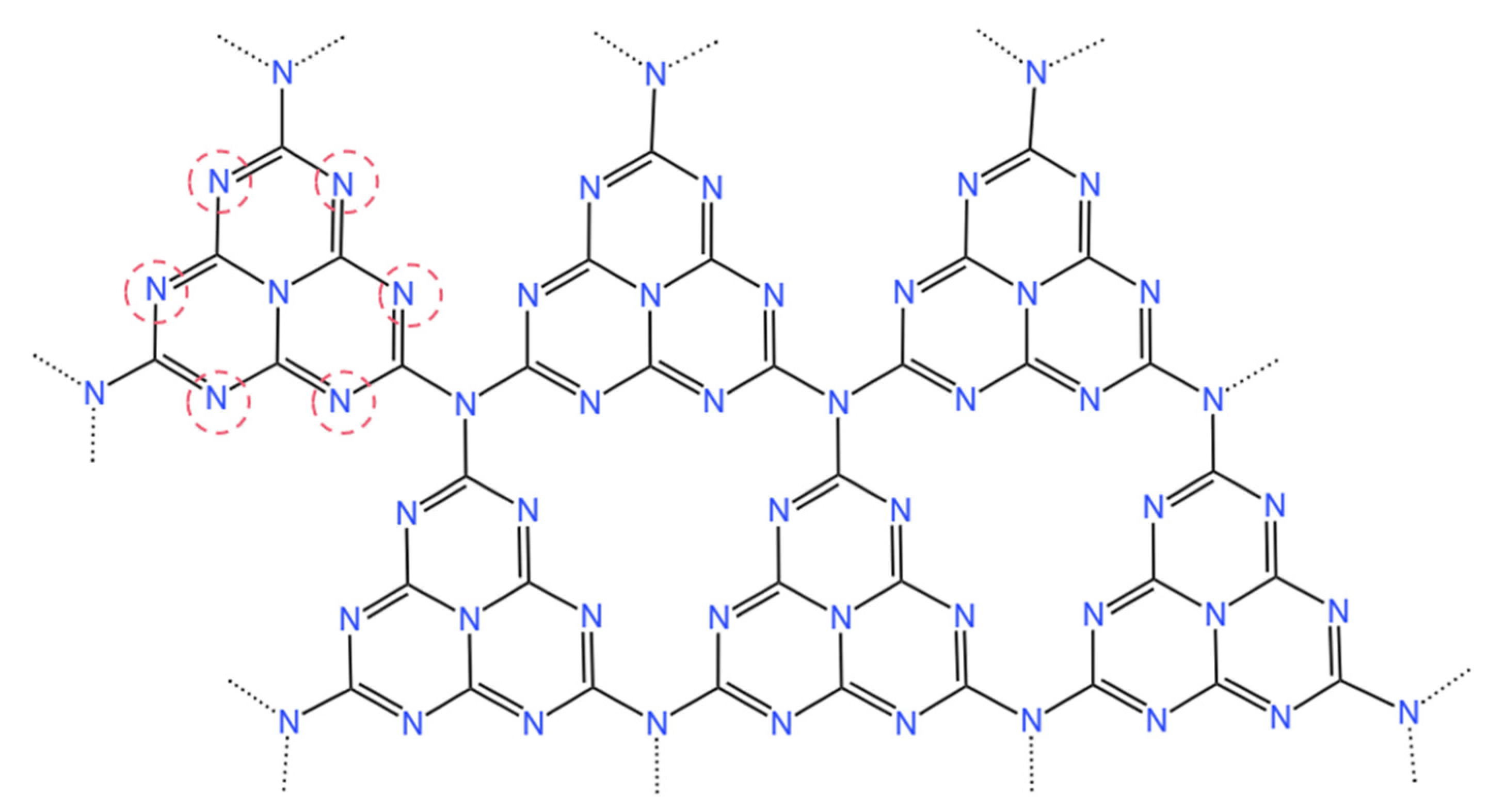
1.2.1. Morphology of g-C3N4
1.2.2. Surface Active Sites
1.2.3. Stability
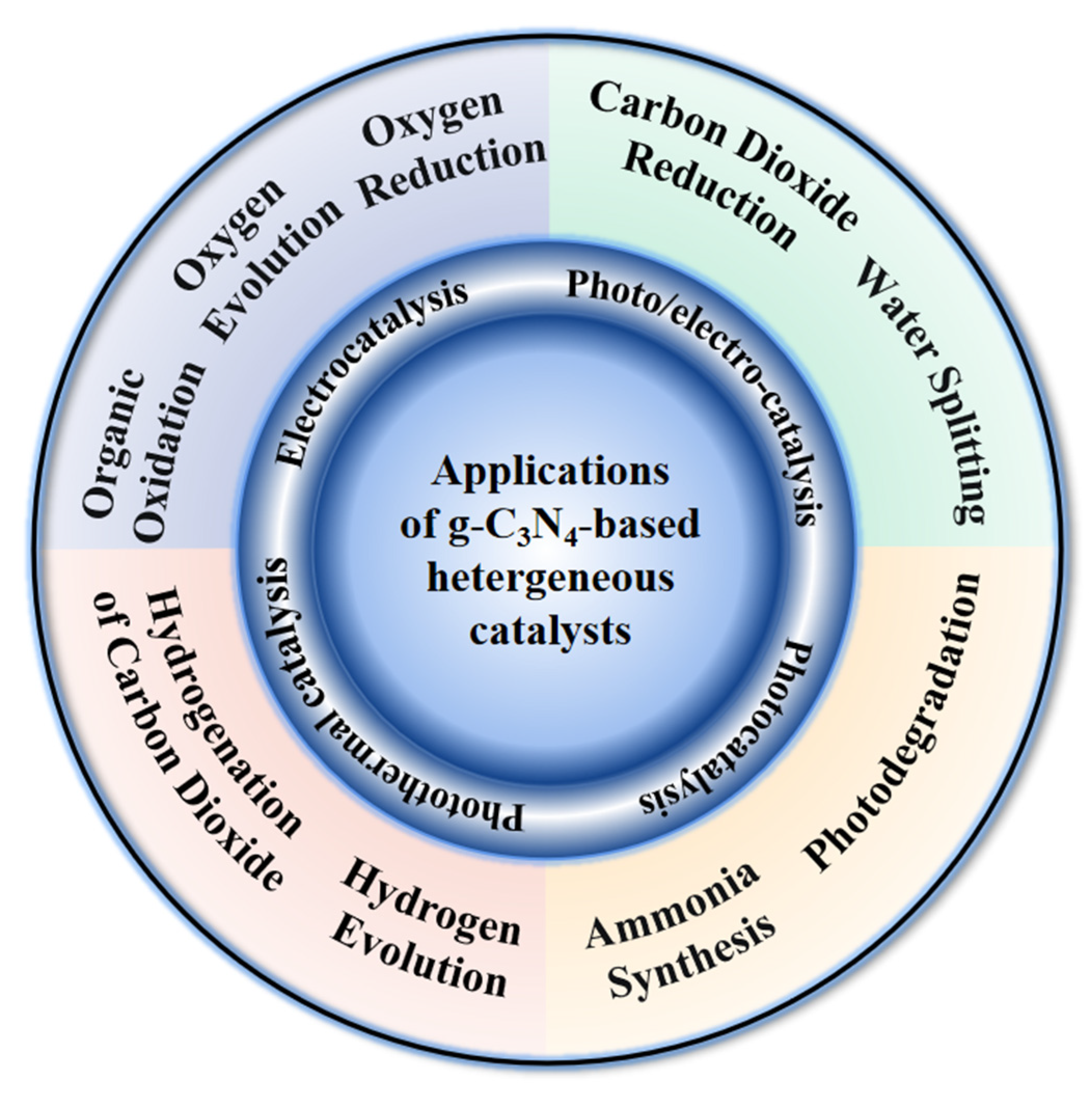
2. Graphitic Carbon Nitride-Based Catalysts for Electrocatalytic CO2RR
2.1. Pristine g-C3N4
2.2. Metal Doped g-C3N4
2.2.1. Single Metal Doped g-C3N4
2.2.2. Bimetallic Doped g-C3N4
2.2.3. Ternary Compound Catalyst
2.3. Non-Metal Doping g-C3N4
3. Method for the Synthesis of g-C3N4-Based Catalysts
3.1. Thermal Polycondensation
3.2. Thermal Decomposition Method
3.3. Hydrothermal Synthesis
3.4. Wet Chemical Reduction
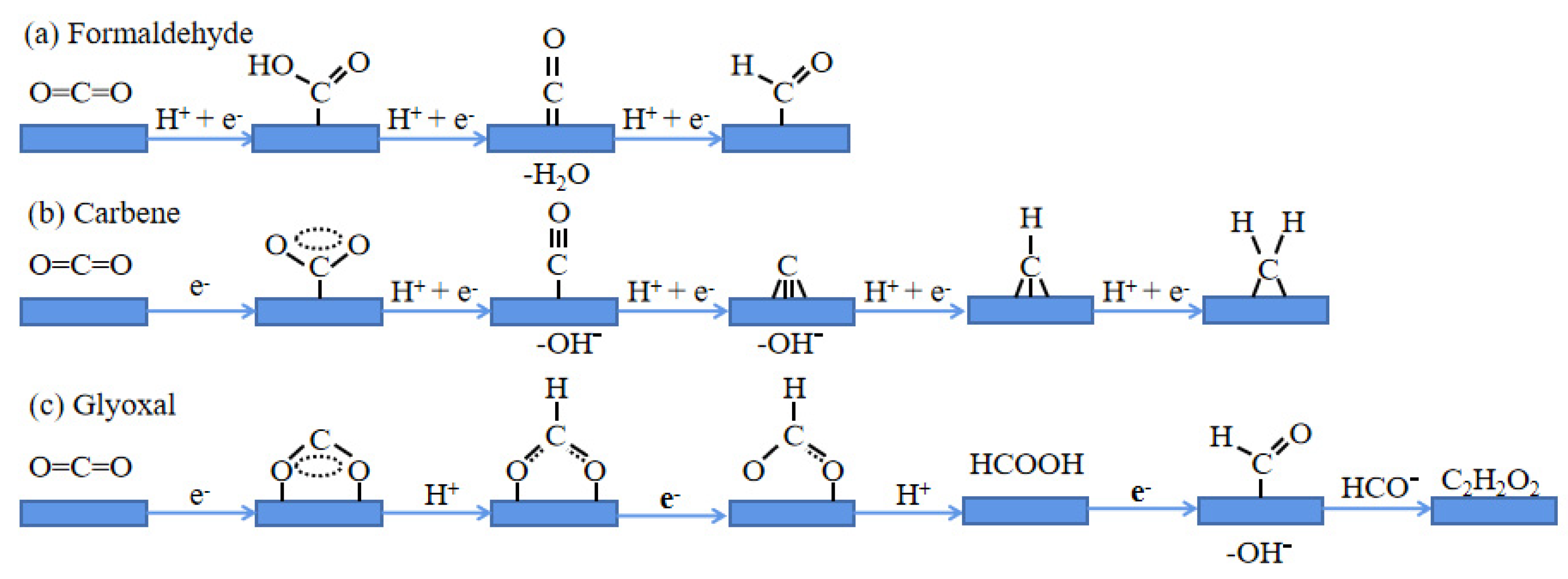
4. Regulation of Reactant Selectivity by g-C3N4-Based Catalyst
5. Summary
6. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S., III; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.; Wang, Z.; Allan, P.; Zhang, F.; Lu, Z. Reticular chemistry in electrochemical carbon dioxide reduction. Sci. China Mater. 2020, 63, 1113–1141. [Google Scholar] [CrossRef]
- Sharma, S.; Basu, S.; Shetti, N.P.; Aminabhavi, T. Waste-to-energy nexus for circular economy and environmental protection: Recent trends in hydrogen energy. Sci. Total. Environ. 2020, 713, 136633. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Kortlever, R.; Shen, J.; Schouten, K.J.P.; Calle-Vallejo, F.; Koper, M.T.M. Catalysts and Reaction Pathways for the Electrochemical Reduction of Carbon Dioxide. J. Phys. Chem. Lett. 2015, 6, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, J.; Jaroniec, M.; Chen, X. Cocatalysts for Selective Photoreduction of CO2 into Solar Fuels. Chem. Rev. 2019, 119, 3962–4179. [Google Scholar] [CrossRef]
- Van Phuc, T.; Kang, S.G.; Chung, J.S.; Hur, S.H. Highly selective metal-organic framework-based electrocatalyst for the electrochemical reduction of CO2 to CO. Mater. Res. Bull. 2021, 138, 111228. [Google Scholar]
- Rafrafi, Y.; Laguillaumie, L.; Dumas, C. Biological Methanation of H2 and CO2 with Mixed Cultures: Current Advances, Hurdles and Challenges. Waste Biomass Valorization 2020, 12, 5259–5282. [Google Scholar]
- Jensen, M.B.; Ottosen, L.D.M.; Kofoed, M.V.W. H2 gas-liquid mass transfer: A key element in biological Power-to-Gas methanation. Renew. Sustain. Energy Rev. 2021, 147, 111209. [Google Scholar]
- Jiao, X.; Zheng, K.; Liang, L.; Li, X.; Sun, Y.; Xie, Y. Fundamentals and challenges of ultrathin 2D photocatalysts in boosting CO2 photoreduction. Chem. Soc. Rev. 2020, 49, 6592–6604. [Google Scholar]
- Wang, G.-B.; Li, S.; Yan, C.-X.; Zhu, F.-C.; Lin, Q.-Q.; Xie, K.-H.; Geng, Y.; Dong, Y.-B. Covalent organic frameworks: Emerging high-performance platforms for efficient photocatalytic applications. J. Mater. Chem. A 2020, 8, 6957–6983. [Google Scholar]
- Le, T.A.; Kang, J.K.; Park, E.D. CO and CO2 Methanation Over Ni/SiC and Ni/SiO2 Catalysts. Top. Catal. 2018, 61, 1537–1544. [Google Scholar] [CrossRef]
- Martínez, J.; Hernández, E.; Alfaro, S.; Medina, R.L.; Aguilar, G.V.; Albiter, E.; Valenzuela, M.A. High Selectivity and Stability of Nickel Catalysts for CO2 Methanation: Support Effects. Catalysts 2018, 9, 24. [Google Scholar]
- Jin, S.; Hao, Z.; Zhang, K.; Yan, Z.; Chen, J. Advances and Challenges for the Electrochemical Reduction of CO 2 to CO: From Fundamentals to Industrialization. Angew. Chem. Int. Ed. 2021, 60, 20627–20648. [Google Scholar] [CrossRef] [PubMed]
- Liebig, J.V. About Some Nitrogen Compounds. 1834. Ann. Pharm. 1834, 10, 1–47. [Google Scholar] [CrossRef]
- Teixeira, I.F.; Barbosa, E.C.; Tsang, S.C.E.; Camargo, P.H. Carbon nitrides and metal nanoparticles: From controlled synthesis to design principles for improved photocatalysis. Chem. Soc. Rev. 2018, 47, 7783–7817. [Google Scholar] [PubMed]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Alamry, K.A.; Sun, X. Ultrathin graphitic C3 N4 nanosheets/graphene composites: Efficient organic electrocatalyst for oxygen evolution reaction. ChemSusChem 2014, 7, 2125–2130. [Google Scholar]
- Ye, S.; Wang, R.; Wu, M.-Z.; Yuan, Y.-P. A review on g-C3N4 for photocatalytic water splitting and CO2 reduction. Appl. Surf. Sci. 2015, 358, 15–27. [Google Scholar]
- Li, X.; Xiong, J.; Gao, X.; Huang, J.; Feng, Z.; Chen, Z.; Zhu, Y. Recent advances in 3D g-C3N4 composite photocatalysts for photocatalytic water splitting, degradation of pollutants and CO2 reduction. J. Alloy. Compd. 2019, 802, 196–209. [Google Scholar]
