Fast and Selective Degradation of Biomass for Xylose, Glucose and Lignin under Mild Conditions
Abstract
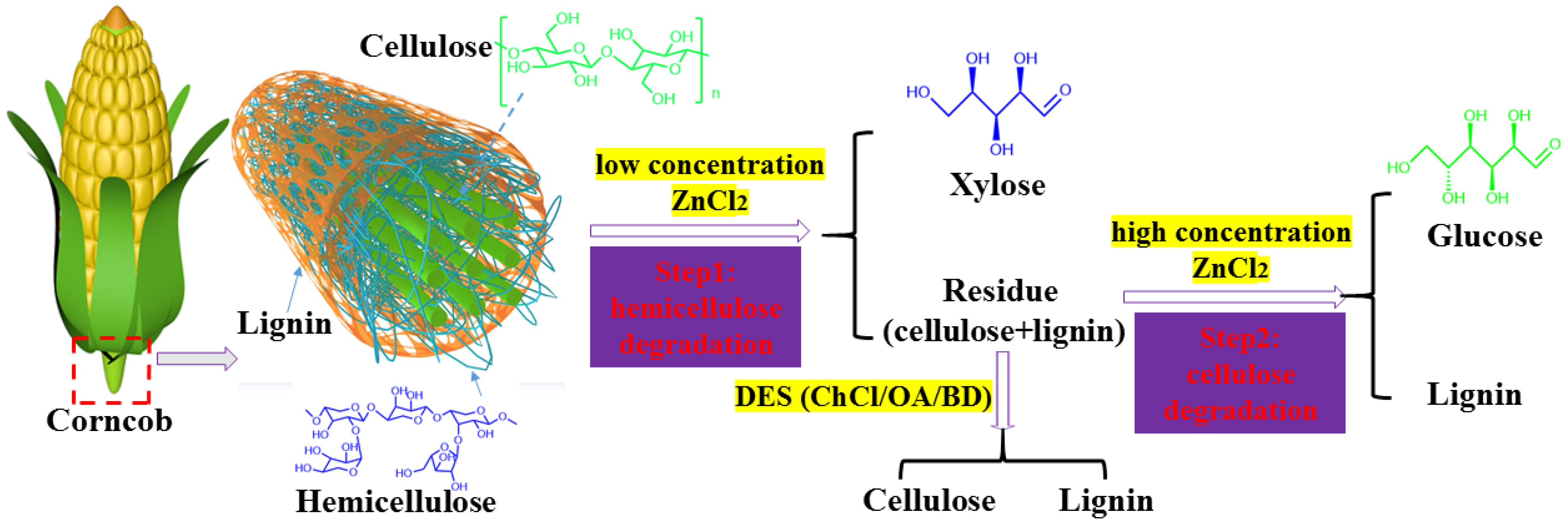
:1. Introduction
2. Result and Discussion
2.1. Effect of ZnCl2 Content
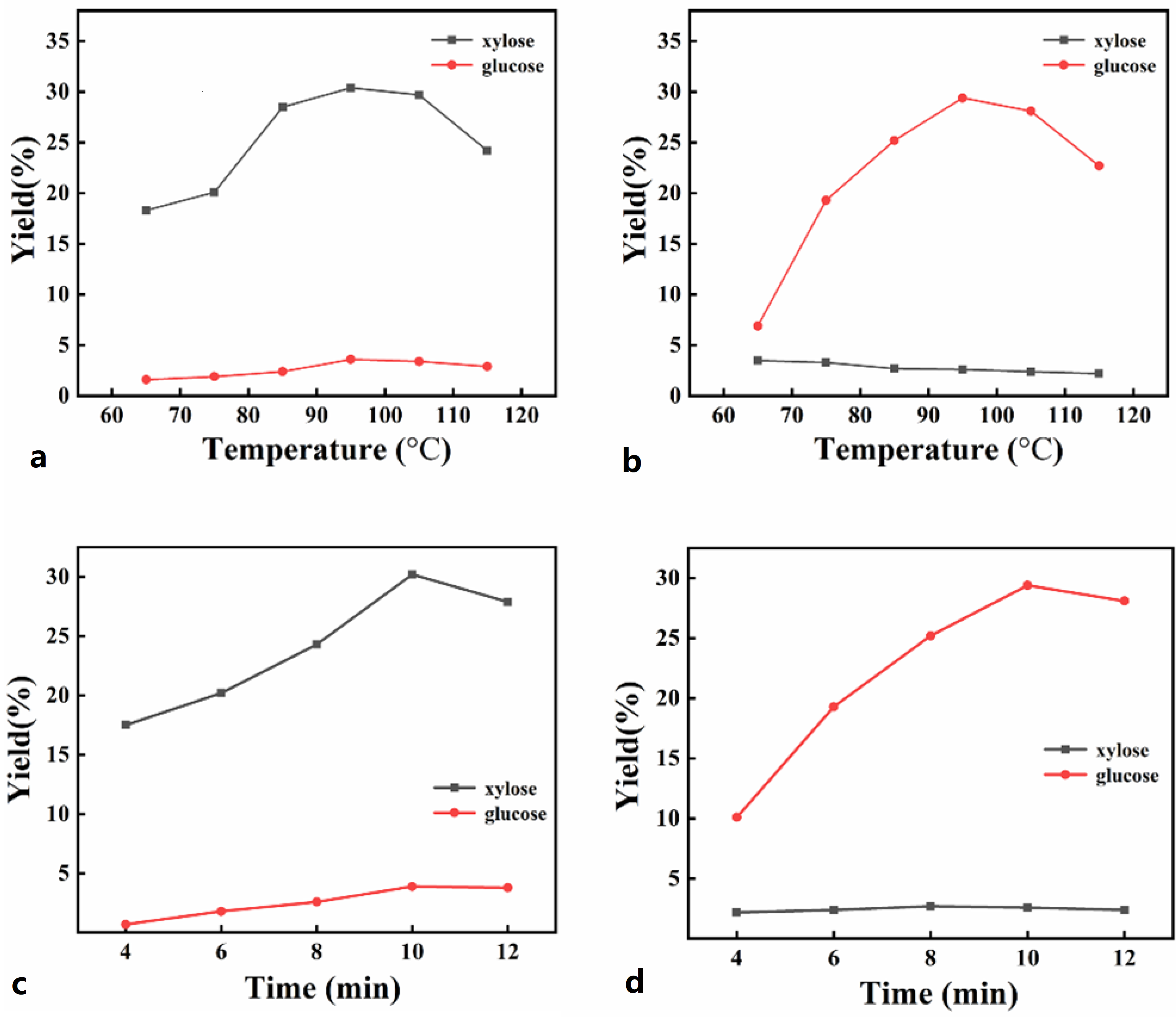
2.2. Effect of Temperature
2.3. Effect of Time
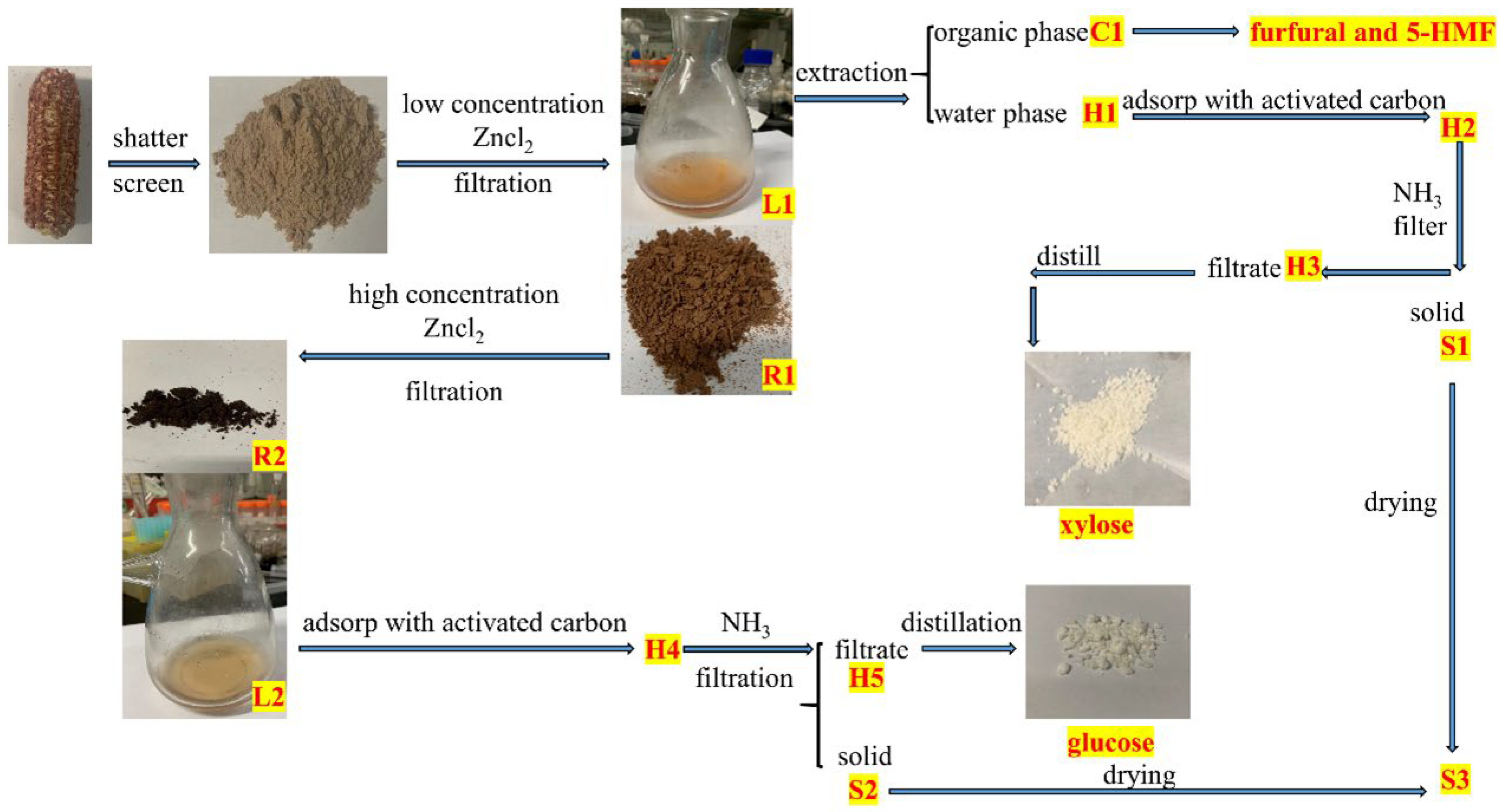
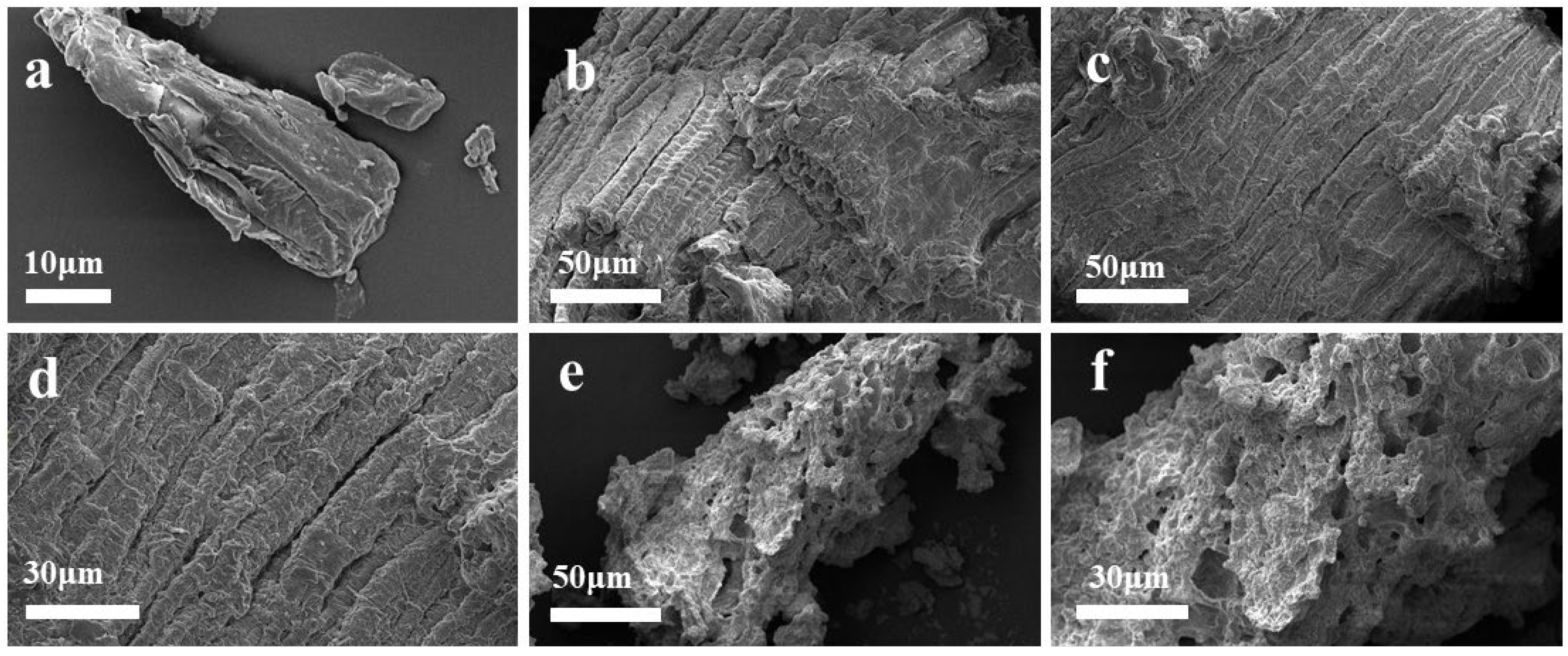
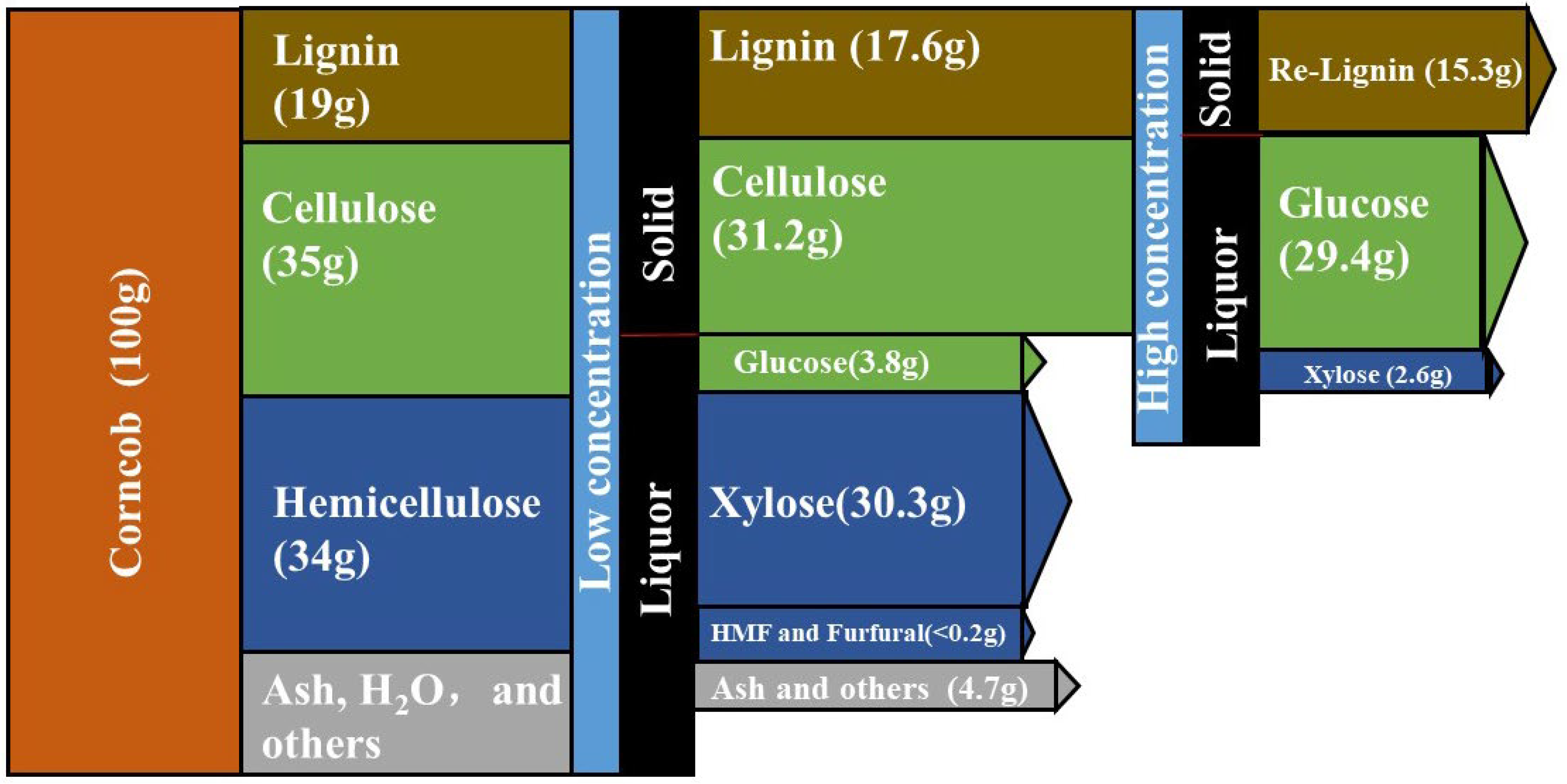
2.4. Characterization of the Intermediates
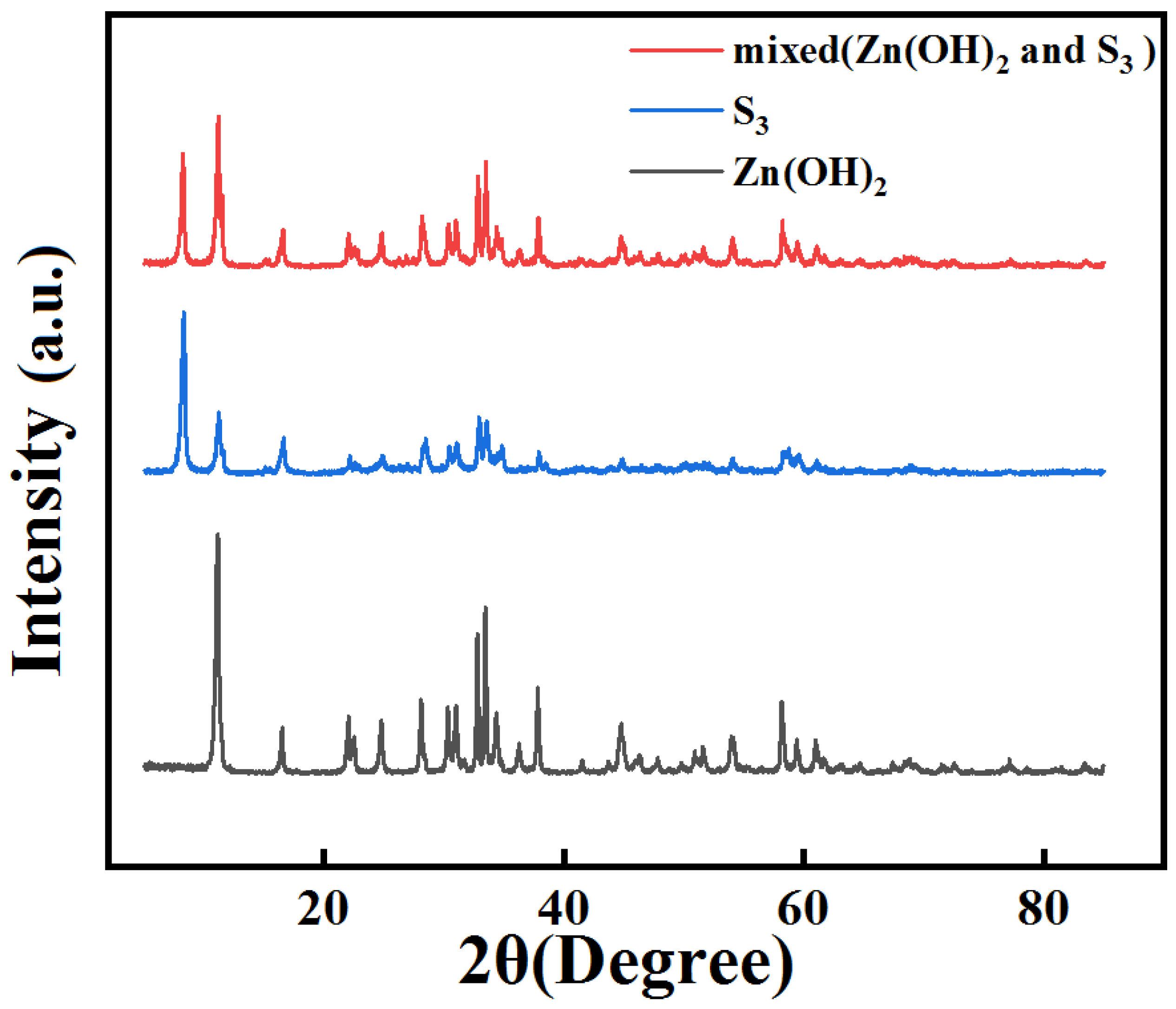
2.5. Recovery of Zinc Ions
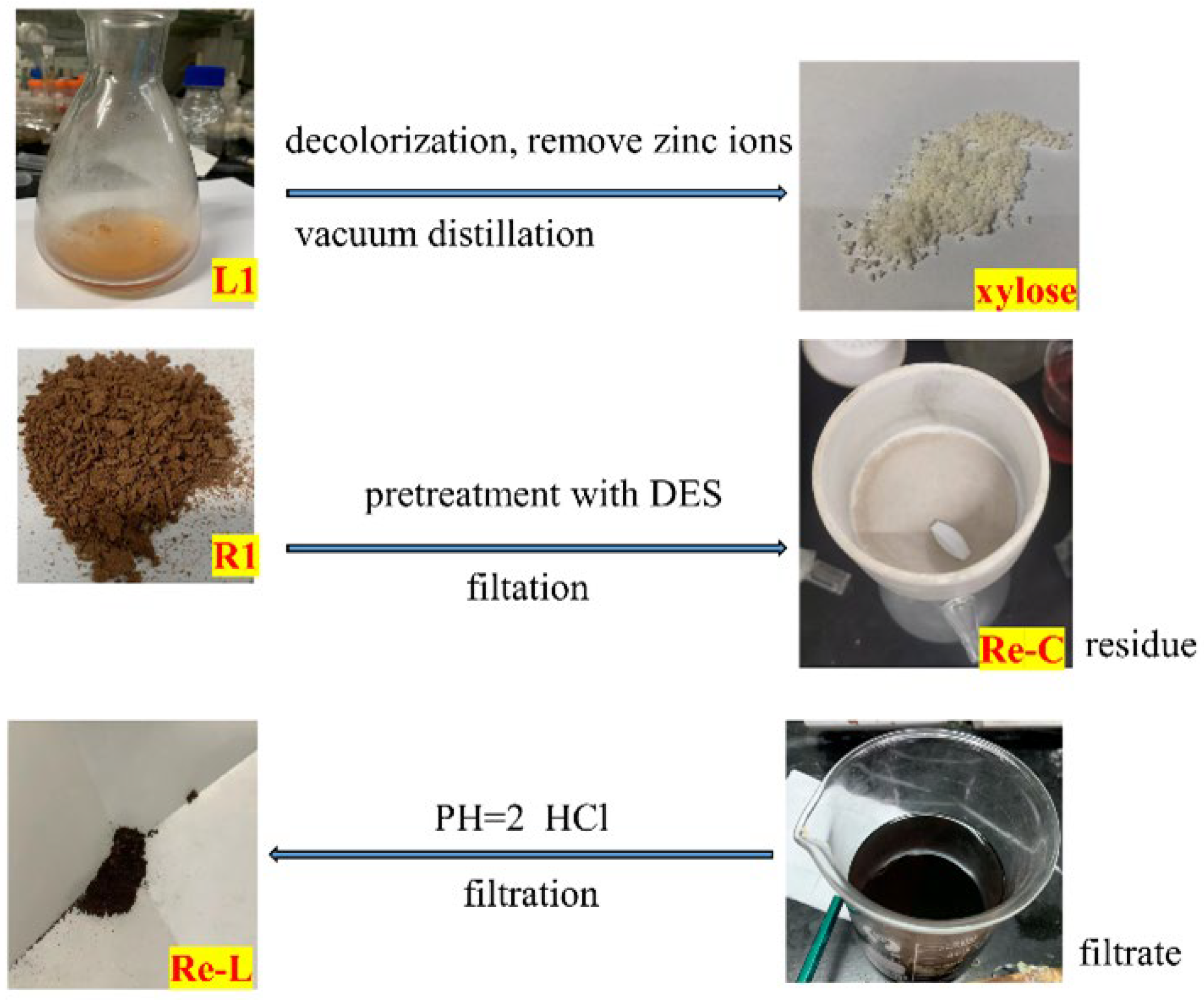
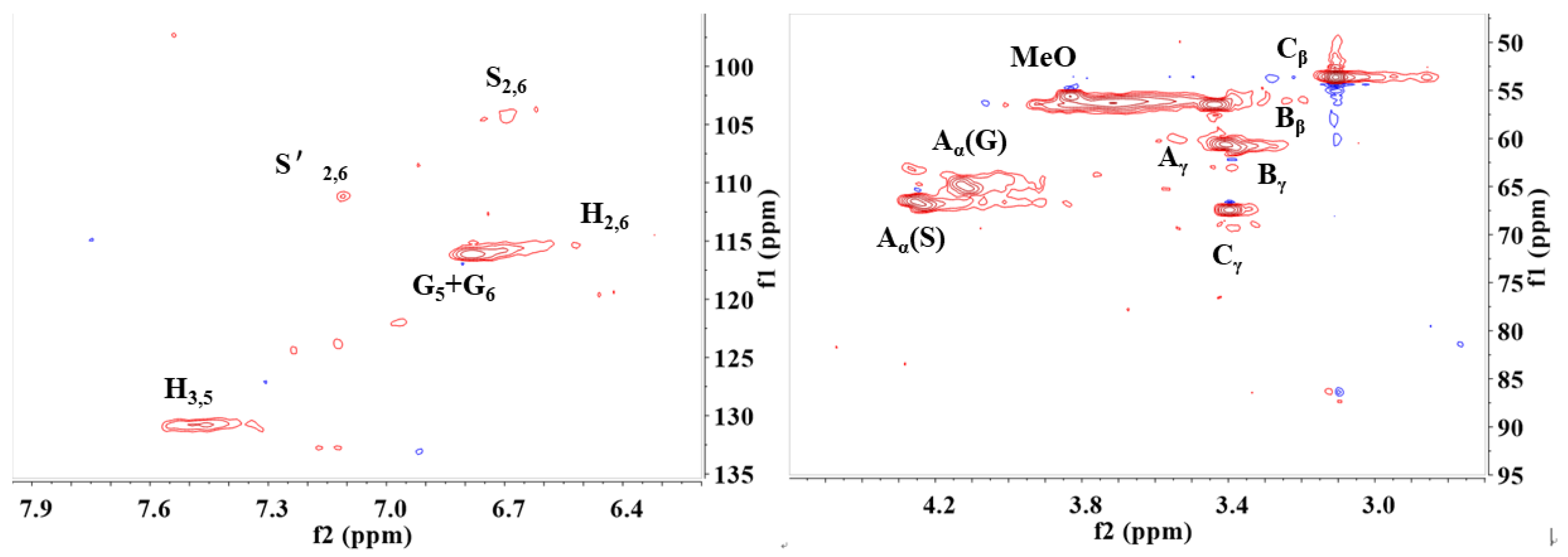
2.6. The Formation of Lignin-R2
2.7. The Formation of Re-C and Re-L
3. Materials and Methods
3.1. Materials
3.2. Selective Degradation of Hemicellulose: Lower Concentration ZnCl2 Solution Treatment
3.3. Selective Degradation of Cellulose: High Concentration ZnCl2 Solution Treatment
3.4. Fractionation of Lignocellulose in DES
3.5. Method of Recovery of Zinc Ion
3.6. Residual Zinc Ions in the Products
3.7. Calculation Method of the Parameters
3.8. SEM Characterization of Corncob and R1, R2
3.9. FT-IR Characterization of Corncob, MCC, R1, and R2
3.10. XRD Characterization of Corncob, MCC, R1, and R2
3.11. Determination of Xylose and Glucose in the Filtrate L1 and L2 by Means of HPLC
3.12. Determination of Furfural and 5-HMF in the C1 by Means of HPLC
3.13. The XRD Characterization of Recovered Zn(OH)2
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhou, C.H.; Xia, X.; Lin, C.X.; Tong, D.S.; Beltramini, J. Catalytic conversion of lignocellulosic biomass to fine chemicals and fuels. Chem. Soc. Rev. 2011, 40, 5588–5617. [Google Scholar] [CrossRef]
- Scapini, T.; Dos Santos, M.S.N.; Bonatto, C.; Wancura, J.H.C.; Mulinari, J.; Camargo, A.F.; Klanovicz, N.; Zabot, G.L.; Tres, M.V.; Fongaro, G.; et al. Hydrothermal pretreatment of lignocellulosic biomass for hemicellulose recovery. Bioresour. Technol. 2021, 342, 126033. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, B.; Zheng, X.; Yu, L.; Li, J. Integrated microwave and alkaline treatment for the separation between hemicelluloses and cellulose from cellulosic fibers. Bioresour. Technol. 2018, 247, 859–863. [Google Scholar] [CrossRef]