- Aggarwal, M.; Basu, S.; Shetti, N.P.; Nadagouda, M.N.; Kwon, E.E.; Park, Y.-K.; Aminabhavi, T.M. Photocatalytic carbon dioxide reduction: Exploring the role of ultrathin 2D graphitic carbon nitride (g-C3N4). Chem. Eng. J. 2021, 425, 131402. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, Z.; Deng, S.; Wang, H.; Wu, Z. Decorating g-C3N4 with alkalinized Ti3C2 MXene for promoted photocatalytic CO2 reduction performance. J. Colloid Interface Sci. 2020, 564, 406–417. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Zhuang, L.; Hu, B.; Chen, J.; Liu, X.; Wang, X. Recent developments of doped g-C3N4 photocatalysts for the degradation of organic pollutants. Crit. Rev. Environ. Sci. Technol. 2021, 51, 751–790. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Xiao, T.; Tian, Y.; Jiang, Z. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Han, Q.; Wu, C.; Jiao, H.; Xu, R.; Wang, Y.; Xie, J.; Guo, Q.; Tang, J. Rational Design of High-Concentration Ti(3+) in Porous Carbon-Doped TiO(2) Nanosheets for Efficient Photocatalytic Ammonia Synthesis. Adv. Mater. 2021, 33, e2008180. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, H.; Liu, Y.; Zhang, S.; Zhang, Y.; Wang, G.; Zhang, H.; Zhao, H. Formation of B—N—C Coordination to Stabilize the Exposed Active Nitrogen Atoms in g-C(3) N(4) for Dramatically Enhanced Photocatalytic Ammonia Synthesis Performance. Small 2020, 16, e1906880. [Google Scholar] [CrossRef]
- Ghane, N.; Sadrnezhaad, S.K. Combustion synthesis of g-C3N4/Fe2O3 nanocomposite for superior photoelectrochemical catalytic performance. Appl. Surf. Sci. 2020, 534, 147563. [Google Scholar] [CrossRef]
- Sagara, N.; Kamimura, S.; Tsubota, T.; Ohno, T. Photoelectrochemical CO2 reduction by a p-type boron-doped g-C3N4 electrode under visible light. Appl. Catal. B Environ. 2016, 192, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Liu, D.; Yang, H.; Huang, H.; Luo, Q.; Huang, Y.; Yu, X.-F.; Chu, P.K. Modification of Layered Graphitic Carbon Nitride by Nitrogen Plasma for Improved Electrocatalytic Hydrogen Evolution. Nanomaterials 2019, 9, 568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, J.T.; Chen, L.; Weng, C.C.; Yuan, G.G.; Yuan, Z.Y. Well-Defined Mo(2)C Nanoparticles Embedded in Porous N-Doped Carbon Matrix for Highly Efficient Electrocatalytic Hydrogen Evolution. ACS Appl. Mater. Interfaces 2018, 10, 33276–33286. [Google Scholar] [CrossRef]
- Li, J.; Banis, M.N.; Ren, Z.; Adair, K.R.; Doyle-Davis, K.; Meira, D.M.; Finfrock, Y.Z.; Zhang, L.; Kong, F.; Sham, T.K.; et al. Unveiling the Nature of Pt Single-Atom Catalyst during Electrocatalytic Hydrogen Evolution and Oxygen Reduction Reactions. Small 2021, 17, e2007245. [Google Scholar] [CrossRef] [PubMed]
- Leal-Rodríguez, C.; Rodríguez-Padrón, D.; Alothman, Z.A.; Cano, M.; Giner-Casares, J.J.; Muñoz-Batista, M.J.; Osman, S.M.; Luque, R. Thermal and light irradiation effects on the electrocatalytic performance of hemoglobin modified Co(3)O(4)-g-C(3)N(4) nanomaterials for the oxygen evolution reaction. Nanoscale 2020, 12, 8477–8484. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S. Electronic and Structural Engineering of Carbon-Based Metal-Free Electrocatalysts for Water Splitting. Adv. Mater. 2018, 31, e1803625. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Kraft, M.; Xu, R. Metal-free carbonaceous electrocatalysts and photocatalysts for water splitting. Chem. Soc. Rev. 2016, 45, 3039–3052. [Google Scholar] [CrossRef] [PubMed]
- Zhi, X.; Jiao, Y.; Zheng, Y.; Qiao, S. Impact of Interfacial Electron Transfer on Electrochemical CO 2 Reduction on Graphitic Carbon Nitride/Doped Graphene. Small 2019, 15, e1804224. [Google Scholar] [CrossRef]
- Guo, C.; Zhang, T.; Deng, X.; Liang, X.; Guo, W.; Lu, X.; Wu, C.M.L. Electrochemical CO(2) Reduction to C(1) Products on Single Nickel/Cobalt/Iron-Doped Graphitic Carbon Nitride: A DFT Study. ChemSusChem 2019, 12, 5126–5132. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Lu, J.; Wang, Q.; Cong, Y. Synergistic photoelectrochemical reduction of Cr (VI) and oxidation of organic pollutants by g-C3N4/TiO2-NTs electrodes. Chemosphere 2016, 162, 55–63. [Google Scholar] [CrossRef]
- Feng, K.; Wang, S.; Zhang, D.; Wang, L.; Yu, Y.; Feng, K.; Li, Z.; Zhu, Z.; Li, C.; Cai, M.; et al. Cobalt Plasmonic Superstructures Enable Almost 100% Broadband Photon Efficient CO 2 Photocatalysis. Adv. Mater. 2020, 32, e2000014. [Google Scholar] [CrossRef]
- Jia, J.; O’Brien, P.G.; He, L.; Qiao, Q.; Fei, T.; Reyes, L.M.; Burrow, T.E.; Dong, Y.; Liao, K.; Varela, M.; et al. Visible and Near-Infrared Photothermal Catalyzed Hydrogenation of Gaseous CO(2) over Nanostructured Pd@Nb(2)O(5). Adv. Sci. 2016, 3, 1600189. [Google Scholar] [CrossRef]
- Li, Y.; Chang, H.; Wang, Z.; Shen, Q.; Liu, X.; Xue, J.; Jia, H. A 3D C@TiO(2) multishell nanoframe for simultaneous photothermal catalytic hydrogen generation and organic pollutant degradation. J. Colloid Interface Sci. 2022, 609, 535–546. [Google Scholar] [CrossRef]
- Hu, S.; Shi, J.; Luo, B.; Ai, C.; Jing, D. Significantly enhanced photothermal catalytic hydrogen evolution over Cu(2)O-rGO/TiO(2) composite with full spectrum solar light. J. Colloid Interface Sci. 2022, 608, 2058–2065. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.J.; Putri, L.K.; Mohamed, A.R. Rational Design of Carbon-Based 2D Nanostructures for Enhanced Photocatalytic CO(2) Reduction: A Dimensionality Perspective. Chemistry 2020, 26, 9710–9748. [Google Scholar] [CrossRef]
- Lu, Q.; Eid, K.; Li, W.; Abdullah, A.M.; Xu, G.; Varma, R.S. Engineering graphitic carbon nitride (g-C3N4) for catalytic reduction of CO2 to fuels and chemicals: Strategy and mechanism. Green Chem. 2021, 23, 5394–5428. [Google Scholar] [CrossRef]
- Chen, Z.; Gao, M.-R.; Zhang, Y.-Q.; Duan, N.; Fan, T.; Xiao, J.; Zhang, J.; Dong, Y.; Li, J.; Yi, X.; et al. Tuning local carbon active sites saturability of graphitic carbon nitride to boost CO2 electroreduction towards CH4. Nano Energy 2020, 73, 104833. [Google Scholar] [CrossRef]
- Chen, D.; Wang, K.; Xiang, D.; Zong, R.; Yao, W.; Zhu, Y. Significantly enhancement of photocatalytic performances via core–shell structure of ZnO@ mpg-C3N4. Appl. Catal. B Environ. 2014, 147, 554–561. [Google Scholar] [CrossRef]
- Piao, H.; Choi, G.; Jin, X.; Hwang, S.-J.; Song, Y.J.; Cho, S.-P.; Choy, J.-H. Monolayer Graphitic Carbon Nitride as Metal-Free Catalyst with Enhanced Performance in Photo- and Electro-Catalysis. Nano-Micro Lett. 2022, 14, 55. [Google Scholar] [CrossRef]
- Weng, G.M.; Xie, Y.; Wang, H.; Karpovich, C.; Lipton, J.; Zhu, J.; Kong, J.; Pfefferle, L.D.; Taylor, A.D. A promising carbon/g-C3N4 composite negative electrode for a long-life sodium-ion battery. Angew. Chem. 2019, 131, 13865–13871. [Google Scholar] [CrossRef]
- Zhang, S.; Mo, Z.; Wang, J.; Liu, H.; Liu, P.; Hu, D.; Tan, T.; Wang, C. Ultra-stable oxygen species in Ag nanoparticles anchored on g-C3N4 for enhanced electrochemical reduction of CO2. Electrochim. Acta 2021, 390, 138831. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Handoko, A.D.; Wei, F.; Yeo, B.S.; Seh, Z.W. Understanding heterogeneous electrocatalytic carbon dioxide reduction through operando techniques. Nat. Catal. 2018, 1, 922–934. [Google Scholar] [CrossRef]
- Zhu, D.D.; Liu, J.L.; Qiao, S.Z. Recent Advances in Inorganic Heterogeneous Electrocatalysts for Reduction of Carbon Dioxide. Adv. Mater. 2016, 28, 3423–3452. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Z.; Bao, A.; Tang, X.; Huang, X.; Yi, H.; Zhao, S.; Sun, T.; Wang, J.; Gao, F. Recent advances on electrocatalytic CO2 reduction to resources: Target products, reaction pathways and typical catalysts. Chem. Eng. J. 2022, 8, 139663. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by Supported Single Metal Atoms. ACS Catal. 2016, 7, 34–59. [Google Scholar] [CrossRef]
- Kuhl, K.P.; Cave, E.R.; Abram, D.N.; Jaramillo, T.F. New insights into the electrochemical reduction of carbon dioxide on metallic copper surfaces. Energy Environ. Sci. 2012, 5, 7050–7059. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Guo, T.; Zhang, S.; Wang, J.; Wu, Y.; Chen, Y. Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction. Nano-Micro Lett. 2019, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Shkrob, I.A.; Dimitrijevic, N.M.; Marin, T.W.; He, H.; Zapol, P. Heteroatom-Transfer Coupled Photoreduction and Carbon Dioxide Fixation on Metal Oxides. J. Phys. Chem. C 2012, 116, 9461–9471. [Google Scholar] [CrossRef]