- Jiang, Z.; Budarin, V.L.; Fan, J.; Remón, J.; Li, T.; Hu, C.; Clark, J.H. Sodium Chloride-Assisted Depolymerization of Xylo-oligomers to Xylose. ACS Sustain. Chem. Eng. 2018, 6, 4098–4104. [Google Scholar] [CrossRef]
- Zhou, X.; Li, W.; Mabon, R.; Broadbelt, L.J. A mechanistic model of fast pyrolysis of hemicellulose. Energy Environ. Sci. 2018, 11, 1240–1260. [Google Scholar] [CrossRef]
- Ding, J.; Yoo, C.G.; Pu, Y.; Meng, X.; Bhagia, S.; Yu, C.; Ragauskas, A.J. Cellulolytic enzyme-aided extraction of hemicellulose from switchgrass and its characteristics. Green Chem. 2019, 21, 3902–3910. [Google Scholar] [CrossRef]
- Hernandez-Perez, A.F.; Costa, I.A.; Silva, D.D.; Dussan, K.J.; Villela, T.R.; Canettieri, E.V.; Carvalho, J.A., Jr.; Soares Neto, T.G.; Felipe, M.G. Biochemical conversion of sugarcane straw hemicellulosic hydrolyzate supplemented with co-substrates for xylitol production. Bioresour. Technol. 2016, 200, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.M.; Zhang, T.; Kumar, R.; Wyman, C.E. Integrated furfural production as a renewable fuel and chemical platform from lignocellulosic biomass. J. Chem. Technol. Biotechnol. 2014, 89, 2–10. [Google Scholar] [CrossRef]
- Monteiro, C.R.M.; Avila, P.F.; Pereira, M.A.F.; Pereira, G.N.; Bordignon, S.E.; Zanella, E.; Stambuk, B.U.; de Oliveira, D.; Goldbeck, R.; Poletto, P. Hydrothermal treatment on depolymerization of hemicellulose of mango seed shell for the production of xylooligosaccharides. Carbohydr. Polym. 2021, 253, 117274. [Google Scholar] [CrossRef]
- Perez, R.F.; Fraga, M.A. Hemicellulose-derived chemicals: One-step production of furfuryl alcohol from xylose. Green Chem. 2014, 16, 3942–3950. [Google Scholar] [CrossRef]
- Kuzmina, O.; Bhardwaj, J.; Vincent, S.R.; Wanasekara, N.D.; Kalossaka, L.M.; Griffith, J.; Potthast, A.; Rahatekar, S.; Eichhorn, S.J.; Welton, T. Superbase ionic liquids for effective cellulose processing from dissolution to carbonization. Green Chem. 2017, 19, 5949–5957. [Google Scholar] [CrossRef]
- FitzPatrick, M.; Champagne, P.; Cunningham, M.F.; Whitney, R.A. A biorefinery processing perspective: Treatment of lignocellulosic materials for the production of value-added products. Bioresour. Technol. 2010, 101, 8915–8922. [Google Scholar] [CrossRef]
- Ghasemi, M.; Tsianou, M.; Alexandridis, P. Assessment of solvents for cellulose dissolution. Bioresour. Technol. 2017, 228, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Li, L.; Liu, C.; Xu, A. Saccharification of cellulose in the ionic liquids and glucose recovery. Renew. Energy 2017, 106, 99–102. [Google Scholar] [CrossRef]
- Wu, T.; Li, N.; Pan, X.; Chen, S.-L. Homogenous hydrolysis of cellulose to glucose in an inorganic ionic liquid catalyzed by zeolites. Cellulose 2020, 27, 9201–9215. [Google Scholar] [CrossRef]
- Sixta, H.; Michud, A.; Hauru, L.; Asaadi, S.; Ma, Y.; King AW, T.; Kilpeläinen, I.; Hummel, M. Ioncell-F: A high-strength regenerated cellulose fibre. Nordic Pulp Paper Res. J. 2015, 30, 43–57. [Google Scholar] [CrossRef]
- Li, Z.; Su, K.; Ren, J.; Yang, D.; Cheng, B.; Kim, C.K.; Yao, X. Direct catalytic conversion of glucose and cellulose. Green Chem. 2018, 20, 863–872. [Google Scholar] [CrossRef]
- Yan, L.; Ma, R.; Wei, H.; Li, L.; Zou, B.; Xu, Y. Ruthenium trichloride catalyzed conversion of cellulose into 5-hydroxymethylfurfural in biphasic system. Bioresour. Technol. 2019, 279, 84–91. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, G.; Gao, M.; Cao, S.; Li, L.; Liu, S.; Xie, C.; Huang, L.; Yu, S.; Ragauskas, A.J. Clean production of 5-hydroxymethylfurfural from cellulose using a hydrothermal/biomass-based carbon catalyst. J. Clean. Prod. 2019, 213, 1096–1102. [Google Scholar] [CrossRef]