- Vinu, A. Two-Dimensional Hexagonally-Ordered Mesoporous Carbon Nitrides with Tunable Pore Diameter, Surface Area and Nitrogen Content. Adv. Funct. Mater. 2008, 18, 816–827. [Google Scholar] [CrossRef]
- Niu, W.; Yang, Y. Graphitic carbon nitride for electrochemical energy conversion and storage. ACS Energy Lett. 2018, 3, 2796–2815. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, Z.; Zhao, H.; Lou, Z.; Liao, Y.; Xue, C. Temperature-controlled morphology evolution of graphitic carbon nitride nanostructures and their photocatalytic activities under visible light. RSC Adv. 2015, 5, 49317–49325. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Sun, Y.; Ho, W.-K.; Zhang, H. Engineering the nanoarchitecture and texture of polymeric carbon nitride semiconductor for enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 401, 70–79. [Google Scholar] [CrossRef]
- Wang, Z.; Guan, W.; Sun, Y.; Dong, F.; Zhou, Y.; Ho, W.-K. Water-assisted production of honeycomb-like g-C3N4 with ultralong carrier lifetime and outstanding photocatalytic activity. Nanoscale 2014, 7, 2471–2479. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Niu, P.; Wang, S.; Shi, J.; Li, L. Porous two-dimensional materials for photocatalytic and electrocatalytic applications. Matter 2020, 2, 1377–1413. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, J.; Zhang, M.; Wang, X. Polycondensation of thiourea into carbon nitride semiconductors as visible light photocatalysts. J. Mater. Chem. 2012, 22, 8083–8091. [Google Scholar] [CrossRef]
- Dong, F.; Sun, Y.; Wu, L.; Fu, M.; Wu, Z. Facile transformation of low cost thiourea into nitrogen-rich graphitic carbon nitride nanocatalyst with high visible light photocatalytic performance. Catal. Sci. Technol. 2012, 2, 1332–1335. [Google Scholar] [CrossRef]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic carbon nitride “reloaded”: Emerging applications beyond (photo) catalysis. Chem. Soc. Rev. 2016, 45, 2308–2326. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Gao, S.; Lei, F.; Xie, Y. Atomically-thin two-dimensional sheets for understanding active sites in catalysis. Chem. Soc. Rev. 2015, 44, 623–636. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.-O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Guo, Y.; Shang, B.; Fan, T.; Lian, X.; Huang, P.; Dong, Y.; Chen, Z.; Yi, X. Unveiling the Synergistic Effect between Graphitic Carbon Nitride and Cu(2) O toward CO(2) Electroreduction to C(2) H(4). ChemSusChem 2021, 14, 929–937. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Lee, H.; Mao, J.; Grimes, C.A.; Liu, C.; Zhang, M.; Lu, Z.; Chen, Y.; Feng, S.P. Efficient electroreduction of CO2 to CO by Ag-decorated S-doped g-C3N4/CNT nanocomposites at industrial scale current density. Mater. Today Phys. 2020, 12, 100176. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, Y.; Mady, M.F.; Mekonnen Tucho, W.; Lou, F.; Yu, Z. Efficient Electrochemical Reduction of CO2 to CO by Ag-Decorated B-Doped g-C3N4, A Combined Theoretical and Experimental Study. Ind. Eng. Chem. Res. 2022, 61, 10400–10408. [Google Scholar] [CrossRef]
- Praus, P.; Svoboda, L.; Ritz, M.; Troppová, I.; Šihor, M.; Kočí, K. Graphitic carbon nitride: Synthesis, characterization and photocatalytic decomposition of nitrous oxide. Mater. Chem. Phys. 2017, 193, 438–446. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, Y.; Mi, L.; Guo, Y.; Wang, H.; Liu, K.; Zhang, K.; Wang, B. Preparation of g-C3N4/TiO2 by template method and its photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126756. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genç, A.; et al. High-yield synthesis and optical properties of gC 3 N 4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Tan, T.H.; Ng, Y.H.; Amal, R. Highly Selective and Stable Reduction of CO2 to CO by a Graphitic Carbon Nitride/Carbon Nanotube Composite Electrocatalyst. Chemistry 2016, 22, 11991–11996. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, W.; Qiao, M.; Wu, G.; Chen, W.; Yuan, T.; Xu, Q.; Chen, M.; Zhang, Y.; Wang, X.; et al. Atomically dispersed Au1 catalyst towards efficient electrochemical synthesis of ammonia. Sci. Bull. 2018, 63, 1246–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Zhao, T.