- Wang, J.; Xi, J.; Wang, Y. Recent advances in the catalytic production of glucose from lignocellulosic biomass. Green Chem. 2015, 17, 737–751. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Ragauskas, A. Perspective on Technical Lignin Fractionation. ACS Sustain. Chem. Eng. 2020, 8, 8086–8101. [Google Scholar] [CrossRef]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Xia, Q.; Liu, Y.; Meng, J.; Cheng, W.; Chen, W.; Liu, S.; Liu, Y.; Li, J.; Yu, H. Multiple hydrogen bond coordination in three-constituent deep eutectic solvents enhances lignin fractionation from biomass. Green Chem. 2018, 20, 2711–2721. [Google Scholar] [CrossRef]
- Yang, Q.; Huo, D.; Si, C.; Fang, G.; Liu, Q.; Hou, Q.; Chen, X.; Zhang, F. Improving enzymatic saccharification of eucalyptus with a pretreatment process using MgCl2. Ind. Crops Prod. 2018, 123, 401–406. [Google Scholar] [CrossRef]
- Kamireddy, S.R.; Li, J.; Tucker, M.; Degenstein, J.; Ji, Y. Effects and Mechanism of Metal Chloride Salts on Pretreatment and Enzymatic Digestibility of Corn Stover. Ind. Eng. Chem. Res. 2013, 52, 1775–1782. [Google Scholar] [CrossRef]
- Nemoto, J.; Nakamata, K. All-cellulose material prepared using aqueous zinc chloride solution. Cellulose 2021, 29, 2795–2803. [Google Scholar] [CrossRef]
- Lin, M.; Shang, X.; Liu, P.; Xie, F.; Chen, X.; Sun, Y.; Wan, J. Zinc chloride aqueous solution as a solvent for starch. Carbohydr. Polym. 2016, 136, 266–273. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, R.J.; Losey, B.P.; Folmer, J.C.; Martin, J.D.; Zeller, M.; Sommer, R. Crystalline and liquid structure of zinc chloride trihydrate: A unique ionic liquid. Inorg. Chem. 2015, 54, 1109–1119. [Google Scholar] [CrossRef]
- Liu, W.; Wu, R.; Wang, B.; Hu, Y.; Hou, Q.; Zhang, P.; Wu, R. Comparative study on different pretreatment on enzymatic hydrolysis of corncob residues. Bioresour. Technol. 2020, 295, 122244. [Google Scholar] [CrossRef]
- Boonchuay, P.; Techapun, C.; Leksawasdi, N.; Seesuriyachan, P.; Hanmoungjai, P.; Watanabe, M.; Takenaka, S.; Chaiyaso, T. An integrated process for xylooligosaccharide and bioethanol production from corncob. Bioresour. Technol. 2018, 256, 399–407. [Google Scholar] [CrossRef]