-J.; Feng, W.-J.; Liu, Y.-X.; Wang, H.-H.; Su, H.; Lv, L.-B.; Li, X.-H.; Chen, J.-S. Polarized few-layer g-C3N4 as metal-free electrocatalyst for highly efficient reduction of CO2. Nano Res. 2018, 11, 2450–2459. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Chen, X.; Takanabe, K.; Domen, K.; Hou, Y.; Fu, X.; Antonietti, M. Polymer semiconductors for artificial photosynthesis: Hydrogen evolution by mesoporous graphitic carbon nitride with visible light. J. Am. Chem. Soc. 2009, 131, 1680–1681. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Zheng, Y.; Chen, P.; Jaroniec, M.; Qiao, S.Z. Molecular Scaffolding Strategy with Synergistic Active Centers To Facilitate Electrocatalytic CO(2) Reduction to Hydrocarbon/Alcohol. J. Am. Chem. Soc. 2017, 139, 18093–18100. [Google Scholar] [CrossRef]
- Sun, Z.; Ma, T.; Tao, H.; Fan, Q.; Han, B. Fundamentals and challenges of electrochemical CO2 reduction using two-dimensional materials. Chem 2017, 3, 560–587. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Hu, W.; Mei, B.; Liu, H.; Yuan, B.; Zang, J.; Chen, T.; Zou, L.; Zou, Z.; Yang, B.; et al. Covalent triazine framework confined copper catalysts for selective electrochemical CO2 reduction: Operando diagnosis of active sites. ACS Catal. 2020, 10, 4534–4542. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, Y.; Li, H.; Cao, L.; Jiang, Z.; Chai, Z.; Wang, X. Molecular Self-Assembly of Oxygen Deep-Doped Ultrathin C3N4 with a Built-In Electric Field for Efficient Photocatalytic H2 Evolution. Inorg. Chem. 2021, 60, 15782–15796. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, W.; Deng, D.; Bao, X. Two-dimensional materials confining single atoms for catalysis. Chin. J. Catal. 2017, 38, 1443–1453. [Google Scholar] [CrossRef]
- Patel, A.M.; Ringe, S.; Siahrostami, S.; Bajdich, M.; Nørskov, J.K.; Kulkarni, A.R. Theoretical approaches to describing the oxygen reduction reaction activity of single-atom catalysts. J. Phys. Chem. C 2018, 122, 29307–29318. [Google Scholar] [CrossRef]
- Li, S.L.; Kan, X.; Gan, L.Y.; Fan, J.; Zhao, Y. Designing efficient single-atomic catalysts for bifunctional oxygen electrocatalysis via a general two-step strategy. Appl. Surf. Sci. 2021, 556, 149779. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, K.; Fu, J.; Cai, C.; Li, H.; Long, Y.; Chen, S.; Liu, B.; Li, H.; Li, W.; et al. Atomically Dispersed s-Block Magnesium Sites for Electroreduction of CO(2) to CO. Angew. Chem. Int. Ed. Engl. 2021, 60, 25241–25245. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, F.; Zheng, L.R.; Wang, H.F.; Yang, X.H.; Yang, H.G. Tuning Metal Catalyst with Metal–C3N4 Interaction for Efficient CO2 Electroreduction. ACS Catal. 2018, 8, 11035–11041. [Google Scholar] [CrossRef]
- Mulik, B.B.; Munde, A.V.; Bankar, B.D.; Biradar, A.V.; Sathe, B.R. Highly efficient manganese oxide decorated graphitic carbon nitrite electrocatalyst for reduction of CO2 to formate. Catal. Today 2021, 370, 104–113. [Google Scholar] [CrossRef]
- Yan, Z.; Wu, T. Highly Selective Electrochemical CO2 Reduction to C2 Products on a g-C3N4-Supported Copper-Based Catalyst. Int. J. Mol. Sci. 2022, 23, 14381. [Google Scholar] [CrossRef]
- Mulik, B.B.; Bankar, B.D.; Munde, A.V.; Chavan, P.P.; Biradar, A.V.; Sathe, B.R. Electrocatalytic and catalytic CO2 hydrogenation on ZnO/g-C3N4 hybrid nanoelectrodes. Appl. Surf. Sci. 2021, 538, 148120. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Zhu, B.; Guo, J.; Wang, D.; Cao, Z.; Chen, L.; Wang, L.; Zhai, C.; Tao, H. Facile Synthesis of Fe@C Loaded on g-C(3)N(4) for CO(2) Electrochemical Reduction to CO with Low Overpotential. ACS Omega 2022, 7, 11158–11165. [Google Scholar] [CrossRef]
- Nazir, R.; Kumar, A.; Saad, M.A.S.; Ashok, A. Synthesis of hydroxide nanoparticles of Co/Cu on carbon nitride surface via galvanic exchange method for electrocatalytic CO2 reduction into formate. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124835. [Google Scholar] [CrossRef]
- Yang, J.; Du, H.; Yu, Q.; Zhang, W.; Zhang, Y.; Ge, J.; Li, H.; Liu, J.; Li, H.; Xu, H. Porous silver microrods by plasma vulcanization activation for enhanced electrocatalytic carbon dioxide reduction. J. Colloid Interface Sci. 2022, 606, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Nian, Y.; Liu, H.; Chen, J.; Wang, Y.; Wang, S.; Xu, J.; Han, Y.; Luo, L. Revealing synergetic structural activation of a CuAu surface during water–gas shift reaction. Proc. Natl. Acad. Sci. USA 2022, 119, e2120088119. [Google Scholar] [CrossRef]
- Hu, C.; Liu, M.T.; Sakai, A.; Yoshida, M.; Lin, K.Y.A.; Huang, C.C. Synergistic effect of Cu and Ru decoration on g-C3N4 for electrocatalytic CO2 reduction. J. Ind. Eng. Chem. 2022, 115, 329–338. [Google Scholar] [CrossRef]
- Zhang, H.; Ouyang, T.; Li, J.; Mu, M.; Yin, X. Dual 2D CuSe/g-C3N4 heterostructure for boosting electrocatalytic reduction of CO2. Electrochim. Acta 2021, 390, 138766. [Google Scholar] [CrossRef]
- Yin, C.; Li, Q.; Zheng, J.; Ni, Y.; Wu, H.; Kjøniksen, A.-L.; Liu, C.; Lei, Y.; Zhang, Y. Progress in regulating electronic structure strategies on Cu-based bimetallic catalysts for CO2 reduction reaction. Adv. Powder Mater. 2022, 100055. [Google Scholar] [CrossRef]
- Woyessa, G.W.; Jay-ar, B.; Rameez, M.; Hung, C.H. Nanocomposite catalyst of graphitic carbon nitride and Cu/Fe mixed metal oxide for electrochemical CO2 reduction to CO. Appl. Catal. B Environ. 2021, 291, 120052. [Google Scholar] [CrossRef]
- Zhao, S.; Tang, Z.; Guo, S.; Han, M.; Zhu, C.; Zhou, Y.; Bai, L.; Gao, J.; Huang, H.; Li, Y.; et al. Enhanced Activity for CO2 Electroreduction on a Highly Active and Stable Ternary Au-CDots-C3N4 Electrocatalyst. ACS Catal. 2017, 8, 188–197. [Google Scholar] [CrossRef]
- Feng, J.; Gao, H.; Zheng, L.; Chen, Z.; Zeng, S.; Jiang, C.; Dong, H.; Liu, L.; Zhang, S.; Zhang, X. A Mn-N(3) single-atom catalyst embedded in graphitic carbon nitride for efficient CO(2) electroreduction. Nat. Commun. 2020, 11, 4341. [Google Scholar] [CrossRef]
- Li, T.T.; Mei, Y.; Li, H.; Qian, J.; Wu, M.; Zheng, Y.Q. Highly Selective and Active Electrochemical Reduction of CO(2) to CO on a Polymeric Co(II) Phthalocyanine@Graphitic Carbon Nitride Nanosheet-Carbon Nanotube Composite. Inorg. Chem. 2020, 59, 14184–14192. [Google Scholar] [CrossRef]
- Hussain, N.; Abdelkareem, M.A.; Alawadhi, H.; Elsaid, K.; Olabi, A.G. Synthesis of Cu-g-C3N4/MoS2 composite as a catalyst for electrochemical CO2 reduction to alcohols. Chem. Eng. Sci. 2022, 258, 117757. [Google Scholar] [CrossRef]
- Ding, C.; Feng, C.; Mei, Y.; Liu, F.; Wang, H.; Dupuis, M.; Li, C. Carbon nitride embedded with transition metals for selective electrocatalytic CO2 reduction. Appl. Catal. B Environ. 2020, 268, 118391. [Google Scholar] [CrossRef]
- Holst, J.R.; Gillan, E.G. From triazines to heptazines: Deciphering the local structure of amorphous nitrogen-rich carbon nitride materials. J. Am. Chem. Soc. 2008, 130, 7373–7379. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, B.; Zhou, K.; Yuen, R.K.; Gui, Z.; Hu, Y.; Jiang, S. Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites. J. Hazard. Mater. 2015, 293, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, C.; Hu, X.; Xue, H.; Pang, H. Metal/Graphitic Carbon Nitride Composites: Synthesis, Structures, and Applications. Chem. Asian J. 2016, 11, 3305–3328. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Gu, J.; Wang, Y.; Tang, Z.; Liu, Z.; Huang, Y.; Zhao, J.; Chen, Z.; Zhi, C. Component Matters: Paving the Roadmap toward Enhanced Electrocatalytic Performance of Graphitic C3N4-Based Catalysts via Atomic Tuning. ACS Nano 2017, 11, 6004–6014. [Google Scholar] [CrossRef]
- Mishra, B.P.; Babu, P.; Parida, K. Phosphorous, boron and sulfur doped g-C3N4 nanosheet: Synthesis, characterization, and comparative study towards photocatalytic hydrogen generation. Mater. Today Proc. 2021, 35, 258–262. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, X.; Wang, X.; Tian, Z.; Yang, X.; Lu, J.; Bai, H.; Jiao, T.; Huang, H.; Hu, J. Construction of Ag decorated P-doped g-C3N4 nanosheets Schottky junction via silver mirror reaction for enhanced photocatalytic activities. Int. J. Hydrog. Energy 2022, 47, 250–263. [Google Scholar] [CrossRef]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, K.; Somorjai, G.A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. ChemCatChem 2012, 4, 1512–1524. [Google Scholar] [CrossRef]
- Bhowmik, S.; Phukan, S.J.; Sah, N.K.; Roy, M.; Garai, S.; Iyer, P.K. Review of Graphitic Carbon Nitride and Its Composite Catalysts for Selective Reduction of CO2. ACS Appl. Nano Mater. 2021, 4, 12845–12890. [Google Scholar] [CrossRef]