- Wen, P.; Liao, H.; Zhu, J.; Xu, Y.; Zhang, J. Production of xylo-oligosaccharides and ethanol from corncob by combined tartaric acid hydrolysis with simultaneous saccharification and fermentation. Bioresour. Technol. 2022, 363, 127977. [Google Scholar] [CrossRef]
- Luo, Y.; Wei, M.; Jiang, B.; Zhang, M.; Miao, Q.; Fu, H.; Clark, J.H.; Fan, J. A hemicellulose and lignin-first process for corn stover valorization catalyzed by aluminum sulfate in γ-butyrolactone/water co-solvent. Green Chem. 2022, 24, 7429–7441. [Google Scholar] [CrossRef]
- Ruzene, D.S.; Silva, D.P.; Vicente, A.A.; Goncalves, A.R.; Teixeira, J.A. An alternative application to the Portuguese agro-industrial residue: Wheat straw. Appl. Biochem. Biotechnol. 2008, 147, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Liao, H.; Li, X.; Lian, Z.; Xu, Y.; Zhang, J. Two-step acetic acid/sodium acetate and xylanase hydrolysis for xylooligosaccharides production from corncob. Bioresour. Technol. 2021, 342, 125979. [Google Scholar] [CrossRef]
- Liao, H.; Xu, Y.; Zhang, J. Efficient production of xylooligosaccharides and fermentable sugars from corncob by propionic acid and enzymatic hydrolysis. Bioresour. Technol. 2021, 342, 125680. [Google Scholar] [CrossRef]
- Hao, X.; Xu, F.; Zhang, J. Effect of pretreatments on production of xylooligosaccharides and monosaccharides from corncob by a two-step hydrolysis. Carbohydr. Polym. 2022, 285, 119217. [Google Scholar] [CrossRef]
- Valladares-Diestra, K.K.; Porto de Souza Vandenberghe, L.; Soccol, C.R. Integrated xylooligosaccharides production from imidazole-treated sugarcane bagasse with application of in house produced enzymes. Bioresour. Technol. 2022, 362, 127800. [Google Scholar] [CrossRef]
- Bachosz, K.; Zdarta, J.; Nghiem, L.D.; Jesionowski, T. Multienzymatic conversion of monosaccharides from birch biomass after pretreatment. Environ. Technol. Innov. 2022, 28, 102874. [Google Scholar] [CrossRef]
- Zhang, F.; Lan, W.; Zhang, A.; Liu, C. Green approach to produce xylo-oligosaccharides and glucose by mechanical-hydrothermal pretreatment. Bioresour. Technol. 2022, 344, 126298. [Google Scholar] [CrossRef]
- Fang, L.; Su, Y.; Wang, P.; Lai, C.; Huang, C.; Ling, Z.; Yong, Q. Co-production of xylooligosaccharides and glucose from birch sawdust by hot water pretreatment and enzymatic hydrolysis. Bioresour. Technol. 2022, 348, 126795. [Google Scholar] [CrossRef]
- Jiang, Z.; Gao, M.; Ding, W.; Huang, C.; Hu, C.; Shi, B.; Tsang, D.C.W. Selective degradation and oxidation of hemicellulose in corncob to oligosaccharides: From biomass into masking agent for sustainable leather tanning. J. Hazard. Mater. 2021, 413, 125425. [Google Scholar] [CrossRef]
- Huo Conceptulization, D.; Sun, Y.; Yang, Q.; Zhang, F.; Fang, G.; Zhu, H.; Liu, Y. Selective Degradation of Hemicellulose and Lignin for Improving Enzymolysis Efficiency via Pretreatment Using Deep Eutectic Solvents. Bioresour. Technol. 2023, 376, 128937. [Google Scholar] [CrossRef]
- Rusanen, A.; Lappalainen, K.; Kärkkäinen, J.; Tuuttila, T.; Mikola, M.; Lassi, U. Selective hemicellulose hydrolysis of Scots pine sawdust. Biomass Convers. Biorefinery 2018, 9, 283–291. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Chen, L.-F. Ultraviolet spectra and structure of zinc-cellulose complexes in zinc chloride solution. J. Appl. Polym. Sci. 1999, 71, 1441–1446. [Google Scholar] [CrossRef]
- Zhang, X.F.; Hou, T.; Chen, J.; Feng, Y.; Li, B.; Gu, X.; He, M.; Yao, J. Facilitated Transport of CO(2) through the Transparent and Flexible Cellulose Membrane Promoted by Fixed-Site Carrier. ACS Appl. Mater. Interfaces 2018, 10, 24930–24936. [Google Scholar] [CrossRef]
- Su, H.; Bi, Z.; Ni, Y.; Yan, L. One-pot Degradation of Cellulose into Carbon Dots and Organic Acids in its Homogeneous Aqueous Solution. Green Energy Environ. 2019, 4, 391–399. [Google Scholar] [CrossRef]