- Cao, J.; Pan, C.; Ding, Y.; Li, W.; Lv, K.; Tang, H. Constructing nitrogen vacancy introduced g-C3N4 pn homojunction for enhanced photocatalytic activity. J. Environ. Chem. Eng. 2019, 7, 102984. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, Y.; Wang, B.; Li, H.; Dong, W. Novel g-C3N4/BiOClxI1-x nanosheets with rich oxygen vacancies for enhanced photocatalytic degradation of organic contaminants under visible and simulated solar light. Appl. Catal. B Environ. 2019, 256, 117789. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, M.; Huang, G.; Chen, Q.; Bi, J. Interlayer Charge Transfer Over Graphitized Carbon Nitride Enabling Highly-Efficient Photocatalytic Nitrogen Fixation. Small 2022, e2205388. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Lu, W.; Huo, Y.; Bian, L. A sulfur vacancy rich CdS based composite photocatalyst with g-C(3)N(4) as a matrix derived from a Cd-S cluster assembled supramolecular network for H(2) production and VOC removal. Dalton Trans. 2018, 47, 4219–4227. [Google Scholar] [CrossRef]
- Shen, H.; Yang, M.; Hao, L.; Wang, J.; Strunk, J.; Sun, Z. Photocatalytic nitrogen reduction to ammonia: Insights into the role of defect engineering in photocatalysts. Nano Res. 2022, 15, 2773–2809. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Z.; Li, B.; Yu, C. Recent Progress of Diatomic Catalysts: General Design Fundamentals and Diversified Catalytic Applications. Small 2022, 18, e2203589. [Google Scholar] [CrossRef]
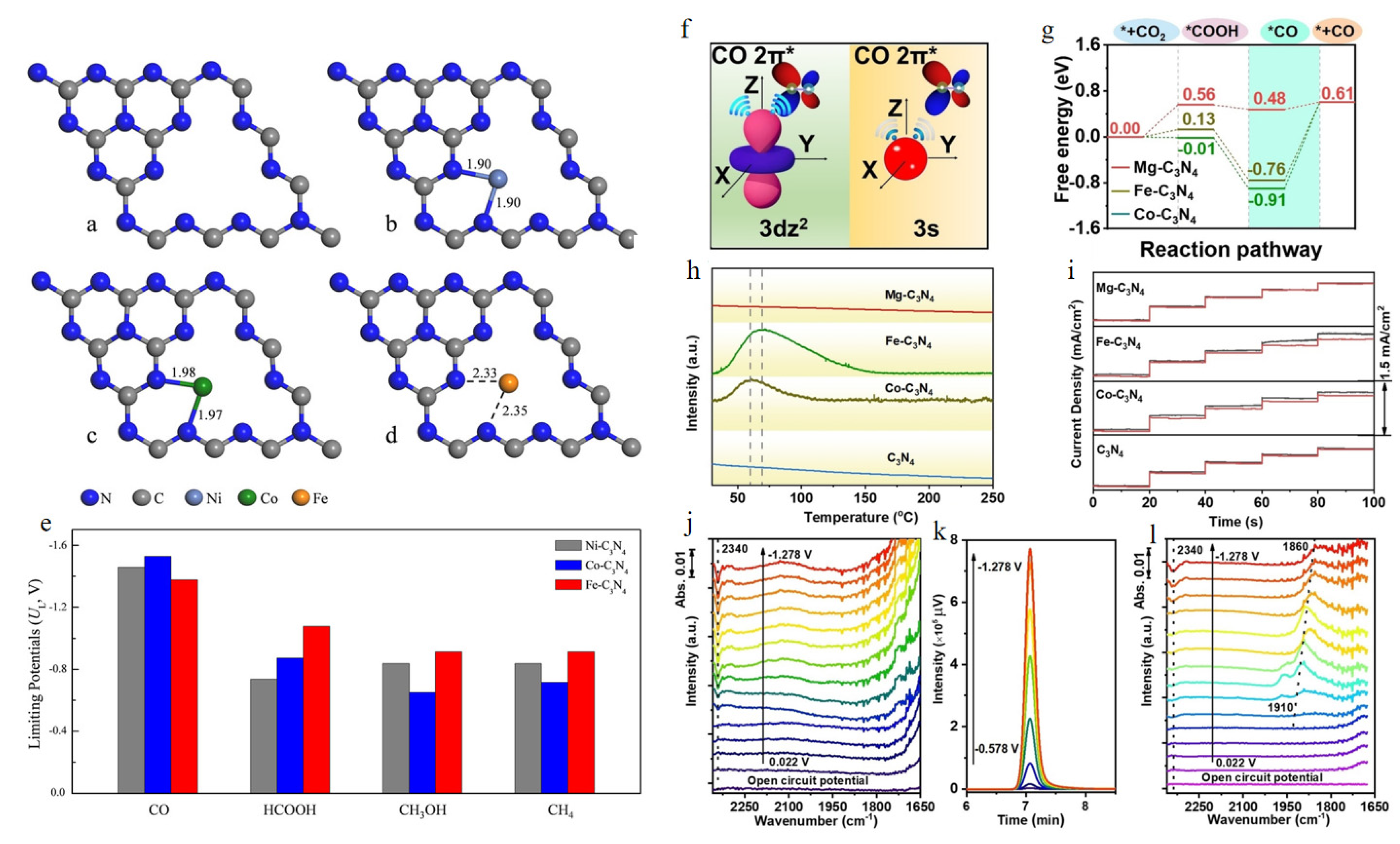
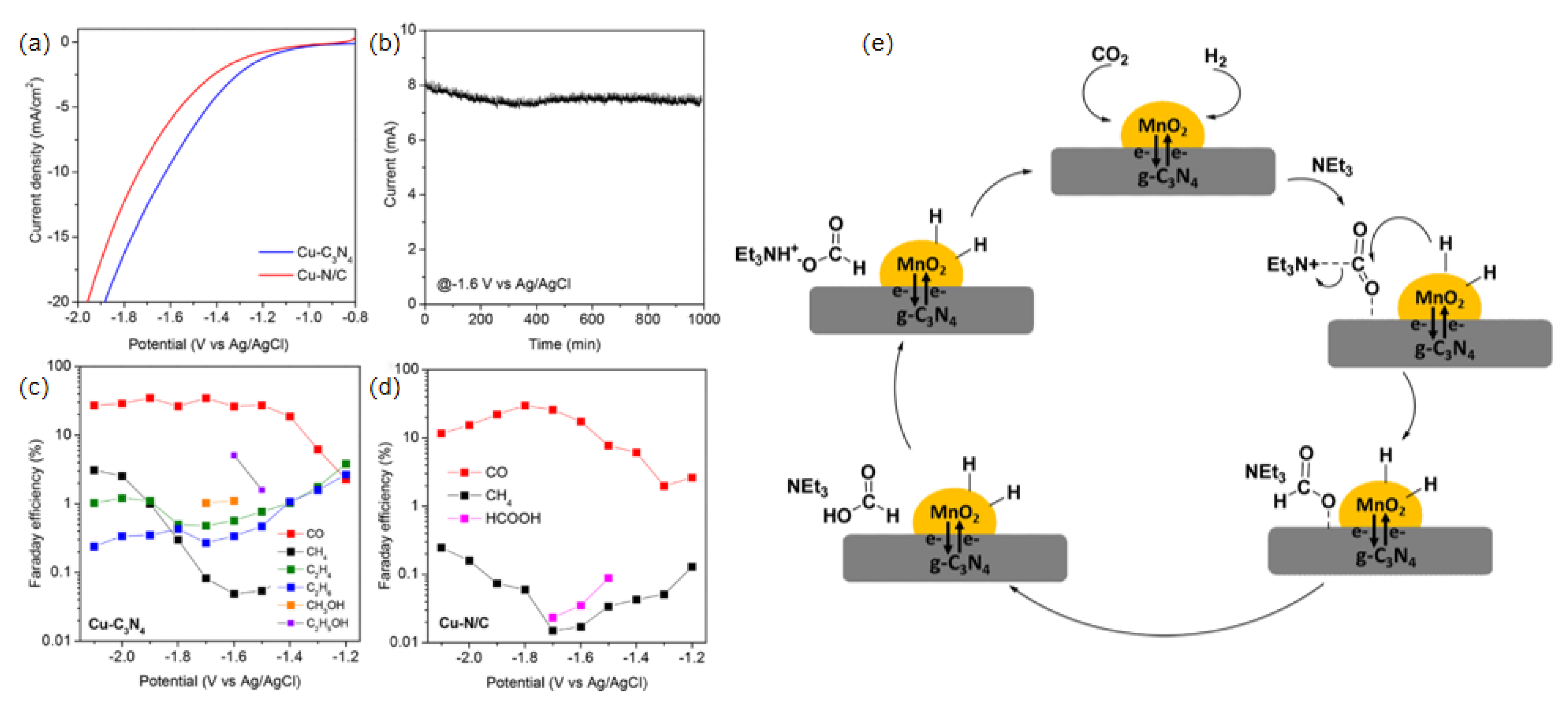

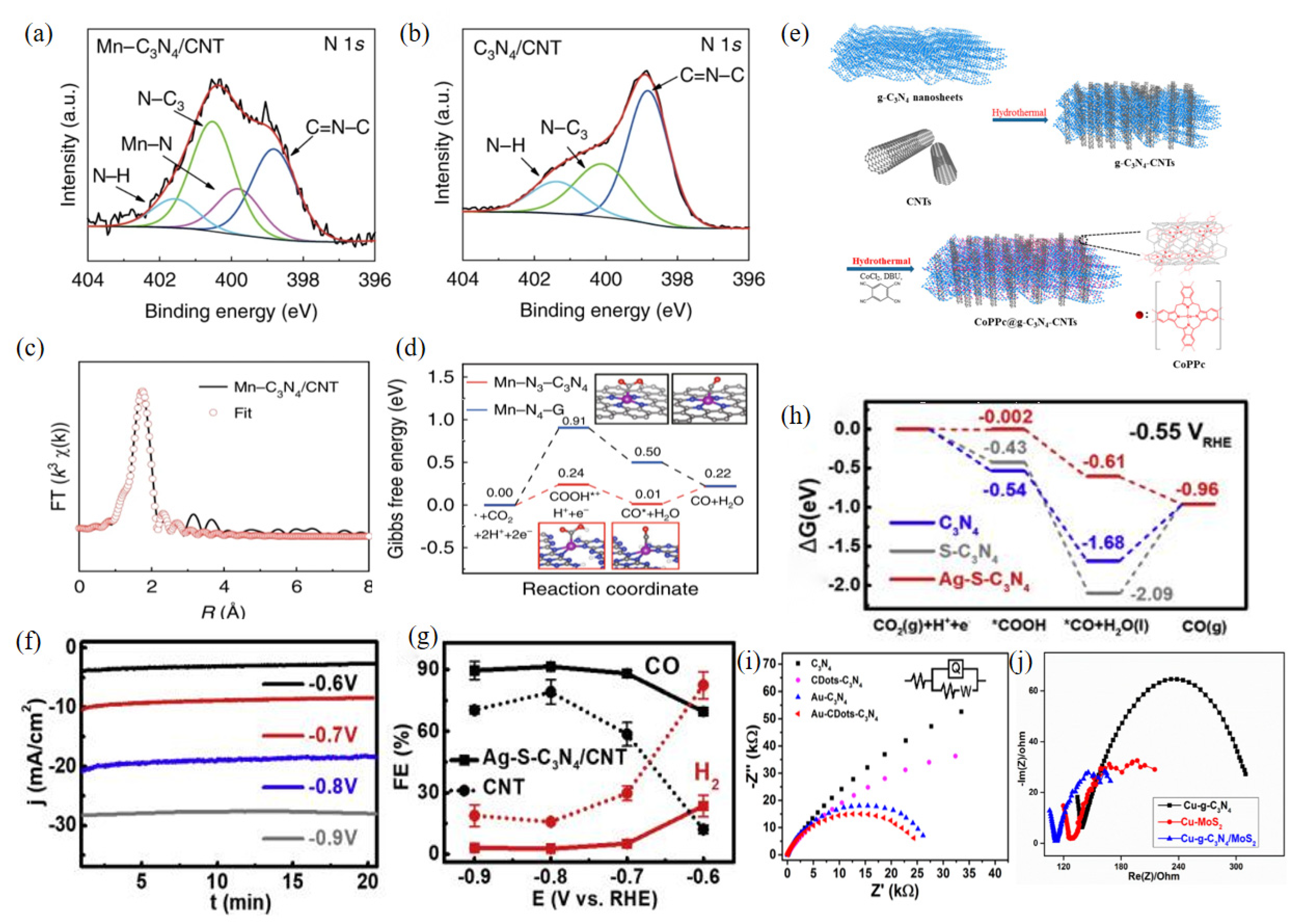
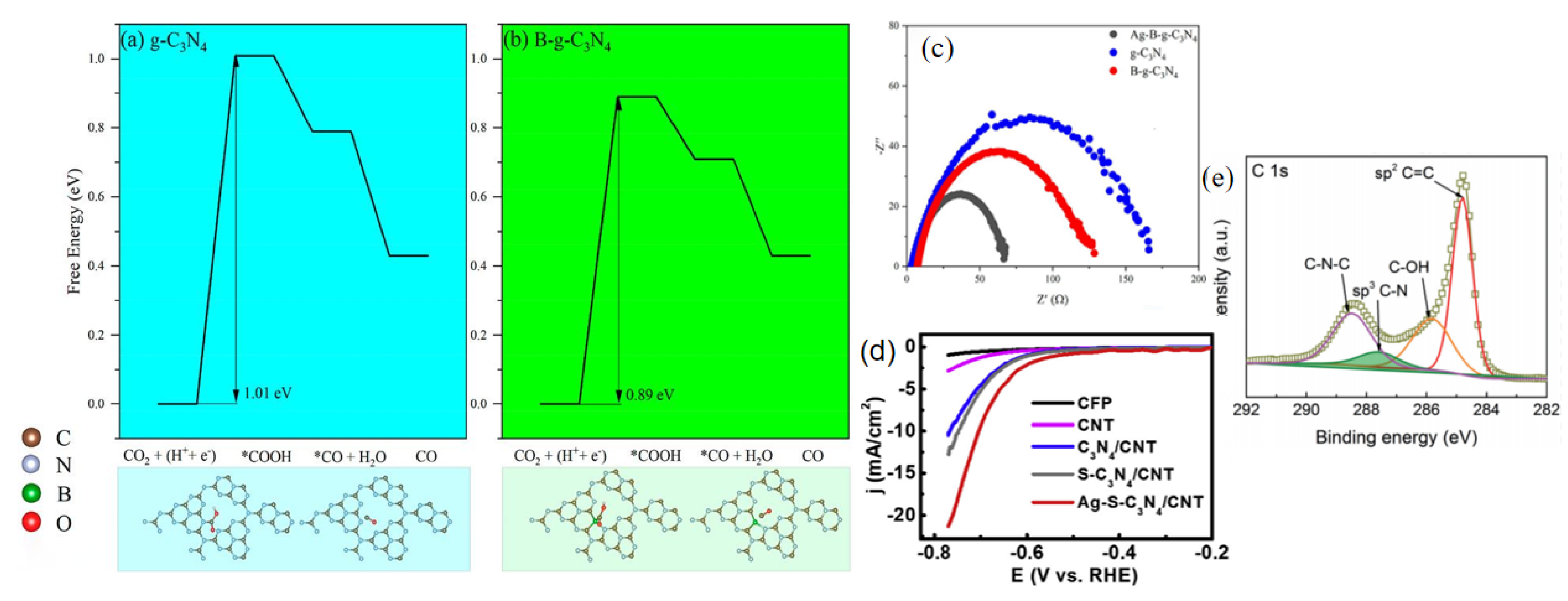
| Chemical Formula and Molecular Formula | Half-Electrochemical Reaction | Potential versus Reversible Hydrogen Electrode (V vs. RHE) | |
|---|---|---|---|
| C1 | HCOOH 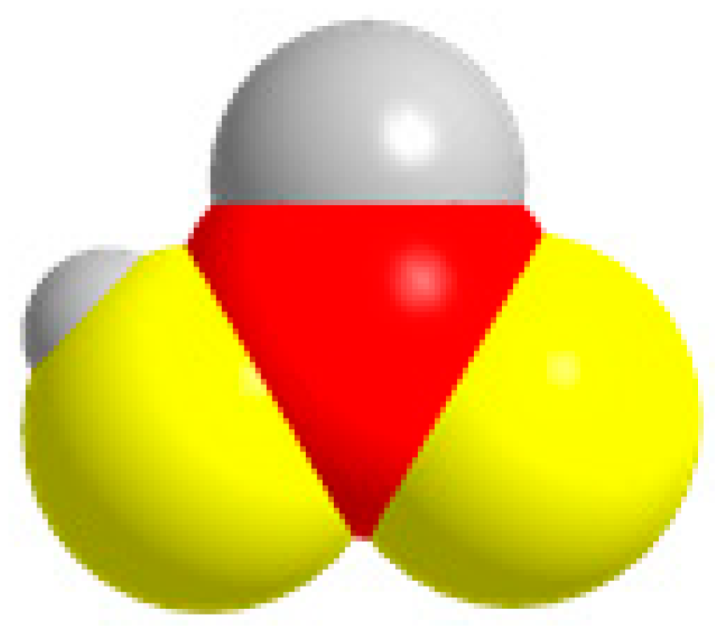 | CO2 + 2H+ + 2e− = HCOOH | −0.651 |
CO 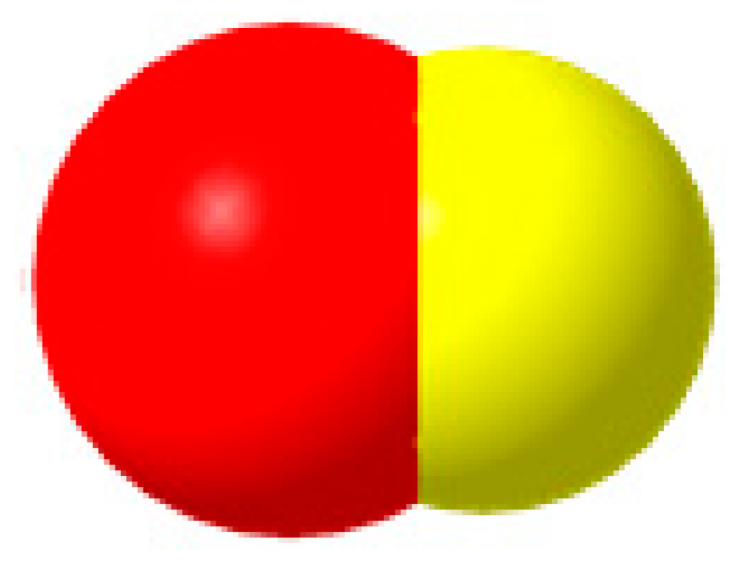 | CO2 + 2H+ + 2e− = CO + H2O | −0.507 | |
CH2O 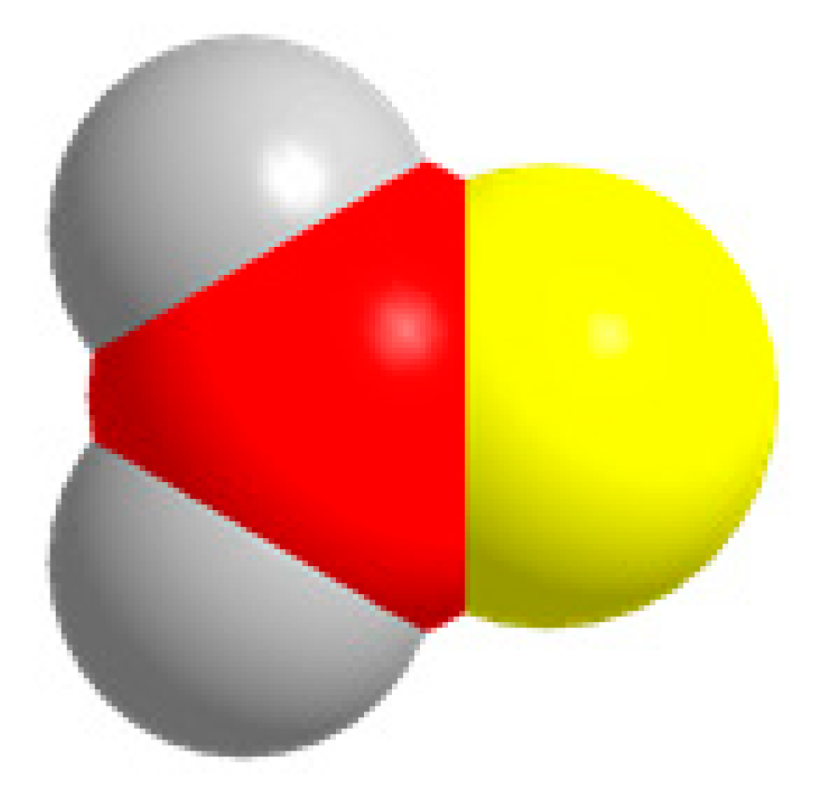 | CO2 + 4H+ + 4e− = CH2O + H2O | −0.471 | |
CH3OH 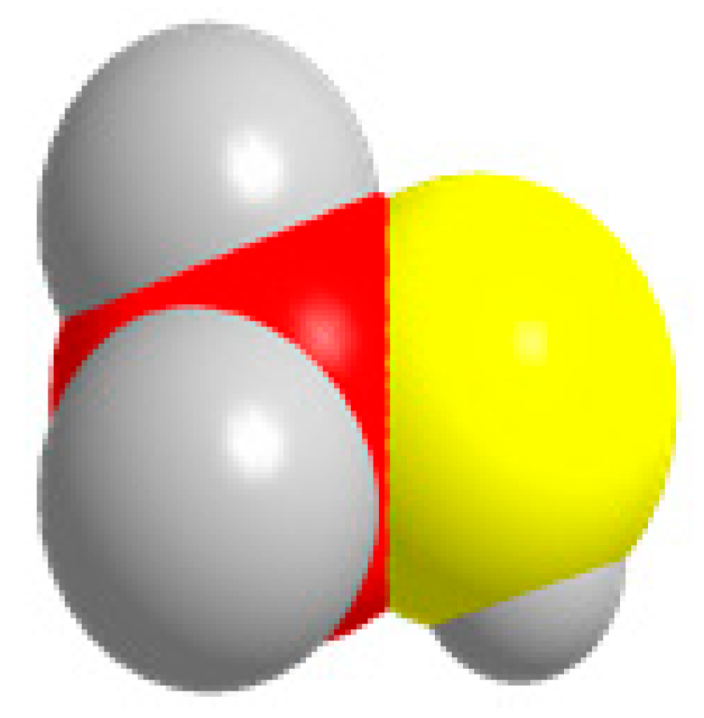 | CO2 + 6H+ + 6e− = CH3OH + H2O | −0.385 | |
CH4  | CO2 + 8H+ + 8e− = CH4 + 2H2O | −0.232 | |
| C2 | C2H2O4 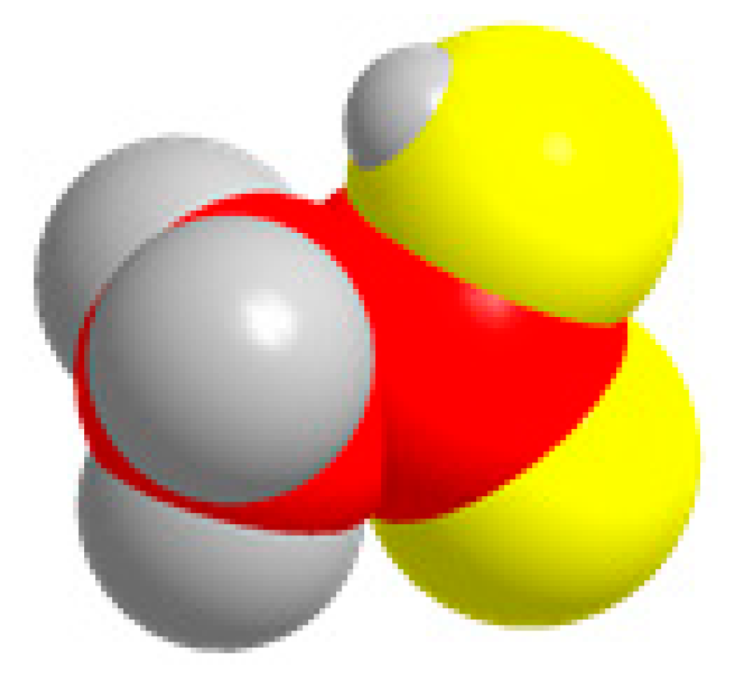 | 2CO2 + 2H+ + 2e− = H2C2O4 | −0.901 |
C2H6 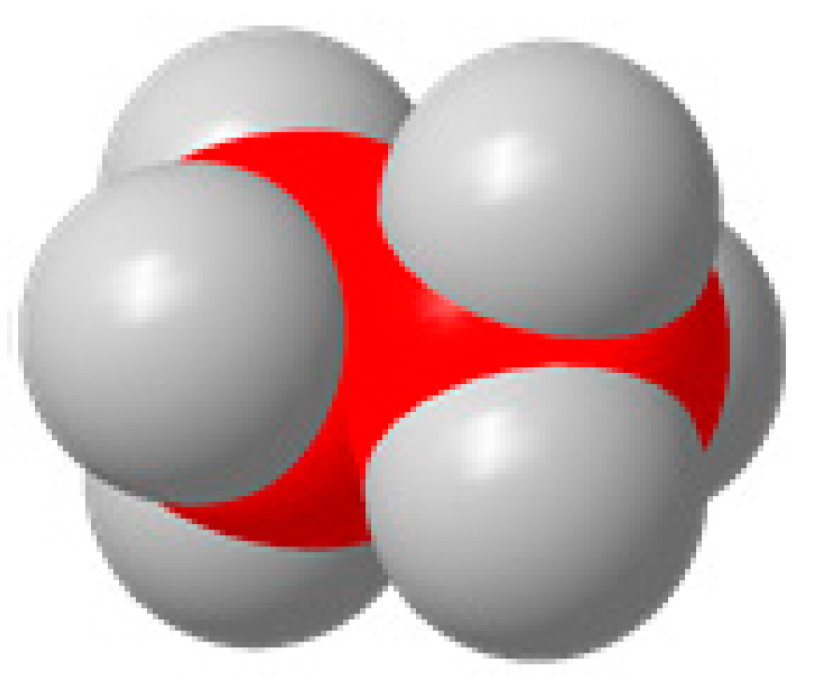 | 2CO2 + 14H+ +14e− = C2H6 + 4H2O | −0.261 | |
C2H4  | 2CO2+ 12H+ + 12e− = CH2CH2 + 4H2O | −0.337 | |
C2H5OH  | 2CO2 + 12H+ + 12e− = CH3CH2OH + 3H2O | −0.3172 |
| Precursors of g-C3N4 | Reaction Method | Specific Surface Area (m2g−1) | Pore Volume (mLg−1) | Pore Diameter (nm) | Reference |
|---|---|---|---|---|---|
| ethylenediamine (EDA) and carbon tetrachloride (CTC) | Hydrothermal synthesis,100 °C | 505 | 0.55 | 4.2 | [57] |
| EDA and CTC | Hydrothermal synthesis,130 °C | 830 | 1.25 | 5.1 | [57] |
| EDA and CTC | Hydrothermal synthesis,150 °C | 650 | 0.89 | 6.4 | [57] |
| Melamine | 470 °C, 2 h, air | 6.0 | 0.02 | 35.2 | [59] |
| Melamine | 500 °C, 2 h, air | 41.5 | 0.14 | 9.2 | [59] |
| Melamine | 520 °C, 2 h, air | 173.6 | 0.77 | 15.6 | [59] |
| Melamine | 540 °C, 2 h, air | 0.77 | 0.94 | 16.5 | [59] |
| urea | 550 °C, 0.5 h, air | 52 | N/A | N/A | [60] |
| urea | 550 °C, 1 h, air | 62 | 0.30 | N/A | [60] |
| urea | 550 °C, 2 h, air | 75 | 0.34 | N/A | [60] |
| urea | 550 °C, 4 h, air | 288 | 1.41 | N/A | [60] |
| Water-assisted urea | 450 °C, 3 h, air | 96 | 0.72 | N/A | [61] |
| Water-assisted urea | 450 °C, 5 h, air | 106 | 0.68 | N/A | [61] |
| Dicyandiamide | 550 °C, 2 h, air | 10 | N/A | N/A | [64] |
| Melamine | 550 °C, 2 h, air | 8.6 | 0.02 | N/A | [59] |
| Thiourea | 550 °C, 2 h, air | 11 | N/A | N/A | [64] |
| Electrode | Product | FE | Potential (V vs. RHE) | Electrolyte | Current Density (mAcm−2) | Ref |
|---|---|---|---|---|---|---|
| Bulk g-C3N4 | CO | 5% | −1.2 | 0.1 M KHCO3 | ca.0 | [70] |
| g-C3N4 | CO | ca.8% | −1.1 | 0.1 M KHCO3 | ca.30 | [75] |
| 2D-pg-C3N4 | CO | 80% | −0.6 | 2 M KHCO3 | 3.05 | [76] |
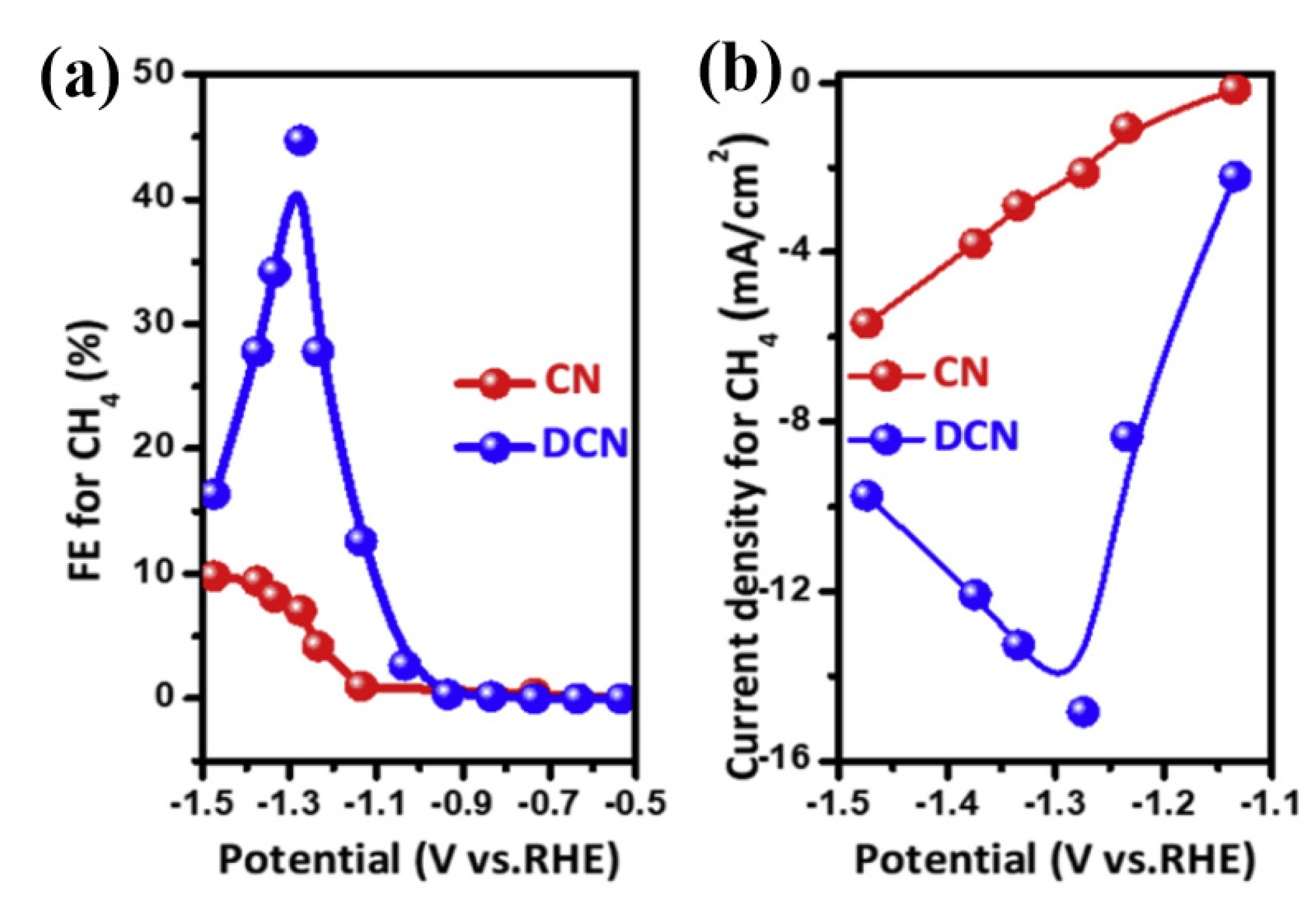
| DCN | CH4 | 44% | −1.27 | 0.5 M KHCO3 | 14.8 | [44] |
| Electrode | Product | FE | Potential (V vs. RHE) | Electrolyte | Current Density (mAcm−2) | Ref |