- Xia, C.; Zhu, S.; Feng, T.; Yang, M.; Yang, B. Evolution and Synthesis of Carbon Dots: From Carbon Dots to Carbonized Polymer Dots. Adv. Sci. 2019, 6, 1901316. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, J.; Deng, Y.; Wang, Y.; Yan, L. Synthesis of Highly Ion-Conductive Lignin Eutectogels in a Ternary Deep Eutectic Solvent and Nitrogen-Doped 3D Hierarchical Porous Carbons for Supercapacitors. Biomacromolecules 2021, 22, 4181–4190. [Google Scholar] [CrossRef]
- Bi, Z.; Lai, B.; Zhao, Y.; Yan, L. Fast Disassembly of Lignocellulosic Biomass to Lignin and Sugars by Molten Salt Hydrate at Low Temperature for Overall Biorefinery. ACS Omega 2018, 3, 2984–2993. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.; Wang, X.; Sang, H.; Hou, Y.; Chen, X.; Feng, X. Dissolution behavior of microcrystalline cellulose in DBU-based deep eutectic solvents: Insights from spectroscopic investigation and quantum chemical calculations. J. Mol. Liq. 2020, 299, 112140. [Google Scholar] [CrossRef]
- Meenatchi, B.; Renuga, V.; Manikandan, A. Cellulose dissolution and regeneration using various imidazolium based protic ionic liquids. J. Mol. Liq. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Yao, L.; Zhou, Z.; Zhang, Z.; Du, X.; Zhang, Q.-L.; Yang, H. Dyeing-Inspired Sustainable and Low-Cost Modified Cellulose-Based TENG for Energy Harvesting and Sensing. ACS Sustain. Chem. Eng. 2022, 10, 3909–3919. [Google Scholar] [CrossRef]
- Ding, Z.-Y.; Chen, Q.-Y.; Yin, Z.-L.; Liu, K. Predominance diagrams for Zn(II)–NH3–Cl––H2O system. Trans. Nonferrous Met. Soc. China 2013, 23, 832–840. [Google Scholar] [CrossRef]
- Yu, Y.; Cheng, W.; Li, Y.; Wang, T.; Xia, Q.; Liu, Y.; Yu, H. Tailored one-pot lignocellulose fractionation to maximize biorefinery toward versatile xylochemicals and nanomaterials. Green Chem. 2022, 24, 3257–3268. [Google Scholar] [CrossRef]
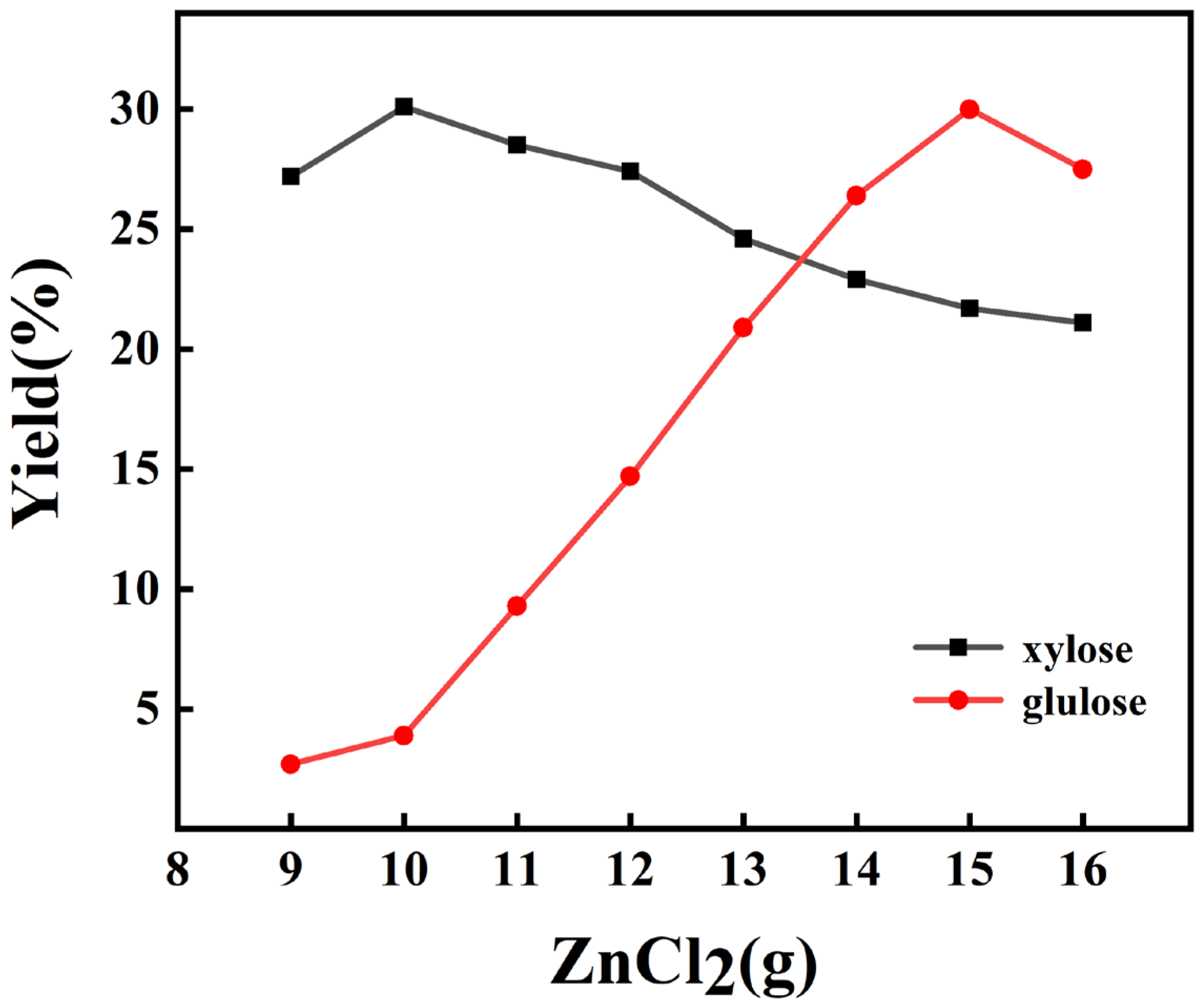



| M(ZnCl2)/g | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
|---|---|---|---|---|---|---|---|---|
| xylose (%) | 27.2 | 30.1 | 28.5 | 27.4 | 24.6 | 22.9 | 21.7 | 21.1 |
| glucose (%) | 2.7 | 3.9 | 9.3 | 14.7 | 20.9 | 26.4 | 30.0 | 27.5 |
| xylose and glucose (%) | 29.9 | 34.0 | 37.8 | 42.1 | 45.5 | 48.3 | 51.7 | 48.6 |