|---|---|---|---|---|---|---|
| Mg-C3N4 | CO | 90% | −1.178 | KHCO3 | 32 | [86] |
| Ag/g-C3N4 | CO | 94% | −0.7 | 1.0 M KHCO3 | 11.5 | [48] |
| Au/C3N4 | CO | 90% | −0.45 | 0.5 M KHCO3 | 2.56 | [87] |
| Ag/C3N4 | CO | 92% | −0.9 | 0.5 M KHCO3 | 22 | [87] |
| Ag-Decorated B-Doped g-C3N4 | CO | 93.20% | −0.8 | 0.5 M KHCO3 | 2.08 | [70] |
| Fe@C/g-C3N4 | CO | 88% | −0.38 | 0.1 M KHCO3 | 5.5 | [91] |
| ZnO/g-C3N4 | formate | 80.99% | −0.934 | 0.5 M KHCO3 | ca.33 | [90] |
| Cu2O/CN | C2H4 | 32.20% | −1.1 | 0.1 M KHCO3 | −4.3 | [68] |
| Cu/C3N4 | CO | ca.30% | ca.−1.0 | 0.1 M KHCO3 | 8 | [78] |
| MnO2/g-C3N4 | formate | 65.28% | −0.54 | 0.5 M KHCO3 | ca.5 | [88] |
| C3N4/(Co/Co(OH)2) | formate | N/A | −0.9 | 0.5 M KHCO3 | 0.08 | [92] |
| Electrode | Product | FE | Potential (V vs. RHE) | Electrolyte | Current Density (mAcm−2) | Ref |
|---|---|---|---|---|---|---|
| CuSe/g-C3N4 | CO | 85.28% | −1.2 | 0.1 M KHCO3 | 11 | [96] |
| CuxRuyCN | N/A | N/A | −0.8 | 0.1 M KHCO3 | 0.3 | [95] |
| g-C3N4/Cu2O-FeO | CO | 84.40% | −0.9829 | 0.1 M KCl | 3.91 | [98] |
| C3N4/(Co(OH)2/Cu(OH)2 | formate | N/A | −0.9 | 0.5 M KHCO3 | 0.23 | [92] |
| Electrode | Product | FE | Potential (V vs. RHE) | Electrolyte | Current Density (mAcm−2) | Ref. |
|---|---|---|---|---|---|---|
| Mn-C3N4/CNT | CO | 98.8% | −0.5 | 0.5 M KHCO3 | 14 | [100] |
| CoPPc@g C3N4-CNTs | CO | 95% | −0.8 | 0.5 M KHCO3 | 21.9 | [101] |
| Ag–S–C3N4/CNT | CO | 91.40% | −0.77 | 0.1 M KHCO3 | 21.3 | [69] |
| NiCu-C3N4-CNT | CO | ca.90% | −0.8 | 0.5 M KHCO3 | ca.14 | [103] |
| NiMn-C3N4-CNT | CO | ca.90% | −0.8 | 0.5 M KHCO3 | ca.12 | [103] |
| Au-CDots-C3N4 | CO | 79.80% | −0.5 | 0.5 M KHCO3 | 0.29 | [99] |
| Cu-g-C3N4/MoS2 | CH3OH | 19.70% | −0.67 | 0.5 M KHCO3 | 78 | [102] |
| Electrode | Product | FE | Potential (V vs. RHE) | Electrolyte | Current Density (mAcm−2) | Ref |
|---|---|---|---|---|---|---|
| S-C3N4 | CO | N/A | −0.77 | 0.1 M KHCO3 | N/A | [69] |
| C3N4/CNT | CO | N/A | −0.77 | 0.1 M KHCO3 | 10 | [69] |
| g-C3N4/MWCNTs | CO | 60% | −0.75 | 0.1 M KHCO3 | ca. 0.55 | [74] |
| Synthesis Method | Advantages and Disadvantages of Catalyst | The Advantages and Shortcomings of the Method |
|---|---|---|
| Thermal polycondensation | Low specific catalyst surface area, high temperature resistance, and good stability | Easy synthesis, high yield, low cost, part of the precursor powder must be uniformly dispersed before participating in the reaction, reaction temperature at (400–600 °C) |
| Thermal decomposition method | Uniform structure, good heat resistance | Simple reaction process, requires specific atmosphere (Air, N2, Ar, H2) and temperature requirements (200–500 °C) |
| Hydrothermal synthesis | Variety of porous catalysts with regular morphology can be produced according to the characteristics of the template, and good heat resistance | Easy to control synthesis, low yields, long preparation cycles, reaction temperatures between (120–200 °C) |
| Wet chemical reduction | Homogeneous morphology, easy formation of nanocluster structure through doped metal elements, high electrochemical performance | The reaction takes place at room temperature and the reaction steps are cumbersome. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, X.; Guo, R.; Chen, Q.; Zhu, H.; Li, H.; Yan, Z.; Guo, Z.; Wu, T. Recent Advances in Graphitic Carbon Nitride Based Electro-Catalysts for CO2 Reduction Reactions. Molecules 2023, 28, 3292. https://doi.org/10.3390/molecules28083292
Mao X, Guo R, Chen Q, Zhu H, Li H, Yan Z, Guo Z, Wu T. Recent Advances in Graphitic Carbon Nitride Based Electro-Catalysts for CO2 Reduction Reactions. Molecules. 2023; 28(8):3292. https://doi.org/10.3390/molecules28083292
Chicago/Turabian StyleMao, Xinyi, Ruitang Guo, Quhan Chen, Huiwen Zhu, Hongzhe Li, Zijun Yan, Zeyu Guo, and Tao Wu. 2023. "Recent Advances in Graphitic Carbon Nitride Based Electro-Catalysts for CO2 Reduction Reactions" Molecules 28, no. 8: 3292. https://doi.org/10.3390/molecules28083292
APA StyleMao, X., Guo, R., Chen, Q., Zhu, H., Li, H., Yan, Z., Guo, Z., & Wu, T. (2023). Recent Advances in Graphitic Carbon Nitride Based Electro-Catalysts for CO2 Reduction Reactions. Molecules, 28(8), 3292. https://doi.org/10.3390/molecules28083292