| Temperature/°C | 65 | 75 | 85 | 95 | 105 | 115 |
|---|---|---|---|---|---|---|
| xylose (%) | 18.3 | 20.1 | 28.5 | 30.4 | 29.7 | 24.2 |
| glucose (%) | 1.6 | 1.9 | 2.4 | 3.6 | 3.4 | 2.9 |
| xylose and glucose (%) | 19.9 | 22.0 | 30.9 | 34.0 | 34.1 | 27.1 |
| Temperature/°C | 65 | 75 | 85 | 95 | 105 | 115 |
|---|---|---|---|---|---|---|
| xylose (%) | 3.5 | 3.3 | 2.7 | 2.6 | 2.4 | 2.2 |
| glucose (%) | 6.9 | 19.3 | 25.2 | 29.4 | 28.1 | 22.7 |
| xylose and glucose (%) | 10.4 | 22.6 | 27.9 | 32.0 | 30.5 | 24.9 |
| Time/min | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|
| xylose (%) | 17.5 | 20.2 | 24.3 | 30.2 | 27.9 |
| glucose (%) | 0.7 | 1.8 | 2.6 | 3.9 | 3.8 |
| xylose and glucose (%) | 18.2 | 22.0 | 26.9 | 34.1 | 31.7 |
| Time/min | 4 | 6 | 8 | 10 | 12 |
|---|---|---|---|---|---|
| xylose (%) | 2.2 | 2.4 | 2.7 | 2.6 | 2.4 |
| glucose (%) | 10.1 | 19.3 | 25.2 | 29.4 | 28.1 |
| xylose and glucose (%) | 12.3 | 21.7 | 27.9 | 33.0 | 30.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Duan, Y.; Teng, C.; Quan, H.; Yang, X.; Li, H.; Li, X.; Yan, L. Fast and Selective Degradation of Biomass for Xylose, Glucose and Lignin under Mild Conditions. Molecules 2023, 28, 3306. https://doi.org/10.3390/molecules28083306
Zhang S, Duan Y, Teng C, Quan H, Yang X, Li H, Li X, Yan L. Fast and Selective Degradation of Biomass for Xylose, Glucose and Lignin under Mild Conditions. Molecules. 2023; 28(8):3306. https://doi.org/10.3390/molecules28083306
Chicago/Turabian StyleZhang, Shangzhong, Yi Duan, Changchang Teng, Hongdong Quan, Xiuguo Yang, Hongyan Li, Xiaohe Li, and Lifeng Yan. 2023. "Fast and Selective Degradation of Biomass for Xylose, Glucose and Lignin under Mild Conditions" Molecules 28, no. 8: 3306. https://doi.org/10.3390/molecules28083306
APA StyleZhang, S., Duan, Y., Teng, C., Quan, H., Yang, X., Li, H., Li, X., & Yan, L. (2023). Fast and Selective Degradation of Biomass for Xylose, Glucose and Lignin under Mild Conditions. Molecules, 28(8), 3306. https://doi.org/10.3390/molecules28083306








